Application of Sequential Combination of Electro-Coagulation/Electro-Oxidation and Adsorption for the Treatment of Hemodialysis Wastewater for Possible Reuse
Abstract
:1. Introduction
2. Materials and Methods
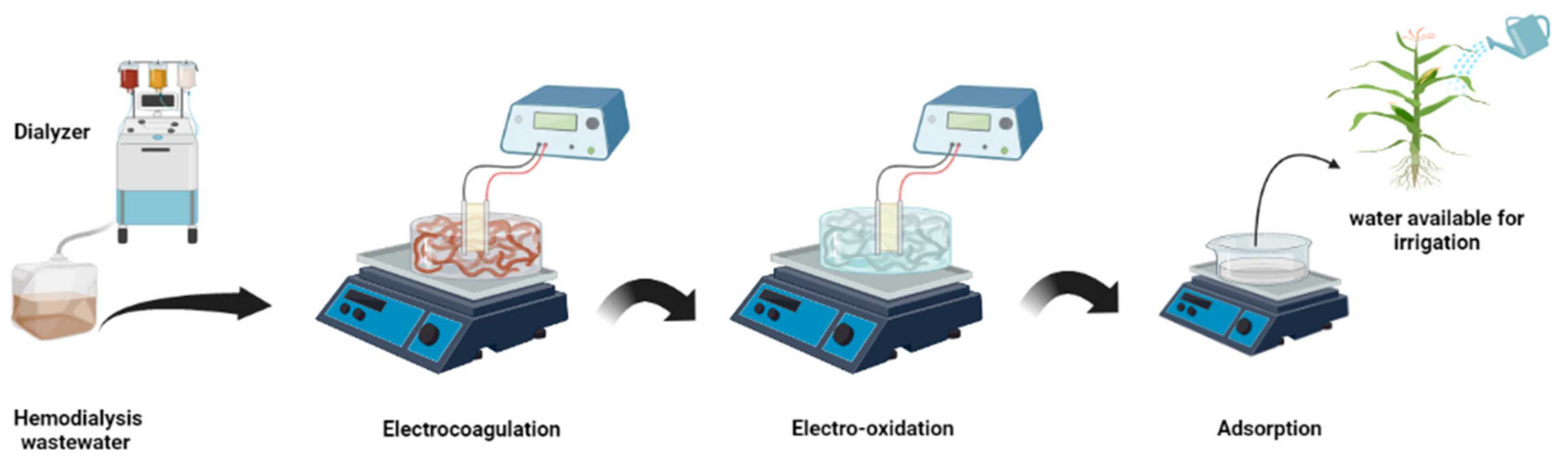
2.1. Experimental Procedure
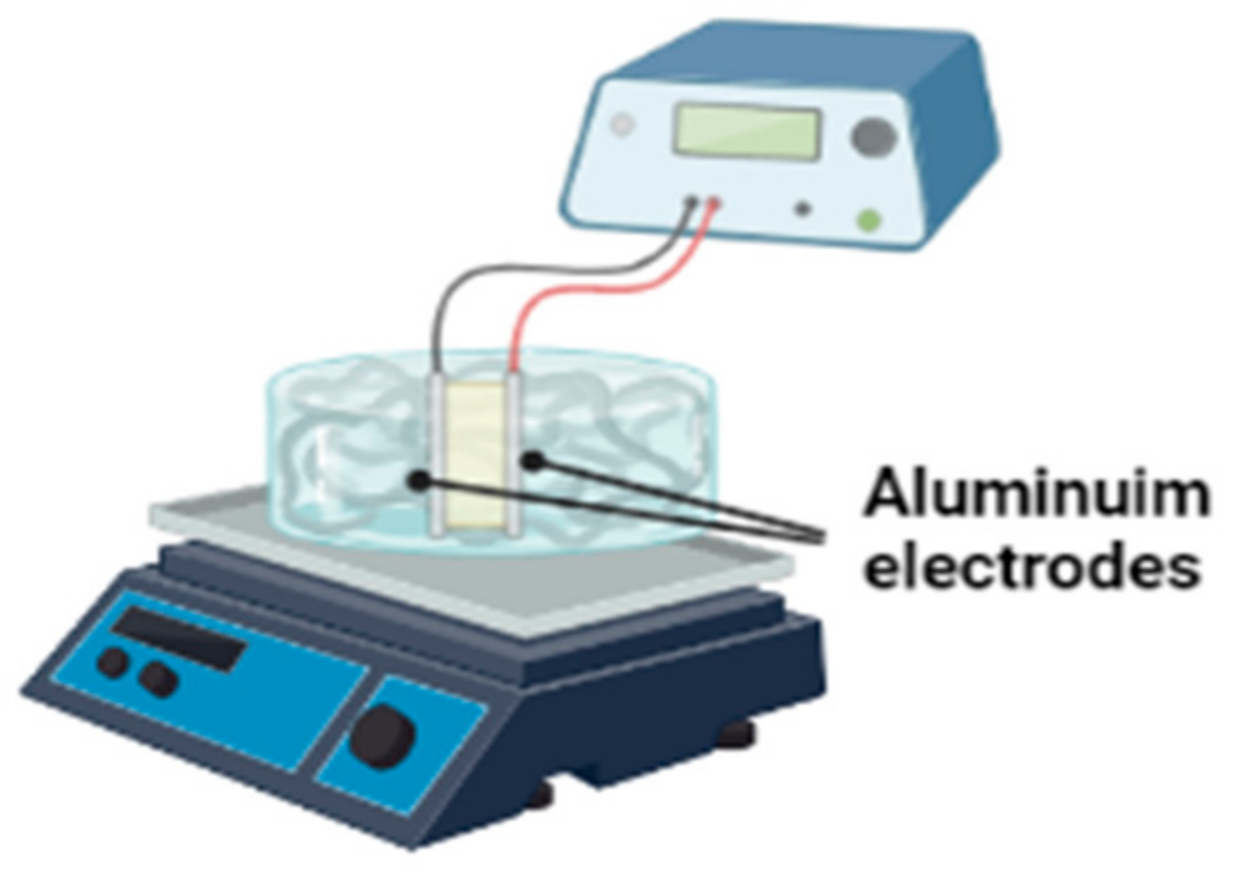
2.1.1. Electro-Chemical Reactors
Electro-Coagulation Reactor
Electro-Oxidation Reactor
2.1.2. Activated Carbon (AC) Process
