Ecotoxicity of Concrete Containing Fine-Recycled Aggregate: Effect on Photosynthetic Pigments, Soil Enzymatic Activity and Carbonation Process
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials
- Strength class I—corresponds to ordinary concrete in strength class C16/20
- Strength class II—corresponds to ordinary concrete in strength class C25/30
- NAC-I, as a reference concrete sample for strength class C16/20
- RMAC-I, as recycled concrete containing RA4, strength class C16/20.
- RCAC-I, as recycled concrete containing RA1, strength class C16/20.
- NAC-II, as a reference concrete sample for the C25/30 strength class
- RMAC-II, as a recycled concrete containing RA4, strength class C25/30
- RCAC-II, as recycled concrete containing RA1, strength class C25/30.
2.2. Methodology
2.3. Ecotoxicology
2.3.1. Chemical and Ecotoxicological Analysis of Leachate
2.3.2. Determination of Photosynthetic Pigments
2.3.3. Soil Enzymatic Test
2.3.4. Statistical Analysis and Data Evaluation
2.4. Carbonation Testing Process
Czech Standard ČSN EN 12390-12 (73 1302)
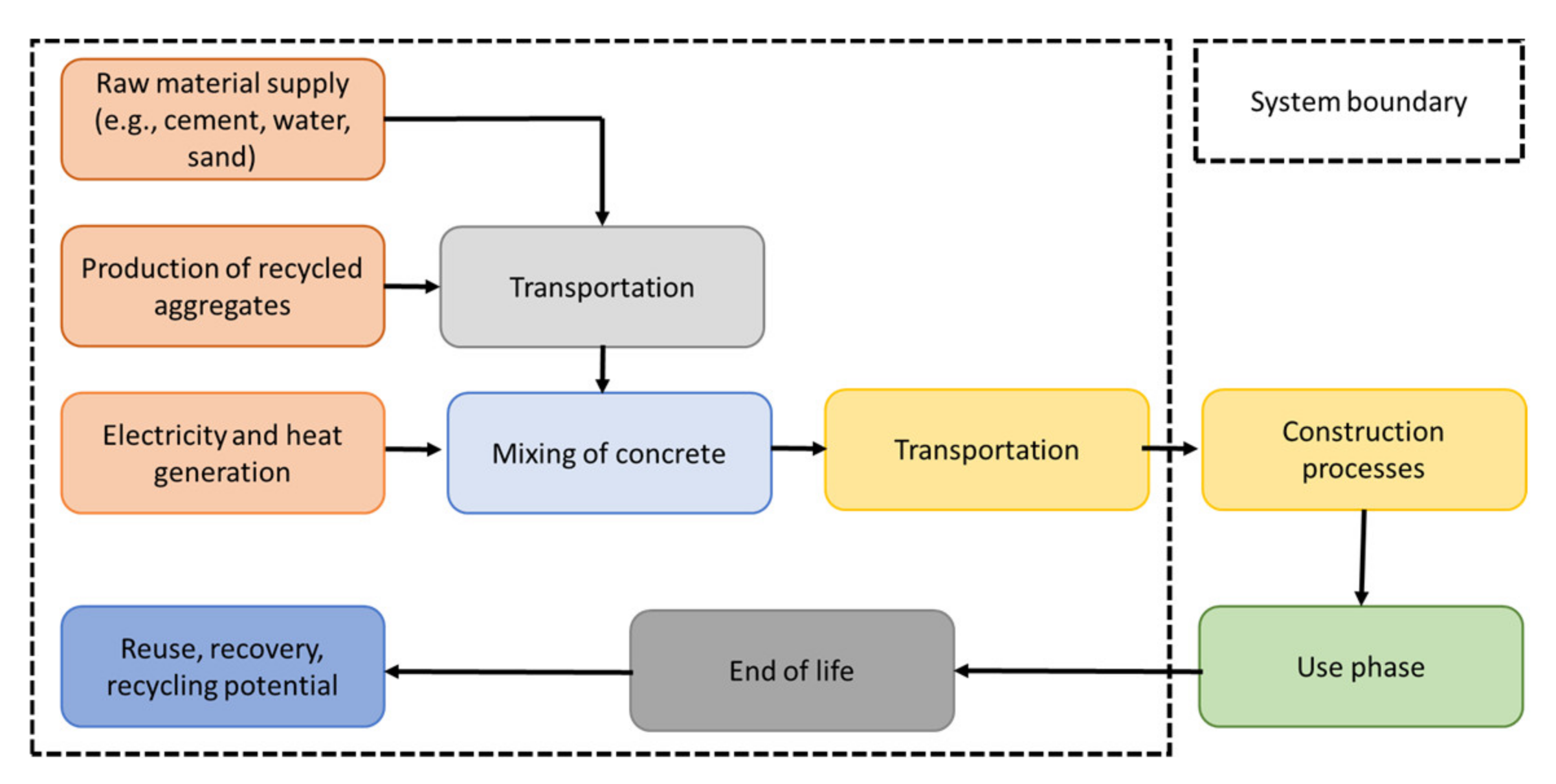
2.5. Life Cycle Assessment
2.5.1. System Boundaries and Functional Unit
2.5.2. Life Cycle Inventory
2.5.3. Influence of Carbonation
2.5.4. Environmental Assessment
2.5.5. Normalization and Weighting
3. Results
3.1. Physicochemical Properties of Concrete Leachates
3.2. Aquatic Ecotoxicity
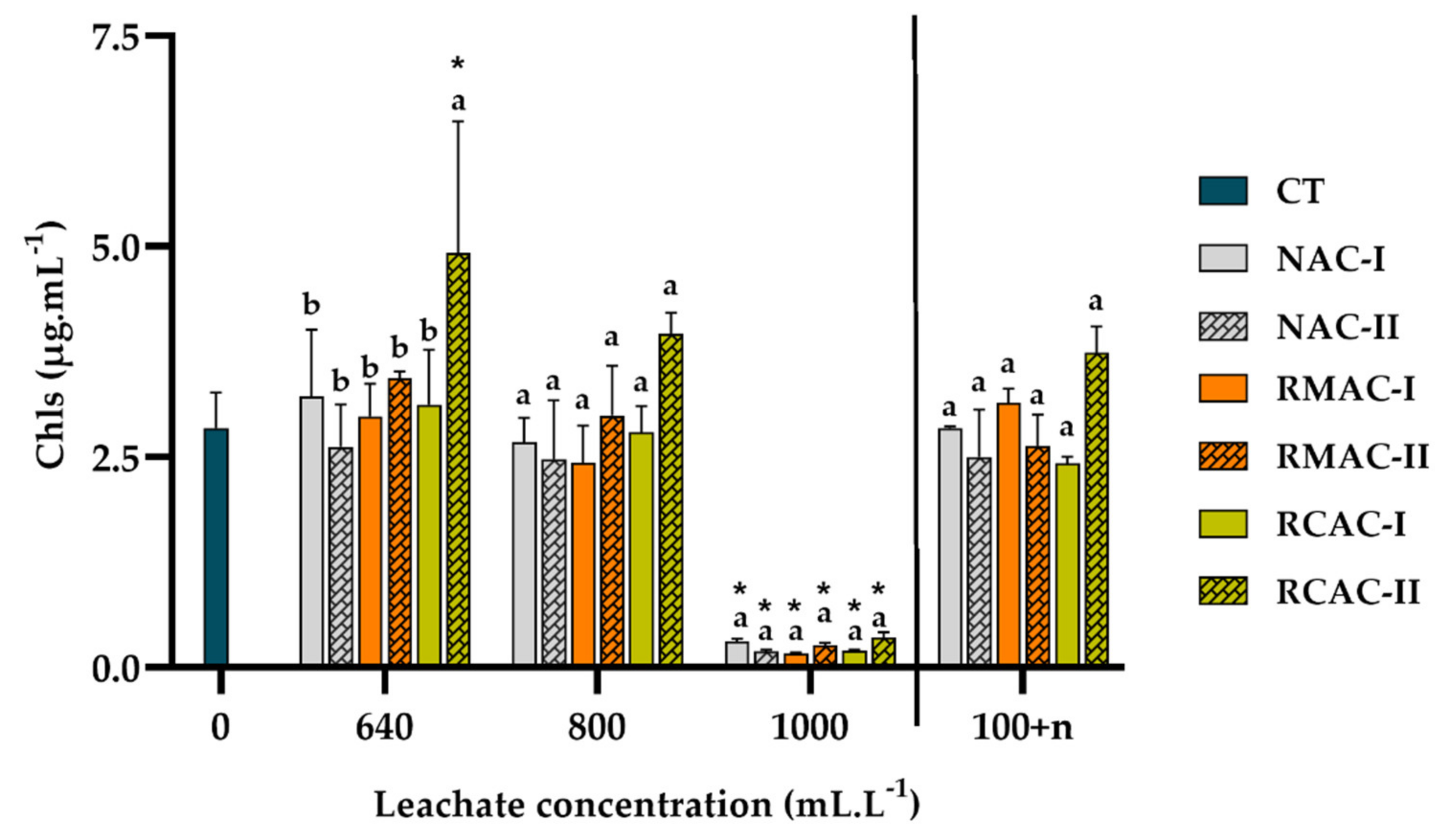
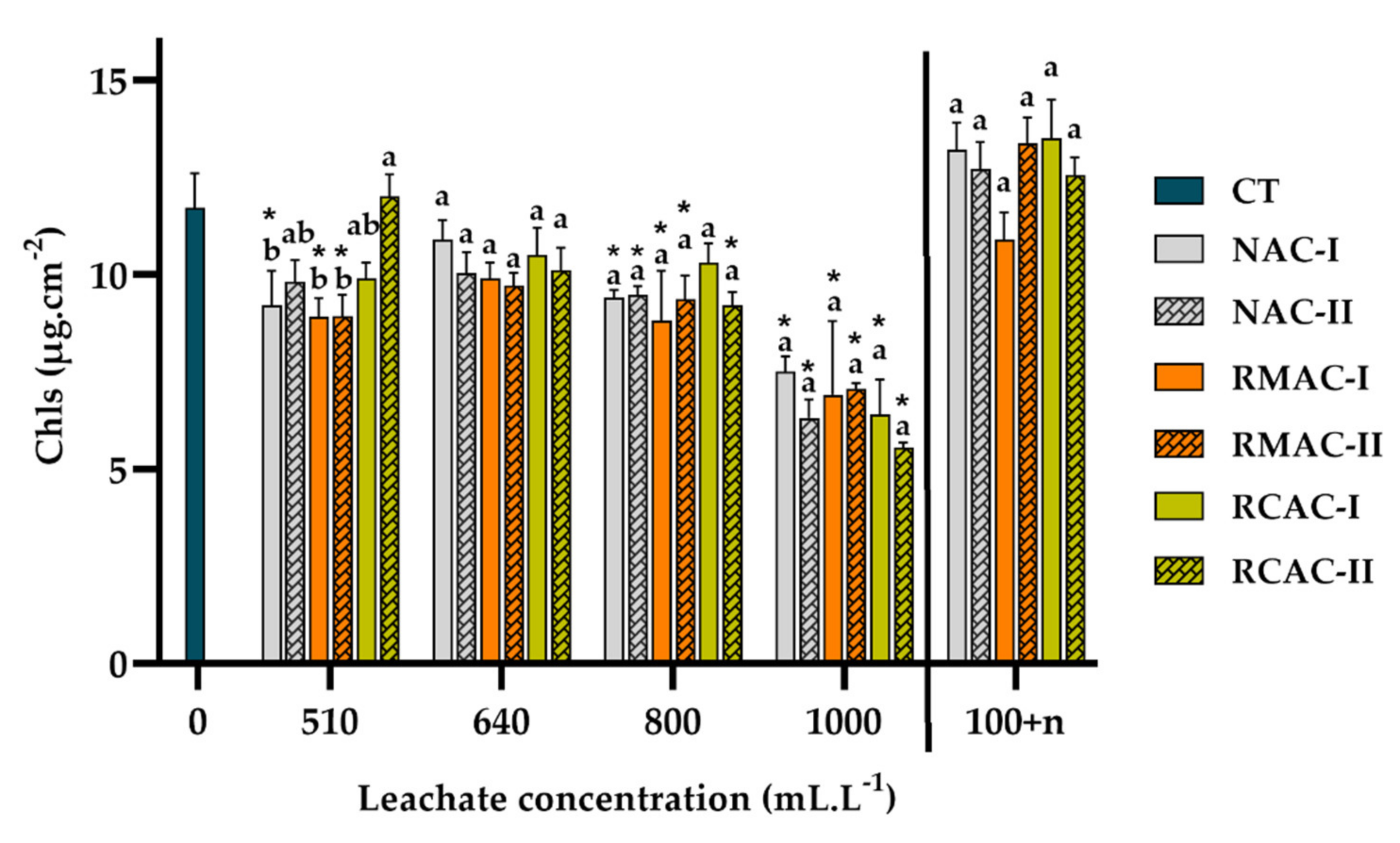
3.3. Photosynthetic Pigments
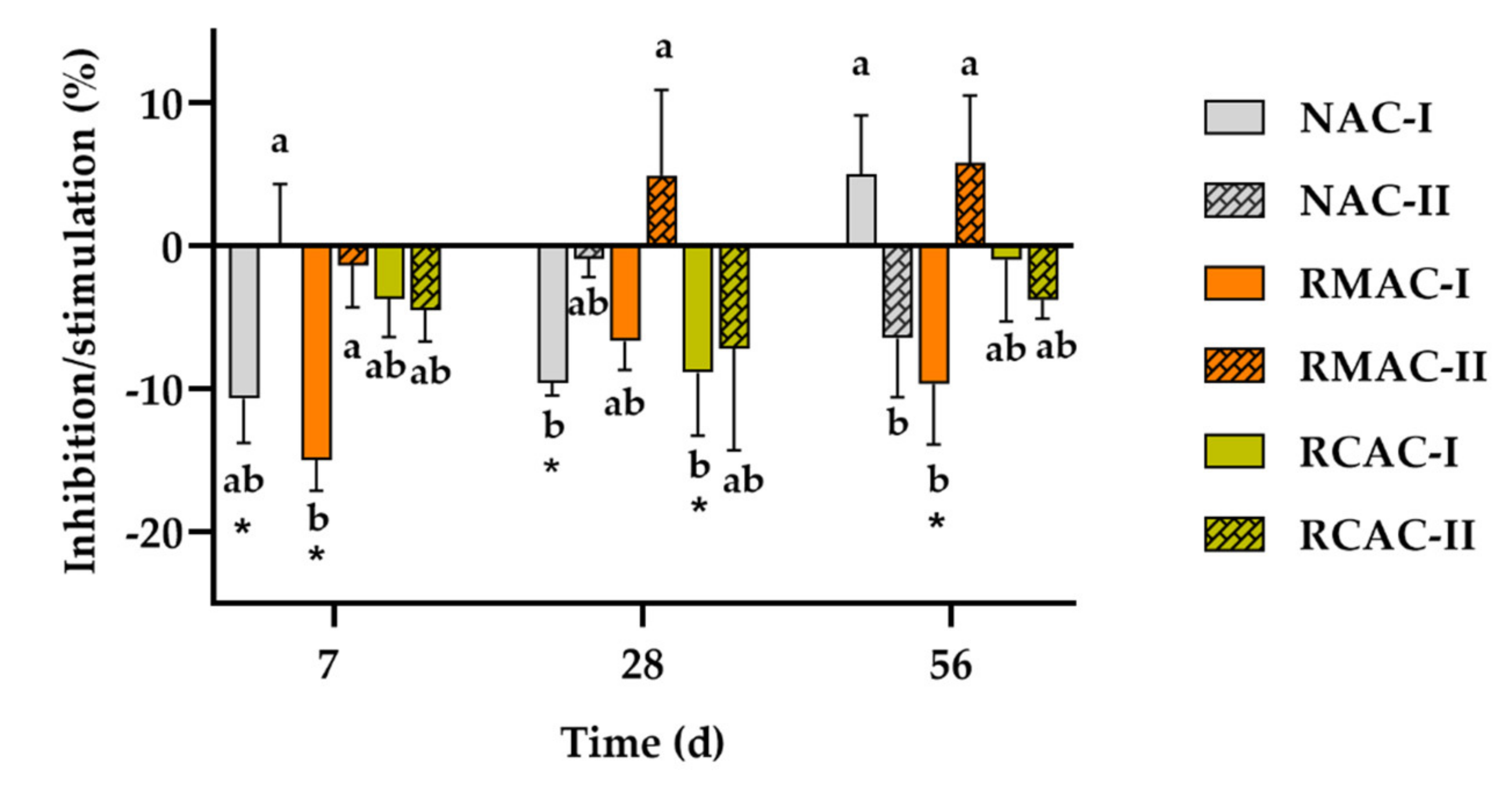
3.4. Soil Dehydrogenase Activity
3.5. Carbonation Effect
3.6. Results of Environmental Assessment
4. Discussion
4.1. Impact of Chemical Composition on Leachate Ecotoxicity
4.2. Selection of Leaching and Ecotoxicity Testing Design
4.3. Photosynthetic Pigment Ratio as Stress Indicators in Aquatic Plants
4.4. Effect of Leachates on Soil Dehydrogenase Activity
4.5. Impact of the Carbonation Process on Concrete
4.6. Environmental Assessment of the Alternative Scenario Considering CO2 Uptake
4.7. Overall Potential Impact on the Environment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vytlacilova, V. Testing ecological suitability for the utilization of recycled aggregates. Green Process. Synth. 2017, 6, 225–234. [Google Scholar] [CrossRef]
