Abstract
In this work, a fuzzy logic model was developed to elucidate the extraction performance of high-pressure CO2 + H2O compared with traditional H2O extraction and aqueous ethanol extraction. The high-pressure CO2 + H2O group acquired the highest comprehensive score considering yield, quality and stability. Both targeted and untargeted metabolomics results proved that the polarity of water was slightly modified; in particular, with the evidence from the untargeted metabolomics data, a higher proportion of water-insoluble compounds (2-methylindole, 3-formylindole, guanine, tyrosine and tryptophan) obtained by high-pressure CO2 + H2O extraction compared with traditional H2O extraction has been reported for the first time. Finally, the “3I” extraction mechanism of high-pressure CO2 + H2O is proposed, which offers an improvement in the solid–liquid mass transfer efficiency of phytochemicals, improving the polarity of solution and the isolation of O2.
1. Introduction
The addition of colorants can improve the appearance of products and attract consumers to buy [1]. Because of their bright color, strong coloring power and good stability, synthetic colorants are widely used in various food, cosmetics and other products [2]. The fatal disadvantage of synthetic colorant is its toxicity to humans [3]; previous studies have found that even if the intake dose of some colorants is low, long-term intake still carries a risk of teratogenicity, carcinogenesis and genotoxicityon in the human population [4,5]. Currently, natural colorants obtained from various animals and plants are being discussed and explored for their physiological activities to alleviate harm caused by synthetic colorants [6,7].
Most natural colorants come from plants and have the characteristics of non-toxic side effects and high safety [8,9]. Anthocyanins are listed as a natural colorant for food adopted by the Codex Alimentarius Commission and approved by the European Union with the E code E163 (EC/2003/822) [10]. The global anthocyanins market is growing at a CAGR of 4.5% for the forecast period 2020–2025 (https://www.mordorintelligence.com/industry-reports/anthocyanin-market, accessed on 15 September 2021). It concerns the polyphenolic phytochemiscals belonging to the group of flavonoids, and these can give different colors, such as red, purple and blue [11]. They are soluble in water, and most are organic solvents but are not soluble in a polar organic solvent and not stable in alkaline or neutral solutions [12]. Anthocyanins are good alternatives to synthetic food dyes as they have health-promoting effects in the prevention of cancer, cardiovascular diseases, neurodegenerative diseases, obesity and diabetes [13,14].
At present, the industrial production of anthocyanins largely relies on H2O or aqueous ethanol extraction and purification from anthocyanin-rich plants. The solvents most commonly used are acidified aqueous solutions [15]. Plenty of studies have shown that aqueous ethanol achieves a higher degree of extraction of anthocyanins than pure water or ethanol extraction but a lower stability of the anthocyanins extracted [11] because aqueous ethanol leads to the higher reactivity of anthocyanins, which lowers the lowest unoccupied molecular orbital energy of anthocyanins and accelerates the nucleophilic attack from water [12]. On the other hand, safety risks and less environmentally friendly processes have strongly stimulated the interest in developing green (environment-friendly) extraction technologies [14].
Water is frequently used as a green solvent for hydrophilic phytochemicals’ extraction, especially when combined with some assisted extraction techniques, such as microwave [16], ultrasound, pulsed electric field [17], high hydrostatic pressure (HHP) [18] and so on. High-pressure CO2 is one promising novel non-thermal technique (its operating pressure does not exceed 50 MPa, and its temperature is below pasteurization temperature), with cell membrane permeabilizing features and minimal effects on the nutritional and sensory qualities of foods [19,20,21]. In our previous study, we proposed that high-pressure CO2 + H2O could be used as a novel extraction technology for anthocyanins for the first time [22]; however, the overall extraction efficiency and deep extraction mechanism are still unclear.
Recently, the field of metabolomics has achieved a very significant improvement in terms of its analytical capability, particularly MS technologies. Metabolomics based on liquid chromatography and ESI-QTOF MS (LC-ESI-QTOF MS), with a high selectivity, sensitivity, and accuracy, has been used to analyze metabolites in agricultural (and food) products in both targeted and untargeted ways [23,24,25].
In this study, fuzzy logic ranking based on the anthocyanins extraction data of yield (total phenolics and total monomeric anthocyanins), quality (Color (L*), Color (a*), Color (b*)) and stability (polymeric color and half-lives at 25 °C) was applied to compare the extraction performances of anthocyanins derived from red cabbage using H2O, aqueous ethanol and high-pressure CO2 + H2O; UPLC-Q-TOF/MS combined with advanced data mining and chemometric tools was used to compare the metabolites obtained from different extraction methods and identify potential biomarkers in high-pressure CO2 + H2O and H2O extractions for further clarification of the extraction mechanism of high-pressure CO2 + H2O.
2. Materials and Methods
2.1. Materials and Reagents
Fresh red cabbage was purchased from a local market in Beijing in January 2021. LC-MS grade acetonitrile and formic acid were purchased from Thermo Fisher scientific (Waltham, MA, USA). Folin–Ciocalteu’s phenol reagent, methanol, KCl, Na2CO3, sodium acetate, citric acid, ethanol and HCl were purchased from DiKMA Technologies (Beijing, China). Water was generated by the Milli-Q integral water purification system from Millipore Billerica (Billerica, MA, USA). The CO2 gas (purity of 99.9%) in this study was purchased from Huanyu Jinghui Capital Gas Technology Co., Ltd. (Beijing, China).
2.2. Extraction Methods
Extractions were performed in the high-pressure CO2 + H2O system described by Liao et al. [26] with or without high-pressure CO2 at a temperature of 60 °C, as in our previous work [22]. Briefly speaking, fresh red cabbage was cut into small pieces, then crushed in a blender (Midea Group Co., Ltd. WBL2501A, Foshan, China). Then 10 g of chopped red cabbage was put into a nylon bag, tied tightly and placed in the vessel with 100 mL of preheated extraction solvent (1.7 g of citric acid was added to extraction solvents of H2O and aqueous ethanol (70%, v/v), respectively, and the extraction solvents were preheated to 60 °C). For H2O and aqueous ethanol extraction, the extraction time was 30 min; for the high-pressure CO2 + H2O extraction, a total 30 min extraction time with three stages was performed, with rising pressure (pressure rise to 10 MPa, 17 min), pressure holding (under 10 M°Pa CO2 pressure for 10 min) and pressure dropping (pressure drop to atmospheric pressure, 3 min). After extraction, the anthocyanin-rich solution was automatically collected from the solid–liquid mixture through filtration of the nylon bag and collected into a sample bottle for further analysis.
