Abstract
Essential oils (EOs) have been reported as a promising group of naturally extracted compounds due to their various reported biological activities. Ipomoea carnea is a widely distributed plant with many traditional uses worldwide. However, although the EOs of various Ipomea species have been reported, I. carnea remains poorly studied. Therefore, the present investigation aimed to characterize the chemical profile of the EO of I. carnea growing in Egypt via gas chromatography/mass spectroscopy (GC-MS) and correlate its profile with other reported species via chemometric analysis using agglomerative hierarchical clustering (AHC) and principal component analysis (PCA). In addition, the aim was to determine the antioxidant and antibacterial activities of the extracted EO. Depending on the GC-MS analysis, 31 compounds were identified, mainly terpenes (94.82), with traces of carotenoid and apocarotenoid-derived compounds. The major compounds were tau-cadinol (35.68%), α-cadinol (26.76%), spathulenol (8.11%), and caryophyllene oxide (6.56%), which were assigned as major compounds. The chemometric studies showed that the Egyptian ecospecies of I. carnea differs in chemical profile from those growing in Brazil, as well as those reported for other Ipomea species. The EO showed significant DPPH and ABTS radical scavenging abilities, with IC50 values of 33.69 and 40.86 mg L−1, respectively. Additionally, the I. carnea EO displayed significant inhibition against the growth of all tested bacterial strains, where it showed an MIC range of 82–1442 mg mL−1. Based on the current results, the I. carnea EO, particularly the major identified compounds, could be used as a potential eco-friendly green resource for antioxidant and antimicrobial activities. Therefore, further study is recommended to evaluate the biological significance of the main compounds, either individually or in combination, as well as assess their modes of action and safety.
1. Introduction
Throughout the ages, medicinal plants have been widely used in traditional medicine because of their viability, safety, low toxicity, and pharmacological potential [1]. Among the overall phytochemicals derived from plants, essential oils (EOs) are considered a promising class that is integrated into food industries, pharmaceutics, and agriculture [2,3,4]. EOs possess several biological activities such as antimicrobial, antiviral, insecticide, herbicide, anti-inflammatory, antiulcerative, antipyretic, anticancer, and antiaging [5,6,7]. The antioxidant activity of EOs is attributed to the bioactivity of terpenoid compounds, particularly oxygenated compounds [8]. On the other hand, several bacterial strains, especially Gram-negative bacteria, have been found to construct resistance against numerous antibiotics due to their many use uses [9]; therefore, scientists are doing their best to explore the use of natural products and EOs from plants as antimicrobial agents [10].
The Ipomoea genus (family: Convolvulaceae) includes more than 600 plant species distributed worldwide, where they are grown as medicinal plants, weeds, and ornamental plants [11]. Plants belonging to the Ipomoea genus have been documented to have nutritional values; among them, I. batatas is well known as sweet potato worldwide [12]. The chemical analysis of Ipomoea plants has shown that they have several metabolites, including terpenes, flavonoids, coumarins, lignans, and alkaloids [13,14,15].
Ipomoea carnea Jacq. is a widely used plant in traditional medicine in several countries worldwide [16]. The different parts of this plant have been reported to be used in folk medicine for the treatment of several disorders such as venereal and skin diseases, immunodeficiency, dysentery, gout, rheumatism, and hypertension, in addition to their roles in menstruation provocation and as a laxative [16,17]. Moreover, several biological evaluations of the different extracts of I. carnea have been documented, including antimicrobial [18], anticancer [19], free radical scavenging [20], antidiabetic [21], immunomodulatory [22], wound healing [23], anticonvulsant, anxiolytic, anti-inflammatory, sedative, and hepatoprotective [16]. Chemically, the reported chemical characterization of the different extracts of I. carnea has revealed the presence of polyphenolic constituents, alkaloids, tannins, amino acids, proteins, terpenoids, carbohydrates, sterols, and saponins [24,25]. Swainsonine and calystegines have been documented as the main components of I. carnea [16].
