Abstract
Among the Mediterranean aromatic plants, Ruta tuberculata Forssk. (Rutaceae) has been widely used as a traditional natural remedy against various disorders resulting from its divers’ pharmacological virtues. The aim of this study is to characterize for the first time the phenolic profile of its ethyl acetate (EtOAcE) and acetonic (AcE) extracts and to screen their in vitro antioxidant, antidiabetic, and neuroprotective activities. Phenolic content was determined using spectrophotometric and cLC-DAD analysis. Pharmacologically, in vitro antioxidant power was evaluated using six different antioxidant methods. Moreover, the antidiabetic and neuroprotective capacities were assessed in vitro by determining the α-amylase, α-glucosidase, and acetylcholinesterase inhibitory activities. Phytochemically, the highest flavonoid content was found in EtOAcE where the major identified compounds were myrecetin, rutin, sylimarin, naringenin, and quercetin. In presence of other phenolic acids, gallic acid was exclusively detected in AcE. Furthermore, both R. tuberculata extracts showed significantly remarkable antioxidant activities, especially the EtOAcE. Interestingly, AcE strongly inhibited the acetylcholinesterase and α-glucosidase, with the respective IC50 values of 20.48 ± 0.2 and 104.5 ± 1.8 µg/mL. In this study, we also reported the nutritional quality associated with the identified phytocompounds. R. tuberculata organic extracts may offer exciting reserves to achieve new anti-diabetic and anti-Alzheimer drugs which have also antioxidant potential.
1. Introduction
Diabetes mellitus (DM) is an epidemic disease, that raises the most public health challenges in the entire world. Considering its current alarming incidence rate, the world health organization predicted that in the year 2030, DM will expand to be the seventh major cause of death worldwide [1]. In this metabolic disorder, the blood glucose level increases markedly as a result of insufficient insulin synthesis or secretion or the inefficiency in binding to its receptor (IR). Indeed, children, adults, and pregnant women were the frequent targets of types (1,2), and gestational diabetes [2,3]. Indeed, it has been documented that hyperglycemia but also fasting hypoglycemia may enhance the Alzheimer’s incidence in T2DM cases [4]. Besides, these distinct illnesses may share the common causes of their progression such as insulin signaling pathway, glucose metabolism relationship, chronic inflammation, and diseases associated with oxidative stress [4,5,6]. Indeed, it was reported that the disruption of insulin signaling in muscle or glucose transport via GLUTs in neurons may be involved in the progression of many neurodegenerative diseases such as AD [4,5]. Indeed, the high circulating levels of glucose and lipids may increase the accumulation of amyloid beta (Aβ) oligomers. These oligomers can markedly inhibit the auto-phosphorylation of insulin receptors (IR) in dendrites of hippocampal neurons, which in return decrease the ability of insulin to stimulate specific signaling pathways in the AD brain, such as the PI3K/AKT/m-TOR pathway, promoting synaptic loss [5].
In addition, chronic inflammatory phenomena and oxidative stress are presumed as the two key risk factors related to diabetes and AD [5,6,7]. However, some undesirable effects may be seen in T2DM and AD patients resulting from the daily consumption of synthetic anti-diabetic and/or anti-cholinesterase inhibitor drugs as a basic treatment. Moreover, the excessive use of some non-steroidal anti-inflammatory and antioxidant drugs, to reduce the complications of those pathologies, often may increase the severity of the medication’s side effects [3,6,8,9].
Recently, many pharmacological investigations demonstrated that natural compounds can display a key role in the prevention of chronic diseases [9]. Indeed, the free radicals’ overproduction or their accumulation may cause or involve complicating factors in several disorders, in particular, Alzheimer’s and diabetes mellitus injuries as well as cancer [9,10,11]. Nowadays, the recent poly-pharmacology trend is to elaborate on new drugs that can target common biological pathways involving at once in AD and T2DM. Indeed, the researchers are showing an increased interest in medicinal plants and their benefic effects as the desired resource of natural drugs which are able to enhance or prevent various chronic illnesses and degenerative diseases [3,12,13]. In fact, herbs have been reported to exhibit potent pharmacological acts including antioxidant, anti-inflammatory, antidiabetic, and anticancer activities [3,6,14]. Moreover, their neuroprotective and anti-cholinesterase capacities were newly documented in the absence of available therapies for the management of progressive neuron impairment and neuronal loss [4,15].
Ruta tuberculata Forssk. is a perennial aromatic herb that belongs to the Ruta (haplopylum) genus from the Rutaceae family which has been growing in all Mediterranean regions, especially the tropical and temperate areas [7,13,16]. Based on green foliage with yellow flowers, more than 1800 plants were classified as Ruta species [9,13,17]. For its safety and benefic effects, Algerian R. tuberculata is traditionally used as a natural therapy in the treatment of skin pathologies, and gastric and intestinal disorders, and as calming and analgesic agents to prevent some neurological problems in children [9,13,16]. Furthermore, this plant was likely prescribed to also treat divers’ metabolic disorders such as diabetes, cardiovascular and hypertension illness and to alleviate rheumatism, tonsillitis, menses disturbance, and infertility in women [18]. In fact, many investigations affirmed that some Ruta species seemed to have various therapeutic virtues and antioxidant potential with an attractive capacity to preserve diverse injuries resulting from oxidative stress, through the inhibition of free radical production mechanisms and enhancement of antioxidant signaling pathways [16,19,20]. Moreover, their extensive anti-inflammatory and neuroprotective effects have also been studied in different neurodegenerative disorders models, particularly, cerebral ischemia, and Parkinson’s and Alzheimer’s diseases [6,13,15,20,21]. In fact, it has been reported that Ruta plants revealed mostly a high level of essential oils [16,20], coumarine derivatives and alkaloids [12], lignanes [22], and polyphenols [6,19,23]. Indeed, the beneficial properties, which Ruta species displayed, were demonstrated to be related to their content in diverse classes of polyphenols [9,13,23,24]. Alternatively, the effective lack of studies that provide information in-depth on the phenolic characterization of Algerian R. tuberculata extracts was noted in literature.
Phenolic compounds could also act as a protective metabolic barrier within the intestinal mucosa where the nutritional quality of them is probably related to their remarkable physicochemical and pharmacological properties, leading to the prevention of several diseases and helping to digest easily complex macromolecules [11].
The aims of this study are to characterize for the first time the phenolic profile of the ethyl acetate and acetonic extracts from Algerian R. tuberculata aerial parts and to assess their in vitro antioxidant, anti-diabetic, and neuroprotective activities.
2. Materials and Methods
2.1. Chemicals
Methanol and other organic solvents (HPLC-grade) used in the present work were purchased from Sigma-Aldrich. Acetylthiocholine iodide (ACI) reagent was purchased from BioChemica. Pure phenolic standards, Acetylcholinesterase (AChE), Yeast α-glucosidase enzyme, Pancreatic α-amylase enzyme (1U), Acarbose, starch, Neocuproine, Galanthamine, 1,1-Diphenyl-2-picryl- hydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS), Butylated hydroxytoluene BHT (C15H24O), Butylate hydroxyanisole BHA (C11H16O2), Vanillin (C8H8O3), Ascorbic Acid (C6H8O6) and the other chemicals were obtained from Sigma-Aldrich and Fluka.
2.2. Plant Material and Extraction
Ruta tuberculata Forssk. aerial parts (Voucher number: Fl.AA 86 86 1775/TRO-28101397; https://www.ipni.org/n/775152-1 accessed on 18 July 2022) were obtained in March 2017 from the southern area Rase El-miaade of Ouled djellal-Algeria (latitude 34.1846; longitude 4.44955).
Acetonic (AcE) and ethyl acetate (EtOAcE) extracts of R. tuberculata aerial parts were prepared according to the previous methods [25,26]. Firstly, the vegetal material was subjected to a cold maceration in petroleum ether for 4 h in ratio 1:10 (w:v) and then was filtrated. Then, the obtained residues from the petroleum ether extraction were macerated in the EtOAc or acetone in ratio 1:10 (w:v) for 48 days. Acetonic and EtOAc crude extracts were filtered under reduced pressure then dried in the oven at 35 °C and stored at −4 °C. Obtained extracts were weighted to determine the extraction yields using the following equation Equation (1):
% Yield = (Weight of dry extract/Weight of taken plant for extraction) × 100.
2.3. Phytochemical Investigation
2.3.1. Spectrophotometric Determination of Total Phenolic Content
The total polyphenols content (TPC) was determined using the standardized Folin-Ciocalteu’s method [27]. The mixture containing 1 mL of Folin-Ciocalteu’s (10% in distilled H2O) and 200 µL of each extract at different concentrations or gallic acid (20–200 µg/mL) was prepared and stored for 5 min before the addition of carbonate sodium (Na2CO3) solution (7.5%, 800 µL). Absorbances were readied at 765 nm after an incubation period of 2 h at room temperature.
Total flavonoid content (TFC) was estimated by following yellowish complex (AlCl3-flavonoid) formation as previously described [28]. A mixture of 1 mL of aluminum chloride (AlCl3) solution and 1 mL of each extract or quercetin as standard (4–20 µg/mL) was prepared and measured at 430 nm. After an incubation period of 10 min in dark, TPC value of the studied extracts was determined according to the linear regression equation of quercetin curve (y = 0.036x + 0.127; R2 = 0.997) and the results were expressed as mg quercetin equivalent (mg QE/g d.E).
The quantification of the total condensed tannins content (CTC) present in R. tuberculata crudes extracts was carried out spectrophotometrically [29]. Briefly, 1.5 mL of vanillin solution (4%) was added to the reaction mixture of 25 µL of each extract or catechin as standard (20–200 µg/mL) and 750 µL of hydrochloric acid HCl (30%). After 15 min of the incubation period, the absorbances of the mixtures were determined at 500 nm. CTC amount was calculated from the calibration equation of catechin curve (y = x 0.0022 + 0.043; R2 = 0.999). Results were expressed in mg catechin equivalent per g of dried extract (mg CE/g d.E).
2.3.2. Characterization of Phenolic Compounds Using cLC-DAD Analysis
The chromatographic screening of the studied extracts was carried out as described by Erenler et al. [30]. The mobile phase consisted of eluent A which contained 0.1% formic acid in water and eluent B, containing methanol (HPLC-grade). A volume of 20 µL from the mobile phase was injected with a flow rate of 0.7 mL/min at 40 °C. The extracts and standards to be analyzed have been solubilized at a dose of 1 mg/mL in a methanol HPLC-grade and then filtrated using a cellulose filter membrane (part no. 3150-0576, 47 mm, pore size 0.45 µm, 100/pk, Germany).