We used the anthocyanin extraction method described by Zhang et al. [27] with some modification by stirring the mixture (10 g of red cabbage mash, 100 mL of 80% (v/v) aqueous methanol solution and acidified by citric acid (1.7 g) at 4 °C for 12 h), which can be used as a reference to detect the relative yield of anthocyanins in red cabbage. The experiments were repeated 12 times for each extraction method.
2.3. Traditional Quality Evaluation Using Targeted Analysis
2.3.1. Total Monomeric Anthocyanins, Relative Yield of Anthocyanins, Total Phenolics Content and Polymeric Color
A Shimadzu UV-1800 spectrophotometer (Shimadzu Co., Tokyo, Japan) was used to determine the total monomeric anthocyanins, total phenolics content and polymeric color of the anthocyanin-rich solution.
The pH differential method was used to calculate the total monomeric anthocyanins in the interfering substance; the method included two buffer systems (potassium chloride with a pH of 1.0 (0.025 mol/L) and sodium acetate with a pH of 4.5 (0.4 mol/L)). Total monomeric anthocyanins were reported as cyanidin-3-glucoside (C3G) equivalents, and the total monomeric anthocyanins content of each solution was calculated using the following Equation (1),
where A = (A520-A700) pH = 1.0–(A520-A700) pH = 4.5; Mw is the molecular weight of anthocyanin (449.2 g mol−1), DF is the dilution factor (10), E is the extinction coefficient (26,900 L cm−1 mol−1) and L is the path length (1 cm).
The relative yield of anthocyanins in red cabbage by different methods was calculated based on the Equation (2),
The method with slight modifications was used to quantify the total phenolics [28]. A total of 0.3 mL of the anthocyanin-rich solution or gallic acid standard was added into brown test tubes, followed by 2 mL of Folin–Ciocalteu reagent. The mixture was incubated in the dark at room temperature for 1 h, then 1.8 mL of 7.5% Na2CO3 solution was added and left in the dark for 15 min. Absorbance was measured in triplicate at 765 nm using the spectrophotometer. Total phenolics content was reported as mg of gallic acid equivalent (GAE) per quality (100 g) of sample (mg GAE/100 g), based on the standard curve (y = 0.0046x − 0.0085, R2 = 0.9977).
Compared with monomeric anthocyanins, polymerized anthocyanins were resistant to bleaching in bisulfite solution. Absorbance of the bisulfite-bleached and control (water diluted) samples were measured at 420 nm, 520 nm and 700 nm by the spectrophotometer. The percentage of polymer color was calculated according to the Equation (3) [29],
2.3.2. Color Property, pH and Half-Life
Color was measured by ColorQuest XE (HunterLab, Inc., Reston, VA, USA), and the CIE color parameters L*, a* and b* were recorded. pH value of each anthocyanin-rich solution was measured by pH meter (FiveEasy Plus, METTLER TOLEDO, Shanghai, China). The classical first-order degradation reaction model Equation (4) was used for evaluating the stability of anthocyanin-rich solution. The anthocyanin-rich solution (12 mL) was stored in the dark at 25 ± 2 °C. The total monomeric anthocyanin content of the extract samples was measured every 7 days. The number of total monomeric anthocyanins in each matrix was fitted into Equation (5) to calculate its half-life, t1/2 [30],
where c0 is the total monomeric anthocyanins concentration at the starting point, ct is the total monomeric anthocyanins concentration after storage for t days, and K is the reaction constant.
2.3.3. Statistical Analysis
Three replicates were performed for each quality character. The data are described as means ± SD (standard deviation). One-way ANOVA and Tukey’s test at α = 0.05 were applied to evaluate the mean differences among different extraction methods. The significances of all terms were evaluated based on an analysis of variance (ANOVA) test with using the F-value at p ≤ 0.05.
2.4. Fuzzy Logic-Based Ranking Function
Fuzzy logic defined by flexible rules can be used to solve complex problems that are hard to describe with clear logics. Fuzzy logic can present complex quality characteristics in the form of scores with the help of simple algebraic steps [31]. A fuzzy ranking function was applied to choose the best extraction system for recovering phenolics from flixweed seeds [32]. We used multiple dimensions to evaluate the red cabbage extract under different extraction methods, but it was difficult to find the best extraction system with a clear formula and value. Therefore, we considered using fuzzy ranking function to select the best extraction system of anthocyanin extract from red cabbage.
2.4.1. Fuzzification
The fuzzification step was obtained from information provided by 20 interviewed experts, including 8 with higher education, 4 senior managers in factories and 8 Ph.D. students in the field of natural colorant research or production. Fuzzification was the first step where crisp values of 7 input variables (Color (L*), Color (a*), Color (b*), total phenolics content, total monomeric anthocyanins, polymeric color and half-lives at 25 °C) and one output variable (score of extraction methods) that were used to rank the various extraction methods were transformed into fuzzy form using triangular-type membership. Triangular-type membership function was according to the Equation (6),
The range of input variables was expressed as very high (VH), high (H), medium (M), and low (L). Figure 1 shows the membership functions for the inputs and flowchart of the fuzzy logic system.
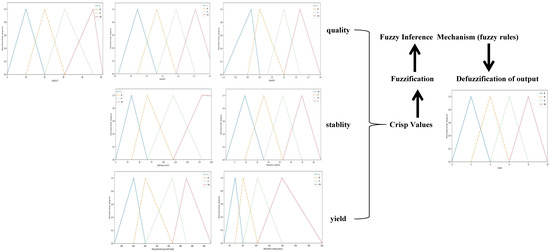
Figure 1.
Flowchart of fuzzy logic system and membership functions for the values of input and output. Input variables including yield (total phenolics, total monomeric anthocyanins), quality (Color (L*), Color (a*), Color (b*)) and stability (polymeric color and half-lives at 25 °C); output is score of extraction system. Four fuzzy sets of input variables were used: low (L), medium high medium (HM), high (H), and very high (VH); Four fuzzy sets of output were used: bad (B), medium (M), good (G) and very good (VG).
2.4.2. Definition of Fuzzy Rules
According to the relevant knowledge of food science, some examples of fuzzy rules for ranking extraction systems are listed (Table 1) by using an “if then” fuzzy rule set, which are used as the basis of modeling.