The chemical profiles of the EOs of some Ipomoea species have been established, such as I. pes-caprae [13,26], I. aquatic [27], I. obscura [28], I. asarifolia, I. setifera [29], I. batatas [30], I. indica, I. amnicola, I. tiliacea, and I. batatas [26]. However, the EO of I. carnea has been reported only for Brazilian ecospecies [26], and to our knowledge, the EO of the Egyptian ecospecies of I. carnea has not been considered yet. Therefore, the present investigation aimed to (i) determine the chemical constituents of the EO of the aerial parts of the Egyptian ecospecies of I. carnea, (ii) compare the EO composition of the Egyptian ecospecies with other reported ecospecies via chemometric analysis, and (iii) evaluate the antioxidant and antimicrobial activities of the I. carnea EO.
2. Materials and Methods
2.1. Plant Materials
Aerial parts of Ipomoea carnea were collected in April 2021 from three populations growing in the wild on a canal bank habitat in cultivated fields around Mansoura City, Egypt (31°04′29.4″ N 31°25′05.3″ E). The collected samples consisted of leaves, stems, and flowers (Figure 1). The samples were air-dried until complete dryness at room temperature, crushed, and stored in a paper bag until further analyses. The plant specimen was identified and authenticated according to the books of the flora of Egypt following Tackholm [31] and Boulos [32]. Additionally, the voucher sample was prepared and dropped into the Mansoura University Herbarium with Voucher Code Mans.0030903009.

Figure 1.
Ipomoea carnea Jacq. Plant: (a) overview of the growing shrub; (b) and (c) close view of the flowering branches.
2.2. EO Extraction Analysis and Characterization
From each sample of I. carnea, 250 g of EO was extracted by hydrodistillation over a Clevenger device for 3 h, separated by n-hexane, and dried with about 0.5 g of anhydrous Na2SO4. The three EO samples were stored in glass vials at 4 °C in a refrigerator for analysis. Chemical profiling of the EOs was established separately using gas chromatography/mass spectrometry (GC/MS) at the National Research Center of Egypt under the same protocols described previously [33]. In detail, the analysis of the extracted EO samples was performed using a GC-MS apparatus combined with the TRACE GC ultra gas chromatograph and Thermo Scientific™ EC quadrupole mass spectrometer unit (Waltham, MA, USA). The dimension and film thickness of the GC-MS column was 30 m × 0.32 mm × 0.25 µm. Helium as a transporter gas was used with a split ratio of 1 to 10 and a flow rate of 1.0 mL per min. The temperature was justified as usual according to the following: 60 °C (1 min), then increased to 240 °C within 4 °C/min. The EO samples were diluted in 1 µL of n-hexane with a ratio of 1:10 (v/v) and then injected in an injector and detector modified at 210 °C. The mass spectral data of components were obtained via electron ionization (EI) with m/z 40–450 as the spectral range at 70 eV. The chemical components were authenticated and identified using Automated Mass Spectral Deconvolution and Identification System (AMDIS) software, NIST library database, the Wiley Spectral Library Collection, and the retention indices closed to n-alkanes (C8–C22).
2.3. Antioxidant Activity
The EO extracted from I. carnea was assayed for its antioxidant activity via the scavenging ability of the free radicals 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Sigma-Aldrich, Darmstadt, Germany). According to Miguel [34], serial concentrations of the EO (5–50 mg L−1) were prepared using 95% methanol as a solvent. In glass tubes, each concentration (2 mL) and freshly prepared DPPH (2 mL of 0.3 mM) were vigorously shaken and left for 30 min in dark conditions at room temperature. At 517 nm, the measurement of the absorbance was performed using a Spectronic 21D model spectrophotometer. For additional confirmation of the antioxidant activity, ABTS scavenging was tested according to Re et al. [35]. In brief, 0.2 mL of each concentration was mixed with 2 mL of 7 mM of freshly prepared ABTS. The mixtures were incubated for 6 min at room temperature, and the absorbance was immediately measured with a spectrophotometer at 734 nm. The positive control was prepared using ascorbic acid (standard antioxidant) with a range of 1–20 mg L−1, while the negative control was treated using methanol similarly to the samples. The calculation of the scavenging potential was established by the following equation:
The IC50 (required EO concentration for 50% scavenging) was calculated based on the exponential curve of the absorbance and concentrations using Microsoft Excel 2019.