2.4. In Vitro Antioxidant Assay
2.4.1. DPPH Free Radical Test
The scavenging activity of its crude EtOAc and acetonic extracts of the plant was assessed using the spectrophotometric method of Blois [31], based on the color turn of the purple solution of the stable free radical 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) to yellow-colored solution in presence of an antioxidant substance. The synthetic antioxidants BHA and BHT (0.78–50 μg/mL) or pure phenolic compounds gallic acid and quercetine (10–200 μg/mL) were used as references. In microplate 96 wells, Methanol DPPH solution 0.004% (160 μL) was added to a volume of 40 μL of each sample (12.5–400 μg/mL) or antioxidant standards. The reaction mixing solution was agitated and preserved in the dark for 30 min at ambient temperature. Absorbance has been read at 517 nm. Decreased absorbance of the mixing solution signified the higher free radical-scavenging capacity of the tested sample where the concentration which provided 50% extinction of the DPPH radical (EC50) was calculated using the Equation (2) resulting from the plotting of the extinction percentage inhibitions:
I% = [(Acontrol − Asample)/Acontrol] × 100
Acontrol is the absorbance of blank and the Asample is that of extract or standard sample.
2.4.2. ABTS Scavenging Assay
The hydrogen donating capacity of AcE and EtOAcE was evaluated even against the ABTS (2,2-azino-bis (3-ethylbenzothiazloine-6-sulfonic acid)) cation radicals as described in the literature [32], with slight modifications. ABTS˙+ solution occurred by mixing ABTS (2 mM in H2O) with 2.45 mM potassium persulfate (K2S2O8). The resulting solution was stored in the dark for 16 h then it was adjusted to obtain an absorbance value of 0.700 ± 0.02 at 734 nm. A mixture consisting of 160 µL of the ABTS cation solution and 40 µL of each extract (12.5–400 μg/mL) or the synthetic antioxidants BHA and BHT (0.78–50 μg/mL) was prepared and incubated for 30 min at room temperature. Absorbance was measured at 734 nm, noting that Equation (2) was used to calculate the inhibition rate.
2.4.3. Total Antioxidant Capacity by Phosphomolybdenum Assay (TAC)
The total antioxidant activity of the studied extracts was investigated by measuring their ability to reduce the molybdate ion Mo (VI) to form a green phosphate/Mo (V) complex at acid pH according to the previous method [33]. A volume of 100 μL of each sample was added to 1 mL of reagent solution containing 4 mM ammonium molybdate and 28 mM sodium phosphate in 0.6 M sulfuric acid. The reaction mixture was incubated for 90 min at 95 °C. After cooling the mixture at room temperature, the absorbance was read at 695 nm. Ascorbic acid (10–300 µg/mL) was used as an antioxidant and its curve was used to estimate the total antioxidant capacity.
2.4.4. Ferric Reducing Antioxidant Power (FRAP) Test
This test was performed according to the Oyaizu [34] method, with minor modifications. Synthetic antioxidants BHA, BHT and ascorbic acid (0.78–50 μg/mL) with pure phenolic compounds gallic acid and quercetin (2–50 μg/mL) were employed as standards. A volume of 1% potassium ferricyanide (K3Fe (CN)6) was added to a mixture consisting of sodium phosphate buffer (0.2 M, pH 6.6) and 10 µL of each extract (12.5–800 μg/mL) or antioxidant standards. The reaction was incubated for 20 min at 50 °C. To acidify the previous mixture, a volume of 50 µL trichloroacetic acid 10% (TCA) was added. After the addition of 40 µL of distilled water and 10 µL of 0.1% FeCl3 to this mixture, the reading of absorbance was done at 700 nm.
2.4.5. Cupric Reducing Antioxidant Capacity (CUPRAC)
The potential of the corresponding extracts to reduce the Copper (II) to copper (I) ions was evaluated using CUPRAC bioassay [35] by mixing a volume of 50 μL of copper (II) chloride solution (10 mM) with 50 μL of neocuprine in ethanol (7.5 mM) and 60 μL of ammonium acetate buffer solution (1 M, pH 7.0). In presence of neocuprine, a stable complex with maximal absorbance at 450 nm resulted from this reduction. To the initial mixture, a volume of 40 μL of each sample (12.5–800 μg/mL) or standard (0.78–50 μg/mL) was added to obtain a final volume of 200 μL. Absorbance was recorded at 450 nm.
2.4.6. β-Carotene/Linoleic Acid Bleaching Assay
The lipid-peroxidation inhibitory activity of the plant extracts was investigated via the previous method previously described [36], whereas, BHT and BHA were used as antioxidant standards. To prepare the β-carotene emulsion, β-carotene (0.5 mg) was dissolved in 1 mL of chloroform in a round-bottomed flask and mixed with 200 mg of Tween 40 and 25 μL of linoleic acid. Under reduced pressure, chloroform was removed in a rotary evaporator then 100 mL of H2O2 was added to this emulsion before vigorous shaking. After adjustment of absorbance to 0.8–0.9 at 470 nm, 160 μL of this emulsion was added to 40 μL of studied extracts (12.5–800 μg/mL) or standards (0.78–50 μg/mL). The reaction mixture was incubated for up to 120 min at 50 °C. β-carotene bleaching was monitored spectrophotometrically at 470 nm. The antioxidant capacity of β-carotene bleaching was calculated according to previous research work [36].
2.5. Anti-Diabetic Test
2.5.1. Anti-α-Amylase Assay
This assay was achieved using the iodine/potassium iodine (IKI) method [17], with minor modifications. A volume of 50 µL of α-amylase solution (1U) prepared in phosphate buffer [PBS (pH 6.9 supplemented with 6 mM sodium chloride)] was mixed with 25 µL of each extract dissolved in MeOH or acarbose, as a positive control, at different concentrations (6.25–400 µg/mL) in 96-well microplate and incubated for 10 min at 37 °C. 50 µL of starch solution (0.1%) was then added to initiate the reaction. The reaction mixture was incubated again for 10 min at 37 °C. The reaction was stopped with the addition of HCl (25 µL, 1M) and 100 µL of IKI solution. Similarly, a blank was prepared by adding the sample solution to all reagents without the enzyme α-amylase solution, and acarbose was used as a positive control. Absorbances were measured at 630 nm, where the absorbance of the blank was subtracted from that recorded by the samples. The α-amylase inhibitory effect was calculated according to Equation (3):
% inhibition = 1 − [(Ac − Ae) − (As − Ab)/(Ac − Ae)]
Ac: Absorbance of the mixture of starch + IKI + HCl + MeOH + phosphate buffer solutions.
Ae: Absorbance of the mixture of the enzyme + starch + IKI + HCL+ MeOH solutions.
As: Absorbance of the mixture of the enzyme + sample + starch + IKI + HCl solutions.
Ab: Absorbance of the mixture of the sample + IKI + phosphate buffer solutions.
2.5.2. Anti-α-Glucosidase Assay
The α-glucosidase inhibitory capacity of EtOAcE and AcE of R. tuberculata was determined according to the developed method [37], where acarbose (78.125–5000 µg/mL) and quercetin (0.48–125 µg/mL) were used as a positive control. A volume of 20 µL of each extract (15.625–1000 µg/mL) or acarbose solution at different concentrations was mixed with 100 µL of α-glucosidase solution (0.1 U) prepared in phosphate buffer (100 mM, pH 6.9) and 80 µL PNPG (5 mM) in 96-well microplate and incubated for 30 min at 37 °C. Similarly, a blank was prepared by the addition of sample solution to all reaction reagents without enzyme solution. The reaction was stopped by the addition of the sodium carbonate solution (0.2 M). The absorbance was then read at 405 nm in initial time (0) and after 30 min.
2.6. Anti-Alzheimer Effect (Anti-Cholinesterase Activity)
The neuroprotective effect of the target extracts was performed by determining Acetylcholinesterase (AChE) inhibition capacity as previously described in the literature [38], using Galantamine as a positive control. Briefly, a volume of 20 µL of AChE enzyme solution (5.32 × 10−3 U) was added to a mixture containing 150 µL of sodium phosphate buffer (0.1 M, pH 8.02) and 10 µL of each extract or standard at different concentrations (3.25–200 µg/mL). After an incubation period of 15 min at 37 °C, a volume of 10 µL of the revealing solution of DTNB (5.50-Dithio-bis (2-nitrobenzoic) acid (0.5 mM)) and 10 µL of the substrate of the reaction (acetylthiocholine iodide ACI (0.73 mM)) were added. A blank was prepared by adding the sample solution to all reaction reagents without enzyme solution. The hydrolysis reaction of the acetylthiocholine iodide by acetylcholinesterase was monitored at 412 nm.
2.7. Statistical Analysis
The actual experiments were carried out in triplicates and the results were expressed as mean ± SD. Graph Pad Prism version 8.0.2 (Graph Pad Software Inc., San Diego, CA, USA) was used for the analysis of variance (One-way ANOVA followed by Tukey’s multiple comparison tests). The statistical significance level was identified at p < 0.05.
3. Results and Discussion
It is commonly acknowledged that herbal polyphenols and flavonoids possess various therapeutic proprieties and exhibit antioxidant, anti-inflammatory, antidiabetic, and neuroprotective activities. Against this background, a moderate number of phytochemical and pharmacological data could be available in the literature on Ruta tuberculata organic fractions compared to the other Ruta species. While no reports concerning the phytochemical composition and the pharmacological proprieties of the acetonic extract were published for all species of genus Ruta, as the best of the author’s acknowledgment. Indeed, most studies of Ruta plants focused mostly on their alkaloid derivatives and essential oil compounds [12,20,22,39], whereas their phenolic contents and pharmacotherapies of them were rarely investigated [17,19,21,40]. That heightened our desire to characterize for the first time the individual phenolic profiles of the EtOAc and acetonic extracts of R. tubercultata aerial parts and to investigate their pharmacological properties.
3.1. Extraction Yield and Spectrophotometric Determination of Total Bioactive Contents
In the recent study, the maceration method of R. tuberculata aerial parts, using different organic solvents, allowed us to obtain greenish semisolid crude EtOAc and acetonic extracts that represented extraction yield values in order of 2.3 ± 0.3 and 1.5 ± 0.2%, respectively. As shown in Table 1, the EtOAc extraction yield was higher than that recorded by the acetonic extraction. In accordance with our results, Zengin et al. [17] proved that ethyl acetate extraction yield (3.5%) of Hapophyllum myrtifolium (Rutaceae) was higher than that registered by the other nonpolar extracts such as petroleum ether solvent. Indeed, diverse factors including the genetic patrimony, the collected plant status, climatic conditions, method, and solvent system used for extraction can significantly affect the extraction yield and have an impact often on the chemical diversity between Ruta species [18,30].

