1. Introduction
Phosphorus (P) deficiency is one of the major limiting factors for plant growth in horticultural crops. Adding phosphorus inputs is necessary for sustainable agricultural crops [
1]. Deficient phosphorus in substrates produces irregular growth, both in the aerial and root systems, which impairs seedling quality [
2]. It is an essential constituent of ATP, which serves as a source of energy for plant metabolism and stimulates the growth of fine roots that are very important for seedling development [
3]. Nutrients in the substrate are essential to the proper growth of seedlings in the nursery in terms of height, diameter, and mass production [
4]. Nevertheless, phosphorus availability is low in soils because of its fixation as insoluble phosphates of iron, aluminum, and calcium in acidic or calcareous soil [
5]. Despite the large amount of total P immobilized on the surfaces of soil mineral particles, the available soluble P is often too small to support good plant growth [
6]. Consequently, small-scale farmers often have difficulty accessing fertilizer supplies due to the high cost of mineral fertilizers.
Phosphate refers to the product of mining and subsequent metallurgical treatment of ores containing phosphorus [
7]. Its low price is very attractive as a phosphate fertilizer, and it is relatively slow to release soluble P compared to industrial P fertilizers [
5]. The direct application of phosphate rocks and their modified forms has been recommended as alternatives for P fertilization.
Additionally, with large quantities deposited in the open air and higher carbonate contents, natural phosphate rock dumps and phosphate sludge have the most damaging effects on the environment. This accumulation of by-products represents a risk and a big problem for storage capacities [
8]. Based on its composition, phosphate sludge might contain characteristics similar to those of phosphate rock [
9]. It consists of fine clayey particles and still contains an important amount of P [
10]. This solid phosphate sludge, generated from phosphate treatment processes at mining sites in agriculture, is an alternative recovery technique for phosphate. SPS can control some soil-borne pathogens and improve the plant growing in the nursery after composting or inoculation with arbuscular mycorrhizal fungi [
11,
12,
13]. Phosphate wash sludge is also rich in mineral elements [