- Rodrigues, P.; Silvestre, J.D.; Flores-Colen, I.; Viegas, C.A.; Ahmed, H.H.; Kurda, R.; De Brito, J. Evaluation of the Ecotoxicological Potential of Fly Ash and Recycled Concrete Aggregates Use in Concrete. Appl. Sci. 2020, 10, 351. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, P.; Silvestre, J.D.; Flores-Colen, I.; Viegas, C.A.; De Brito, J.; Kurad, R.; Demertzi, M. Methodology for the Assessment of the Ecotoxicological Potential of Construction Materials. Materials 2017, 10, 649. [Google Scholar] [CrossRef] [Green Version]
- Couvidat, J.; Diliberto, C.; Meux, E.; Cotelle, S.; Bojic, C.; Izoret, L.; Lecomte, A. Greening effect of slag cement-based concrete: Environmental and ecotoxicological impact. Environ. Technol. Innov. 2021, 22, 101467. [Google Scholar] [CrossRef]
- Barbosa, R.; Lapa, N.; Dias, D.; Mendes, B. Concretes containing biomass ashes: Mechanical, chemical, and ecotoxic performances. Constr. Build. Mater. 2013, 48, 457–463. [Google Scholar] [CrossRef]
- Naaz, S.; Pandey, S.N. Effects of industrial waste water on heavy metal accumulation, growth and biochemical responses of lettuce (Lactuca sativa L.). J. Environ. Biol. 2010, 31, 273–276. [Google Scholar]
- Gao, Z.; Han, J.; Mu, C.; Lin, J.; Li, X.; Lin, L.; Sun, S. Effects of Saline and Alkaline Stresses on Growth and Physiological Changes in Oat (Avena sativa L.) Seedlings. Not. Bot. Horti Agrobot. 2014, 42, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Zhou, J.; Hao, W.; Gu, F.; Liu, Q.; Li, H.; Xia, X.; Mao, L. Germination, Growth, Chlorophyll Fluorescence and Ionic Balance in Linseed Seedlings Subjected to Saline and Alkaline Stresses. Plant Prod. Sci. 2014, 17, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Radić, S.; Stipaničev, D.; Cvjetko, P.; Mikelić, I.L.; Rajčić, M.M.; Širac, S.; Pevalek-Kozlina, B.; Pavlica, M. Ecotoxicological assessment of industrial effluent using duckweed (Lemna minor L.) as a test organism. Ecotoxicology 2009, 19, 216–222. [Google Scholar] [CrossRef]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. (Eds.) Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 1996; ISBN 9783642646331. [Google Scholar]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil Enzyme Activities and Biodiversity Measurements as Integrative Microbiological Indicators. In SSSA Special Publications; Wiley: Hoboken, NJ, USA, 2015; pp. 247–271. ISBN 978-0-89118-944-2. [Google Scholar]
- Delreux, T.; Ohler, F. Climate Policy in European Union Politics. In Oxford Research Encyclopedia of Politics; Oxford University Press: Oxford, UK, 2019; ISBN 9780190228637. [Google Scholar]