Table 1.
Colorimetric determination of total polyphenol, total flavonoid, and condensed tannin contents in R. tuberculata extracts.
The phenolic content of EtOAc and acetonic crude extracts from R. tuberculata aerial parts were estimated and illustrated in Table 1. Based on the obtained results, both target extracts showed to be rich in various classes of bioactive phenols but with varying levels. In evidence, EtOAc extract revealed the highest significant value (p < 0.05) of total polyphenols (51.0± 0.1 mg GAE/g d.E) and condensed tannin (43.2 ± 0.6 mg CE/g d.E) than that recorded by acetonic extract. However, no significant difference has been noted in FTC values between the studied extracts which means that they showed a similar amount of total flavonoids.
Our results accord with that reported by Hamdi et al. [40], who proved with overwhelming evidence that the highest TPC and FTC of Tunisian R. tuberculata aerial parts were found in their EtOAc fraction with the respective values of 262 mg GAE/g d.E and 99.1 mg QE/g d.E. In fact, they are most three times considerable than our findings. In contrast, the monitored CTC by Hamdi et al. [40] in the same extract is almost four times lower than that obtained. According to this result, Zengin et al. [17] indicated that ethyl acetate extract of H. myrtifolium showed nearly a similar TPC value of 52.5 ±1.5 mg GAE/g d.E to that obtained in R. tuberculata EtOAc extract, but it was the richest one in flavonoids (25.6 ± 0.5 mg QE/g d.E). However, the TPC value obtained in our study was also lower than that investigated by Kacem et al. [26] in the same extract of Tunisian R. chalepensis aerial parts (142.5 mg GAE/g d.E), as well as the phenolic content was further much smaller in hexane extract (15 mg GAE/g d.E) than that one obtained in the studied AcE. Moreover, the finding results showed that R. tuberculata EtOAc extract is richer even in FTC and CTC compared to that found by Kacem et al. [26] in R. chalepensis EtOAc extract. Overall, it has also been reported [6] that the EtOAc fraction from Algerian R. chalepensis was the second richest extract in TPC (175.23 ± 5.64 GAE/g d.E) and FTC (78.95 ± 1.02 mg QE/g d.E) amounts comparing to the other fractions.
On the whole, several reports offered by Eissa et al. [19], Kacem et al. [26], and Gali and Bedjou [6] indicated the same distribution of the total phenolic and flavonoids contents between the solvent systems but with higher amounts when compared with the obtained results. Indeed, these components revealed differences in their contents depending to the solvent’s polarities, whether TPC and FTC contents increase depending on the polarity of the solvent, which reached the maximum in the ethyl acetate and decreased gradually with the nonpolar solvents as suggesting by previous reports [9,11,17]. Based on our findings, we could assume further that ethyl acetate is the most adequate solvent able to extract a wide range of the middle and nonpolar classes of polyphenols.
3.2. Phenolic Characterization Using cLC-DAD Analysis
Depending on the molecular segregation of the phenolic compounds at four wavelengths and the retention time, the phenolic profiles of the corresponding extracts of Algerian R. tubercutata were assessed in Figure 1 and Figure 2, while the results of the individual phenolic quantification were summarized in Table 2. Our findings revealed that various classes of polyphenols were detected in both extracts of R. tubercutata aerial parts. However, EtOAc extract showed high amounts of polyphenols, mainly flavonoids. Furthermore, greater amounts of cinnamic derivatives were found in this extract such as trans-cinnamic, p-coumaric, caffeic, and trans-ferulic acids, respectively (Table 2). More than benzoic derivatives as hydroxyl-benzoic acid, vanillin acid was also identified in both studied extracts, as well as gallic acid being exclusively detected in the acetonic extract. Moreover, the most abundant phenolic acids in EtOAc extract were trans-cinnamic acid (87.01 mg/100 g d.E) followed by cinnamic acid (74.95 mg/100 g d.E), p-hydroxybenzoic acid (53.85 mg/100 g d.E), vanillic acid (37.03 mg/100 g d.E) and p-coumaric acid (28.17 mg/100 g d.E) with the nonappearance of gallic acid. Likewise, it was noted that the cinnamic acid (97.41 mg/100 g d.E) is the main phenolic acid in the acetonic extract followed by gallic acid (64.025 mg/100 g d.E), trans-cinnamic aid (86.47 mg/100 g d.E), p-coumaric acid (35.97 mg/100 g d.E), vanillin acid (18.20 mg/100 g d.E) and p-hydroxybenzoic acid (14.06 mg/100 g d.E).
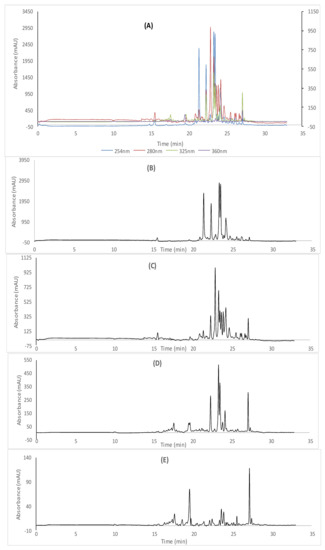
Figure 1.
cLC−DAD chromatogram of ethyl acetate extracts; (A) combined chromatograms, (B) 254 nm, (C) 280 nm (D) 325 nm (E) 360 nm.
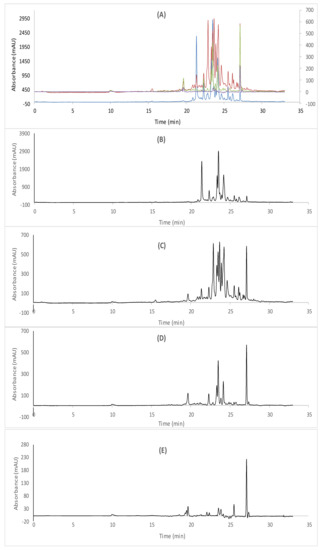
Figure 2.
cLC−DAD chromatogram of acetonic extracts; (A) combined chromatograms, (B) 254 nm, (C) 280 nm (D) 325 nm (E) 360 nm.

Table 2.
Characterization of polyphenols in R. tuberculate organic extracts using cLC-DAD analysis.
Interestingly, both studied extracts showed to contain diverse flavonoid classes in particular EtOAc extract which presented the highest levels of myricetin and quercetin as flavonols, naringenin as flavonones, catechin as flavanol, rutin as flavone, and silymarin as flavanonol derivate. Relying on cCL-DAD analysis results (Table 2), the most abundant flavonoid detected in EtOAc extract was myricetin (4253.86 mg/100 g d.E) followed by rutin (645.59 mg/100 g d.E), silymarin (345.61 mg/100 g d.E) and catechin (147.02 mg/100 g d.E). In the same order, myricetin was considerably the major compound in acetonic acid with the highest amount of 2111.03 mg/100 g d.E, followed by rutin (172.77 mg/100 g d.E), silymarin (149.29 mg/100 g d.E), and catechin (22.24 mg/100 g d.E). However, naringenin was found in both extracts, in which the higher values were depicted in the EtOAcE extract. Otherwise, it is important to note that a high level of quercetin was observed in AcE compared to EtOAcE.
Hamdi et al. [40] identified both vanillin and caffeic acids in the EtOAc fraction of Tunisian R. tuberculate (or H. tuberculatum) aerial parts using a UPLC-ESI-MS. According to our findings, the phenolic screening data [19] using LC-MS depicted closely the same diversity of small phenolic compounds which were ferulic, caffeic, and p-OH cinnamic acids as cinnamic acids derivatives and vanillic acid as benzoic acids derivative.
Using different analytical techniques, several phytochemical studies demonstrated that alcoholic extracts of many Ruta species, such as Algerian R. tuberculate [9,13] and Tunisian R. graveolens L. [18], and R. chalepensis [19,41,42], showed their richness in diverse flavonoid categories that affirmed effectively our findings. Align with these previous reports, our suggestion on the wide phenolic diversity contained in R. tuberculata aerial parts could be certainly confirmed but supposedly with some variation in biocompound levels compared to the other Ruta species. Indeed, the chemical structure of phenolic compounds and their amounts in Ruta plants can vary under diverse conditions as documented in previous data [18,30,40]. When refereeing to literature, the present study is the first report that characterized the flavonoid compounds mainly myricetin, catechin, sylimarin naringenin, and quercetin in both EtOAc and acetonic extracts from the aerial parts of Algerian R. tuberculata.
3.3. Antioxidant Activity
Various universal bioassays have been practiced as in vitro models to assess the likely antioxidant potential of samples, but without specifying details about their mode of action. Among those methods, six approaches were carried out in the actual work to evaluate the eventual antioxidant activities of R. tuberculata EtOAc and acetonic extracts. The obtained results were expressed as the respective values of ascorbic acid equivalents, IC50 and A0.5 (mean ± SD) as shown in Table 3. Noting that standards values have been reported in our previous study [9].

Table 3.
Antioxidant activities of AcE and EtOAcE from R. tuberculata aerial parts.
3.3.1. Radicals Scavenging Activity
A high percentage of radical scavenging was recorded at a low concentration of the tested samples which reflects their strong antioxidant activity. For this reason, we can suggest that remarkable antiradical capacities were shown by R. tuberculata EtOAcE and AcE against both DPPH and ABTS stable radicals. Statistically, AcE was seen to be (p < 0.001) the stronger DPPH free radical scavenger agent, with an IC50 value of 82.6 ± 1.06 μg/mL which is 2 times lower than that exhibited by EtOAc extract (186.86 ± 1.01 μg/mL) when its phenolic content is relatively the lowest one (Table 3). It was interested to underline that its DPPH scavenging activity seems to be similar to that exerted by quercetin, but it remains significantly lower than that recorded by the other antioxidants drugs such as the synthetic standards (BHT, BHA) and the natural pure compounds (ascorbic acid and gallic acid). However, the anti-radical activity of EtOAcE against the DPPH radicals was statistically (p < 0.001) weaker than that shown by all antioxidant standards. Among these references, BHA expressed significantly (p < 0.001) the potent antiradical capacity with the weakest IC50 value (15.74 ± 0.5 μg/mL) tracked by the ascorbic acid (26.38 ± 0.5 μg/mL). Whereas, no statistical difference was noted between the BHT (49.77 ± 0.1 μg/mL), gallic acid (53.03 ± 0.0 μg/mL), and quercetin (60.77 ± 0.0 μg/mL) which scavenged similarly the DPPH radicals. Compared to that, EtOAcE seemed to have the most significant (p < 0.001) quenching activity against the ABTS cation radical than that recorded by the AcE with IC50 values of 62.65 ± 1.5 and 123.58 ± 1.4 μg/mL, respectively. It is important to underline that BHT (1.55 ± 0.3 μg/mL) showed to be the best antioxidant drug against ABTS cation radical with no significant difference compared to BHA (7.54 ± 0.7 μg/mL).