2.4. Antibacterial Activity
The EO extracted from the aerial parts of I. carnea was evaluated for its bacterial inhibitory potential via the agar diffusion method [36]. The activity was tested against four Gram-negative bacterial isolates (Escherichia coli ATCC 10536, Klebsiella pneumoniae ATCC 10031, Pseudomonas aeruginosa ATCC 9027, and Salmonella typhimurium ATCC 25566) and four Gram-positive bacterial isolates (Bacillus cereus EMCC 1080, Staphylococcus epidermidis ATCC 12228, Staphylococcus haemolyticus ATCC 29970, and Staphylococcus xylosus NCCP 10937). These bacterial isolates were purchased from the Cairo Microbiological Resources Centre (Cairo MIRCEN), Agriculture College, Ain Shams University, Egypt. To test the antibacterial activity of the EO, 10 mg mL−1 EO was prepared using 1% (CH3)2SO (DMSO), and filter paper discs (0.5 cm) were impregnated with 50 μL of EO. Petri plates with the medium of nutrient agar were incubated with 1 × 108 colony forming units (CFU)/mL of each bacterial isolate, and the discs saturated with EO were placed over the medium within the plates. The plates were immediately sealed with Parafilm® tape (Sigma, St. Louis, MO, USA) and incubated in an incubator modified at 37 °C. After 24 h, the diameter of the clear (inhibition) zone around the disc was measured in millimeters from three points. In addition, DMSO was used as a negative control, where it was prepared and treated as previously mentioned for the EO, as it did not show any antibacterial activity. Cephalexin, tetracycline, ofloxacin, and ampicillin were tested as the positive control (standard antibiotic). The minimum inhibitory concentration (MIC) was calculated for each bacterial isolate based on the exponential curve of the inhibition zone diameter and various concentrations of the EO.
2.5. Data Analysis
The antioxidant and antibacterial activity experiments were repeated with 3 replicas, and the data were expressed as average ± standard error. To test the significance among treatments, the data of either antioxidant or antibacterial activities, with replications, were subjected to a one-way ANOVA, followed by Duncan’s post-hoc test at a 0.05 probability level using CoStat software (version 6.311, CoHort Software, Monterey, CA, USA). To correlate the ecospecies studied in this paper with other reported species, we used two chemometric analyses: agglomerative hierarchical clustering (AHC) and principal component analysis (PCA). A dataset of 22 Ipomea ecospecies was constructed, consisting of (1) I. carnea (leaf) collected from Egypt, (2) I. pes-caprae (fresh leaf) collected from Mauritius, (3) I. pes-caprae (dry leaf) collected from Mauritius [13], (4) I. obscura (dry leaf) collected from India [28], (5) I. aquatica (aerial parts) collected from Japan [27], (6) I. asarifolia (leaf) collected from Brazil, (7) I. setifera (leaf) collected from Brazil [29], (8) I. batatas (leaf) collected from Nigeria [30], (9) I. batatas (leaf) collected from Brazil, (10) I. batatas (flower) collected from Brazil, (11) I. carnea (leaf) collected from Brazil, (12) I. carnea (flower) collected from Brazil, (13) I. pes-caprae (leaf) collected from Brazil, (14) I. pes-caprae (flower) collected from Brazil, (15) I. alba (leaf) collected from Brazil, (16) I. alba (flower) collected from Brazil, (17) I. indica (leaf) collected from Brazil, (18) I. indica (flower) collected from Brazil, (17) I. indica (leaf) collected from Brazil, (18) I. indica (flower) collected from Brazil, (19) I. tiliacea (leaf) collected from Brazil, (20) I. tiliacea (flower) collected from Brazil, (21) I. amnicola (leaf) collected from Brazil, and (22) I. amnicola (flower) collected from Brazil [26]. The dataset contains 46 major compounds (>3%) from 22 Ipomea ecospecies (see Supplementary Materials Table S1). The dataset matrix was subjected to AHC and PCA.