- Monteiro, P.J.M.; Miller, S.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef]
- GREEN Solution, s.r.o. Green Solution s.r.o. Program Předcházení Vzniku Odpadu; Publisher Ministry of the Environment: Prague, Czech Republic, 2017. [Google Scholar]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M. Experimental investigation and mathematical modeling of the concrete carbonation problem. Chem. Eng. Sci. 1991, 46, 1333–1338. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Assessment of mechanical properties of concrete incorporating carbonated recycled concrete aggregates. Cem. Concr. Compos. 2016, 65, 67–74. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental Modeling and Experimental Investigation of Concrete Carbonation. ACI Mater. J. 1991, 88, 363–373. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Physical and Chemical Characteristics Affecting the Durability of Concrete. ACI Mater. J. 1991, 88, 186–196. [Google Scholar] [CrossRef]
- Parrott, L.; Killoh, D. Carbonation in a 36 year old, in-situ concrete. Cem. Concr. Res. 1989, 19, 649–656. [Google Scholar] [CrossRef]
- Collepardi, M. The Influence of Slag and Fly Ash on the Carbonation of Concrete. In Proceedings of the Eighth CANMET/ACI International Conferance on Fly Ash, Silica Fume, Slag, and Natural Pozzolans in Concrete, Las Vegas, NV, USA, 23–29 May 2004; Volume 2004, pp. 221–229. [Google Scholar]
- Matthews, S. Design of Durable Concrete Structures; BRE Trust: Glasgow, UK, 2014. [Google Scholar] [CrossRef]
- Chang, C.-F.; Chen, J.-W. The experimental investigation of concrete carbonation depth. Cem. Concr. Res. 2006, 36, 1760–1767. [Google Scholar] [CrossRef]
- ČSN EN 12390-12 (73 1302). Testing Hardened Concrete—Part 12: Determination of the Resistance of Concrete to Carbonation—Accelerated Carbonation Method; Office for Technical Standardization, Metrology and State Testing: Prague, Czech Republic, 2020. [Google Scholar]
- Mariaková, D.; Mocová, K.A.; Fořtová, K.; Ryparová, P.; Pešta, J.; Pavlů, T. Ecotoxicity and Essential Properties of Fine-Recycled Aggregate. Materials 2021, 14, 463. [Google Scholar] [CrossRef]
- Mariaková, D.; Mocová, K.A.; Fořtová, K.; Pavlů, T.; Hájek, P. Waste Glass Powder Reusability in High-Performance Concrete: Leaching Behavior and Ecotoxicity. Materials 2021, 14, 4476. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- ISO 10390:2021. Soil, Treated Biowaste and Sludge—Determination of PH; ISO: Geneva, Switzerland, 2021; p. 8. [Google Scholar]