2.2. Analytical Techniques
2.3. Energy Consumption Calculation
2.4. Phytotoxicity Test
3. Results and Discussions
3.1. Characteristics of HWW
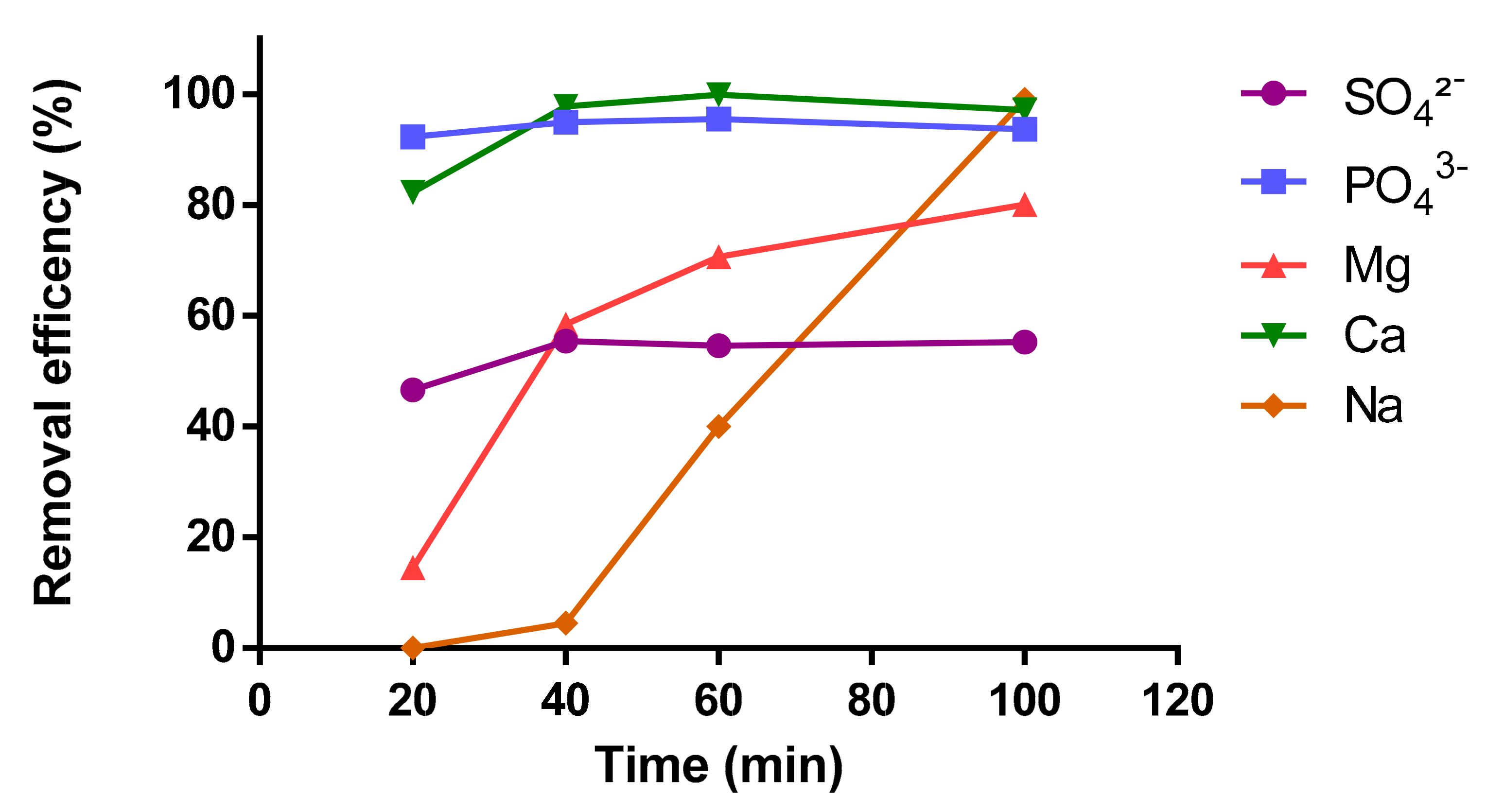
3.2. Effect of Electrolysis Time on Contaminants Removal
3.3. Effect of Electro-Oxidation Treatment on HWW Characteristics
3.4. Efficiency of HWW Treatment by Adsorption onto Activated Carbon
3.5. SAR Hazard of HWW for Possible Reuse in Irrigation