8]. We hypothesize that using sludge in a nursery can recover some of the mineral elements that are essential substrates for growing plants, including phosphorus. Thus, this study aims to assess the effect of solid phosphate sludge amendments on the growth of fruit and forest trees in a nursery.
4. Discussion
Plant height and plant diameter are the main parameters observed when assessing plant growth, as are leaf chlorophyll content index (SPAD) values, where higher values can be indicators of a higher yield [
15], photosynthetic rate [
16], and important biochemical indicators for plants [
17].
The solid phosphate sludge amendment used contains fluorapatite (44%), carbonates (22%), quartz (17%), and smectite-like swelling clay (montmorillonite) [
18], which resulted in a very high pH (8.2) and made these amendments unsuitable for tree cultivation, which requires a pH of 6–7 [
19]. This study showed that the application of amendments such as Maamora soil or sand gradually decreases the pH over concentrations.
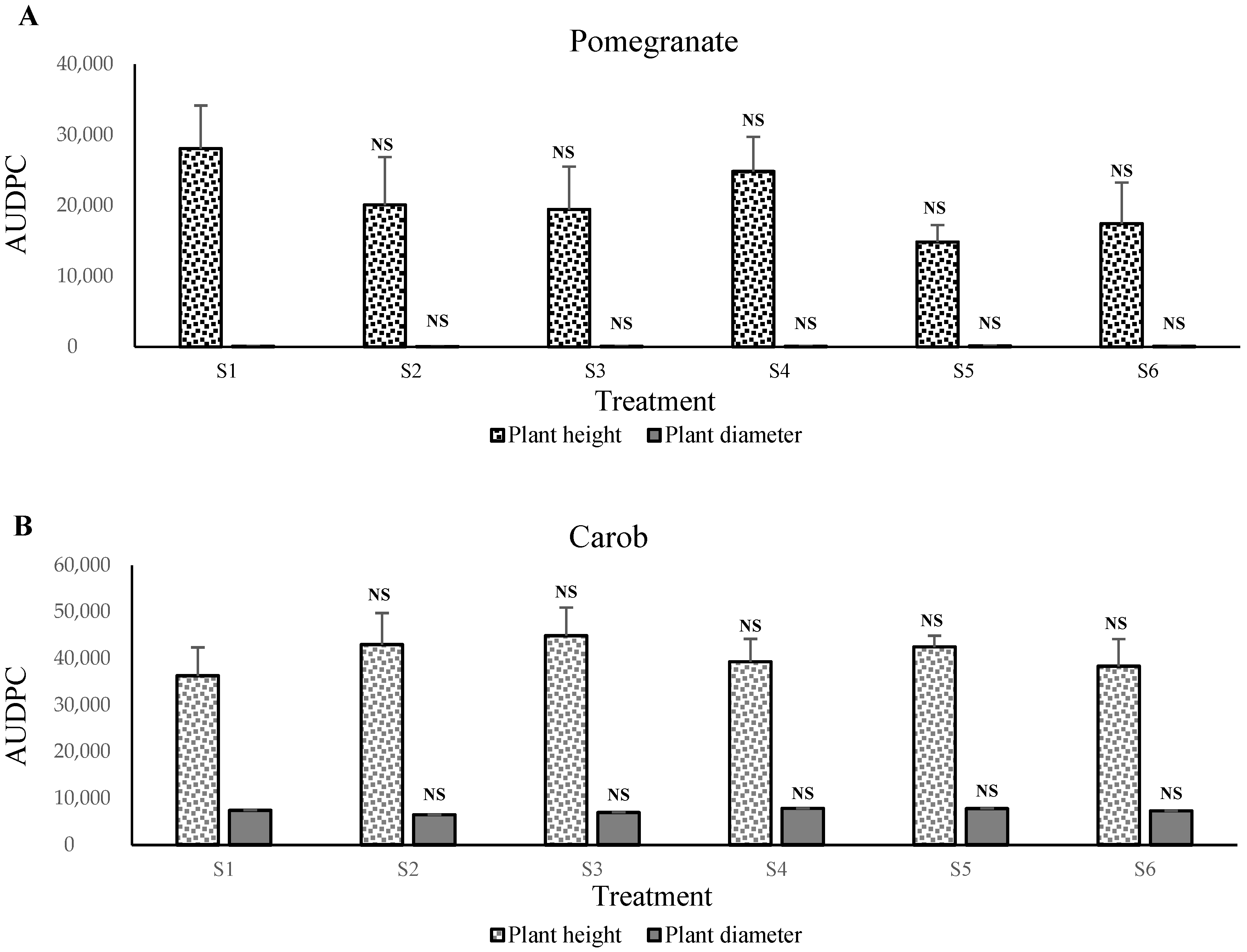
The first experiment showed that the control soil (S1 mixture) presented the highest growth for pomegranate,
C. volkameriana, and acacia plants. This means that Maamora soil is rich soil with a high level of organic matter. The latter plays a vitally important role in P solubilization through the acidifying and chelation mechanisms. The soil’s low level of organic matter may also be an essential factor for the overall low mineralization trend of P observed in this study [
20]. These results are consistent with the research conducted by Benjelloun [
21], where he confirms that the cork oak soils are relatively more stable and better provided with organic matter (greater than 1.10%). According to Provencher [
22], organic matter positively influences the assimilation of phosphorus by the plant. On the other hand, as the acidity increases, more phosphorus can bind energetically with the hydroxides of iron (Fe) or aluminum (Al) and then not be released in the soil solution and consequently not be available for vegetation [
23,
24].
The pomegranate and
C. volkameriana plants might also prefer the pH of the Maamora soil, which varies between 4.8 and 6.4 with an average of 5.68, which places it in the category of acid soils [
25]. Of these plants, trees prefer this interval of pH for nutrient uptake. If the pH of a substrate exceeds 6.5, mineral deficiencies may occur, and the solubility of nutrients in the soil is affected [
26]. The application of phosphate sludge increases the pH, which might affect plant growth. The supply of clay phosphate sludge can also affect the growth of these young plants.