- ISO 23753-1:2005. Soil Quality—Determination of Dehydrogenase Activity in Soils—Part 1: Method Using Triphenyltetrazolium Chloride (TTC); ISO: Geneva, Switzerland, 2005; p. 5. [Google Scholar]
- ČSN EN 12390-2. Testing Hardened Concrete—Part 2: Making and Curing Specimens for Strength Tests; Office for Technical Standardization, Metrology and State Testing: Prague, Czech Republic, 2020. [Google Scholar]
- Guinée, J. Handbook on life cycle assessment—Operational guide to the ISO standards. Int. J. Life Cycle Assess. 2001, 6, 255. [Google Scholar] [CrossRef]
- International Organization for Standardization. Environmental Management. Life Cycle Assessment. Principles and Framework; Standard ISO 14040; International Organization for Standardization: Geneva, Switzerland, 2006; Available online: https://www.iso.org/standard/37456.html (accessed on 27 March 2020).
- International Organization for Standardization. ISO 14040 Environmental Management-Life Cycle Assessment-Principles and Framework. Available online: https://www.iso.org/standard/38498.html (accessed on 13 December 2019).
- CEN EN 15804:2012+A2:2019 Sustainability of Construction Works—Environmental Product Declarations—Core Rules for the Product Category of Construction Products. Available online: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT:70014&cs=1CFE7BDC38149F238F05C4C13E0E3B4C2 (accessed on 27 November 2020).
- Life Cycle Assessment LCA Software: Gabi Software. Available online: http://www.gabisoftware.com/ (accessed on 30 October 2020).
- Kupfer, T.; Baitz, M.; Colodel, C.M.; Kokborg, M.; Schöll, S.; Rudolf, M.; Thellier, L.; Bos, U.; Bosch, F.; Gonzalez, M.; et al. GaBi Database and Modelling Principles. Available online: http://www.gabi-software.com/fileadmin/gabi/Modelling_Principles/Modeling_Principles_-_GaBi_Databases_2020_2.pdf (accessed on 1 December 2020).
- ČSN EN 16757. Sustainability of Construction Works—Environmental Product Declarations—Product Category Rules for Concrete and Concrete Elements; Office for Technical Standardization, Metrology and State Testing: Prague, Czech Republic, 2018. [Google Scholar]
- Product Environmental Footprint (PEF) Guide. Available online: https://ec.europa.eu/environment/archives/eussd/pdf/footprint/PEF%20methodology%20final%20draft.pdf (accessed on 7 October 2020).
- Environmental Footprint 3.0. Available online: https://eplca.jrc.ec.europa.eu//EnvironmentalFootprint.html (accessed on 1 November 2021).
