Copper and Zinc Removal from Wastewater Using Alum Sludge Recovered from Water Treatment Plant
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
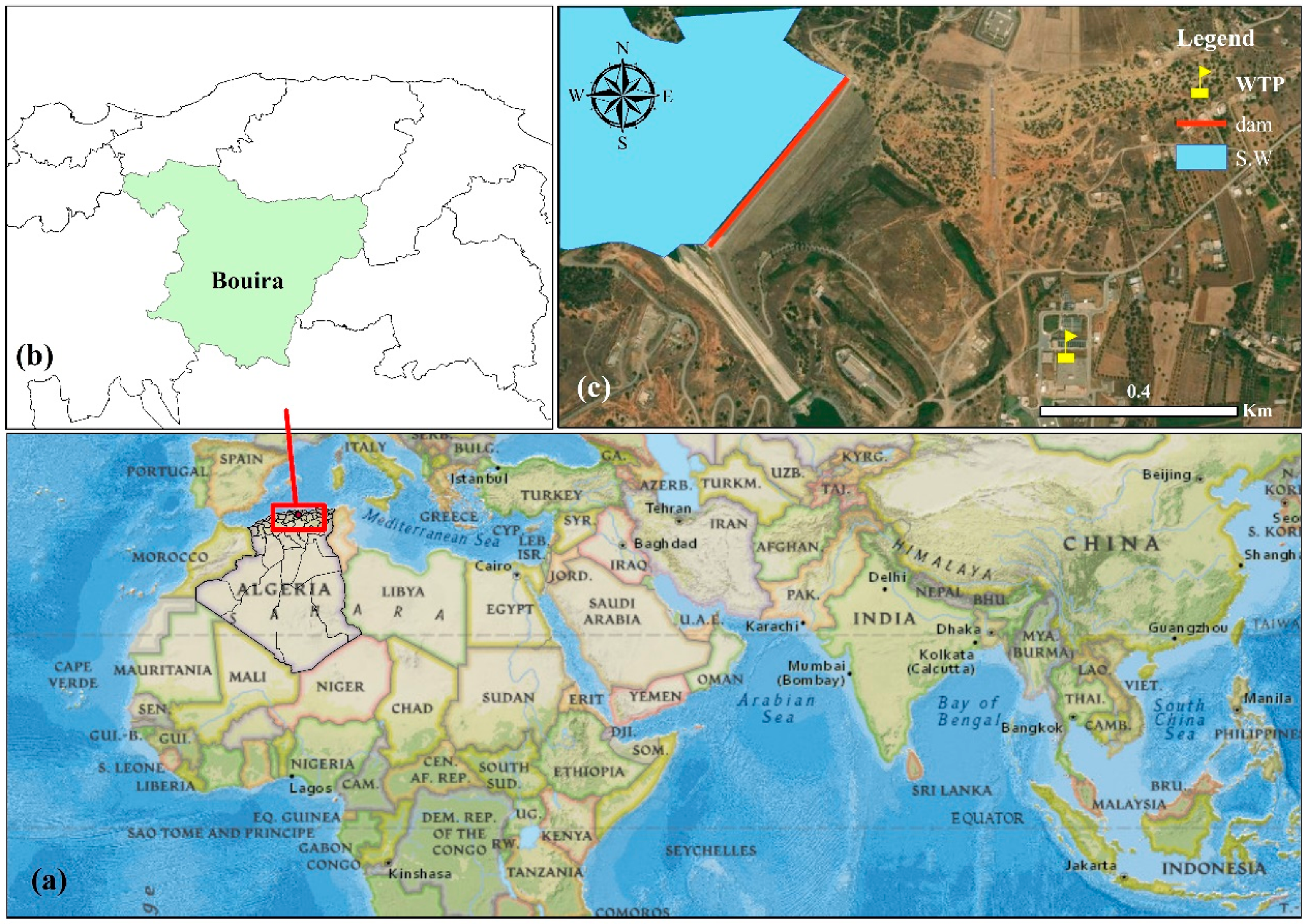
2.1.1. Sludges Sampling and Laboratory Preparation
- Sampling and conservation
- Sludge characterisation
2.1.2. Synthetic Wastewater Preparation
3. Results and Discussion