3.6. Evaluation of the Phytotoxicity of RO, Raw HWW and Salty Water on the Germination Test in Petri Dishes
3.7. Germination Tests on Pots
3.7.1. Germination Rate Index “GRI”
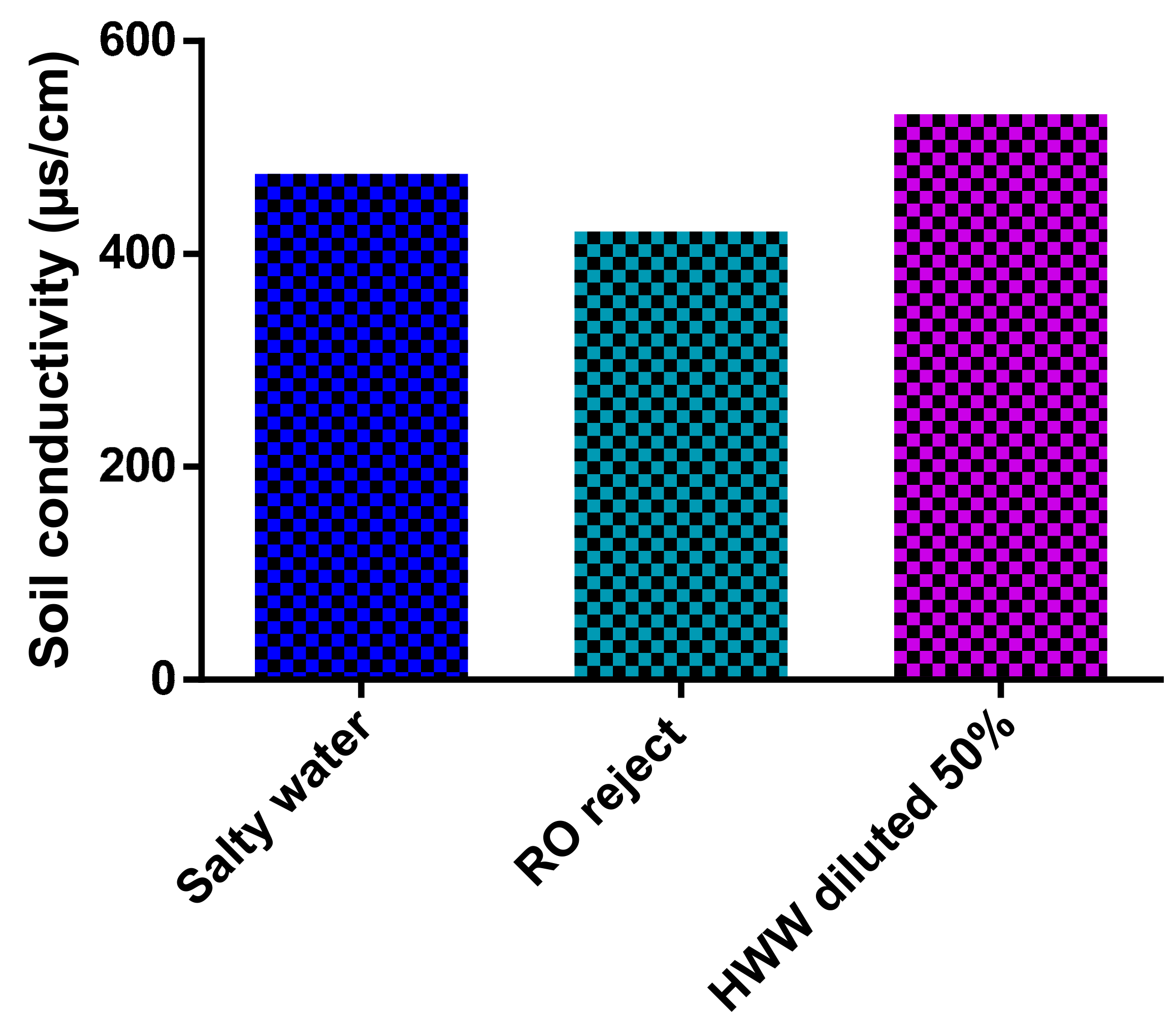
3.7.2. Effect of Irrigation with Treated HWW on the Soil Salinity
3.7.3. Progress towards Zero Waste Fluid in Hemodialysis
4. Conclusions
- 1.
- With the EO process, the removal performance of COD, total nitrogen, and Mg was significantly better and reached 100, 83, and 89%, respectively, after 100 min of treatment, compared to sulfate and phosphate being only 12 and 23%, respectively.
- 2.
- With the EC process, the removal efficiency of Mg and Na increased with increasing electrolysis time and achieved 80% and 99%, respectively, after 100 min. The optimal time was 40 min for the Ca, sulfate, and phosphate removal efficiency. A high-rate of performance reduction was after 20 min for COD.
- 3.