Our results are in complete agreement with those reported by Hamdi et al. [40], who demonstrated that the EtOAc fraction of H. tuberculatum, from Egypt, has the highest scavenging capacity against both DPPH and ABTS than the other studied fractions, including chloroform and ether petroleum fractions, with IC50 values ranged from 20 to 29 μg/mL, respectively. While these reported IC50 values of the same extract against both ABTS and DPPH were mostly lower than those presented by the analyzed extracts in our study. However, a similar DPPH scavenging activity was found in the literature [17] by EtOAc extract of R. chalepensis L., another species of Ruta genus [26]. Otherwise, EtOAc extract from H. myrtifolium seemed to be most effective against ABTS than DPPH free radical. When compared to previous report data on Algerian R. chalepensis [6], the EtOAc fraction from its aerial parts seems to be more effective in DPPH and ABTS reduction process than R. tuberculata EtOAc extract, with IC50 values reached to 54.98 ± 0.5 and 31.75± 0.95 μg/mL, respectively. Moreover, the IC50 values produced by the nonpolar fractions of R. chalepensis, in particular n-hexane and chloroform fractions showed to be closely higher than that one obtained by the acetonic extract against both DPPH and ABTS radical that makes it the most effective one against free radicals [7,9,19,41,42].
Regarding the current results, we supposed that the remarkable scavenging activity of R. tuberculata extracts might be due to their richness in several phenolic biocompounds, particularly in the presence of the cinnamic, trans-cinnamic, and gallic acids. This last one, which was detected only in acetonic extract, has been reported as a potent antiradical agent [43,44]. Among the diver antioxidant mechanisms, the hydrogen or hydroxyl groups contained in the phenolic structures succeed easily in the transfer of the acidic protons to the free radicals, which leads to stabilizing them effectively. Corollary, the formation of a stable phenoxyl radical resulting from the delocalization of the unpaired electron, as documented par several data [2,30,45]. Furthermore, the significant antioxidant effect of the studied extracts against the free radical may be correlated to the flavonoid compounds previously detected in them. Several investigations presumed that the structural features of flavonoids, particularly the binding of hydroxyl groups on C3 and C4 in the B ring and the double bound between C2 and C3 in the C ring, provided further evidence about their interest in antiradical ability [10,30,46,47].
3.3.2. Reducing Power
The ability of R. tuberculata extracts to reduce iron and copper ions was assessed using based ferric reducing antioxidant power (FRAP) and copper reducing antioxidant power (CUPRC) assays, which lead to measuring the reducers contained in samples. The data was presented in Table 3 as A0.5 values estimated from absorbance curves at corresponding wavelengths, 700 and 450 nm, respectively as previously described in Section 2. The reduction capacity exhibited by R. tuberculata extracts evolved in concentration depending on both assays.
Compared to BHT (A0.5 > 50 μg/mL), EtOAc extract reduced effectively the iron ions with the lowest A0.5 value of 111.13 ± 0.3 μg/mL. Thus, the acetonic extract showed to be (p < 0.001) the fewer one with an A0.5 value of 120.93 ± 0.9 μg/mL. Statistically, the iron reducing capacity of R. tuberculata extracts remains to be weaker than that registered by the antioxidant standards, which used as a positive control. In contrast to the FRAP bioassay, the high copper reducing capacity was recorded by acetonic extract (A0.5 = 184.13 ± 0.7 μg/mL) despite its few phenolic contents compared to that presented by EtOAc extract. With no statistical difference (p > 0.05) between them, BHA and BHT as, synthetic antioxidants, exhibited interestingly the best Cu+2 reduction effect (p < 0.001) with the weakest A0.5 values ranging from 3.64 ± 0.2 and 9.62 ± 0.9 μg/mL, respectively, compared to the corresponding extracts.
As far as is known to the authors, no data about the possible reducing power of R. tuberculata organic extracts were documented which means that this study is the first investigation of their eventual reducing power using FRAP and CUPRAC approaches. Our results were appreciable but not significant to that reported data [6], where it is indicated that the EtOAc fraction of Algerian R. chalepensis presents interesting copper and iron reducing power capacities with weakest values of A0.5 (40.7 ± 0.9 and 45.28 ± 0.4 μg/mL, respectively) than n-hexane and chloroform fractions, which showed the low reduction ability in both tests with A0.5 values > 200 μg/mL. However, both studied extracts showed a potent reducing capacity with the lowest FRAP values when compared to those cultivated and spontaneous Tunisian R. chalepensis obtained from its flowers and leaves. Furthermore, the reducing power capacities of EtOH extracts from Tunisian R. mentana and R. chalepensis were investigated by Khadhri et al. [7], who affirmed that the highest iron reducing ability was found with R. mentana extracts, mainly in EtOH extract from its stem and leaves.
The electron-donating power acted by R. tuberculata extracts is presumably ascribed to the redox proprieties of their polyphenols content which are considered natural redactors able to donate easily the electron and hydrogen atoms. Similarly to our suggestion, it has been documented that the small phenols and their related compounds in plants are the main antioxidants that are responsible for their reducing power based on their most effective ability to deliver more free atoms [11,13,45,47]. Indeed, these natural biocompounds may act through many transfer mechanisms to scavenge free radicals, chelate metal ions, and modulate the endogenous antioxidant systems [45].
3.3.3. Total Antioxidant Activity (TAC)
The phospho-molybdenum assay is frequently performed to determine the total antioxidant activity of samples. Mo(VI) is reduced to Mo(V) by the antioxidants contained in extracts which form a stable green-colored phosphate/Mo(V), this complex has a maximal absorbance at 695 nm [33]. Results are expressed as ascorbic acid equivalent and registered in Table 3. Relevantly, EtOAc extract of R. tuberculata aerial parts showed to have a lower TAC value (216.6 ± 1.0 AAE mg/g E) than that established by acetonic extract (295.62 ± 2.2 AAE mg/g E), which reflect significantly (p < 0.001) the best TAC rate of EtOAc extract.
Our findings disagree with ones reported on R. chalepensis by Gali and Bedjou [6], who demonstrated that the EtOAc fraction presents the highest TAC compared to the nonpolar fractions, as well as n-hexane and chloroform fractions which gave the lower TAC values of 108.1 ± 3.8 and 49.30 ± 1.4 AAE mg/g d.E, respectively. However, Zengin et al. [17] showed that the EtOAc fraction of H. myrtifolium had almost 1.7 times better efficiency than its MeOH extract. In addition, Kacem et al. [26] found that the TAC rates were decreased in the order of EtOH extract > MeOH (50%) extract > MeOH (100%) extract > EtOAc extract (92.38 ± 4.0 AAE mg/g E) > water extract > n-hexane extract (3.05 ± 0.1 AAE mg/g E), which affirmed resolutely the positive correlation between the polyphenols amount in sample and its total antioxidant capacity. Furthermore, the variation in antioxidant capacity could be correlated with the high amount of the same small phenolic acids in EtOAc extract than in acetonic extracts, such as p-hydroxybenzoic, vanillic acid, caffeic acid, and trans-ferulic acid that may act as great reducer agents. Besides, a high amount of gallic acid was found in the acetonic extract, which is an excellent antioxidant agent [48], that can elucidate often the great bioactivity of this letter. On the other hand, it is interesting to highlight the correlation between TAC rate and the richness of the studied plant extracts in flavonoids mainly rutin, myricetin, sylimarin, and naringenin, which are known as potent antioxidants compounds [24,44,48].
3.3.4. Lipid Peroxidation Inhibition Capacity
This bioassay, which is considered a hydrogen transfer-based test, is widely used to assess the ability of samples to neutralize lipid free radicals by preventing the decomposition of hydro-peroxides into free radicals and the continued hydrogen abstraction [6,47]. Indeed, β-carotene is a natural vitamin A precursor with a red-orange color. This pigment is quickly decolorized when is oxidized in presence of radicals or oxidizing ingredients. Once the reaction medium is enriched up with oxygen during the β-carotene bioassay, the linoleic acid well is autoxidized to lipid radical, which oxidases and relatively decolorizes this pigment.
The obtained results showed that both acetonic and EtOAc extracts of R. tuberculata exhibited similarly a remarkable inhibitory effect on β-carotene blanching phenomenon in a dose-dependent manner with maximal inhibition rate values of 95.31 ± 2.0 and 96.97 ± 0.7% for the highest tested concentration, at the end of the test. As shown in Table 3, no significant difference was statistically noted (p > 0.05) between the inhibition activities of these extracts on linoleic acid oxidation. Apparently, AcE seemed to be slightly more effective than EtOAc extract, nearly with equal IC50 values of 60.93 ± 0.5 and 62.19 ± 1.8 μg/mL, respectively. Nevertheless, their inhibitory activity remains weaker than that exerted by the synthetic antioxidants BHT and BHA which present close IC50 values of 1.24 ± 0.00 and 1.26 ± 0.00 μg/mL, respectively.
Our results agree with the findings data [40], which demonstrated that the best β-carotene blanching inhibitory effect was exerted by the EtOAc fraction of Egyptian R. tuberculata (22 μg/mL) which is similar to that elaborated by the ascorbic acid (21 μg/mL). Nevertheless, their chloroform and petroleum ether fractions showed lower inhibitory activity. According to the phyto-pharmacological report of Gali and Bedjou [6], the EtOAc fraction of R. chalepensis revealed an appreciable inhibition of lipid peroxidation followed closely by n-hexane fraction with the respective IC50 values of 38.52 ± 0.7 and 46.76 ± 2.2 μg/mL. Moreover, Kacem et al. [26] reported that the n-hexane and EtOAc extracts of R. chalepensis showed an interesting β-carotene blanching inhibition rate than alcoholic extracts. In contrast, Loizzo et al. [41] affirmed that MeOH extract from R. chalepensis leaf has a promising capacity to inhibit lipid peroxidation with an IC50 value of 16.9 μg/mL. In addition, we suggested that the antiradical proprieties of R. tuberculata extracts are intensively correlated with their phenolic biocompounds may arrest the reaction chain of free radicals production and prevent lipid peroxidation. Hence, our finding agreed with previous data that found a high correlation between the antioxidant activities of plants and their phenolic contents [9,11,21,26,40]. On the other hand, many approaches were approved by the scientific community to assess the capacity of samples to inhibit free radicals involved in lipid membranes oxidation, among those methods, the ORAC assay which was performed by Eissa et al. [19], who documented that EtOH (70%) fraction of Egyptian R. tuberculata expressed a remarkable peroxyl radical-trap activity. This important information confirms that Ruta species, in particular tuberculata, could contain hydrogen donors able to prevent lipid peroxidation such as polyphenols.