3.1. Physical and Chemical Characteristics of Alum Sludge
3.2. Characterisation of Alum Sludge
3.2.1. Morphological Characterisation
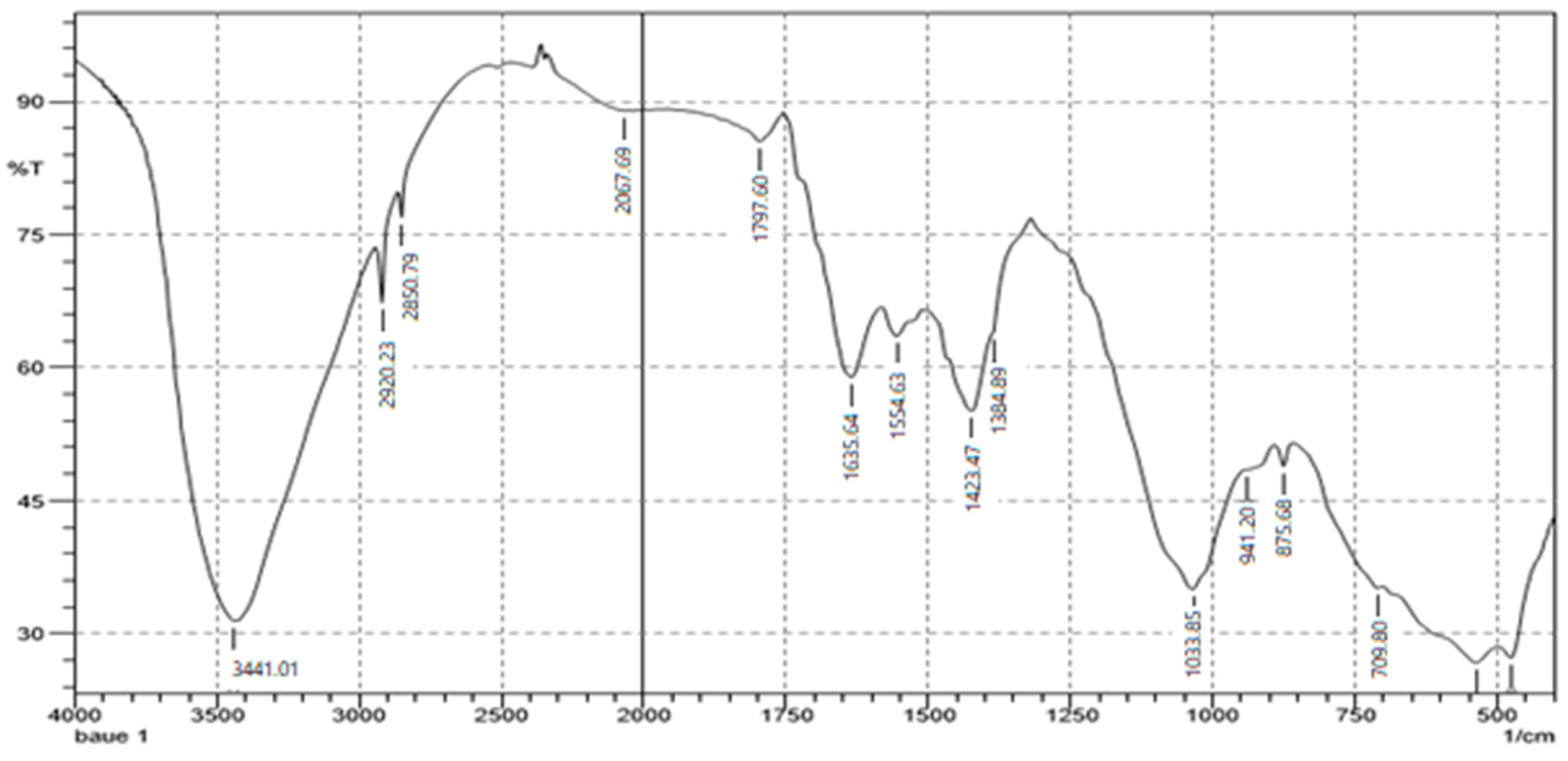
3.2.2. Structural and FTIR Characterisation
3.3. Efficiency of Alum Sludge on Heavy Metal Removal
- (1)
- pH effect
- (2)
- Sludge amount effect
4. Conclusions
- A small amount of Alum sludge with pH = 4 is destined for industrial wastewater.