- The increased amount of activated carbon from 0.5 to 1 g increased the removal efficiency of calcium and a slight increase of phosphate.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertrand-Krajewski, J.-L.; Barraud, S.; Chocat, B. Need for improved methodologies and measurements for sustainable management of urban water systems. Environ. Impact Assess. Rev. 2000, 20, 323–331. [Google Scholar] [CrossRef]
- Mahdavi, M.; Mahvi, A.H.; Salehi, M.; Sadani, M.; Biglari, H.; Tashauoei, H.R.; Ebrahimi, A.; Yengejeh, R.J.; Fatehizadeh, A. Wastewater reuse from hemodialysis section by combination of coagulation and ultrafiltration processes: Case study in Saveh-Iran Hospital. Desalination Water Treat. 2020, 193, 274–283. [Google Scholar] [CrossRef]
- Kumari, A.; Maurya, N.S.; Tiwari, B. Hospital Wastewater Treatment Scenario around the Globe. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 549–570. ISBN 978-0-12-819722-6. [Google Scholar]
- Khan, M.T.; Shah, I.A.; Ihsanullah, I.; Naushad, M.; Ali, S.; Shah, S.H.A.; Mohammad, A.W. Hospital wastewater as a source of environmental contamination: An overview of management practices, environmental risks, and treatment processes. J. Water Process Eng. 2021, 41, 101990. [Google Scholar] [CrossRef]
- Timraz, K.; Xiong, Y.; Al Qarni, H.; Hong, P.-Y. Removal of bacterial cells, antibiotic resistance genes and integrase genes by on-site hospital wastewater treatment plants: Surveillance of treated hospital effluent quality. Environ. Sci. Water Res. Technol. 2016, 3, 293–303. [Google Scholar] [CrossRef]
- Okuhama, A.; Ishikane, M.; Katagiri, D.; Kanda, K.; Nakamoto, T.; Kinoshita, N.; Nunose, N.; Fukaya, T.; Kondo, I.; Katano, H.; et al. Detection of SARS-CoV-2 in Hemodialysis Effluent of Patient with COVID-19 Pneumonia, Japan. Emerg. Infect. Dis. 2020, 26, 2757–2760. [Google Scholar] [CrossRef]
- Jmii, H.; Gharbi-Khelifi, H.; Assaoudi, R.; Aouni, M. Detection of SARS-CoV-2 in the sewerage system in Tunisia: A promising tool to confront COVID-19 pandemic. Futur. Virol. 2021, 16, 751–759. [Google Scholar] [CrossRef]
- Machado, C.K.; Pinto, L.H.; Del Ciampo, L.F.; Lorenzi, L.; Correia, C.H.G.; Häder, D.P.; Erzinger, G.S. Potential environmental toxicity from hemodialysis effluent. Ecotoxicol. Environ. Saf. 2014, 102, 42–47. [Google Scholar] [CrossRef]
- Resolução CONAMA No 430 DE 13/05/2011-Federal-LegisWeb. Available online: https://www.legisweb.com.br/legislacao/?id=114770 (accessed on 2 June 2022).
- Dehghani, M.; Seresht, S.S.; Hashemi, H. Treatment of hospital wastewater by electrocoagulation using aluminum and iron electrodes. Int. J. Environ. Health Eng. 2014, 3, 15. [Google Scholar] [CrossRef]
- Abarkan, A.; Grimi, N.; Métayer, H.; Houssaïni, T.S.; Legallais, C. Electrodialysis Can Lower the Environmental Impact of Hemodialysis. Membranes 2021, 12, 45. [Google Scholar] [CrossRef]