Nevertheless, no correlation is observed in our study between the inhibitory effect of the studied extracts on the β-carotene blanching phenomena and their phenolic constituents, that accord with several data. Therefore, it depends mainly on the chemical features of the solvents used to extract effectively the bio-compounds that are able to neutralize free radicals during this assay [6,26]. Indeed, the linoleic acid emulsion constitutes the β-carotene reaction medium that leads the lipophilic biomolecules, as well as triterpenoids, to easily attack the lipid radicals [6,11]. It was important to note that the ethyl acetate can dissolve the flavonoids aglycones and diterpenes, which are previously well documented [11,44], while triterpanoids are often extracted with non-polar solvents, such as acetone. These biocompounds showed an important inhibition of lipid peroxidation that may explain the effectiveness of the studied extracts against the β-carotene blanching phenomena.
3.4. Anti-Diabetic Activity
One of the most important treatment strategies for type 2 Diabetes (T2D) is the management of postprandial hyperglycemia by delaying of the release glucose from the digestive tract through the inhibition of carbohydrates hydrolase enzymes, mainly pancreatic α-amylase (EC 3.2.1.1.) and intestinal α-glucosidase (EC 3.2.1.21.). This mechanism may retard the gastrointestinal absorption of dietary carbohydrates and decreases consequently the circulating glucose blood levels [1,17,49,50]. For these reasons, the antidiabetic capacity of our plant was investigated in the present work by assessment of their inhibitory effect on α-amylase and α-glucosidase enzymatic activity. The results are expressed as mean ± SD and provided in Table 4.

Table 4.
Anti-diabetic and anti-Alzheimer’s activities of R. tuberculata extracts.
The obtained results evidenced that both studied extracts exhibited a strong inhibitory effect against both enzymes in a dose-depending manner with maximum inhibition rates > 75.0% at the high tested dose of 400 µg/mL against α-glucosidase and 800 µg/mL against α-amylase, respectively (Figure 3 and Figure 4). As shown in Table 4, the inhibition effect of EtOAc extract (146.7 ± 1.6 μg/mL) α-amylase has been significantly (p < 0.001) almost 1.6 times higher than that exerted by acetonic extract (IC50 value reached 233.04 ± 0.9 μg/mL) and even 25 times than that one of acarbose which was used as a positive control. Notably, the inhibitory activity of acarbose against α-amylase is almost 15 times weaker (3650.9 ± 1.7 μg/mL) than that registered by the acetonic extract. However, the natural pure quercetin inhibited effectively α-amylase and established the lowest IC50 value (4.3 ± 0.2 μg/mL). Indeed, this activity is higher than that one recorded against the α-amylase. Likewise, EtOAc extract inhibits remarkably the α-glucosidase (165. 4 ± 1.1 μg/mL) which exerted significantly a high inhibition effect compared to acorbose (275.4 ± 1.6 μg/mL). These results can reflect the potent antidiabetic effect of both R. tuberculata studied extracts.
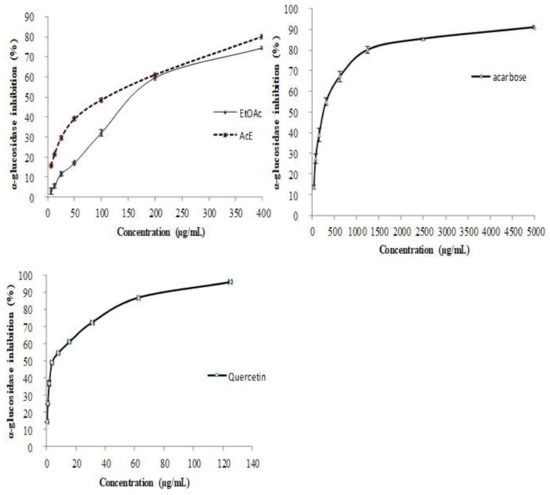
Figure 3.
Inhibitory effect of R. tuberculata organic extracts, quercetin and acarbose against α-glucosidase. Values are expressed as mean± SD (n = 3).
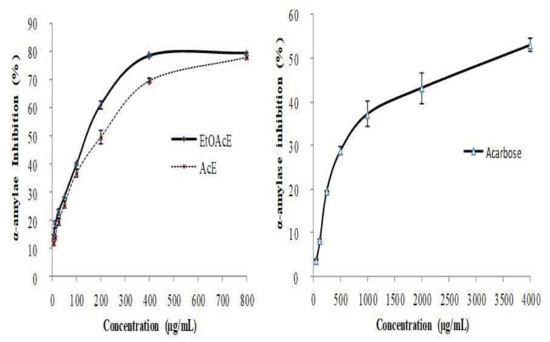
Figure 4.
Inhibitory effect of R. tuberculata organic extracts and acarbose against α-amylase. Values are expressed as mean± SD (n = 3).
Unfortunately, insufficient data about the possible inhibitory activities of Ruta species against α-amylase and α-glucosidase were available in the literature, while no reports on R. tuberculata inhibitory activities were published. Interestingly, Zengin et al. [17] indicated that EtOAc extract from H. myrtifulum leaves showed a higher inhibition effect against both tested enzymes followed by petroleum ether and MeOH extracts, respectively. Similarly, a previous study [41] focused on R. chalepensis aerial parts affirmed that its MeOH extract is able to inhibit more effectively α-amylase than α-glucosidase with respective IC50 values of 69.0 and 85.5 μg/mL. Indeed, these findings data allows as to suggest that the richest extract in phenolic biocompounds, especially in flavonoids, could exhibit the potent inhibitory effect on both α-amylase and α-glucosidase enzymes, while, other bioactive compounds could be involved to enhance or increase the enzyme inhibition activity of extracts as small phenolic acids, tannins and terpenoids [17,50]. Furthermore, one of the interesting antidiabetic mechanisms of those bioactive compounds is their capacity to regulate carbohydrate digestion through the inhibition of these two enzymes, to enhance glucose uptake and its metabolism in the liver [3,49,51,52]. Moreover, previous studies in vivo reported that flavonoid compounds improve significantly the uptake of blood glucose by accelerating insulin sensitivity and CLUT-4 glucose transporters function [3,14,49,51,52].
Compelling evidence noticed even that naringerin, which was detected in R. tuberculata extracts, inhibits significantly in vitro the catalytic activity of intestinal α-glucosidase that decreases the intestinal carbohydrate absorption, and reduce therefore the postprandial blood glucose level [49]. On the other hand, the based-plant flavonoid catechin is known also as the occurring antidiabetic agent which is able to inhibit probably the carbohydrate hydrolyzing enzymes via its virtuous capacity to bind with those enzymatic proteins or with enzyme-substrate complex [10]. Based on the report of Oboh et al. [53], rutin and quercetin, which were also found in both R. tuberculata extracts, have a potent hypoglycemia impact and could inhibit strongly both α-amylase and α-glucosidase. In an enzyme kinetic study performed by Limanto et al. [48], rutin showed to be an excellent competitive inhibitor of α-glucosidase than acarbose by binding to the active site of the free enzyme through non-covalent interactions, while quercetin inhibits this enzyme in a mixed type of competitive and non-competitive inhibition phenomena. Indeed, the chemical structure of flavonoids and the number of free phenolic hydroxyl in the B ring exhibited significantly a positive effect on their enzymatic inhibitory capacity. In fact, the flavonoid aglycones were reported to be the strangest inhibitor of α-glucosidase than glycosylated flavonoids. On the other hand, the large molecular size of those previously cited phytoconstituents may hinder their access to the active site of this enzyme [53,54,55,56,57,58]. This important information may explain the high efficacy of pure quercetin used as a reference to the studied extracts on α-glucosidase. Thus, R. tuberculata acetonic extract, as the richest extract of quercetin, inhibited more effectively this enzyme.
Furthermore, Limanto and its collaborators [48] suggested that both rutin and quercetin exerted their hypoglycemic effect compounds via multiple mechanisms including the improvement of insulin sensitivity, the increase of β-cell proliferation, and the stimulation of insulin release. Moreover, it was demonstrated that the free radical scavenging ability of these bioflavonoids and their lipid peroxidation inhibition power assist to alleviate streprozolocin-induced oxidative damage in diabetic rats and protecting their pancreatic β cells that improve insulin secretion and decrease lipid profile, hemoglobin A1c (HbA1c) and blood glucose levels [52,54,55,56]. As the same, the anti-diabetic effect of these natural flavonoids in a patient with T1DM may be mediated through the reduction of % change of fasting blood glucose level via diverse mechanisms including the decrease of the intestinal carbohydrate absorption and gluconeogenesis, prevention of the Langerhans islets cells against degeneration, stimulation of insulin release and increase tissue glucose uptake [57]. In addition, the treatment of the diabetic rats with quercetin and hesperitin, the derive aglycone of the flavanone hesperidin, improved significantly the hepatic transaminases, α-amylase, and lipase enzymes function and modulated their serum glucose, glucagon and insulin levels [58]. Indeed, these health benefits were evidenced and expressed by their ability to increase the antioxidant defenses system and decrease lipid profile and oxidative stress indicators [54,57,58]. Furthermore, these bioflavanones could enhance GLUT-2 and 4 transporters expression and attenuate the 6-P dehydrogenase, 6-PG dehydrogenase, hexokinase, and glucokinase function in pancreatic tissue. In addition, they may down regulate the insulin receptor (IR), PI3kinase, AMPKinase and IL-1β expression levels in diabetic rats which alters the IP/PI3K and AMPK signaling pathways and remedies insulin resistance [2,3,58].
Several pharmacological studies have postulated the biological proprieties of small phenolic acids in the preventing or treating of certain metabolic disorders linked to oxidative stress phenomena like cardiovascular disease, cancer, and diabetes. Indeed, these natural bioactive compounds act as antioxidants and could modulate effectively the oxidative stress in the body, thus may prevent often those diseases [49,50]. Moreover, Oboh et al. [59] and Abdel-Moneim et al. [60] reported that gallic and p-coumaic acids mostly detected in the studied acetonic extract exhibited a significant inhibitory effect on both target enzymes. This information may explain the great inhibition effect of acetonic extract against α-glucosidase. Furthermore, it was demonstrated that gallic acid is a strong competitive inhibitor of α-glucosidase when compared to acarbose, thus, this inhibitory capacity was resulting from the binding to the active site of this enzyme in free status [48].