Table 1.
Some examples of fuzzy rules for ranking of extraction systems.
2.4.3. Defuzzification of Output
The centroid method such as the following equation was used for defuzzification that converts the fuzzy output to a single clear number to help sort the extraction methods and make decisions [33].
: a fuzzy set of output and Y: crisp value of output.
The test environment for fuzzy logic was python3.7.0 with matplotlib and numPY (NumFOCUS, a 501(c) (3) nonprofit charity, Austin, TX, USA).
2.5. Metabolomics Analysis
2.5.1. Sample Pretreatment and Data Acquisition
A volume of 30 mL of anthocyanin-rich solution was put into a 50 mL brown test tube and stored in refrigerator at −20 °C for 24 h. The sample was then transferred to freeze dryer (Virtis Ultra 25 XL, New York, NY, USA) for 108 h to completely freeze dry. The temperature and vacuum of the freeze dryer were set as −60 °C and 10 Pa. Lyophilized samples were redissolved with 3 mL of extraction solvent and filtered with 0.22 μm filter membrane before they were placed in the vials UPLC-ESI-QTOF-MS (6600, Agilent, CA, USA) for analysis.
Chromatographic separation was achieved on an SB-C18 column (Poroshell 120 SB-C18 2.7 μm, 3.0 × 100 mm, Agilent, CA, USA) using a flow rate of 0.3 mL/min at 30 °C. The mobile phases consisted of 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in acetonitrile (B). The gradient program was as follows: 5% B to 35% B (0–8 min); 35% B to 100% B (8–11 min); 100% B (12–15 min); 100% B to 5% B (15–15.01 min); 5% B (15.01–18 min). The mass data were acquired in negative and positive mode using parameters as follows: ion source gas 1 and gas 2 (50 arbitrary units); curtain gas (25 arbitrary units); drying gas temperature (500 °C); ion spray voltage floating, declustering potential and collision energy were 5500 V, 80 eV and 35 eV (for positive mode); mass ranges of TOF MS were m/z 100–1500 and product ion scans were m/z 50–1000. The LC-MS data were acquired using Analyst TF Version1.7.1 (SCIEX, Redwood City, CA, USA).
2.5.2. Individual Anthocyanins and Phenolics Identification
Firstly, the mass spectrum information of 23 anthocyanins [34,35] and 33 polyphenols [36,37] reported to be in red cabbage was sorted into a list according to existing literature reports. Import the list containing the relevant information (name, formula) of the known target compounds into the MasterView module of PeakView software (SCIEX, Redwood City, CA, USA), and then input the additive ions. At this time, the software automatically generated the accurate mass according to the molecular formula. The mass resolution was set to 1.0 m/z. The sample data collected by the instrument were also imported into the MasterView module.
According to the given molecular formula of the target, ionized adduct ions and retention time, MasterView determined whether there may be target ions in the MS1 data (MS1 tolerance < 10 ppm) and searched the MS2 (MS2 tolerance < 10 ppm) diagram corresponding to the target ions found in the sample in the spectrum library. Further, the MS2 of the sample target was calculated with the standard spectrum. Then, the system matched the presence and content of the sample according to the information in the import list.
2.5.3. Data Processing and Potential Marker Putative Annotation
The raw data were converted to mzXML format using MSConvert (Version 3.0, http://proteowizard.sourceforge.net, accessed on 28 September 2021). Then, the extracted peak table was generated using MS-DIAL Version 4.38 (Agilent, CA, USA) software after performing peak detection, peak alignment and primary identification. Principal component analysis (PCA), Student’s t-test, fold change (FC) and the latent structure discriminant analysis (OPLS-DA) were generated by MetaboAnalyst website (https://www.metaboanalyst.ca/, accessed on 30 September 2021). The MS1 and MS2 information of predefined potential markers was imported into MS-FINDER (Version 3.24) software for putative annotation by matching with the in-built databases. MS-FINDER calculates the possible molecular formula according to the accurate mass number of MS1 mass spectrum of the ion (MS1 tolerance < 10 ppm) and the isotope abundance ratio. At the same time, the molecular formula was verified by the accurate mass number of MS2 mass spectrum (MS2 tolerance < 15 ppm) according to a certain mass spectrum cleavage law. A list of potential markers for the discrimination of the different groups was obtained.
3. Results and Discussion
3.1. Traditional Quality Characters
In this study, relative yields of anthocyanins obtained by the three extraction methods are shown in Figure 2A. The relative yield of anthocyanins in the high-pressure CO2 + H2O extraction group was 1.62 and 1.56 times that of the pure H2O and aqueous ethanol extraction groups, respectively. As shown in Table 2, the content of total monomeric anthocyanins was 157.71 ± 0.98 mg/100 g in high-pressure CO2 + H2O extraction, 92.10 ± 1.08 mg/100 g in aqueous ethanol extraction and 73.88 ± 0.54 mg/100 g in H2O extraction. Similarly, the maximum yields of total phenols were observed in high-pressure CO2 + H2O extraction (355.32 ± 3.47 mg/100 g), followed by aqueous ethanol (314.26 ± 1.53 mg/100 g) and the lowest in H2O (185.83 ± 0.77 mg/100 g).
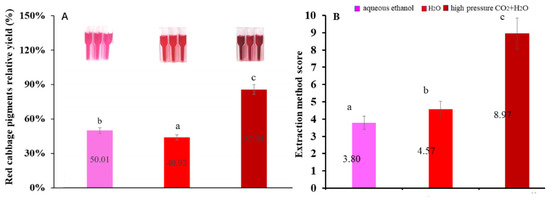
Figure 2.
The relative yields of anthocyanins (A) and fuzzy logic score (B) in the three groups. The three extraction methods are high-pressure CO2 + H2O, aqueous ethanol and H2O extraction. Different letters (a, b, c) indicate significant differences (p < 0.05).

Table 2.
The color, quality and stability properties of red cabbage pigment extracts recovered.
The finding that the high-pressure CO2 + H2O has great advantages in anthocyanin extraction is consistent with previous reports. Z. Xu et al. optimized the extraction condition and found that high-pressure CO2 + H2O produced 5–10% more anthocyanins from frozen red cabbage than H2O [22]. F. Lao et al. found that high pressure CO2 + H2O (10 MPa, 60 °C) produced 50% and 25% more colorants from purple sweet potato than water and aqueous ethanol extraction [38]. Nunes et al. found that high-pressure CO2 + H2O (10 MPa 40 °C and 20% volume ratio) produced more 60% colorants than H2O extraction from Opuntia spp. [39]. On the whole, the extraction yields of high-pressure CO2 + H2O were significantly higher than those of water and ethanol.