- Gharbi, M.B. Renal replacement therapies for end-stage renal disease in North Africa. Clin. Nephrol. 2011, S17–S19. [Google Scholar] [CrossRef]
- Barraclough, K.A.; Agar, J.W.M. Green Nephrology. Nat. Rev. Nephrol. 2020, 16, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Counil, É.; Cherni, N.; Kharrat, M.; Achour, A.; Trimech, H. Trends of Incident Dialysis Patients in Tunisia Between 1992 and 2001. Am. J. Kidney Dis. 2008, 51, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Chaabouni, Y.; Yaich, S.; Khedhiri, A.; Zayen, M.A.; Kharrat, M.; Kammoun, K.; Jarraya, F.; Hmida, M.B.; Damak, J.; Hachicha, J. Profil Épidémiologique de l’insuffisance Rénale Chronique Terminale Dans La Région de Sfax. Pan Afr. Med. J. 2018, 29. [Google Scholar] [CrossRef] [PubMed]
- Cherni, Y.; Elleuch, L.; Messaoud, M.; Kasmi, M.; Chatti, A.; Trabelsi, I. Recent Technologies for Leachate Treatment: A Review. Euro-Mediterr. J. Environ. Integr. 2021, 6, 79. [Google Scholar] [CrossRef]
- Jallouli, S.; Wali, A.; Buonerba, A.; Zarra, T.; Belgiorno, V.; Naddeo, V.; Ksibi, M. Efficient and sustainable treatment of tannery wastewater by a sequential electrocoagulation-UV photolytic process. J. Water Process Eng. 2020, 38, 101642. [Google Scholar] [CrossRef]
- Aniyikaiye, T.E.; Oluseyi, T.; Odiyo, J.O.; Edokpayi, J.N. Physico-Chemical Analysis of Wastewater Discharge from Selected Paint Industries in Lagos, Nigeria. Int. J. Environ. Res. Public Health 2019, 16, 1235. [Google Scholar] [CrossRef] [Green Version]
- Qasim, W.; Mane, A. Characterization and treatment of selected food industrial effluents by coagulation and adsorption techniques. Water Resour. Ind. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Boujelben, R.; Ellouze, M.; Sayadi, S. Detoxification Assays of Tunisian Tannery Wastewater under Nonsterile Conditions Using the Filamentous Fungus Aspergillus niger. BioMed Res. Int. 2019, 2019, 9020178. [Google Scholar] [CrossRef] [Green Version]
- Taleshi, M.S.A.; Nejadkoorki, F. Characterization of Hemodialysis Reverse Osmosis Wastewater From Yazd Educational Hospitals. Avicenna J. Environ. Health Eng. 2016, 3, 5067. [Google Scholar] [CrossRef]
- Tarrass, F.; Benjelloun, M.; Benjelloun, O. Recycling Wastewater After Hemodialysis: An Environmental Analysis for Alternative Water Sources in Arid Regions. Am. J. Kidney Dis. 2008, 52, 154–158. [Google Scholar] [CrossRef]
- Pescod, M.B. Wastewater Treatment and Use in Agriculture; FAO irrigation and drainage paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; ISBN 978-92-5-103135-3. [Google Scholar]
- WHO. Guidelines for the safe use of wastewater, excreta and greywater. In Volume 2: Wastewater Use in Agriculture; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent—A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Helmy, E.; Nassef, E.; Hussein, M. Study on the Removal of Water Hardness by Electrocoagulation Technique. Int. J. Chem. Biochem. Sci. 2017, 17, 1–17. [Google Scholar]