3.5. Cholinesterase Inhibitory Activity (Anti-Alzheimer Activities)
Alzheimer’s disease (AD) is one of the common neurodegenerative illnesses which affects more than 4% of people over the age of 65% and the excessive hydrolysis of acetylcholine, a key neurotransmitter in nerve terminations, may be considered the principal cause of this disease [6,7]. Actually, the pharmacological research on natural inhibitors of AChE from plants gained the attention to elaborate novel active ingredients that could enhance cognitive function and alleviate other symptoms related to this disease [9]. In this context, the AChE inhibition capacity of the studied plant was screened in vitro as previously described in Section 2, compared to Galanthamine, the natural anti-AChE drug which is notably used in therapeutics. The results are reported in Table 4.
In a dose-depending manner, the target extracts and Galantamine exhibited a significant inhibition activity (p < 0.001) on AChE with inhibitory rates maximally reached to 79.6 ± 1.2, 86.7 ± 0.5, and 94.77 ± 0.3% at the high tested dose (200 μg/mL) as depicted in Figure 5. Notably, the acetonic extract showed to have a potent anti-AChE capacity with a weaker IC50 value (20.48 ± 0.2 μg/mL) when compared to EtOAc extract, which inhibited moderately the AChE with an IC50 value ranging from 45.62 ± 0.8 μg/mL which is c almost seven times lower than Galanthamine (6.27 ± 1.1 μg/mL). However, the inhibitory activity of acetonic extract rests almost three times lower than that of Galanthamine. These findings allow us to presume that the organic solvents effectively extract the high level of the natural AChE inhibitor drugs. This agrees with that found by Zengin et al. [17], who indicated that petroleum ether extract of H. myrtifulum has the best anti-AChE activity followed by EtOAc and MeOH extracts. Moreover, other previous data [6] affirmed that organic extracts of some plants of the genus Ruta possess a significant capacity to inhibit AChE, for example, the chloroform fraction of Algerian R. chalepensis was the most active against AChE (IC50 value of 41.14 ± 2.8 μg/mL) despite of its low phenolic content, compared to the other analyzed fractions, in particular, EtOAc fraction which presents a higher IC50 value of 108 ± 4.6 μg/mL. Moreover, n-hexane extract of R. graveolens, as an organic extract, showed a remarka-ble anti-AChE activity [61,62]. However, EtOH extract of R. chalepensis leaves demon-strated a great inhibitory effect (12 ± 1.1 μg/mL) than the same one of R. montena which present an IC50 value of 76 ± 1.6 μg/mL [7].
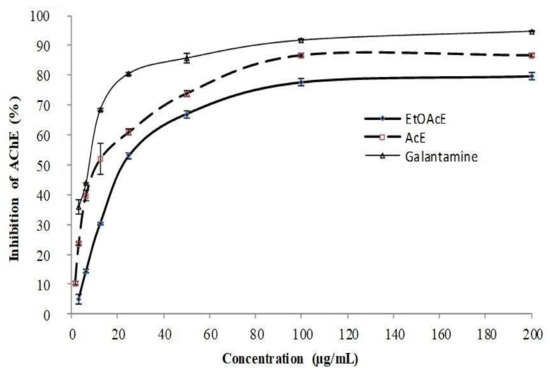
Figure 5.
Inhibitory effect of R. tuberculata organic extracts and Galantamine against acetylcholinesterase. Values are expressed as mean ± SD (n = 3).
Based on our results, we share even suggestions with Eissa et al. [19] and Saidi et al. [9], who signaled that R. tuberculata (or H. tuberculatum) may have substantial therapy features that could prevent Alzheimer’s and Parkinson’s diseases as neurodegenerative disorders or reduce often the behavior changes in AD sufferers via its main polyphenols content. Indeed, many reports underlined that diverse small phenolic acids which is particularly detected in acetonic extract of R. tuberculata, whereas, its antioxidant power was also documented [8,43]. That can elucidate the interesting Anti-AChE effect of this extract. Moreover, the significant neuroprotective effect of vanillic acid was demonstrated as evidence of LPS-induced neurotoxicity by regulating the JNK signaling pathway in the mouse brain. Therefore, this phytophenolic compound can preserve also the neuro-inflammatory process and improve the memory impairment phenomena [63]. Noting also that p-coumarin acid can alleviate cerebral ischemia-reperfusion injuries via its antioxidant potential that interestingly prevent hippocampal neuronal death by enhancing catalase and dismutase activity and decreasing malondialdehyde (MDA) level [64]. It was also proved that p-hydroxybenzoic acid inhibited potently the AChE with an IC50 value of 20.07 μg/mL which can affirm their ability to maintain the cognitive function at a normal level in T2DM patients [65]
A study realized by Hernandez and his collaborators [66] showed that the bioflavonoids rutin and quercetin, which were also found in both target extracts, exhibited an interesting anti-AChE activity with the respective IC50 values of 86.0 and 62.0 μg/mL that reflects their ability to prevent considerably many neurodegenerative illnesses such as Alzheimer and Parkinson diseases. However, Khandare et al. [67] and Zhao et al. [68] proved that quercetin exerted a potential neuroprotective effect on streprozolocin-induced diabetic neuropathy in rats resulting from its capacity to modulate oxidative stress indicators and reduce the release of the anti-inflammatory cytokine as well as TNF-α and IL-1β via the TLR4/My88/NF-κB signaling pathway down-regulation. Moreover, it was proved that kaempferol exhibited a noticeable neuroprotective effect on chlorpyrifos-induced oxidative stress and memory deficit in rats which inhibited significantly the GSK3β gene expression through the modulating of the Nrf2 signaling pathway in cerebral tissue [69]. On the other hand, the neuroprotective activity of sylimarin was investigated in various models of Alzheimer’s and Parkinson’s diseases where this health benefit is considerably assigned to its ability to enhance the IR1-AKT signaling pathway and to decrease effectively the oxidative stress and the inflammatory cytokines genes expression in cerebral tissue that frequently associated with the probable alteration of the cellular apoptosis machinery in there [15,44]. It was important to note that catechin revealed also a great anti-AChE and anti-BuChE activities that lead to restoring effectively the synaptic acetylcholine level, thus, it may be considered a great candidate to up-regulate the Alzheimer’s disease progression [70,71,72].
3.6. Nutritional Importance of the Identified Compounds
Besides the pharmacological potential of Ruta species, these plants are also well known for their importance at the nutritional level. Indeed, it has been reported that the nutritional status of rats can considerably be improved following a daily oral administration of R. graveolens extracts for a period of three weeks, especially regarding total food intake and protein efficiency ratio [73]. The same research team also reported that this plant can significantly ameliorate both kidney and liver functions and maintain a coherent Albumin/globulin ratio, which could be partially linked to the non-toxic character of Ruta species [74].
Myricetin is an important phyto-compound, well recognized for its nutraceuticals value, and already integrated as a key ingredient for various foods and beverages. In this study, the phytochemical investigation revealed that this compound is present in both tested extracts with a considerable value, considered the best. This information is crucial since many health benefits are attributed to myricetin since this compound is effective for the treatment of obesity, diabetes, hypertension, and metabolic syndrome [75]. Indeed, an in vivo investigation made by Akindehin et al. [76] revealed that myricetin can reduce body weight by 11% but may also improve glucose tolerance, and fatty acid consumption and significantly decrease mitochondrial proteins acetylation in adipose tissue.
In our study, we identified a key flavonoid in a high amount called rutin, which is well known for its strong antioxidant properties but may also help the human body produce collagen and correctly use vitamin C, which suggests that this flavonoid can improve the immune system by stimulating the activity of white blood cells [77]. Noting that this bio-compound has also been identified in R. graveolen and revealed a non-negligible neuroprotective effect, especially to enhance spatial memory and learning performance of animals [78], but may also be involved in the regulation of energy metabolism and methane production in dairy cows [79]. Chalepensis leaves could also be considered healthy food ingredients since it contains a high amount of hesperidin and rutin, and their combination can generate beneficial impacts on blood vessels and thus maintain a healthy circulation [80].
Green tea, chocolate, and blueberries are gaining immense popularity among consumers in the world due to their considerable antioxidant, and relaxing effect but also to limit the risk of cardiovascular disease and stroke [81,82]. The fact that these aliments contain a high amount of catechin could explain their pharmacological aspect. Indeed, several studies reported that catechin can increase the resistance of LDL to oxidation and promotion of gut health but also inhibit the production and activation of harmful bacteria [83,84]. It is also interesting to underline [85], which may help provide energy for the contracting muscles. Noting that catechin has also been identified in our study with an important amount. These compounds may also lower blood sugar and cholesterol levels but may also improve memory, prevent neurodegenerative diseases, and decrease inflammatory pain by inhibiting neutrophil recruitment [86,87,88].
4. Conclusions
R. tuberculata organic extracts exhibited remarkable antioxidant activities through different antioxidant mechanisms. Moreover, they showed potent anti-diabetic and anti-AChE activities that may be due to their natural biocompounds which were identified for the first time in them, mainly naringinin, myricetin, kaempferol, catechin, sylimarin, and hesperidin. Indeed, these pharmacological acts may justify the traditional use of this plant and its involvement in Mediterranean traditional medicine. Therefore, it may offer an exciting reserve of natural bioactive drugs which able to avoid or treat many disorders especially diabetes and Alzheimer’s diseases. However, additional phytochemical studies are required to purify the biocompounds contained in this plant and to investigate their pharmacological activities using in vivo and in vitro bioassays.
Author Contributions
Data curation, A.S., F.G.E., A.A.S., A.E., S.A. and L.H.; Formal analysis, A.S., M.M.R., M.D.A., R.S.A., A.E., S.A., R.S.B. and M.S.B.; Funding acquisition, R.S., Z.D.A., A.E., S.A., R.S.B., A.E., S.A., F.G.E., A.A.S. and L.H.; Investigation, A.S., A.E., S.A., I.E.K., C.B. and R.S.; Methodology, A.S., M.S.B., C.B., I.E.K. and S.A.; Supervision, L.H., A.E., R.S.B. and R.S.; Visualization, R.S., A.E., S.A., R.S.B. and S.A. Writing—review and editing, A.S., A.E., S.A., R.S.B., M.S.B., R.S., I.E.K., S.A. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors are grateful to the Algerian Ministry of Higher Education and Scientific Research and the directorate general of Scientific Research and technological development (MESRS, DGRSDT) for supporting this work. The authors would like to thank also the biochemistry laboratory in Research Center in Biotechnology (CRBT, Constantine, Algeria) for providing us to perform the cLC-DAD analysis and to curry out the In vitro antioxidant and anti-enzymatic investigations. The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4290372DSR03). Taif University Researchers Supporting Project Number (TURSP-2020/269), Taif University, Taif, Saudi Arabia. This work was supported by Deanship of Scientific Research, King Khalid University, KSA (Research group project, Grant number: RGP.2/113/43).