The total number of monomeric anthocyanins may be lost due to polymerization during extraction. As shown in Table 2, the polymeric color of high-pressure CO2 + H2O extract was the lowest (10.27 ± 0.04%), followed by H2O extract (15.42 ± 2.72%), and the color of aqueous ethanol extract was the highest (32.93 ± 3.30%). A high polymeric color of an aqueous ethanol extract may be associated with polymerization reactions. It has been demonstrated that the degradation of anthocyanins in purple rice bran can be delayed under limited oxygen [40]. The treatment of high-pressure CO2 + H2O could also protect anthocyanins against polymerization through removing oxygen from the solution [41]. Compared with water extraction, the content of O2 in high-pressure CO2 + H2O extraction was lower, indicating that the treatment of high-pressure CO2 + H2O prevents polymers from forming through removing oxygen from the solution, which could inhibit the polymerization of anthocyanins.
The color of the anthocyanin-rich solution extracted by aqueous ethanol extraction was light red and had more blue tones. The L* value of extraction by high-pressure CO2 + H2O was the lowest (33.51 ± 0.26), followed by aqueous ethanol (37.53 ± 0.55), and the highest in H2O (42.08 ± 0.72); the a* value of extraction by high-pressure CO2 + H2O was the highest (17.8 ± 0.57), followed by aqueous ethanol (14.3 ± 0.92), and the lowest in water (10.1 ± 1.14); the b* value of extraction by high-pressure CO2 + H2O was the highest (1.27 ± 0.77), followed by H2O (1.48 ± 1.16), and the lowest in aqueous ethanol (0.41 ± 1.54) (Table 2). The most likely reason for the light color of the aqueous ethanol extract (3.41) is that its ratio of total phenols to total monomeric anthocyanins was much higher than that of the aqueous matrix (H2O, 2.52; high-pressure CO2 + H2O, 2.25) (Table 2) [15]. In addition, the pH of the ethanol extract, which was higher than that of the aqueous matrix, also has a certain impact [40]. The color of the aqueous matrix extract was red, while the color of the high-pressure CO2 + H2O extract was darker. When the ratio of total phenols to total anthocyanins was similar, the increase in total anthocyanin concentration was the main reason for the darker color of the extract.
Further experiments confirmed this by using the classical first-order degradation reaction model for evaluating the degradation kinetics of anthocyanins described in 2.3.5; it was found that the half-life of the anthocyanin-rich solution extracted by high-pressure CO2 + H2O (139 days) was longer than that of traditional water extraction (59 days) and ethanol extraction (46 days). Studies showed that ethanol can reduce the stability of anthocyanins and accelerate their degradation, while high-pressure CO2 + H2O can improve the half-life of anthocyanins [42] and showed that although the number of anthocyanins extracted by ethanol was greater than that extracted by H2O, the degradation rate of ethanol-extracted anthocyanins was much faster than that of anthocyanins extracted by H2O.
Polyphenols are good co-pigments of anthocyanins, which was conducive to stabilizing the color of the high-pressure CO2 + H2O crude extract [43]. Palmira et al. demonstrated that there is a co-pigmentation phenomenon between cyanidin 3,5-diglucoside and ferulic acid by π-stacking interactions [44]. Moreover, condensation reactions of ferulic acids with anthocyanins have also been shown to occur in strawberry and raspberry juices [45]. As well as in combination with anthocyanins, their sole presence in the solution can prevent the invasion of light and heat and reduce the damage of anthocyanins [46]. It has been proved that the half-life of the anthocyanin-rich solution from purple sweet potato was prolonged by more than 3 times after using high-pressure CO2 + H2O technology [38]. Anthocyanins are vulnerable to degradation due to a nucleophilic attack by water, resulting in the partial destruction of molecular structure [47]. Acylated anthocyanins show higher stability through acyl accumulation reduces the probability of a water nucleophilic attack and generates steric hindrance [48]. However, with the degree of acylation and distribution uniformity increasing, a nucleophilic attack by water is no longer the main factor affecting the stability of anthocyanins [49]. Moreover, the foldable sugar chain of acylated anthocyanins can wrap and fix organic acids on the carbon skeleton, which shows that this accumulation is more resistant to the nucleophilic attack of water and has a certain defense against other types of degradation [50]. High-pressure CO2 + H2O was helpful in obtaining the anthocyanin-rich solution with higher stability at room temperature [51].
3.2. Fuzzy Logic Ranking
For a manufacturer, the extraction efficiency and the stability of anthocyanins are the most important criteria. The ranking of extraction methods is based on three dimensions including yield, quality and stability of anthocyanins. Thus, it is difficult to give a precise evaluation in a separate evaluation. The fuzzy logic evaluation comprehensively considered the three dimensions of the different extraction methods, making the evaluation results more scientific.
The results of the defuzzification score of each method by fuzzy rules are shown in Figure 2B. The high-pressure CO2 + H2O extraction method acquired the highest score (8.97), followed by aqueous ethanol extraction (4.36) and H2O extraction (3.25) (a score > 8 can be considered as a “very good” extraction method).
The high-pressure CO2 + H2O method has the advantages of a high total phenolic content and total monomeric anthocyanin content, the longest half-life and the least polymer color, which have attracted the attention of experts in this evaluation. In general, high-pressure CO2 + H2O exhibited great advantages in anthocyanin extraction from plants compared to aqueous ethanol extraction and H2O from a comprehensive perspective.
3.3. Targeted Metabolomics Analysis
As shown in Table 3, 19 anthocyanins were found in red cabbage. Compared with aqueous ethanol extraction, the contents of four non-acylated anthocyanins were higher in H2O extraction, while the contents of five mono-acylated anthocyanins and nine di-acylated anthocyanins were higher in high-pressure CO2 + H2O extraction. Specifically, for mono-acylated anthocyanins, the extraction effect of high-pressure CO2 + H2O was significantly enhanced (about 30% higher than that of aqueous ethanol extraction), and the effect of H2O extraction was about 10% lower than that of aqueous ethanol extraction; for di-acylated anthocyanins, the extraction effect of high-pressure CO2 + H2O was excellent (about 60% higher than that of aqueous ethanol extraction), and the performance of H2O extraction was about 30% lower than that of aqueous ethanol extraction (Figure 3A).