3. Results and Discussion
3.1. Chemical Profile of I. carnea EO
The hydrodistillation of the I. carnea aerial parts provided 0.034% (v/w) golden-yellow EO. The yield of EOs from other reported Ipomea species such as I. carnea, I. pes-caprae, I. alba, I. batatas, and I. indica was 0.01–0.13% for fresh leaves and 0.01–0.03 for fresh flowers [26]. The extracted oil was subjected to GC-MS analysis (Figure 2), where 31 compounds were identified with a relative concentration of 97.58%. The compound names, molecular formulas, retention times, relative concentrations (%), and Kovats indices (KIs) of all identified compounds are presented in Table 1.
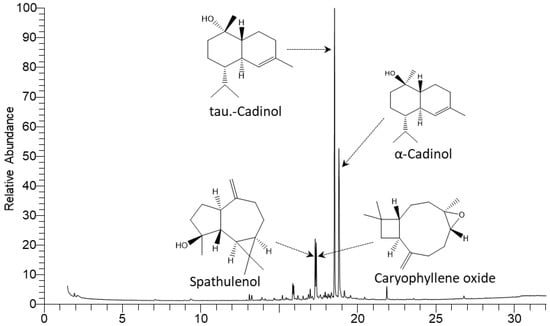
Figure 2.
GC-MS of the EO extracted from Ipomoea carnea aerial parts.

Table 1.
Identified components of essential oil of Ipomoea carnea assigned by GC-MS.
The identified compounds can be grouped into six classes: oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, diterpene hydrocarbons, carotenoid, and apocarotenoid-derived compounds (Table 1). Sesquiterpene represented the abundant EO components with a relative concentration of 92.71% comprising mainly oxygenated sesquiterpene (82.88%) and a low relative concentration of sesquiterpene hydrocarbons (9.83%). The abundance of the sesquiterpenoids was common in the EOs of several Ipomoea plants such as I. batatas (46.5%) [30], I. pes-caprae (70.4%) [13], and I. obscura (84.9%) [28].
Tau-cadinol (35.68%), α-cadinol (26.76%), spathulenol (8.11%), and caryophyllene oxide (6.56%) (Figure 2) represented the major constituents of oxygenated sesquiterpene compounds, while 6-epishyobunone (0.35%) was determined as a minor compound. On the other hand, ar-curcumene (1.83%), γ-muurolene (1.76%), and α-calacorene (1.04%) were identified as the main components of sesquiterpene hydrocarbons. The preponderance of the cadinene and cadinol isomers was documented in numerous Ipomoea plants such as I. carnea collected from Brazil [26] and I. pes-caprae collected from Belgium [13]. In addition, spathulenol and caryophyllene oxide were documented as major sesquiterpenoids in the EOs of Brazilian I. pes-caprae [13] and Nigerian I. batatas [30].
On the other hand, monoterpenes were assigned as trace components and represented only by oxygenated compounds (1.75%) and no monoterpene hydrocarbons were detected (Table 1). Three oxygenated monoterpenes, 4-terpineol (0.69%), α-fenchyl alcohol (0.57%), and linalool (0.49%), were only assigned. The scarcity of the monoterpenes is common in the reported EO profiles of many studied Ipomoea species [13,27,28,30].
In the extracted EO of I. carnea in the present study, only one diterpene compound, beyerene, was identified with a very low relative concentration (0.36%). The scarcity of the diterpenes in the EOs derived from medicinal and aromatic plants is common, with a few exceptions [8]. In addition, the EOs of Ipomoea plants have been reported to contain a low relative concentration of diterpenoid compounds [13,27,28,30]. In addition, three non-terpenoid compounds were characterized that can be categorized into carotenoid (0.95%), including E-β-damascenone (0.77%), E-α-ionone (0.18%), and apocarotenoid (1.81%), comprising compounds derived from hexahydrofarnesyl acetone (1.81%). These three compounds were identified as trace components in the EOs of some I. pes-caprae [13].