- Matoušek, M.; Drochytka, R. Atmosférická Koroze Betonu; ČKAIT a IKAS: Prague, Czech Republic, 1998. [Google Scholar]
- Teplý, B.; Chromá, M.; Rovnanik, P. Durability assessment of concrete structures: Reinforcement depassivation due to carbonation. Struct. Infrastruct. Eng. 2010, 6, 317–327. [Google Scholar] [CrossRef]
- Pašek, J.; Vejvara, L. Karbonatace Betonových a Železobetonových Obvodových Konstrukcí. In Proceedings of the Poruchy a Obnova Obalových Konštrukcií Budov, Vysoké Tatry, Slovakia, 4 April 2004; pp. 86–91. [Google Scholar]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Carbonation of concrete made with high amount of fly ash and recycled concrete aggregates for utilization of CO2. J. CO2 Util. 2018, 29, 12–19. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Cao, Z.; Guo, M.; Zheng, J. Effect of carbonated coarse recycled concrete aggregate on the properties and microstructure of recycled concrete. J. Clean. Prod. 2019, 233, 421–428. [Google Scholar] [CrossRef]
- Liang, C.; Lu, N.; Ma, H.; Ma, Z.; Duan, Z. Carbonation behavior of recycled concrete with CO2-curing recycled aggregate under various environments. J. CO2 Util. 2020, 39, 101185. [Google Scholar] [CrossRef]
- Bold, H.C. The Morphology of Chlamydomonas chlamydogama, Sp. Nov. Bull. Torrey Bot. Club 1949, 76, 101. [Google Scholar] [CrossRef]
- Ebert, D.; Zschokke-Rohringer, C.D.; Carius, H.J. Within- and between-population variation for resistance of Daphnia magna to the bacterial endoparasite Pasteuria ramosa. Proc. R. Soc. B Boil. Sci. 1998, 265, 2127–2134. [Google Scholar] [CrossRef]
- ISO (International Organisation for Standardization). 2005 ISO 20079 Water Quality—Determination of Toxic Effect of Water Constituents and Waste to Duckweed (Lemna Minor)—Duckweed Growth Inhibition Test; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- Mor, S.; Ravindra, K.; Dahiya, R.P.; Chandra, A. Leachate Characterization and Assessment of Groundwater Pollution Near Municipal Solid Waste Landfill Site. Environ. Monit. Assess. 2006, 118, 435–456. [Google Scholar] [CrossRef]
- Vriens, B.; Voegelin, A.; Hug, S.J.; Kaegi, R.; Winkel, L.H.E.; Buser, A.M.; Berg, M. Quantification of Element Fluxes in Wastewaters: A Nationwide Survey in Switzerland. Environ. Sci. Technol. 2017, 51, 10943–10953. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Cañedo-Argüelles, M.; Kefford, B.J.; Piscart, C.; Prat, N.; Schäfer, R.B.; Schulz, C.-J. Salinisation of rivers: An urgent ecological issue. Environ. Pollut. 2013, 173, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.S.; Cañedo-Argüelles, M.; Hintz, W.D.; Dyack, B.; Birk, S.; Relyea, R.A. Regulations are needed to protect freshwater ecosystems from salinization. Philos. Trans. R. Soc. B Biol. Sci. 2018, 374, 20180019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baberschke, N.; Irob, K.; Preuer, T.; Meinelt, T.; Kloas, W. Potash mining effluents and ion imbalances cause transient osmoregulatory stress, affect gill integrity and elevate chronically plasma sulfate levels in adult common roach, Rutilus rutilus. Environ. Pollut. 2019, 249, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.C.; Entrekin, S.A.; Evans-White, M.A.; Clay, N.A. Salty water and salty leaf litter alters riparian detrital processes: Evidence from sodium-addition laboratory mesocosm experiments. Sci. Total Environ. 2021, 806, 151392. [Google Scholar] [CrossRef]
- Bandow, N.; Gartiser, S.; Ilvonen, O.; Schoknecht, U. Evaluation of the impact of construction products on the environment by leaching of possibly hazardous substances. Environ. Sci. Eur. 2018, 30, 14. [Google Scholar] [CrossRef] [Green Version]
- Heisterkamp, I.; Ratte, M.; Schoknecht, U.; Gartiser, S.; Kalbe, U.; Ilvonen, O. Ecotoxicological evaluation of construction products: Inter-laboratory test with DSLT and percolation test eluates in an aquatic biotest battery. Environ. Sci. Eur. 2021, 33, 1–14. [Google Scholar] [CrossRef]
- Choi, J.B.; Bae, S.M.; Shin, T.Y.; Ahn, K.Y.; Woo, S.D. Evaluation of Daphniamagna for the Ecotoxicity Assessment of Alkali Leachate from Concrete. Int. J. Ind. Èntomol. 2013, 26, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Jalal, K.; Shamsuddin, A.; Rahman, M.; Nurzatul, N.; Rozihan, M. Growth and Total Carotenoid, Chlorophyll a and Chlorophyll b of Tropical Microalgae (Isochrysis sp.) in Laboratory Cultured Conditions. J. Biol. Sci. 2012, 13, 10–17. [Google Scholar] [CrossRef] [Green Version]