Table 3.
The red cabbage anthocyanin and phenolic compositional profiles.
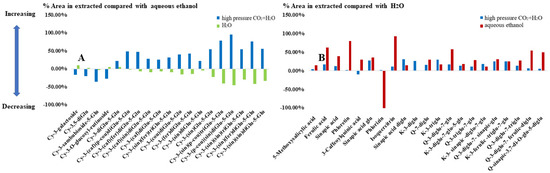
Figure 3.
Relative peak area of anthocyanins (compared with aqueous ethanol group) in water and high-pressure CO2 + H2O groups (A) and relative peak area of phenolic compounds (compared with H2O group) in aqueous ethanol and high-pressure CO2 + H2O groups (B). Abbreviation: Cy, cyanidin; glu, glucoside; caf, caffeic; p-cou, p−coumaric; fer, ferulic; sin, sinapic acid; K, kaempferol; Q, quercetin.
As shown in Table 3, 21 polyphenols were found in red cabbage. Aqueous ethanol has been known to be a good solvent for phenolic extraction. Based on Chem YQ (http://www.chemyq.com/xz.htm, accessed on 15 October 2021), the extracted phenolics are divided into the following three categories: (1) Substances insensitive to solvent polarity, such as kaempferol, quercetin and glycoside, can be well dissolved in water and ethanol. The increase in their content is mainly due to full contact with the extractant; (2) substances sensitive to solvent polarity, such as sinapic acid glucose, and the increase in content is mainly due to the change of solvent polarity. The content of strongly polar or non-polar substances such as phloretin and phlorizin remains basically unchanged because high-pressure CO2 + H2O treatment has little influence on solvent polarity; [52] and (3) substances sensitive to solvent temperature, such as 3-caffeoylquinic acid, which is greatly affected by temperature [53] and has high solubility in hot water and low solubility in cold water, decreased in content by high-pressure CO2 + H2O treatment, which may have an opposite or ineffective influence on the two main factors (solvent polarity and temperature), and the comprehensive effect is not obvious. Specifically, although CO2 promotes the entry of the extractant into cells and fully extracts the substance, due to the evaporation and the endothermic heat of liquid CO2 during high-pressure CO2 + H2O decompression, the solution temperature suddenly decreases [54,55], and the solubility decreases sharply.
3.4. Untargeted Metabolomics Analysis
3.4.1. PCA Analysis
In total, the three groups share 4394 compounds, the high-pressure CO2 + H2O and aqueous ethanol share 322 compounds, the high-pressure CO2 + H2O and H2O share 2827 compounds and the aqueous ethanol and H2O share 146 compounds, respectively (Figure 4A). PCA was carried out to determine whether the three extraction methods could be differentiated. The PCA score plots generated for the anthocyanin-rich solution by the three methods show a separation into distinctive clusters of two groups; one group was high-pressure CO2 + H2O and H2O, and the other group was aqueous ethanol (Figure 4B). The results show that there were significant differences in the composition of anthocyanin-rich solutions with H2O as a solvent and aqueous ethanol as a solvent.
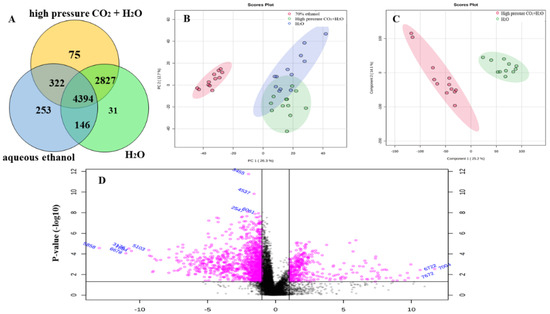
Figure 4.
Differential compounds analysis: Venn plots of high-pressure CO2 + H2O, H2O and aqueous ethanol (A); PCA score plots of high-pressure CO2 + H2O, H2O and aqueous ethanol (B); OPLS-DA score scatter plot of high-pressure CO2 + H2O and H2O (C). Volcano plots of high-pressure CO2 + H2O and H2O (D).
3.4.2. Differential Analysis and Potential Marker Annotation
Compounds with differential abundances are defined as compounds with a p less than 0.05 and an FC no less than 2 or no more than 0.5 (Figure 4D). By calculating FC, 217 compounds were defined. Considering the similarity of the extraction solvent between high-pressure CO2 + H2O and H2O extraction, the orthogonal projection to latent structure discriminant analysis (OPLS-DA) model was built to find potential characteristic markers for these two groups (Figure 4C). Fifteen potential markers were selected based on VIP values (>2), including six alkaloids, one nucleotide, six amino acids and two fatty acids (Table 4).

Table 4.
Annotation of differential compounds in red cabbage between high-pressure CO2 + H2O and H2O.
These potential markers could also be divided into the three categories as described in Section 3.3: (1) soluble in water (betaine (Figure 5A), phenylalanine betaine (Figure 5B), lenticin (Figure 5C), phenylalanine (Figure 5D), isoleucine (Figure 5E), triethyl phosphate (Figure 5F), triethylcitrate (Figure 5G) and tri-isobutylphosphate (Figure 5H); (2) insoluble in water (2-methylindole (Figure S1I), 3-formylindole (Figure S1J), guanine (Figure S1K), tyrosine (Figure S1L) and tryptophan (Figure S1M); and (3) sensitive to temperature (indole (Figure S1N) and proline (Figure S1O).
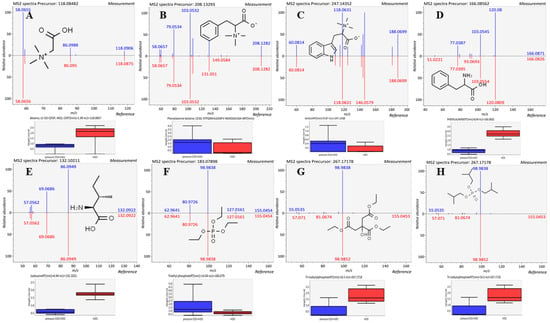
Figure 5.
The boxplots of the abundance and structures of (A) betaine, (B) phenylalanine betaine, (C) lenticin, (D) phenylalanine, (E) isoleucine, (F) triethyl phosphate, (G) triethylcitrate and (H) tri-isobutylphosphate. The blue bar represents high-pressure CO2 + H2O extraction and the red bar represents H2O extraction.