- Djuricic, T.; Malinovic, B.; Bjelić, D. The Phosphate Removal Efficiency Electrocoagulation Wastewater Using Iron and Aluminum Electrodes. Bull. Chem. Technol. Bosnia Herzegovina 2016, 47, 32–38. [Google Scholar]
- Hamada, M.; Abu Ghalwa, N.; Farhat, N.B.; Al Mahllawi, K.; Jamee, N. Optimization of Electrocoagulation on Removal of Wastewater Pollutants. Int. J. Waste Resour. 2018, 8, 4–9. [Google Scholar] [CrossRef]
- Hamdan, S.S.; El-Naas, M.H. An electrocoagulation column (ECC) for groundwater purification. J. Water Process Eng. 2014, 4, 25–30. [Google Scholar] [CrossRef]
- Lan, Y.; Coetsier, C.; Causserand, C.; Serrano, K.G. Feasibility of Micropollutants Treatment by Coupling Nanofiltration and Electrochemical Oxidation: Case of Hospital Wastewater. Int. J. Chem. React. Eng. 2015, 13, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, U.; Jang, M.; Jung, S.P.; Park, D.; Park, S.J.; Yu, H.; Oh, S.-E. Electrochemical Removal of Ammonium Nitrogen and COD of Domestic Wastewater using Platinum Coated Titanium as an Anode Electrode. Energies 2019, 12, 883. [Google Scholar] [CrossRef] [Green Version]
- Rossrucker, L.; Samaniego, A.; Grote, J.-P.; Mingers, A.M.; Laska, C.A.; Birbilis, N.; Frankel, G.S.; Mayrhofer, K.J.J. The PH Dependence of Magnesium Dissolution and Hydrogen Evolution during Anodic Polarization. J. Electrochem. Soc. 2015, 162, C333–C339. [Google Scholar] [CrossRef] [Green Version]
- Sajjadi, B.; Chen, W.-Y.; Egiebor, N.O. A Comprehensive Review on Physical Activation of Biochar for Energy and Environmental Applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater Reuse in Irrigation: A Microbiological Perspective on Implications in Soil Fertility and Human and Environmental Health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef] [Green Version]
- Berrada, S.; Squalli, F.Z.; Squalli, H.T.; Hannin, M.; Oualti, A.E.; Lalami, A.E.O. Recyclage des effluents du service d’hémodialyse de l’hôpital Al Ghassani de la ville de Fès: Caractérisation avant et après traitement (Effluent recycling of hemodialysis service of Al Ghassani hospital of Fez: Characterization before and after treatment). J. Mater. Environ. Sci. 2014, 14, 2265–2277. [Google Scholar]
- Salian, R.; Wani, S.; Reddy, R.; Patil, M. Effect of Brewery Wastewater Obtained from Different Phases of Treatment Plant on Seed Germination of Chickpea (Cicer Arietinum), Maize (Zea Mays), and Pigeon Pea (Cajanus Cajan). Environ. Sci. Pollut. Res. 2018, 25, 9145–9154. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elwahed, M.S. Influence of long-term wastewater irrigation on soil quality and its spatial distribution. Ann. Agric. Sci. 2018, 63, 191–199. [Google Scholar] [CrossRef]
- Gautham, D. Effect of Dairy Effluent on Wheat (Triticumaestivum). J. Ecobiol. 1992, 4, 111–115. [Google Scholar]