- ISO 11348 (2007–12). Water Quality—Determination of the Inhibitory Efect of Water Samples on the Light Emission of Vibrio Fscheri (Luminescent Bacteria Test)—Part 1: Method Using Freshly Prepared Bacteria, Part 2: Method Using Liquid-Dried Bacteria, Part 3: Method Using Freeze-Dried Bacteria; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Ladislas, S.; El-Mufleh, A.; Gérente, C.; Chazarenc, F.; Andrès, Y.; Béchet, B. Potential of Aquatic Macrophytes as Bioindicators of Heavy Metal Pollution in Urban Stormwater Runoff. Water Air Soil Pollut. 2011, 223, 877–888. [Google Scholar] [CrossRef]
- Leblebici, Z.; Aksoy, A.; Duman, F. Influence of nutrient addition on growth and accumulation of cadmium and copper in Lemna gibba. Chem. Speciat. Bioavailab. 2010, 22, 157–164. [Google Scholar] [CrossRef]
- Varga, M.; Horvatić, J.; Čelić, A. Short term exposure of Lemna minor and Lemna gibba to mercury, cadmium and chromium. Open Life Sci. 2013, 8, 1083–1093. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Kabiel, H.F.; Fawzy, M. Duckweed as heavy metal accumulator and pollution indicator in industrial wastewater ponds. Desalination Water Treat. 2009, 12, 400–406. [Google Scholar] [CrossRef]
- Thomas, G.; Stärk, H.-J.; Wellenreuther, G.; Dickinson, B.C.; Küpper, H. Effects of nanomolar copper on water plants—Comparison of biochemical and biophysical mechanisms of deficiency and sublethal toxicity under environmentally relevant conditions. Aquat. Toxicol. 2013, 140-141, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO. ISO In Water Quality—Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae; ISO: Geneva, Switzerland, 2012; p. 21. [Google Scholar]
- OECD. OECD Test No. 201: Alga, Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems; OECD Publishing: Paris, France, 2006; ISBN 978-92-64-06992-3. [Google Scholar]
- ISO 10260:1992. Water Quality—Measurement of Biochemical Parameters—Spectrometric Determination of the Chlorophyll-a Concentration; ISO: Geneva, Switzerland, 1992; p. 6. [Google Scholar]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments Content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of Different Commercial Dried Algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Ivashchenko, K.V.; Sushko, S.V. Microbial Indicators of Urban Soils and Their Role in the Assessment of Ecosystem Services: A Review. Eurasian Soil Sci. 2021, 54, 1517–1531. [Google Scholar] [CrossRef]
- Goodwin, D. Urban soils and functional trees. In The Urban Tree; Taylor and Francis Group: London, UK, 2017; pp. 56–89. [Google Scholar] [CrossRef]
- Qi, D.; Wieneke, X.; Tao, J.; Zhou, X.; DeSilva, U. Soil pH Is the Primary Factor Correlating with Soil Microbiome in Karst Rocky Desertification Regions in the Wushan County, Chongqing, China. Front. Microbiol. 2018, 9, 1027. [Google Scholar] [CrossRef] [Green Version]
- Kuwano, B.H.; Nogueira, M.A.; Santos, C.A.; Fagotti, D.S.; Santos, M.B.; Lescano, L.E.; Andrade, D.S.; Barbosa, G.M.; Tavares-Filho, J. Application of Landfill Leachate Improves Wheat Nutrition and Yield but Has Minor Effects on Soil Properties. J. Environ. Qual. 2017, 46, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Shailaja, G.S.J.; Srinivas, N.; Rao, P.P.V.V. Effect of Municipal Solid Waste Leachate on Soil Enzymes. Nat. Environ. Pollut. Technol. 2021, 20. [Google Scholar] [CrossRef]
- Kazlauskaitė-Jadzevičė, A.; Volungevičius, J.; Gregorauskienė, V.; Marcinkonis, S. The role of PH in heavy metal contamination of urban soil. J. Environ. Eng. Landsc. Manag. 2014, 22, 311–318. [Google Scholar] [CrossRef]
- Gholizadeh-Vayghan, A.; Bellinkx, A.; Snellings, R.; Vandoren, B.; Quaghebeur, M. The effects of carbonation conditions on the physical and microstructural properties of recycled concrete coarse aggregates. Constr. Build. Mater. 2020, 257, 119486. [Google Scholar] [CrossRef]
- Ho, D.W.S.; Lewis, R.K. Carbonation of concrete and its prediction. Cem. Concr. Res. 1987, 17, 489–504. [Google Scholar] [CrossRef]
- Malati, M.A. Experimental Inorganic/Physical Chemistry; Woodhead Publishing Limited, Abington Hall: Cambridge, UK, 1999; p. 338. [Google Scholar]
- Bernal, S.A.; San Nicolas, R.; Myers, R.J.; Mejía de Gutiérrez, R.; Puertas, F.; van Deventer, J.S.J.; Provis, J.L. MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem. Concr. Res. 2014, 57, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 systems: The management of reaction products and designing of precursors. Mater. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kuroda, Y. Carbon Dioxide Uptake in Demolished and Crushed Concrete. J. Adv. Concr. Technol. 2011, 9, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Dodoo, A.; Gustavsson, L.; Sathre, R. Carbon implications of end-of-life management of building materials. Resour. Conserv. Recycl. 2009, 53, 276–286. [Google Scholar] [CrossRef]