Conflicts of Interest
The authors declare no conflict of interest.
References
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Bensaad, M.; Dassamiour, S.; Hambaba, L.; Bensouici, C.; Karima, O.; Kahoul, M. HPLC-DAD phenolics screening and in vitro investigation of haemostatic, antidiabetic, antioxidant and photoprotective properties of Centaurea tougourensis Boiss. & Reut. Herba Pol. 2021, 67, 16–31. [Google Scholar]
- Bensaad, M.S.; Dassamiour, S.; Hambaba, L.; Saidi, A.; Melakhsou, M.A.; Nouicer, F.; Baghiani, A.; Khennouf, S.; Kahoul, M.A.; Kadrine, N. In vivo investigation of antidiabetic, hepatoprotective, anti-inflammatory and antipyretic activities of Centaurea tougourensis Boiss. & Reut. J. Physiol. Pharmacol. 2021, 72, 439–449. [Google Scholar]
- Kubis-Kubiak, A.; Dyba, A.; Piwowar, A. The Interplay between Diabetes and Alzheimer’s Disease—In the Hunt for Biomarkers. Int. J. Mol. Sci. 2020, 21, 2744. [Google Scholar] [CrossRef] [PubMed]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediat. Inflamm. 2015, 17, 105828–105844. [Google Scholar] [CrossRef] [PubMed]
- Gali, L.; Bedjou, F. Antioxidant and anticholinesterase effects of the ethanol extract, ethanol extract fractions and total alkaloids from the cultivated Ruta chalepensis. S. Afr. J. Bot. 2018, 120, 163–169. [Google Scholar]
- Khadhri, A.; Bouali, I.; Belkhir, S.; Mokded, R.; Smiti, S.; Falé, P.; Araújo, M.E.M.; Serralheiro, M.L.M. In vitro digestion, antioxidant and antiacetylcholinesterase activities of two species of Ruta: Ruta chalepensis and Ruta montana. Pharm. Biol. 2016, 55, 101–107. [Google Scholar] [CrossRef]
- Nag, G.; De, B. Acetylcholinesterase inhibitory activity of Terminalia chebula, Terminalia bellerica and Emblica officinalis and some phenolic compounds. Int. J. Pharm. Pharm. Sci. 2011, 3, 121–124. [Google Scholar]
- Saidi, A.; Hambaba, L.; Bensaad, M.S.; Melakhessou, M.A.; Bensouici, C.; Ferhat, N.; Kahoul, M.A.; Helal, M.; Sami, R.; Alharthy, S.A.; et al. Phenolic Characterization Using cLC-DAD Analysis and Evaluation of in vitro and in vivo Pharmacological Activities of Ruta tuberculata Forssk. Antioxidants 2022, 11, 1351. [Google Scholar] [CrossRef]
- Geng, S.; Shan, S.; Ma, H.; Liu, B. Antioxidant Activity and α-Glucosidase Inhibitory Activities of the Polycondensate of Catechin with Glyoxylic Acid. PLoS ONE 2016, 11, e0150412. [Google Scholar] [CrossRef]
- Sami, R.; Khojah, E.; Mansour, A.M.A.; Al-Mushhin, A.A.M.; Elhakem, A.; El-Sherif, D.M.; Alkaltham, M.S.; Salamatullah, A.M. Nutritional Values, Microbial Population and Bioactive Components of Pomegranate (Punica granatum L.) Peel Extracts. Int. J. Pharmacol. 2021, 17, 208–216. [Google Scholar] [CrossRef]
- Carvalho, L.S.A.d.; Queiroz, L.S.; Junior, I.J.A.; Almeida, A.d.C.; Coimbra, E.S.; Pinto, P.d.F.; Silva, M.P.N.d.; Moraes, J.D.; Filho, A.A.D.S. In vitro Schistosomicidal Activity of the Alkaloid-Rich Fraction from Ruta graveolens L. (Rutaceae) and Its Characterization by UPLC-QTOF-MS. Evid. Based Complement Altern. Med. 2019, 16, 7909137–7909144. [Google Scholar] [CrossRef] [PubMed]
- Saidi, A.; Hambaba, L.; Kucuk, B.; Cacan, E.; Erenler, R. Phenolic Profile, Acute Toxicity, and Hepatoprotective and Antiproliferative Activities of Algerian Ruta tuberculata Forssk. Curr. Bioact. Compd. 2022, 18, 1–12. [Google Scholar] [CrossRef]
- Zahiruddin, G.S.; Parveen, B.; Ibrahim, M.; Sharma, I.; Sharma, S.; Sharma, A.K.; Parveen, R.; Ahmad, S. TLC-MS Bioautography-Based Identification of Free-Radical Scavenging, α-Amylase and α-Glucosidase Inhibitor Compounds of Antidiabetic Tablet BGR-34. ACS Omega 2020, 5, 29688–29697. [Google Scholar]
- Ullah, H.; Khan, H. Anti-Parkinson Potential of Silymarin: Mechanistic Insight and Therapeutic Standing. Front. Pharmacol. 2018, 9, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Al-Brashdi, A.S.; Al-Ariymi, H.; Hashmi, M.A.; Khan, S.A. Evaluation of antioxidant potential, total phenolic content and phytochemical screening of aerial parts of a folkloric medicine, Haplophyllum tuberculatum (Forssk.) A. Juss. J. Coast. Life Med. 2016, 4, 315–319. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crops. Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Aguila, L.; Ruedlinger, J.; Mansilla, K.; Ordenes, J.; Salvatici, R.; Campos, R.R.d.; Romero, F. Relaxant effects of a hydroalcoholic extract of Ruta graveolens on isolated rat tracheal rings. Biol. Res. 2015, 48, 28–33. [Google Scholar] [CrossRef]
- Eissa, T.F.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Biological activity of HPLC-characterized ethanol extract from the aerial parts of Haplophyllum tuberculatum. Pharm. Biol. 2014, 52, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Eissa, T.F.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Compositional analysis and in vitro protective activity against oxidative stress of essential oils from egyptian plants used in traditional medicine. Nat. Prod. Commun. 2014, 9, 1377–1382. [Google Scholar] [CrossRef]
- Hamdi, A.; Majouli, K.; Abdelhamid, A.; Marzouk, B.; Belghith, H.; Chraief, I.; Bouraoui, A.; Marzouk, Z.; Heyden, Y.V. Pharmacological activities of the organic extracts and fatty acid composition of the petroleum ether extract from Haplophyllum tuberculatum leaves. J. Ethnopharmacol. 2018, 216, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A.; Öztürk, M. Alkaloids, Coumarins and Lignans from Haplophyllum Species. Rec. Nat. Prod. 2008, 2, 54–69. [Google Scholar]
- Richardson, J.S.M.; Sethi, G.; Lee, G.S.; Malek, S.N.A. Chalepin: Isolated from Ruta angustifolia L. Pers induces mitochondrial mediated apoptosis in lung carcinoma cells. BMC Complement Med. Ther. 2016, 16, 389–415. [Google Scholar] [CrossRef] [PubMed]
- Nayki, C.; Nayki, U.; Cimen, F.K.; Kulhan, M.; Yapca, O.E.; Kurt, N.; Ozbek, A.B. The effect of rutin on ovarian ischemia-reperfusion injury in a rat model. Gynecol. Endocrinol. 2018, 34, 809–814. [Google Scholar] [CrossRef]
- Kasimala, M.B.; Tukue, M.; Ermias, R. Phytochemical Screening and Antibacterial Activity of Two Common Terrestrial Medicinal Plants Ruta chalepensis and Rumex nervosus. Bali Med. J. 2014, 3, 116–121. [Google Scholar] [CrossRef]
- Kacem, M.; Kacem, I.; Simon, G.; BenMansour, A.; Chaabouni, S.; Feki, A.f.E.; Bouaziz, M. Phytochemicals and biological activities of Ruta chalepensis L. growing in Tunisia. Food Biosci. 2015, 12, 73–83. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analyse: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Bahorun, T.; Grinier, B.; Trotin, F.; Brunet, G.; Pin, T.; Luncky, M.; Vasseur, J.; Cazin, M.; Cazin, C.; Pinkas, M. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimitt. Forsch. 1996, 46, 1086–1089. [Google Scholar]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 99, 464–469. [Google Scholar] [CrossRef]
- Erenler, R.; Sen, O.; Yaglioglu, A.S.; Demirtas, I. Bioactivity-Guided Isolation of Antiproliferative Sesquiterpene Lactones from Centaurea solstitialis L. ssp. Solstitialis. Comb. Chem. High Throughput Screen. 2016, 19, 66–72. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable Free Radical. Nature 1958, 4617, 1119–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cationdecoloriza-tionassay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Marco, G.J. A rapid method for evaluation of antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The alpha-amylase and alpha-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, A., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sabry, O.M.M.; Sayed, A.M.E.; Alshalmani, S.K. GC/MS Analysis and Potential Cytotoxic Activity of Haplophyllum tuberculatum Essential Oils against Lung and Liver Cancer Cells. Pharmacogn. J. 2016, 8, 66–69. [Google Scholar] [CrossRef]
- Hamdi, A.; Viane, J.; Mahjoub, M.A.; Majouli, K.; Gad, M.H.H.; Kharbach, M.; Demeyer, K.; Marzouk, Z.; Heyden, Y.V. Polyphenolic contents, antioxidant activities and UPLCndash ESI-MS analysis of Haplophyllum tuberculatum A. Juss leaves extracts. Int. J. Biol. Macromol. 2017, 106, 1071–1079. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Falco, T.; Bonesi, M.; Sicari, V.; Tundis, R.; Bruno, M. Ruta chalepensis L. (Rutaceae) leaf extract: Chemical composition, antioxidant and hypoglicaemic activities. Nat. Prod. Res. 2018, 32, 521–528. [Google Scholar] [PubMed]
- Ouerghemmi, I.; Rebey, I.B.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Tounsi, M.S. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [PubMed]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological Effects of Gallic Acid in Health and Disease: A Mechanistic Review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [PubMed]
- Rha, C.S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.O. Antioxidative, anti-inflammatory, and anticancer effects of purified flavonol glycosides and aglycones in green tea. Antioxidants 2019, 8, E278–E290. [Google Scholar] [PubMed]
- Obrenovich, M.E.; Li, Y.; Parvathaneni, K.; Yendluri, B.B.; Palacios, H.H.; Leszek, J.; Aliev, G. Antioxidants in health, disease and aging. CNS Neurol. Disord. Drug Targets 2011, 10, 192–207. [Google Scholar]
- Boudjada, A.; Touil, A.; Bensouici, C.; Bendif, H.; Rhouati, S. Phenanthrene and dihydrophenanthrene derivatives from Dioscorea communis with anticholinesterase, and antioxidant activities. Nat. Prod. Res. 2018, 33, 3278–3282. [Google Scholar]