The potential markers with good water solubility can be divided into two groups: the contents of phenylalanine betaine, lenticin, triethyl phosphate, and triethylcitrate are higher in high-pressure CO2 + H2O; and the contents of phenylalanine, betaine, isoleucine are higher in H2O. During the pressure relief process, dissolved CO2 may impinge on and shear cells at gas-expanding velocity [56]. The “explosion” effect of high-pressure CO2 can enhance the damage to the cellular structure of the extracted substrate, reduce the mass transfer resistance of target materials and improve the extraction efficiency. This fact shows that the soluble compounds of phenylalanine betaine, lenticin, triethyl phosphate, triethylcitrate and guanine are higher in high-pressure CO2 + H2O extraction compared to H2O extraction. This confirms that the explosion effect caused by high-pressure CO2 causes greater contact between the solvent and solute.
Generally, the polarity of water is strong, so it is difficult to extract substances with weak polarity. Surprisingly, in this work, the content of some insoluble compounds including 2-methylindole, 3-formylindole, guanine, tyrosine and tryptophan were higher, and the soluble compounds of phenylalanine, betaine, isoleucine were lower in high-pressure CO2 + H2O extraction compared to H2O extraction. The increase in the content of substances which are sensitive to solvent polarity was mainly due to the change in solvent polarity [57]. During high-pressure CO2 + H2O extraction, CO2 gas is pressurized to a liquid, and the polarity of the solvent is changed. The weak polarity of CO2 fluid improves the extraction efficiency of weakly polar substances. Indole and proline are easily soluble in hot water and almost insoluble in cold water. We highly suspect that the reason for its lower content in the high-pressure CO2 + H2O extraction is that the solution temperature decreases at that stage of pressure relief.
3.5. Extraction Mechanism of High-Pressure CO2 + H2O
Based on our early experiments, the results of this paper and relevant literature, we propose the “3I” extraction mechanism of pressure CO2 + H2O as follows: (1) By improving the isolation of O2, the existence of high-pressure CO2 ensures the whole system is in a high-pressure, acidic and O2-free environment, which effectively prevents the degradation of natural products and oxygen reactions during the extraction process [58]. It has been demonstrated that the degradation of anthocyanins can be delayed under the condition of limited oxygen. Additionally, high-pressure CO2 + H2O protects against polymerization of anthocyanins by removing oxygen from the solution [40,41]. (2) By improving the polarity of the solution, increased CO2 solubility (and pH) in aqueous solutions under high pressure leads to the formation of more carbonic acid (H2CO3), which changes the polarity of solution. That is why high-pressure CO2 + H2O extraction is conducive to the extraction of acylated anthocyanins and water-insoluble substances (i.e., 2-methylindole, 3-formylindole, guanine, tyrosine and tryptophan); (3) By improving the solid–liquid mass transfer efficiency of phytochemicals, the “explosion” effect of high pressure can enhance the damage to the cellular structure and reduce the mass transfer resistance in target materials, thus improving the extraction efficiency. The higher content of water-soluble phenolics (kaempferol, quercetin and glycoside) and potential water-soluble markers (phenylalanine betaine, lenticin, triethyl phosphate, triethylcitrate) in high-pressure CO2 + H2O treatment can be attributed to the improvement in solid–liquid mass transfer efficiency.
4. Conclusions
The “3I” extraction mechanism of high-pressure CO2 + H2O is proposed. It is interesting to note that direct evidence for the modification of the polarity of H2O combined with high-pressure CO2 has been reported for the detection of the water-insoluble compounds (2-methylindole, 3-formylindole, guanine, tyrosine and tryptophan) in a anthocyanin-rich solution obtained by high-pressure CO2 + H2O, while less amounts of these compounds were found in the H2O extract group. Additionally, direct evidence for reduced temperatures during the pressure relief process has also been provided by the discovery of a lower content of indole and proline (temperature-sensitive compounds) for the first time. Based on the above conclusion, we propose that high-pressure CO2 + H2O could be used as a promising technology for phytochemical extraction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su14031369/s1, Figure S1. Mirror images, structures and content of quantified compounds. (I) 2-methylindole; (J) 3-formylindole; (K) guanine; (L) tyrosine; (M) tryptophan; (N) indole; (O) proline. The blue bar represents high pressure CO2 + H2O extraction and the red bar represents H2O extraction.
Author Contributions
Conceptualization, Z.X.; methodology, L.X.; software, B.W.; validation, B.W.; investigation, X.W.; data curation, B.W.; writing—original draft preparation, B.W.; writing—review and editing, S.Y.; data curation, L.M. and K.W.; funding acquisition, X.L. and Z.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Nature Science Foundation of China (NSFC) (grant number: 31930087).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare there is no conflict of interest.