- Yang, K.-H.; Seo, E.-A.; Tae, S.-H. Carbonation and CO2 uptake of concrete. Environ. Impact Assess. Rev. 2014, 46, 43–52. [Google Scholar] [CrossRef]
- Marinković, S.; Dragaš, J.; Ignjatović, I.; Tošić, N. Environmental assessment of green concretes for structural use. J. Clean. Prod. 2017, 154, 633–649. [Google Scholar] [CrossRef]
- Colangelo, F.; Navarro, T.G.; Farina, I.; Petrillo, A. Comparative LCA of concrete with recycled aggregates: A circular economy mindset in Europe. Int. J. Life Cycle Assess. 2020, 25, 1790–1804. [Google Scholar] [CrossRef]
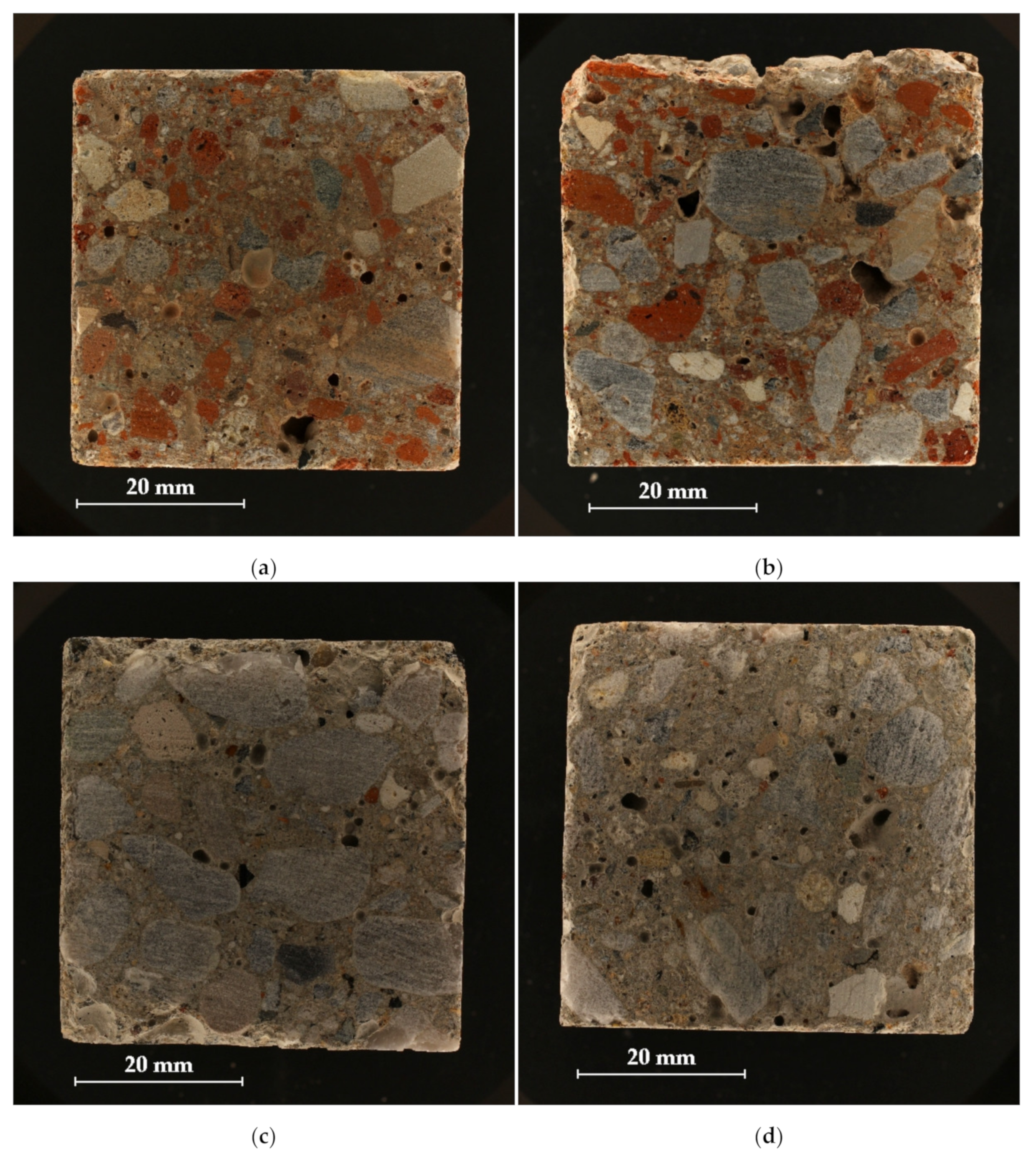





| Material (kg) | I | II | ||||
|---|---|---|---|---|---|---|
| NAC | RMAC | RCAC | NAC | RMAC | RCAC | |
| Cement | 260 | 260 | 260 | 300 | 300 | 300 |
| Nature Sand | 709 | - | - | 671 | - | - |
| Gravel 4/8 | 38 | - | - | 28 | - | - |
| Gravel 8/16 | 1092 | 766 | 949 | 1139 | 822 | 994 |
| Recycled Aggregate 0/4 | - | 971 | 843 | - | 920 | 800 |
| Water | 169 | 187 | 186 | 165 | 182 | 181 |
| Element (mg.L−1) | I | II | ||||
|---|---|---|---|---|---|---|
| NAC | RMAC | RCAC | NAC | RMAC | RCAC | |
| Na | 3.04 ± 0.05 | 3.96 ± 0.09 | <2.5 | <2.5 | 4.16 ± 0.17 | <2.5 |
| Mg | 0.20 ± 0.01 | 0.29 ± 0.01 | 0.24 ± 0.01 | 0.18 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.01 |
| Al | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| K | 17.27 ± 0.32 | 15.66 ± 0.63 | 14.20 ± 0.46 | 14.03 ± 0.49 | 19.19 ± 0.32 | 12.82 ± 0.11 |
| Ca | 28.85 ± 0.23 | 29.44 ± 0.95 | 24.68 ± 0.49 | 21.19 ± 0.59 | 19.75 ± 0.33 | 22.13 ± 0.49 |
| Cr 1 | <0.5 | 0 | 0 | 0 | 0 | 0 |
| Fe | ~0.04 | ~0.08 | ~0.03 | ~0.02 | ~0.04 | ~0.02 |
| Zn 2 | ~0.008 | 0.182 ± 0.007 | 0.016 ± 0.001 | 0.033 ± 0.001 | 0.055 ± 0.002 | 0.015 ± 0.001 |
| Sr | <0.03 | ~0.08 | ~0.03 | ~0.03 | ~0.03 | ~0.03 |
| pH | 10.7 ± 0.1 | 10.6 ± 0.1 | 10.7 ± 0.1 | 10.6 ± 0 | 10.6 ± 0.1 | 10.5 ± 0.1 |
| el. conductivity (µS.cm−2) | 225 ± 12 | 232 ± 24 | 191 ± 11 | 183 ± 6 | 211 ± 38 | 162 ± 17 |
| Concrete Mix | Value | Water Flea | Algae GR | Duckweed GR | Toxicity Level |
|---|---|---|---|---|---|
| NAC I | EC50 | 931 | ˃1000 | 870 | |
| CI 95% | 890–n.c. | - | 833–912 | ||
| R2 | 0.89 | 0.80 | 0.94 | ||
| NOEC | 800 | 800 | 640 | ||
| TC | NT-1 | NT-1 | NT-2 | Non-toxic | |
| RMAC I | EC50 | 929 | ˃1000 | 896 | |
| CI 95% | 894–n.c. | - | 838–966 | ||
| R2 | 0.96 | - | 0.86 | ||
| NOEC | 800 | 800 | 640 | ||
| TC | NT-1 | NT-1 | NT-2 | Non-toxic | |
| RCAC I | EC50 | ˃1000 | ˃1000 | 911 | |
| CI 95% | n.c. | - | 864–971 | ||
| R2 | 0.69 | 0.77 | 0.92 | ||
| NOEC | 800 | 800 | 640 | ||
| TC | NT-1 | NT-1 | NT-2 | Non-toxic | |
| NAC II | EC50 | ˃1000 | ˃1000 | 844 | |
| CI 95% | - | - | 829–861 | ||
| R2 | 0.11 | 0.82 | 0.99 | ||
| NOEC | 640 | 800 | 510 | ||
| TC | NT-2 | NT-1 | NT-2 | Non-toxic | |
| RMAC II | EC50 | 992 | ˃1000 | 926 | |
| CI 95% | 976–n.c. | n.c. | 909–943 | ||
| R2 | 0.94 | 0.76 | 0.99 | ||
| NOEC | 800 | 800 | 640 | ||
| TC | NT-1 | NT-1 | NT-2 | Non-toxic | |
| RCAC II | EC50 | ˃1000 | ˃1000 | 928 | |
| CI 95% | - | - | 895–966 | ||
| R2 | 0.65 | 0.77 | 0.95 | ||
| NOEC | 800 | 800 | 640 | ||
| TC | NT-1 | NT-1 | NT-2 | Non-toxic |
| mL.L−1 | I | II | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAC | RMAC | RCAC | NAC | RMAC | RCAC | |||||||
| 0 | A | 5.6 ± 0.4 | A | 5.6 ± 0.4 | A | 5.6 ± 0.4 | A | 5.6 ± 0.4 | A | 5.6 ± 0.4 | A | 5.6 ± 0.4 |
| 640 | A | 5.5 ± 0.1 a | A | 6.0 ± 0.2 a | A | 5.4 ± 0.2 a | A | 6.2 ± 0.1 a | A | 5.4 ± 0.7 a | A | 6.3 ± 0.1 a |
| 800 | A | 5.1 ± 0.2 a | A | 5.8 ± 0.8 a | A | 5.1 ± 0.2 a | A | 5.8 ± 0.3 a | A | 5.6 ± 0.1 a | A | 5.9 ± 0.1 a |
| 1000 | B | 1.9 ± 0.1 a | B | 1.7 ± 0.2 a | B | 1.8 ± 0.1 a | B | 2.0 ± 0.1 a | B | 1.9 ± 0.2 a | B | 1.8 ± 0.2 a |
| 100 + n | A | 5.4 ± 0.2 a | A | 5.7 ± 0.2 a | A | 5.2 ± 0.0 a | A | 5.9 ± 0.1 a | A | 5.5 ± 0.1 a | A | 5.9 ± 0.1 a |
| mL.L−1 | I | II | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAC | RMAC | RCAC | NAC | RMAC | RCAC | |||||||
| 0 | A | 7.9 ± 0.2 | A | 7.9 ± 0.2 | A | 7.9 ± 0.2 | A | 7.9 ± 0.2 | A | 7.9 ± 0.2 | A | 7.9 ± 0.2 |
| 510 | A | 7.0 ± 0.3 a | A | 7.0 ± 0.5 a | A | 7.6 ± 0.6 a | A | 7.2 ± 0.2 a | A | 7.0 ± 0.3 a | A | 8.1 ± 0.4 a |
| 640 | A | 7.9 ± 1.0 a | A | 7.4 ± 0.3 a | A | 7.7 ± 0.5 a | A | 7.1 ± 0.3 a | A | 7.1 ± 0.4 a | A | 7.0 ± 0.3 a |