The present chemical characterization of I. carnea EO deduced some similarities with the other Ipomoea species, which may be ascribed to the climatic, genetic, and environmental conditions [4,37,38].
3.2. Chemometric Analysis of the EOs of Ipomea Specie
The chemometric analysis of the EO profile of the Egyptian I. carnea ecospecies and the other reported Ipomea species revealed considerable variation (Figure 3).
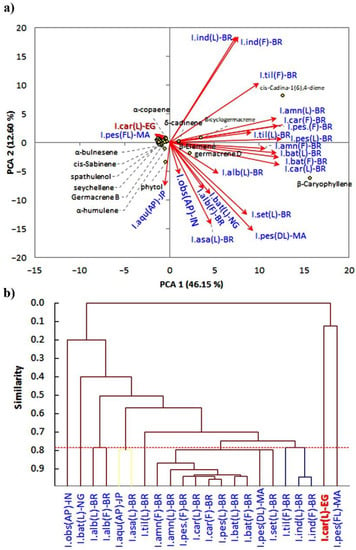
Figure 3.
Chemometric analysis of the EOs of various Ipomoea carnea and various reported Ipomoea species: (a) principal component analysis (PCA); (b) agglomerative hierarchical clustering (AHC). I.car: I. carnea, I.pes: I. pes-caprae, I.obs: I. obscura, I.Aqu: I. aquatica, I. asa: I. asarifolia, I.set: I. setifera, I.bat: I. batatas, I.alb: I. alba, I.ind: I. indica, I.til: I. tiliacea, I.amn: I. amnicola, AP: aerial parts, L: leaf, F: flower, DL: dry leaf, FL: fresh leaf, IN: India, NG: Nigeria, BR: Brazil, JP: Japan, MA: Mauritius, EG: Egypt.
However, the present results showed a positive correlation with the profile of the EO of the I. pes-caprae collected from Mauritius [13]. The cluster analysis showed that each Ipomea ecospecies has its own specific chemical profile signature of the EO compounds. These data could be ascribed to the genetic characteristics of each species [38,39]. In addition, the only studied I. carnea from Brazil did not correlate with those reported in the Egyptian ecospecies of the present study. This could be attributed to the variation in the environmental and climatic conditions [40]. The extraction techniques have been reported to have a great effect on the composition of the extracted EO [41,42,43]; therefore, this variation could be a reasonable factor for the determined variation between the Egyptian and Brazilian ecospecies of I. carnea, where, for the Brazilian ecospecies, the EO was extracted with steam distillation, while for the Egyptian ecospecies, the EO was extracted upon hydrodistillation.
The application of PCA on the dataset of the 22 Ipomea ecospecies showed that present Egyptian I. carnea and I. pes-caprae collected from Mauritius are correlated with each other, where they did not show specific correlation with specific compounds. On the other side of the PCA, the Brazilian ecospecies of Ipomoea amnicola, I. indica, I.alba, I. pes-caprae, I. carnea, I. tiliacea, and I. batatas were separated at the right side of the PCA and showed a correlation with β-caryophyllene, cis-cadina-1(6),4-diene, germacrene, β-elemene, bicyclogermacren, and δ-cadinene. On the other hand, the Japanese ecospecies of I. aquatica were separated alone and revealed a correlation with the phytol compound.
3.3. Free Radical Scavenging Activity of I. carnea EO
The free radical scavenging potentiality of I. carnea EO was evaluated using DPPH and ABTS assays with respect to ascorbic acid as a reference antioxidant agent. The present data showed that the EO of I. carnea has strong antioxidant capability in comparison to the standard antioxidant (ascorbic acid) (Table 2). The activity of scavenging increased with the increment of the concentration. At a concentration of 20 mg L−1, the EO showed 31.05% and 24.15% DPPH and ABTS scavenging, respectively, while ascorbic acid showed 73.17% and 69.06% scavenging, respectively. According to the IC50 values, the EO of I. carnea displayed IC50 of 33.69 mg L−1 for DPPH and 40.86 mg L−1 for ABTS, whereas ascorbic acid showed an IC50 of 11.51 and 12.94 mg L−1.