References
- Kamatar, M.Y. Natural food colouring: A healthier alternative to artificial food colouring. In Proceedings of the Global Milling Conference: Saftey, Sustainability and Food Supply for the 21st Century, Chennai, India, February 2013. [Google Scholar]
- Christensen, C.M. Effects of Color on Aroma, Flavor and Texture Judgments of Foods. J. Food Sci. 1983, 48, 787–790. [Google Scholar] [CrossRef]
- Gao, H.-W.; Xu, Q.; Chen, L.; Wang, S.-L.; Wang, Y.; Wu, L.-L.; Yuan, Y. Potential Protein Toxicity of Synthetic Pigments: Binding of Poncean S to Human Serum Albumin. Biophys. J. 2008, 94, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Titova, N. 300 The Method of Antigen Specific Damage of Leucocytes by Food Additives in Patients with Bronchial Asthma. World Allergy Organ. J. 2012, 5, S97–S114. [Google Scholar] [CrossRef]
- Weber, R.W.; Hoffman, M.; Raine, A.D.; Nelson, H.S.; Rainejr, D. Incidence of bronchoconstriction due to aspirin, azo dyes, non-azo dyes, and preservatives in a population of perennial asthmatics. J. Allergy Clin. Immunol. 1979, 64, 32–37. [Google Scholar] [CrossRef]
- Rana, B.; Bhattacharyya, M.; Patni, B.; Arya, M.; Joshi, G.K. The Realm of Microbial Pigments in the Food Color Market. Front. Sustain. Food Syst. 2021, 5, 584605. [Google Scholar] [CrossRef]
- Chou, P.-H.; Matsui, S.; Misaki, K.; Matsuda, T. Isolation and Identification of Xenobiotic Aryl Hydrocarbon Receptor Ligands in Dyeing Wastewater. Environ. Sci. Technol. 2007, 41, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Nazarpoor, K.; Karimi, L. Eco-friendly dyeing of wool using natural dye from weld as co-partner with synthetic dye. J. Clean. Prod. 2011, 19, 1045–1051. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Jackman, R.L.; Yada, R.; Tung, M.A.; Speers, R.A. Anthocyanins as food colorants—A review. J. Food Biochem. 1987, 11, 201–247. [Google Scholar] [CrossRef]
- Andersen, O.M.; Jordheim, M. Basic anthocyanin chemistry and dietary sources. Anthocyanins Health Dis. 2013, 13–90. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Markakis, P. Stability of Anthocyanins in Foods—ScienceDirect. Anthocyanins Food Colors 1982, 163, 180. [Google Scholar]
- Tsuda, T. Anthocyanins as Functional Food Factors—Chemistry, Nutrition and Health Promotion—. Food Sci. Technol. Res. 2012, 18, 315–324. [Google Scholar] [CrossRef]
- Turturică, M.; Oancea, A.M.; Râpeanu, G.; Bahrim, G. Anthocyanins: Naturally occuring fruit pigments with functional properties. Ann. Univ. Dunarea De Jos Galati. Fascicle VI Food Technol. 2015, 39, 9–24. [Google Scholar]
- Jafari, S.; Mahdavee, K.; Assadpour, E. Production of a natural color through microwave—Assisted extraction of saffron tepal’s anthocyanins. Food Sci. Nutr. 2019, 7, 1438–1445. [Google Scholar] [CrossRef]
- He, Y.; Wen, L.; Liu, J.; Li, Y.; Zheng, F.; Min, W.; Yue, H.; Pan, P. Optimisation of pulsed electric fields extraction of anthocyanin from Beibinghong Vitis Amurensis Rupr. Nat. Prod. Res. 2017, 32, 23–29. [Google Scholar] [CrossRef]
- Tiberio de Jesus, A.L.; Cristianini, M.; dos Santos, N.N.; Marostica Junior, M.R. Effects of high hydrostatic pressure on the microbial inactivation and extraction of bioactive compounds from acai (Euterpe oleracea Martius) pulp. Food Res. Int. 2020, 130, 108856. [Google Scholar] [CrossRef]
- Knorr, D. Impact of non-thermal processing on plant metabolites. J. Food Eng. 2003, 56, 131–134. [Google Scholar] [CrossRef]
- Briongos, H.; Illera, A.E.; Sanz, M.; Melgosa, R.; Beltrán, S.; Solaesa, A. Effect of high pressure carbon dioxide processing on pectin methylesterase activity and other orange juice properties. LWT 2016, 74, 411–419. [Google Scholar] [CrossRef][Green Version]
- Tiwari, B.K.; O’Donnell, C.P.; Cullen, P.J. Effect of non thermal processing technologies on the anthocyanin content of fruit juices. Trends Food Sci. Technol. 2009, 20, 137–145. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, J.; Zhang, Y.; Hu, X.; Liao, X.; Wang, Z. Extraction of anthocyanins from red cabbage using high pressure CO2. Bioresour. Technol. 2010, 101, 7151–7157. [Google Scholar]
- Castro-Puyana, M.; Herrero, M. Metabolomics approaches based on mass spectrometry for food safety, quality and traceability. TrAC Trends Anal. Chem. 2013, 52, 74–87. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Huang, X.; Wang, X.; Yang, R.; Mao, J.; Wang, X.; Wang, X.; Zhang, Q.; Li, P. Identification of Nutritional Components in Black Sesame Determined by Widely Targeted Metabolomics and Traditional Chinese Medicines. Molecules 2018, 23, 1180. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R. Grape and Wine Metabolomics to Develop New Insights Using Untargeted and Targeted Approaches. Fermentation 2018, 4, 92. [Google Scholar] [CrossRef]
- Liao, H.; Hu, X.; Liao, X.; Chen, F.; Wu, J. Inactivation of Escherichia coli inoculated into cloudy apple juice exposed to dense phase carbon dioxide. Int. J. Food Microbiol. 2007, 118, 126–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, X.; Chen, F.; Wu, J.; Hu, X. Isolation, identification, and color characterization of cyanidin-3-glucoside and cyanidin-3-sophoroside from red raspberry. Eur. Food Res. Technol. 2007, 226, 395–403. [Google Scholar] [CrossRef]
- Zhang, J.; Iqbal, A.; Murtaza, A.; Zhou, X.; Xu, X.; Pan, S.; Hu, W. Effect of high pressure carbon dioxide on the browning inhibition of sugar-preserved orange peel. J. CO2 Util. 2021, 46, 101467. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. The effect of pigment matrix, temperature and amount of carrier on the yield and final color properties of spray dried purple corn (Zea mays L.) cob anthocyanin powders. Food Chem. 2017, 227, 376–382. [Google Scholar] [CrossRef]
- Zou, H.; Lin, T.; Bi, X.; Zhao, L.; Wang, Y.; Liao, X. Comparison of high hydrostatic pressure, high-pressure carbon dioxide and high-temperature short-time processing on quality of mulberry juice. Food Bioproc. Technol. 2016, 9, 217–231. [Google Scholar] [CrossRef]
- Rahul, K.; Payel, G.; Srinivasa Rao, P.; Rana, S.S.; Rahul, V.; Kambhampati, V. Sensory evaluation of microwave assisted ultra-sound treated soymilk beverage using fuzzy logic. J. Saudi Soc. Agric. Sci. 2021, 20, 257–264. [Google Scholar]