- An average amount of Alum sludge with pH = 6 is destined for drinking water.
- A high amount of Alum sludge with pH = 8 is destined for wastewater.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, N.; Qiu, Y.; Hu, K.; Huang, C.; Xiang, J.; Li, H.; Xiao, T. One-step synthesis of cake-like biosorbents from plant biomass for the effective removal and recovery heavy metals: Effect of plant species and roles of xanthation. Chemosphere 2021, 266, 129129. [Google Scholar] [CrossRef]
- Ghorpade, A.; Ahammed, M.M. Water treatment sludge for removal of heavy metals from electroplating wastewater. Environ. Eng. Res. 2018, 23, 92–98. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Haynes, R.J. Removal of Pb (II), Cr (III) and Cr (VI) from aqueous solutions using alum-derived water treatment sludge. Water Air Soil Pollut. 2011, 215, 631–643. [Google Scholar] [CrossRef]
- Seehamoke, C.; Sungsitthisawad, W. Efficiency of Removing Heavy Metals from Chemical Oxygen Demand Test Wastewater by Using Alum Sludge from a Surface Water Supply Treatment Plant. Asia-Pac. J. Sci. Technol. 2011, 16, 435–443. [Google Scholar]
- Billah, R.E.K.; Abdellaoui, Y.; Anfar, Z.; Giácoman-Vallejos, G.; Agunaou, M.; Soufiane, A. Synthesis and Characterisation of Chitosan/Fluorapatite Composites for the Removal of Cr (VI) from Aqueous Solutions and Optimised Parameters. Water Air Soil Pollut. 2020, 231, 163. [Google Scholar] [CrossRef]
- Elouahli, A.; Zbair, M.; Anfar, Z.; Ahsaine, H.A.; Khallok, H.; Chourak, R.; Hatim, Z. Apatitic tricalcium phosphate powder: High sorption capacity of hexavalent chromium removal. Surf. Interfaces 2018, 13, 139–147. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Guo, X.; Huang, H. Adsorption of chromium (III) on lignin. Bioresour. Technol. 2008, 99, 7709–7715. [Google Scholar] [CrossRef] [PubMed]
- Cimá-Mukul, C.A.; Abdellaoui, Y.; Abatal, M.; Vargas, J.; Santiago, A.A.; Barrón-Zambrano, J.A. Eco-efficient biosorbent based on leucaena leucocephala residues for the simultaneous removal of Pb (II) and Cd (II) ions from water system: Sorption and mechanism. Bioinorg. Chem. Appl. 2019, 2019, 2814047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abatal, M.; Olguin, M.T.; Abdellaoui, Y.; El Bouari, A. Sorption of Cd (II), Ni (II) and Zn (II) on natural, sodium-, and acid-modified clinoptilolite-rich tuff. Environ. Prot. Eng. 2018, 44, 41–59. [Google Scholar] [CrossRef]
- Teh, C.Y.; Wu, T.Y. The potential use of natural coagulants and flocculants in the treatment of urban waters. Chem. Eng. Trans. 2014, 10, 1603–1608. [Google Scholar]
- Yang, Y.; Tomlinson, D.; Kennedy, S.; Zhao, Y.Q. Dewatered alum sludge: A potential adsorbent for phosphorus removal. Water Sci. Technol. 2006, 54, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babatunde, A.O.; Zhao, Y.Q.; Burke, A.M.; Morris, M.A.; Hanrahan, J.P. Characterisation of aluminium-based water treatment residual for potential phosphorus removal in engineered wetlands. Environ. Pollut. 2009, 157, 2830–2836. [Google Scholar] [CrossRef] [Green Version]