Table 2.
DPPH and ABTS radicals scavenging activity percentage and IC50 values by the EO of the Ipomoea carnea and ascorbic acid as standard.
The current findings were in total agreement with the fact of the significant antioxidant activities of the different extracts of the Ipomoea species. The alcoholic extract of the Indian I. carnea was reported to exhibit significant in vitro antioxidant activity using DPPH and ABTS assays [20]. In addition, the different extracts of the leaves of I. batatas, including EOs, were described to have strong antioxidant potential [13,14,15]. Alam and coworkers described that the methanolic extract of I. mauritiana has potent DPPH scavenging activity compared with ascorbic acid [44]. Moreover, the phenolic-enriched alcoholic extract of the halophyte, I. pes-caprae, was reported to have strong reducing power on DPPH radicals, better than butylated hydroxyanisole and butylated hydroxytoluene [45].
The antioxidant activity of I. carnea EO might be ascribed to the chemical constituents characterized mainly as terpenes. The reproducible free radical scavenging potential of the EOs was documented to be strongly correlated with the overbalance of the terpenoid compounds [6,8,46]. In addition, the oxygenated compounds are responsible for more antioxidant activity due to their functions in the oxygenation of the components and scavenging the free radicals [8,47,48]. The chemical profiling of the I. carnea EO showed that 83.63% of overall oil mass are oxygenated compounds that could cause significant observed antioxidant activity.
Among the oxygenated compounds, the oxygenated sesquiterpene compounds, tau-cadinol, α-cadinol, spathulenol, and caryophyllene oxide, represented the main compounds in the I. carnea EO. These compounds might have played an effective role as antioxidant agents either individually or synergistic with others. The EO derived from Cullen plicata has been reported to possess a strong antioxidant ability due to the abundance of tau-cadinol, α-cadinol, and caryophyllene oxide [49]. In addition, the EO of Algerian Teucrium polium was reported to have significant antioxidant activity due to its high content of spathulenol and tau-cadinol [50]. Caryophyllene oxide represented one of the most common compounds in the plants’ EOs with important biological actions, especially antioxidant activity, due to the presence of lone pairs of electrons that increase the free radicals trapping [51]. This compound was reported to be of the main contributors to the increase in the antioxidant activity of the EOs of Cannabis sativa [51], Salvia palaestina, S. ceratophylla [52], and Heliotropium curassavicum [53]. Additionally, α-cadinol was promoted to act as a significant antioxidant mediator in the EO of several herbs such as Tabernaemontana catharinensis [54] and Xenophyllum poposum [55]. Furthermore, the minor constituents could be contributed to the antioxidant activity via the synergetic effect [56].
3.4. Antibacterial Activity of I. carnea EO
The inhibitory effects of the EO derived from I. carnea were estimated against eight bacterial strains (four Gram-negative and four Gram-positive strains). The results showed that the EO displayed significant inhibitory effects against all strains. For Gram-negative bacteria, the activity can be ordered as follows: K. pneumoniae > E. coli > P. aeruginosa > S. typhimurium, where they showed MIC values of 84, 94, 124, and 158 mg mL−1, respectively (Table 3). However, the EO exhibited inhibition against the four Gram-positive bacteria strains in the following order: S. xylosus > B. cereus > S. epidermidis > S. haemolyticus, with MIC values of 82, 84, 1442, and 82 mg mL−1, respectively. Previously, different extracts and EOs of several Ipomoea plants were stated to have inhibitory effects against different bacterial strains. The crude alcoholic extract of I. mauritiana was described to significantly inhibit the growth of several bacteria strains [44]. Moreover, the whole plant extracts of I. pes-caprae have been reported to inhibit a set of Gram-positive and Gram-negative bacterial strains [57]. In addition, some compounds isolated from different extracts and oils of I. pes-caprae, such as (-) mullein [58] and E-phytol [59], have been described to have the potential for inhibiting some bacterial strains such as Escherichia coli and Staphylococcus sp. In agreement with our data, Yuan et al. [60] described that the EO derived from the waste materials of I. batatas can strongly stop the growth of Pseudomonas aeruginosa, Escherichia coli, and Bacillus cereus.