- Bazine, I.; Cheraiet, Z.; Bensegueni, R.; Bensouici, C.; Boukhari, A. Synthesis, antioxidant and anticholinesterase activities of novel quinoline-aminophosphonate derivatives. J. Heterocycl. Chem. 2020, 57, 1–11. [Google Scholar]
- Limanto, A.; Simamora, A.; Santoso, A.W.; Timotius, K.H. Antioxidant, a-Glucosidase Inhibitory Activity and Molecular Docking Study of Gallic Acid, Quercetin and Rutin: A Comparative Study. Mol. Cell Biol. Sci. 2019, 3, 67–74. [Google Scholar]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibitsa-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem.-Biol. Interact. 2014, 210, 77–85. [Google Scholar]
- Deo, P.; Hewawasam, E.; Karakoulakis, A.; Claudie, D.J.; Nelson, R.; Simpson, B.S.; Smith, N.M.; Semple, S.J. In vitro inhibitory activities of selected Australian medicinal plant extracts against protein glycation, angiotensin converting enzyme (ACE) and digestive enzymes linked to type II diabetes. BMC Complement Altern. Med. 2016, 16, 435–445. [Google Scholar]
- Hartogh, D.J.D.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenols. Biomolecules 2019, 9, E99–E119. [Google Scholar]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [PubMed]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin onα-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidationin rat pancreas. Comp. Clin. Pathol. 2015, 24, 1103–1110. [Google Scholar]
- Tian, R.; Yang, W.; Xue, Q.; Gao, L.; Huo, J.; Ren, D. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur. J. Pharmacol. 2016, 15, 84–92. [Google Scholar]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar]
- Dong, B.; Shi, Z.; Dong, Y.; Chen, J.; Wud, Z.; Wua, W.; Chen, Z.; Han, C. Quercetin ameliorates oxidative stress-induced cell apoptosis of seminal vesicles via activating Nrf2 in type 1 diabetic rats. Biomed. Pharmacother. 2022, 151, 113108–113113. [Google Scholar]
- Ragheb, S.R.; Wakeel, L.M.E.; Nasr, M.S.; Sabri, N.A. Impact of Rutin and Vitamin C combination on oxidative stress and glycemic control in patients with type 2 diabetes. Clin. Nutr. ESPEN 2019, 35, 128–135. [Google Scholar]
- Abdou, H.M.; Hamaad, F.A.; Ali, E.Y.; Ghoneum, M.H. Antidiabetic efficacy of Trifolium alexandrinum extracts hesperetin and quercetin in ameliorating carbohydrate metabolism and activating IR and AMPK signaling in the pancreatic tissues of diabetic rats. Biomed. Pharmacother. 2022, 149, 112838–112847. [Google Scholar]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar]
- Abdel-Moneim, A.; El-Twab, S.; Yousef, A.I.; Reheim, E.S.A.; Ashour, M.B. Modulation of hyperglycemia and dyslipidemia in experimental type 2 diabetes by gallic acid and p-coumaric acid: The role of adipocytokines and PPARγ. Biomed. Pharmacother. 2018, 105, 1091–1097. [Google Scholar]
- Wszelaki, N.; Kuciun, A.; Kiss, A.K. Screening of traditional European herbal medicines for acetylcholinesterase and butyrylcholinesterase inhibitory activity. Acta Pharm. 2010, 60, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Talić, S.; Dragičević, I.; Ćorajević, L.; Bevanda, A.M. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of extracts from medicinal plants. Bull. Chem. Technol. Bosnia Herzeg. 2014, 43, 11–14. [Google Scholar]
- Ullah, R.; Ikram, M.; Park, T.J.; Ahmad, R.; Saeed, K.; Alam, S.I.; Rehman, I.U.; Khan, A.; Khan, I.; Jo, M.G.; et al. Vanillic Acid, a Bioactive Phenolic Compound, Counteracts LPS-Induced Neurotoxicity by Regulating c-Jun N-Terminal Kinase in Mouse Brain. Int. J. Mol. Sci. 2020, 22, 361. [Google Scholar] [CrossRef] [PubMed]
- Sakamula, R.; Thong-Asa, W. Neuroprotective effect of p-coumaric acid in mice with cerebral ischemia reperfusion injuries. Metab. Brain Dis. 2018, 33, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, Y.J.; Hyun, S.K.; Min, B.S.; Kim, D.W.; Jung, J.H.; Choi, J.S. Selective cholinesterase inhibitory activities of a new monoterpene diglycoside and other constituents from Nelumbo nuciferastamens. Biol. Pharm. Bull. 2010, 33, 267–272. [Google Scholar] [CrossRef]
- Hernandez, M.F.; Falé, P.L.V.; Araújo, M.E.M.; Serralheiro, M.L.M. Acetylcholinesterase inhibition and antioxidant activity of the water extracts of several Hypericum species. Food Chem. 2010, 120, 1076–1082. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Raygude, K.S.; Kumar, V.S.; Rajmane, A.R.; Visnagri, A.; Ghule, A.E.; Ghosh, P.; Badole, S.L.; Bodhankar, S.L. Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin-induced diabetic neuropathy in rats. Biomed. Aging Pathol. 2012, 2, 173–186. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Q.; Liang, X.; Xie, J.; Sun, Q. Quercetin reduces inflammation in a rat model of diabetic peripheral neuropathy by regulating the TLR4/MyD88/NF-κB signalling pathway. Eur. J. Pharmacol. 2021, 912, 174607–174612. [Google Scholar] [CrossRef]
- Hussein, R.M.; Mohamed, W.R.; Omar, H.A. A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3β-Nrf2 signaling pathway. Pestic. Biochem. Physiol. 2018, 152, 29–37. [Google Scholar] [CrossRef]
- Okello, E.J.; Mather, J. Comparative Kinetics of Acetyl- and Butyryl-Cholinesterase Inhibition by Green Tea: Catechins|Relevance to the Symptomatic Treatment of Alzheimer’s Disease. Nutrients 2020, 12, 1090. [Google Scholar] [CrossRef]
- Cardoso-Lopes, E.M.; Maier, J.A.; Silva, M.R.d.; Regasini, L.O.; Simote, S.Y.; Lopes, N.P.; Pirani, J.R.; Bolzani, V.d.S.; Young, M.C.M. Alkaloids from stems of Esenbeckia leiocarpa Engl. (Rutaceae) as potential treatment for Alzheimer Disease. Molecules 2010, 15, 9205–9213. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed]
- Al-Okbi, S.Y.; El-Sayed, E.M.; Ammar, N.M.; El-Sayed, N.K.; Abou-El Kassem, L.T. Effect of Ruta graveolens L. and Euphorbia peplus L. anti-inflammatory extracts on nutritional status of rats and the safety of their use. Indian J. Exp. Biol. 2002, 40, 45–48. [Google Scholar]
- Coimbra, A.T.; Ferreira, S.; Duarte, A.P. Genus Ruta: A natural source of high value products with biological and pharmacological properties. J. Ethnopharmacol. 2020, 260, 113076–113156. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Akindehin, S.; Jung, Y.S.; Kim, S.N.; Son, Y.H.; Lee, I.; Seong, J.K.; Jeong, H.W.; Lee, Y.H. Myricetin Exerts Anti-Obesity Effects through Upregulation of SIRT3 in Adipose Tissue. Nutrients 2018, 10, 1962. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017–6241034. [Google Scholar] [CrossRef]
- Asgharian, S.; Hojjati, M.R.; Ahrari, M.; Bijad, E.; Deris, F.; Lorigooini, Z. Ruta graveolens and rutin, as its major compound: Investigating their effect on spatial memory and passive avoidance memory in rats. Pharm. Biol. 2020, 58, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Stoldt, A.K.; Derno, M.; Das, G.; Weitzel, J.M.; Wolffram, S.; Metges, C.C. Effects of rutin and buckwheat seeds on energy metabolism and methane production in dairy cows. J. Dairy Sci. 2016, 99, 2161–2168. [Google Scholar] [CrossRef]
- Alotaibi, S.M.; Saleem, M.S.; Al-Humaidi, J.G. Phytochemical contents and biological evaluation of Ruta chalepennsis L. growing in Saudi Arabia. Saudi Pharm. J. 2018, 26, 504–508. [Google Scholar] [CrossRef]
- Li, Y.; Rokayya, S.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Almatrafi, M.; Benajiba, N.; Helal, M. Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 2021, 11, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Chandra Das, P.; Sami, R.; Ismail, K.; Almushhin, A.; Iqbal, A.; Vasudevan, R.T. Effect of Spent Green Tea Leaf Extracts, Butylated Hydroxytoluene and Repeated Freezing-Thawing on Physicochemical and Oxidative Properties of Chevon. J. Biobased Mater. Bioenergy 2022, 22, 56–67. [Google Scholar] [CrossRef]
- Baranwal, A.; Aggarwal, P.; Rai, A.; Kumar, N. Pharmacological Actions and Underlying Mechanisms of Catechin: A Review. Mini Rev. Med. Chem. 2022, 22, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.K.; Sami, R.; Algarni, E.; Al-Mushhin, A.; Benajiba, N.; Almasoudi, A.; Almasoudi, A.G.; Mekawi, E. Phytochemical Profile and Antioxidant Activity of Sesame Seed (Sesamum indicum) By-Products for Stability and Shelf Life Improvement of Refined Olive Oil. Antioxidants 2022, 11, 338. [Google Scholar] [CrossRef]
- Hursel, R.; Viechtbauer, W.; Dulloo, A.G.; Tremblay, A.; Tappy, L.; Rumpler, W.; Westerterp-Plantenga, M.S. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: A meta-analysis. Obes. Rev. 2011, 12, e573–e581. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity in vitro (O/W Emulsion Systems) along with Their in vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Bensaad, M.S.; Dassamiour, S.; Hambaba, L.; Bensouici, C.; Haba, H. In vitro assessment of antioxidant, anti-inflammatory, neuroprotective and antimicrobial activities of Centaurea tougourensis Boiss. & Reut. J. Pharm. Pharmacogn. Res. 2021, 9, 790–802. [Google Scholar]
- Figueiredo-González, M.; Reboredo-Rodríguez, P.; González-Barreiro, C.; Simal-Gándara, J.; Valentão, P.; Carrasco-Pancorbo, A.; Andrade, P.B.; Cancho-Grande, B. Evaluation of the neuroprotective and antidiabetic potential of phenol-rich extracts from virgin olive oils by in vitro assays. Food Res. Int. 2018, 106, 558–567. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).