- Blakemore, R.; Chandler, R.; Surrey, T.; Ogilvie, D.; Walmsley, N. Management of Water Treatment Plant Residuals in New Zealand; Water Supply Managers’ Group, New Zealand Water and Wastes Association: Auckland, New Zealand, 1998. [Google Scholar]
- Muisa, N.; Hoko, Z.; Chifamba, P. Impacts of alum residues from Morton Jaffray Water Works on water quality and fish, Harare, Zimbabwe. Phys. Chem. Earth Parts A/B/C 2011, 36, 853–864. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Murcia, M.D.; Gómez, M.; Gómez, E.; García-Izquierdo, C.; Solano, C. Possible Uses for Sludge from Drinking Water Treatment Plants. J. Environ. Eng. 2017, 143, 04016088. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Alexandre, J.; Margem, J.I.; Sánchez, R.; Vieira, C.M.F. Incorporation of sludge waste from water treatment plant into red ceramic. Constr. Build. Mater. 2008, 22, 1281–1287. [Google Scholar] [CrossRef]
- Dassanayake, K.B.; Jayasinghe, G.Y.; Surapaneni, A.; Hetherington, C. A review on alum sludge reuse with special reference to agricultural applications and future challenges. Waste Manag. 2015, 38, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Barakwan, R.A.; Trihadiningrum, Y.; Bagastyo, A.Y. Characterisation of alum sludge from Surabaya Water Treatment Plant, Indonesia. J. Ecol. Eng. 2019, 20, 7–13. [Google Scholar] [CrossRef]
- Ooi, T.Y.; Yong, E.L.; Din, M.F.M.; Rezania, S.; Aminudin, E.; Chelliapan, S.; Rahman, A.A.; Park, J. Optimisation of aluminium recovery from water treatment sludge using Response Surface Methodology. J. Environ. Manag. 2018, 228, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ruziqna, D.P.; Suwartha, N.; Moersidik, S.S.; Adityosulindro, S. Aluminium Recovery from Water Treatment Sludge as Coagulant by Acidification. IOP Conf. Ser. Earth Environ. Sci. 2020, 448, 12045. [Google Scholar] [CrossRef] [Green Version]
- Mazari, L.; Abdessemed, D.; Szymczyk, A. Evaluating Reuse of Alum Sludge as Coagulant for Tertiary Wastewater Treatment. J. Environ. Eng. 2018, 144, 04018119. [Google Scholar] [CrossRef]
- Ren, B.; Lyczko, N.; Zhao, Y.; Nzihou, A. Alum sludge as an efficient sorbent for hydrogen sulfide removal: Experimental, mechanisms and modeling studies. Chemosphere 2020, 248, 126010. [Google Scholar] [CrossRef] [PubMed]
- Rozhkovskaya, A.; Rajapakse, J.; Millar, G.J. Optimisation of zeolite LTA synthesis from alum sludge and the influence of the sludge source. J. Environ. Sci. 2021, 99, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Meng, P.; Pei, H.; Hu, W.; Chen, Y. Phosphorus adsorption characteristics of alum sludge: Adsorption capacity and the forms of phosphorus retained in alum sludge. Mater. Lett. 2018, 229, 31–35. [Google Scholar] [CrossRef]
- Zahari, N.M.; Hua, C.K.; Mohd Sidek, L. Behaviour of Waterworks Alum Sludge for Phosphate Removal. Adv. Mater. Res. 2015, 1113, 764–769. [Google Scholar]
- Tony, M.A. Zeolite-based adsorbent from alum sludge residue for textile wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 2485–2498. [Google Scholar] [CrossRef]
- Ngatenah, S.N.I.; Kutty, S.R.M.; Isa, M.H. Optimization of Heavy Metal Removal from Aqueous Solution Using Groundwater Treatment Plant Sludge (Gwtps). Civ. Eng. 2010, 2010, 1–9. [Google Scholar]