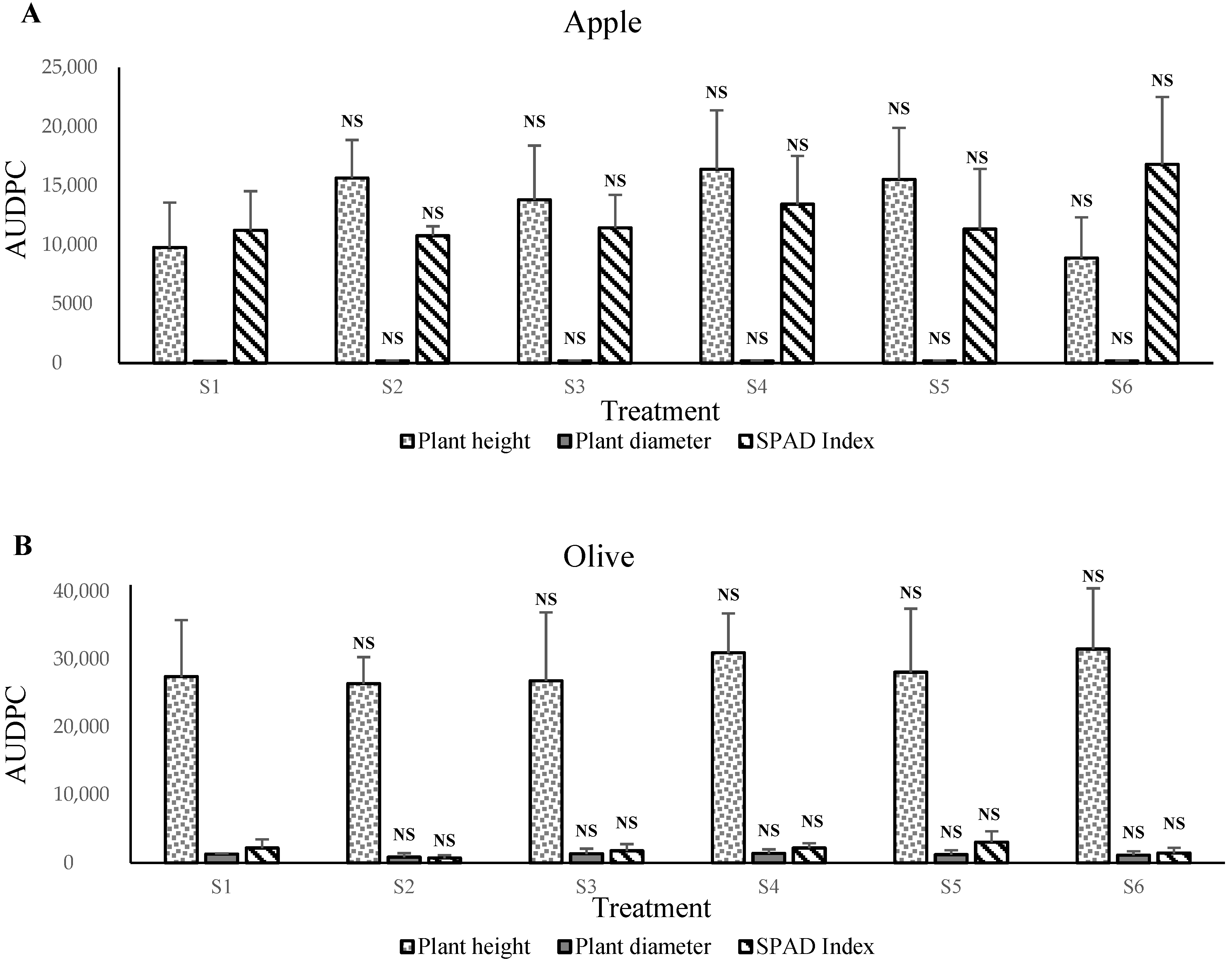
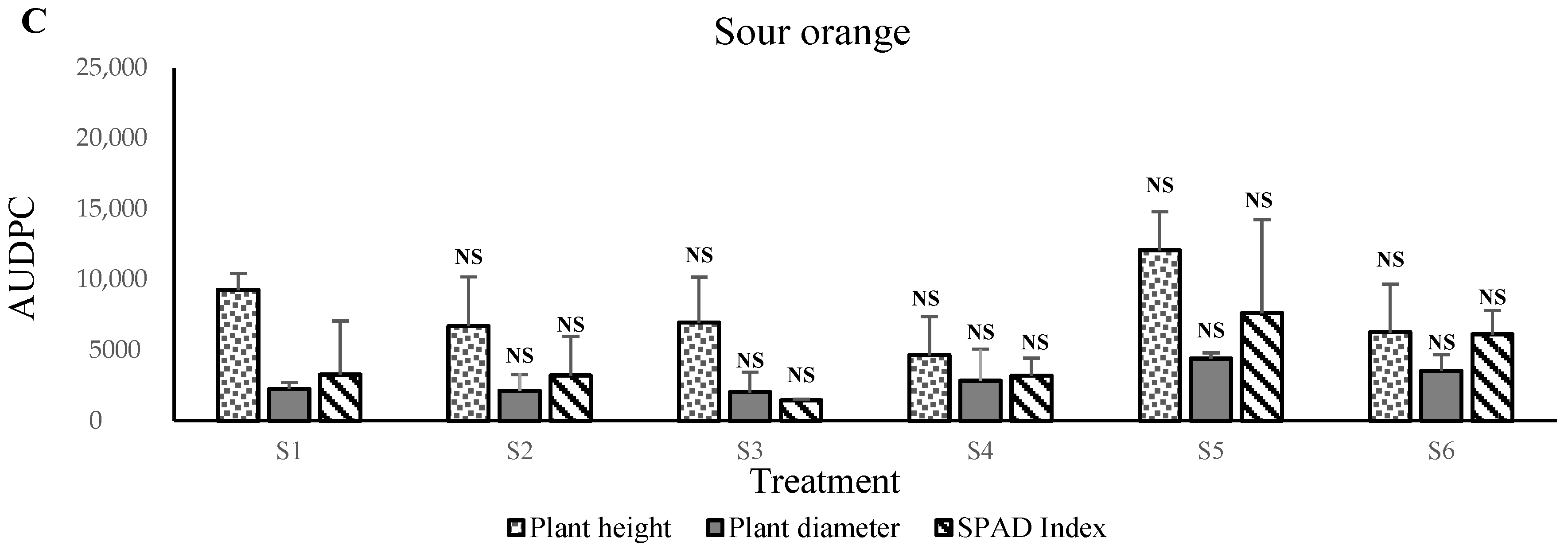
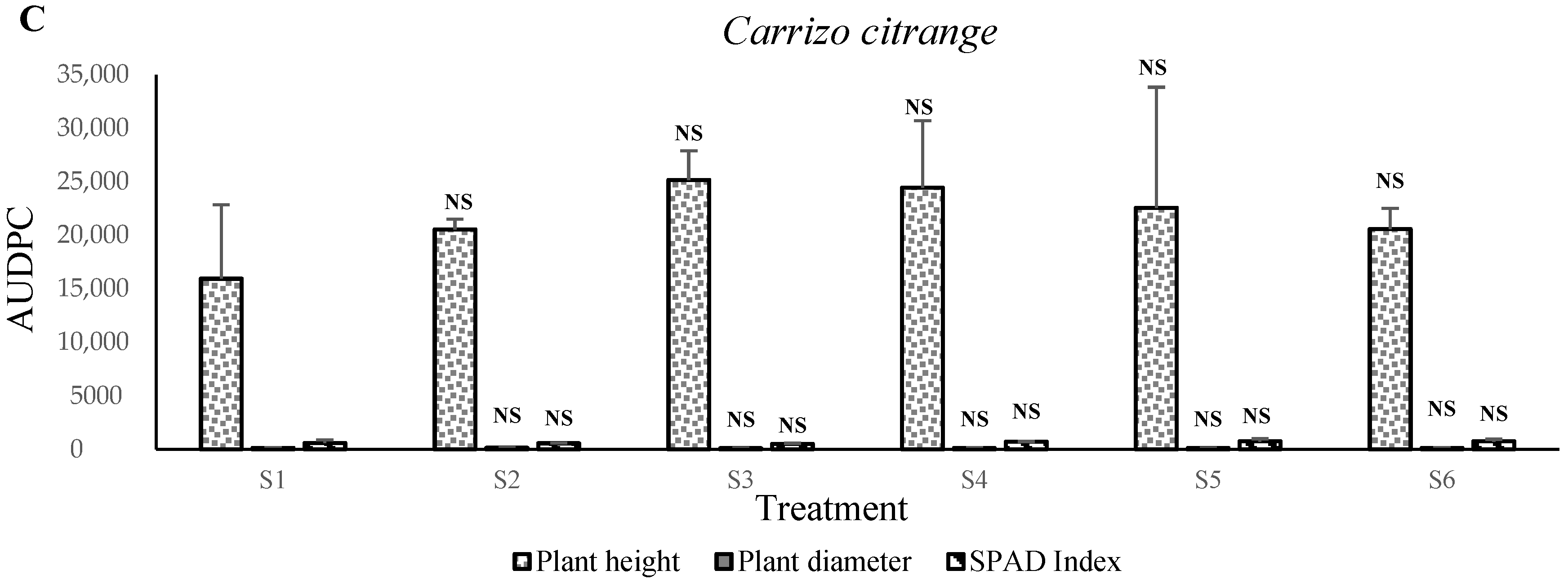
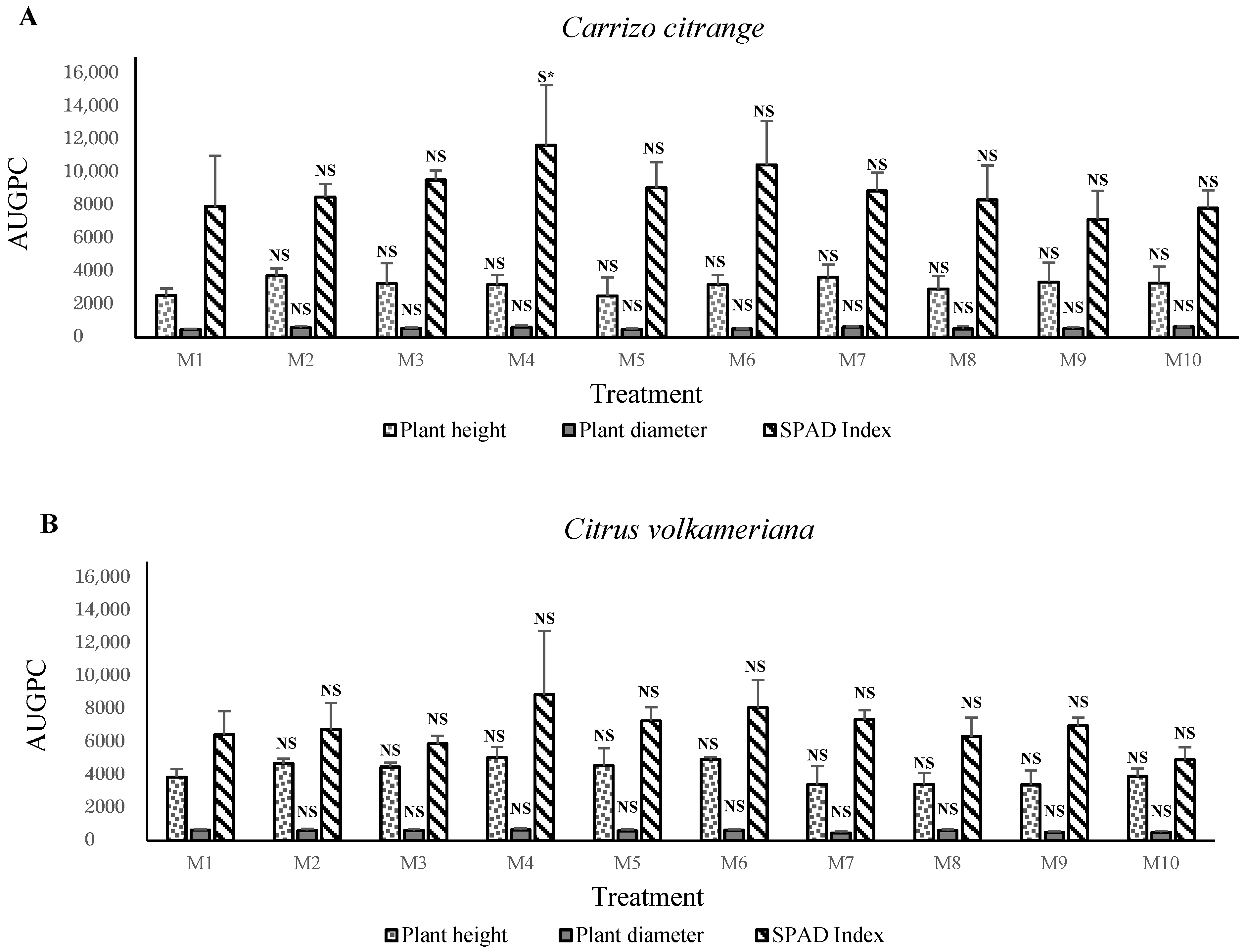
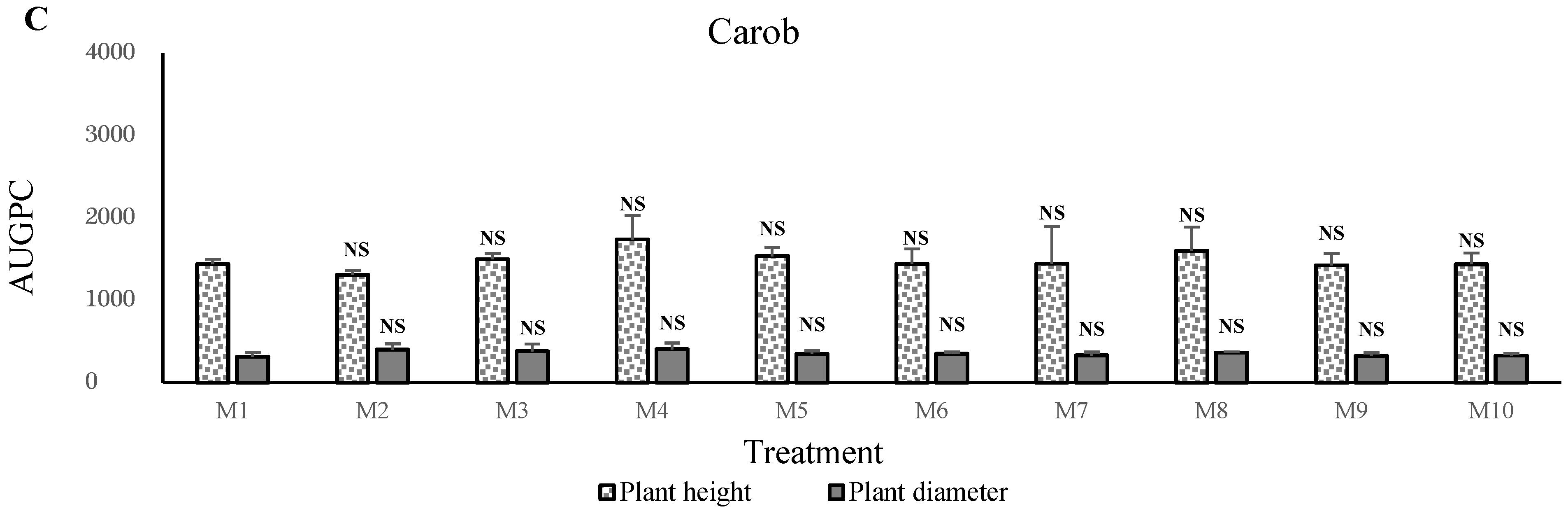
In contrast, the apple, olive,
Carrizo citrange,
C. macrophylla, sour orange, carob, and argan plants prefer growth in S3 and S4 mixtures, that is, between 20% and 40% of solid phosphate sludge amendments. The obtained results indicated that the vigor of plants corresponded positively with the nutritional status of substrates. We assume that phosphate sludge and Maamora soil material stimulate plant growth and increase mineral nutrient uptake rates. The concentrations increases the pH level to between 7.0 and 7.7 (results not shown). This level can be the optimum for these species’ plant growth. This positive effect of phosphate sludge application suggests that natural P works best in acidic soils, while it shows poor efficiency in alkaline soils [
20]. Under acidic conditions such as Maamora soil, organic acid anions with oxygen-containing carboxylic groups can form stable complexes with cations such as calcium, aluminum, and iron commonly bound with phosphate [
27]. Organic acid anions loosen cation–oxygen bonds by complexing with cations on the mineral surface and catalyzing the release of cations to solution [
28]. Natural P is more effective under acidic conditions [
20].