| 800 | A | 6.9 ± 1.0 a | A | 7.0 ± 0.9 a | B | 5.1 ± 0.1 b | B | 4.6 ± 0.2 b | B | 4.4 ± 0.1 b | B | 4.4 ± 0.1 b |
| 1000 | B | 1.5 ± 0.1 b | B | 3.8 ± 0.5 a | C | 3.9 ± 0.4 a | B | 3.2 ± 0.7 a | B | 3.8 ± 0.1 a | C | 2.8 ± 0.2 ab |
| 100 + n | A | 8.2 ± 0.1 a | A | 7.7 ± 0.2 a | A | 8.6 ± 0.3 a | A | 8.1 ± 0.3 a | A | 8.3 ± 0.3 a | A | 8.1 ± 0.1 a |
| Mean Carbonation Depth (mm) | I | II | ||||
|---|---|---|---|---|---|---|
| NAC | RMAC | RCAC | NAC | RMAC | RCAC | |
| d1 | 2.99 | 12.69 | 9.66 | 2.50 | 1.18 | 2.56 |
| d2 | 6.82 | 8.41 | 7.99 | 5.25 | 3.74 | 1.87 |
| d3 | 3.66 | 7.35 | 6.27 | 0.34 | 3.37 | 4.10 |
| d4 | 4.17 | 11.73 | 12.14 | 2.50 | 6.25 | 1.30 |
| dk | 4.40 ± 1.45 | 10.04 ± 2.22 | 9.01 ± 2.16 | 2.65 ± 1.74 | 3.37 ± 1.79 | 2.45 ± 1.05 |
| I | II | |||||
|---|---|---|---|---|---|---|
| NAC | RMAC | RCAC | NAC | RMAC | RCA | |
| Acidification (Mole of H+ eq.) | 9.96 × 10−1 | 8.99 × 10−1 | 8.64 × 10−1 | 1.06 | 9.66 × 10−1 | 9.34 × 10−1 |
| Climate Change—total (kg CO2 eq.) | 3.21 × 102 | 2.59 × 102 | 2.21 × 102 | 3.54 × 102 | 2.95 × 102 | 2.59 × 102 |
| Climate Change, biogenic (kg CO2 eq.) | 3.62 × 10−1 | 3.18 × 10−1 | 3.69 × 10−1 | 3.88 × 10−1 | 3.47 × 10−1 | 3.95 × 10−1 |
| Climate Change, fossil (kg CO2 eq.) | 3.20 × 102 | 2.58 × 102 | 2.20 × 102 | 3.53 × 102 | 2.94 × 102 | 2.58 × 102 |
| Climate Change, LULUC (kg CO2 eq.) | 6.12 × 10−1 | 6.35 × 10−1 | 6.95 × 10−1 | 6.29 × 10−1 | 6.51 × 10−1 | 7.09 × 10−1 |
| Ecotoxicity, freshwater—total (CTUe) | 1.71 × 103 | 1.39 × 103 | 1.57 × 103 | 1.77 × 103 | 1.47 × 103 | 1.65 × 103 |
| Eutrophication, freshwater (kg P eq.) | 1.07 × 10−3 | 7.31 × 10−4 | 8.75 × 10−4 | 1.12 × 10−3 | 7.91 × 10−4 | 9.28 × 10−4 |
| Eutrophication, marine (kg N eq.) | 3.42 × 10−1 | 3.25 × 10−1 | 3.30 × 10−1 | 3.59 × 10−1 | 3.43 × 10−1 | 3.48 × 10−1 |
| Eutrophication, terrestrial (Mole of N eq.) | 3.76 | 3.59 | 3.66 | 3.95 | 3.78 | 3.85 |
| Human toxicity, cancer—total (CTUh) | 9.03 × 10−8 | 4.41 × 10−8 | 2.19 × 10−8 | 9.24 × 10−8 | 4.87 × 10−8 | 2.77 × 10−8 |
| Human toxicity, non-cancer—total (CTUh) | 6.75 × 10−6 | 5.62 × 10−6 | 5.31 × 10−6 | 7.12 × 10−6 | 6.05 × 10−6 | 5.76 × 10−6 |
| Ionising rad., human health (kBq U235 eq.) | 6.49 | 4.83 | 6.29 | 6.99 | 5.41 | 6.80 |
| Land use (Pt) | 5.57 × 102 | 4.66 × 102 | 5.30 × 102 | 5.88 × 102 | 5.01 × 102 | 5.63 × 102 |
| Ozone depletion (kg CFC-11 eq.) | 3.73 × 10−7 | 2.53 × 10−7 | 3.13 × 10−7 | 3.85 × 10−7 | 2.71 × 10−7 | 3.28 × 10−7 |
| Particulate matter (Disease incidences) | 1.12 × 10−5 | 6.37 × 10−6 | 5.74 × 10−6 | 1.20 × 10−5 | 7.43 × 10−6 | 6.83 × 10−6 |
| Photochem. ozone form., hum. health (kg NMVOC eq.) | 8.52 × 10−1 | 7.98 × 10−1 | 7.80 × 10−1 | 9.02 × 10−1 | 8.51 × 10−1 | 8.35 × 10−1 |
| Resource use, fossils (MJ) | 2.08 × 103 | 1.31 × 103 | 9.71 × 102 | 2.17 × 103 | 1.44 × 103 | 1.12 × 103 |
| Resource use, mineral and metals (kg Sb eq.) | 3.05 × 10−5 | 6.43 × 10−5 | 1.81 × 10−4 | 3.30 × 10−5 | 5.68 × 10−5 | 1.67 × 10−4 |
| Water use (m3 world equiv.) | 1.27 × 103 | 7.67 × 102 | 8.40 × 102 | 1.31 × 103 | 8.35 × 102 | 9.03 × 102 |
| NAC I | RMAC I | RCAC I | NAC II | RMAC II | RCAC II | |
|---|---|---|---|---|---|---|
| Total CO2 potential uptake (kg CO2 per cube) | 4.21 | 4.21 | 4.21 | 3.53 | 3.53 | 3.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariaková, D.; Mocová, K.A.; Pešta, J.; Fořtová, K.; Tripathi, B.; Pavlů, T.; Hájek, P. Ecotoxicity of Concrete Containing Fine-Recycled Aggregate: Effect on Photosynthetic Pigments, Soil Enzymatic Activity and Carbonation Process. Sustainability 2022, 14, 1732. https://doi.org/10.3390/su14031732
Mariaková D, Mocová KA, Pešta J, Fořtová K, Tripathi B, Pavlů T, Hájek P. Ecotoxicity of Concrete Containing Fine-Recycled Aggregate: Effect on Photosynthetic Pigments, Soil Enzymatic Activity and Carbonation Process. Sustainability. 2022; 14(3):1732. https://doi.org/10.3390/su14031732
Chicago/Turabian StyleMariaková, Diana, Klára Anna Mocová, Jan Pešta, Kristina Fořtová, Bhavna Tripathi, Tereza Pavlů, and Petr Hájek. 2022. "Ecotoxicity of Concrete Containing Fine-Recycled Aggregate: Effect on Photosynthetic Pigments, Soil Enzymatic Activity and Carbonation Process" Sustainability 14, no. 3: 1732. https://doi.org/10.3390/su14031732
APA StyleMariaková, D., Mocová, K. A., Pešta, J., Fořtová, K., Tripathi, B., Pavlů, T., & Hájek, P. (2022). Ecotoxicity of Concrete Containing Fine-Recycled Aggregate: Effect on Photosynthetic Pigments, Soil Enzymatic Activity and Carbonation Process. Sustainability, 14(3), 1732. https://doi.org/10.3390/su14031732