- Ahmad, T.; Ahmad, K.; Ahad, A.; Alam, M. Characterisation of water treatment sludge and its reuse as coagulant. J. Environ. Manag. 2016, 182, 606–611. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Wang, L.; Ren, Y.X.; Han, Y. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep. Purif. Technol. 2006, 51, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Berkowitz, J.; Anderson, M.A.; Graham, R.C. Laboratory investigation of aluminum solubility and solid-phase properties following alum treatment of lake waters. Water Res. 2005, 39, 3918–3928. [Google Scholar] [CrossRef]
- Awab, H.; Paramalinggam, P.T.; Yusoff, A.R.M. Characterisation of Alum Sludge for Reuse and Disposal. Malays. J. Fundam. Appl. Sci. 2012, 8, 251–255. [Google Scholar] [CrossRef]
- Tantawy, M.A. Characterisation and pozzolanic properties of calcined alum sludge. Mater. Res. Bull. 2015, 61, 415–421. [Google Scholar] [CrossRef]
- Ling, Y.P.; Tham, R.-H.; Lim, S.-M.; Fahim, M.; Ooi, C.-H.; Krishnan, P.; Matsumoto, A.; Yeoh, F.-Y. Evaluation and reutilization of water sludge from fresh water processing plant as a green clay substituent. Appl. Clay Sci. 2017, 143, 300–306. [Google Scholar] [CrossRef]
- Desta, W.M.; Bote, M.E. Wastewater treatment using a natural coagulant (Moringa oleifera seeds): Optimisation through response surface methodology. Heliyon 2021, 7, e08451. [Google Scholar] [CrossRef] [PubMed]








| Parameters | Sludge A |
|---|---|
| Temperature (°C) | 17.20 |
| pH | 7.12 |
| Electrical Conductivity (dS/m) | 0.96 |
| TDs (PPM) | 482 |
| Salinity (PSU) | 0.48 |
| Copper (mg/g) | 0.000324 |
| Zinc (mg/g) | 0.00125 |
| Specific surface (m2/g) | 176.7934 |
| Pore size (nm) | 12.1570 |
| Pore volume (cm3/g) | 0.522754 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abba, A.B.; Saggai, S.; Touil, Y.; Al-Ansari, N.; Kouadri, S.; Nouasria, F.Z.; Najm, H.M.; Mashaan, N.S.; Eldirderi, M.M.A.; Khedher, K.M. Copper and Zinc Removal from Wastewater Using Alum Sludge Recovered from Water Treatment Plant. Sustainability 2022, 14, 9806. https://doi.org/10.3390/su14169806
Abba AB, Saggai S, Touil Y, Al-Ansari N, Kouadri S, Nouasria FZ, Najm HM, Mashaan NS, Eldirderi MMA, Khedher KM. Copper and Zinc Removal from Wastewater Using Alum Sludge Recovered from Water Treatment Plant. Sustainability. 2022; 14(16):9806. https://doi.org/10.3390/su14169806
Chicago/Turabian StyleAbba, Alia Besma, Sofiane Saggai, Youcef Touil, Nadhir Al-Ansari, Saber Kouadri, Fatima Zohra Nouasria, Hadee Mohammed Najm, Nuha S. Mashaan, Moutaz Mustafa A. Eldirderi, and Khaled Mohamed Khedher. 2022. "Copper and Zinc Removal from Wastewater Using Alum Sludge Recovered from Water Treatment Plant" Sustainability 14, no. 16: 9806. https://doi.org/10.3390/su14169806
APA StyleAbba, A. B., Saggai, S., Touil, Y., Al-Ansari, N., Kouadri, S., Nouasria, F. Z., Najm, H. M., Mashaan, N. S., Eldirderi, M. M. A., & Khedher, K. M. (2022). Copper and Zinc Removal from Wastewater Using Alum Sludge Recovered from Water Treatment Plant. Sustainability, 14(16), 9806. https://doi.org/10.3390/su14169806