The mineral nutrition of plants is one of the major constraints that can affect plants’ main physiological processes (accumulation of organic matter, amount of chlorophyll, and intensity of photosynthesis) [
29]. The main elements in chlorophyll synthesis are nitrogen, copper, zinc, iron, and manganese [
30]. The significant increase of these elements can improve the nutritional condition of the soil, which can reflect the growth of the plant and especially the chlorophyll content [
31,
32]. The application of phosphate sludge increases the capacities of these nutrient elements in the mixtures. It has been previously demonstrated that legumes could immediately benefit from the application of natural phosphate [
33]. In citrus trees, rootstocks can improve many soil constraints, such as saline stress [
34] and low nutrient availability [
35]. The ability to acquire P from the soil of these rootstocks is known as an essential variability [
36], which might explain differences in responses of citrus trees to uptake P, as demonstrated by other authors and as observed in our study [
37,
38]. However, the specific mechanisms that explain the differential responses of cultivated citrus to P fertilization are not yet fully understood [
38] because root growth is limited by low-P availability [
39]. In this context, the effects of P rates and placement might be more pronounced in young trees than in mature ones because the former have a relatively greater nutrient demand and lower P absorption capacity due to their limited root systems [
40]. Supplying phosphate sludge enhances citrus plant growth (except for
C. volkameriana rootstock in the first experiment). It might arise from the fact that P moves in the soil through diffusion and roots create depletion zones that hinder P uptake [
41].
Relative growth for apple plants is related to phosphorus concentrations; Fortuna [
42] confirmed this, which showed that the only plants fertilized with high amounts of phosphate showed renewal of shoot apical growth. They concluded that the renewal of shoot apical growth was related to tissue P concentration. In addition, among the numerous elements influencing plant development during forest establishment are the effects of water availability in the soil on plant growth and how these compromise plant performances prevail [
43]. Sandy soils contain large pores that positively allow more water movement and air circulation but negatively cause water stress and nutrient leaching [
44]. Adding water stress, this medium can be challenging to plant growth and expansion. Plants need water for their growth and development. It is the transporter of mineral nutrients from the soil into the plants’ vascular system [
45]. Water is indispensable for plant growth, especially in the early stages. A water deficit during this phase reduces growth and increases death rates and disease susceptibility [
46,
47]. Plants take up P in its inorganic form as phosphate [
48]. Since inorganic P is usually depleted in soil solutions, P is often the first limiting macronutrient for plant growth under natural conditions [
49,
50] because nutrient availability is generally considered the major resource factor limiting growth in many forest tree species [
51]. We can suggest that the application of phosphate sludge increases forest plant growth with an increase in nutrient elements and water retention.
These results were confirmed with the second experiment using SPS amendment in the poor sand. These results showed higher growth in M3 and M4 mixtures with 20% to 30% phosphate sludge. The supply of SPS positively affects plant growth. This effect was evident in this experiment with the use of poor sand. We can suggest that natural phosphate in the solid phosphate plays an essential role in the growth of fruit and forest plants in the nurseries, but at concentrations of 20–30%. Direct application of ground natural phosphate was proven beneficial to crops on soils [
52]. The natural P has the potential and some benefits of being used as an alternative to mineral fertilizers [
1]. The direct application is available in many countries as a substitutional solution to the expensive water-soluble P fertilizers used for crop production in tropical soils [
1,
53]. The partial acidulation can make the natural P more effective, especially as a superphosphate for some soils and crops. The solubilization of this P can be influenced by oxygen, water, or complexing agents [
54,
55,
56]. Therefore, it is likely that smectite may be the dominant clay mineral present in soil composition that adsorbs high H
2PO
4−. The other possibility may be the fixation of some of the applied or native P on the surface of the clay particles, given the 28% clay content of the soil used in the study [
20]. Furthermore, high concentrations of solid phosphate sludge can negatively affect the growth of fruit and forest plants. This is confirmed by our results with the concentrations of 40 and 50% and might be due to the high level of pH and the clay rate in sludge.
Phosphate is applied continuously because plants only utilize small amounts of phosphate fertilizers. The rest (about 70%) converted rapidly into insoluble complexes (calcium phosphate, aluminum phosphate, and iron phosphate) in the soil [
57,
58,
59]. Moreover, P is often considered one of the main nutrients in agricultural soils [
60]. The major P sources to produce seedlings are the nutrients released slowly from rock phosphate and organic fertilizers in organic nursery production [
61,
62]. The production of quality seedlings also requires intensive fertilization in high-quality nurseries, especially in the early growth stages [
63].