- GilPavas, E.; Correa-Sanchez, S. Assessment of the optimized treatment of indigo-polluted industrial textile wastewater by a sequential electrocoagulation-activated carbon adsorption process. J. Water Process Eng. 2020, 36, 101306. [Google Scholar] [CrossRef]
- Moore, F. Impacts of Waste Water Irrigation on Soils and Crops in Suburban Area of Shiraz, Iran. Acta Geochimica 2006, 25, 214. [Google Scholar]
- Wang, Z.; Teng, X.; Xie, M.; Cheng, X.; Li, J. Pretreatment of polyvinyl alcohol by electrocoagulation coupling with catalytic oxidation: Performance, mechanism and pathway. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]

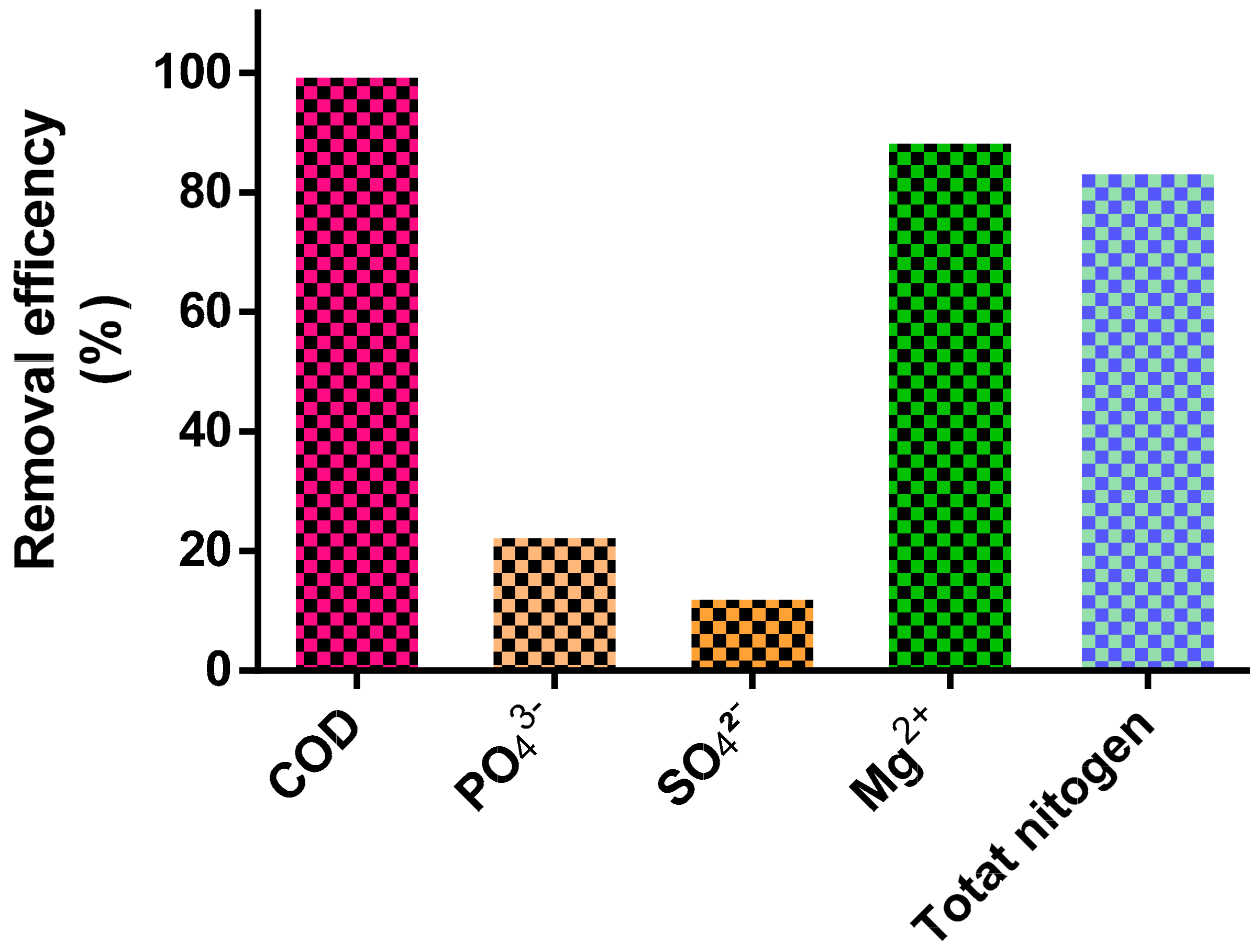


| Parameters | Current Study | HWW from Brazil [8] | HWW from Iran [21] | HWW from Morocco [22] | FAO/WHO Standards for Irrigation Water [23,24] |
|---|---|---|---|---|---|
| pH | 7.46 | 7.49 | 7.84 | 7.84 | 6–8.5 |
| Conductivity (mS/cm) | 13.53 | 4.08 | 0.854 | 13.2 | 0.3–0.7 |
| Salinity (g/L) | 9.113 | 9.42 | - | - | - |
| COD (mg/L) | 262.033 | 832 | 16.10 | - | 5–45 |
| Cl− (mg/L) | 3976 | - | 25.93 | 289 | 30 |
| Total nitrogen (mg N/L) | 143 | 126.7 | - | - | - |
| PO43− (mg/L) | 6.472 | 53.95 | - | - | - |
| SO42− (mg/L) | 110.67 | 23 | 133.86 | 80.4 | 0–20 |
| Mg2+ (mg/L) | 13.88 | - | - | - | - |
| Ca2+ (mg/L) | 21.091 | - | - | - | - |
| Na+(mg/L) | 3757 | - | - | - | - |
| SAR Values (meq/L) | Sodium Hazard to Soil |
|---|---|
| 0–10 | Low |
| 10–18 | Medium |
| 18–26 | High |
| >26 | Very high |
| Water Type | HWW | HWW Treated | Salty Water | RO Reject |
|---|---|---|---|---|
| SAR | 157.13 | 1.78 | 1253 | 27 |
| Na (%) | 97 | 1.84 | 99 | 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jallouli, S.; Chouchene, K.; Ben Hmida, M.; Ksibi, M. Application of Sequential Combination of Electro-Coagulation/Electro-Oxidation and Adsorption for the Treatment of Hemodialysis Wastewater for Possible Reuse. Sustainability 2022, 14, 9597. https://doi.org/10.3390/su14159597
Jallouli S, Chouchene K, Ben Hmida M, Ksibi M. Application of Sequential Combination of Electro-Coagulation/Electro-Oxidation and Adsorption for the Treatment of Hemodialysis Wastewater for Possible Reuse. Sustainability. 2022; 14(15):9597. https://doi.org/10.3390/su14159597
Chicago/Turabian StyleJallouli, Sameh, Khawla Chouchene, Mohamed Ben Hmida, and Mohamed Ksibi. 2022. "Application of Sequential Combination of Electro-Coagulation/Electro-Oxidation and Adsorption for the Treatment of Hemodialysis Wastewater for Possible Reuse" Sustainability 14, no. 15: 9597. https://doi.org/10.3390/su14159597
APA StyleJallouli, S., Chouchene, K., Ben Hmida, M., & Ksibi, M. (2022). Application of Sequential Combination of Electro-Coagulation/Electro-Oxidation and Adsorption for the Treatment of Hemodialysis Wastewater for Possible Reuse. Sustainability, 14(15), 9597. https://doi.org/10.3390/su14159597