- Shahidi, B.; Sharifi, A.; Nasiraie, L.R.; Niakousari, M.; Ahmadi, M. Phenolic content and antioxidant activity of flixweed (Des-curainia sophia) seeds extracts: Ranking extraction systems bsed on fuzzy logic method. Sustain. Chem. Pharm. 2020, 16, 100245. [Google Scholar] [CrossRef]
- Balezentiene, L.; Streimikiene, D.; Balezentis, T. Fuzzy decision support methodology for sustainable energy crop selection. Renew. Sustain. Energy Rev. 2013, 17, 83–93. [Google Scholar] [CrossRef]
- Gachovska, T.; Cassada, D.; Subbiah, J.; Hanna, M.; Thippareddi, H.; Snow, D. Enhanced Anthocyanin Extraction from Red Cabbage Using Pulsed Electric Field Processing. J. Food Sci. 2010, 75, E323–E329. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Clevidence, B.A.; Britz, S.J.; Novotny, J.A. Effect of dose size on bioavailability of acylated and nonacylated an-thocyanins from red cabbage (Brassica oleracea L. var. capitata). J. Agric. Food Chem. 2007, 55, 5354–5362. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiao, Z.; Lin, L.-Z.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling Polyphenols in Five Brassica Species Microgreens by UHPLC-PDA-ESI/HRMSn. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization, Quantification, and Yearly Variation of the Naturally Occurring Polyphenols in a Common Red Variety of Curly Kale (Brassica oleracea L. convar. acephala var. sabellica cv. ‘Redbor’). J. Agric. Food Chem. 2010, 58, 11346–11354. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Cheng, H.; Wang, Q.; Wang, X.; Liao, X.; Xu, Z. Enhanced water extraction with high-pressure carbon dioxide on purple sweet potato pigments: Comparison to traditional aqueous and ethanolic extraction. J. CO2 Util. 2020, 40, 101188. [Google Scholar] [CrossRef]
- Nunes, A.N.; Carmo, C.S.D.; Duarte, C.M.M. Production of a natural red pigment derived from Opuntia spp. using a novel high pressure CO2 assisted-process. RSC Adv. 2015, 5, 83106–83114. [Google Scholar] [CrossRef]
- Klaykruayat, S.; Mahayothee, B.; Khuwijitjaru, P.; Nagle, M.; Müller, J. Influence of packaging materials, oxygen and storage temperature on quality of germinated parboiled rice. LWT 2019, 121, 108926. [Google Scholar] [CrossRef]
- Hart, A.; Kleinig, A. The role of oxygen in the ageing of bottled wine. Aust. NZ Wine Industry J. 2005, 20, 46–50. [Google Scholar]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.; de Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Teixeira, N.; Cruz, L.; Brás, N.F.; Mateus, N.; Ramos, M.J.; De Freitas, V. Structural Features of Copigmentation of Oenin with Different Polyphenol Copigments. J. Agric. Food Chem. 2013, 61, 6942–6948. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Lima, J.C.; Freitas, A.; Shimizu, K.; Macanita, A.; Quina, F. Charge-Transfer Complexation as a General Phenomenon in the Copigmentation of Anthocyanins. J. Phys. Chem. A 2005, 109, 7329–7338. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Ollilainen, V.; Vahermo, M.; Yli-Kauhaluoma, J.; Heinonen, M. Identification of novel pyranoanthocyanins in berry juices. Eur. Food Res. Technol. 2005, 220, 239–244. [Google Scholar] [CrossRef]
- Eiro, M.J.; Heinonen, M. Anthocyanin Color Behavior and Stability during Storage: Effect of Intermolecular Copigmentation. J. Agric. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef]
- Xie, F.-y.; Li, F.-f.; Zhang, S.; Bi, W.-w.; Zhang, X.-l.; Zhang, X.-n. Analysis of acylation modification of black rice anthocyanins using fourier transform infrared spectroscopy (FTIR). Spectrosc. Spect. Anal. 2018, 38, 2386–2389. [Google Scholar]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Sources and relative stabilities of acylated and nonacylated anthocyanins in bev-erage systems. J. Food Sci. Technol. 2021, 1–15. [Google Scholar] [CrossRef]
- Fenger, J.-A.; Moloney, M.; Robbins, R.J.; Collins, T.M.; Dangles, O. The influence of acylation, metal binding and natural anti-oxidants on the thermal stability of red cabbage anthocyanins in neutral solution. Food Funct. 2019, 10, 6740–6751. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.H.; Könighofer, L.; Hogg, M.; Scharinger, A.; Buchweitz, M. Impact of B-Ring Substitution and Acylation with Hydroxy Cinnamic Acids on the Inhibition of Porcine α-Amylase by Anthocyanin-3-Glycosides. Foods 2020, 9, 367. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; LaRocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Zeng, F.; Zeng, H.; Ye, Y.; Zheng, S.; Zhuang, Y.; Liu, J.; Fei, P. Preparation of acylated blueberry anthocyanins through an en-zymatic method in an aqueous/organic phase: Effects on their colour stability and pH-response characteristics. Food Funct. 2021, 12, 6821–6829. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Azevedo, J.; Teixeira, N.; Araújo, P.; de Freitas, V.; Basílio, N.; Pina, F. On the Limits of Anthocyanins Co-Pigmentation Models and Respective Equations. J. Agric. Food Chem. 2021, 69, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Zhang, C.L.; Cheng, X.L.; Zhao, J.H.; Wang, L.C. Solubility of 3-Caffeoylquinic Acid in Different Solvents at 291–340 K. Russ. J. Phys. Chem. A 2017, 91, 2512–2517. [Google Scholar] [CrossRef]
- Santos, P.F.D.; André, L.; Ducousso, M.; Contamine, F.; Cézac, P. Experimental Measurements of CO2 solubility in aqueous MgCl2 solution at temperature between 323.15 and 423.15 K and pressure up to 20 MPa. J. Chem. Engi. Data 2021, 66, 4166–4173. [Google Scholar] [CrossRef]
- Wang, L.; Pan, J.; Xie, H.; Yang, Y.; Lin, C. Inactivation of Staphylococcus aureus and Escherichia coli by the synergistic action of high hydrostatic pressure and dissolved CO2. Int. J. Food Microbiol. 2010, 144, 118–125. [Google Scholar] [CrossRef]
- Lockwood, S.F.; O’Malley, S.; Mosher, G.L. Improved aqueous solubility of crystalline astaxanthin (3,3′-dihydroxy-beta, be-ta-carotene-4,4′-dione) by Captisol (sulfobutyl ether beta-cyclodextrin). J. Pharm. Sci. 2003, 92, 922–926. [Google Scholar] [CrossRef]
- Mantell, C.; Rodríguez, M.; de la Ossa, E.M. A Screening Analysis of the High-Pressure Extraction of Anthocyanins from Red Grape Pomace with Carbon Dioxide and Cosolvent. Eng. Life Sci. 2003, 3, 38–42. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).