Table 3.
Antibacterial activity of the Ipomoea carnea essential oil and some selected reference antibiotics (10 mg mL−1).
The bacterial inhibition capability of the I. carnea EO was stronger than that described for other plants’ EOs as Kickxia aegyptiaca [46], Teucrium polium [41], and Deverra tortuosa [6]. The observed antibacterial activity of the I. carnea EO in the present study could be attributed to the major compounds such as caryophyllene oxide, tau-cadinol, α-cadinol, and spathulenol. The two main compounds, tau-cadinol and α-cadinol, in the current study were also reported in high concentrations of the EO of various plants that showed strong bacterial growth inhibition such as Eugenia chlorophylla [61], Litsea acutivena [62], Diospyros discolor [63], and Teucrium polium [41]. Spathulenol and caryophyllene oxide represented common compounds in EOs derived from the plant kingdom [4]. Many EOs were documented to have strong antibacterial activity due to the preponderance of spathulenol and caryophyllene oxide such as Baccharis dracunculifolia [64], Salvia hydrangea [65], Satureja coerulea [66], and Kickxia aegyptiaca [46]. Schmidt and coworkers described that caryophyllene oxide is one of the most active antibacterial agents as a single compound against Escherichia, Klebsiella, and Salmonella strains [67]. The major compound, tau-cadinol, in the present study was described as a potent antibacterial agent against S. aureus [68]. In addition, caryophyllene and its oxygenated derivatives were documented to have bactericidal effects against several bacterial strains, including B. cereus, B. subtilis, E. coli, S. aureus, and P. aeruginosa [69]. Spathulenol has been reported in high concentrations (36.6%) of Helichrysum amorginum EO, where it showed strong inhibitory effects against S. aureus and S. epidermidis [70]. Moreover, the other minor components might act in synergetic interaction with other compounds as antibacterial agents [4].
The Gram-negative bacteria were well-known bacteria with strong resistance to antibiotics due to their outer rigid membrane with a high content of lipopolysaccharide [9]. Several documented data have revealed that the EOs and their bioactive constituents could attach to the bacterial cell surface and therefore perforate the cell membrane phospholipid bilayer, then accumulate with mischievous effects on the cell metabolism, causing bacterial cell death [71].
4. Conclusions
The present study showed for the first time the chemical profile of the EO extracted from the Egyptian ecospecies of I. carnea. The identified compounds were mainly sesquiterpenes with caryophyllene oxide, tau-cadinol, α-cadinol, and spathulenol as major compounds. The chemometric analysis revealed that the EO of the presently studied I. carnea is different from that reported from other species of Ipomoea. The extracted EO exhibited substantial antioxidant activity and strong inhibition against the tested Gram-negative and Gram-positive bacteria. The observed activities of this oil in the current study might be ascribed to the major identified constituents, which could be integrated as eco-friendly natural resources with antioxidant and antibacterial potential. Further study is recommended to evaluate the various activities of the major identified compounds, either individually or in combination, and determine their potential, modes of action, and safety.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su14159504/s1, Table S1: A dataset of the major compounds (>3%) of the present studied Ipomea carnea and 21 other reported Ipomea ecospecies.
Author Contributions
Conceptualization, A.M.A.-E. and A.I.E.; methodology, A.M.A.-E., Y.A.E.-A., A.E.-N.G.E.G. and A.I.E.; validation, A.M.A.-E. and A.I.E.; investigation, A.M.A.-E., Y.A.E.-A., A.M.E., E.M.H., S.F.A., A.E.-N.G.E.G., K.M.E. and A.I.E.; visualization, A.M.A.-E.; writing—original draft preparation, A.M.A.-E. and A.I.E.; writing—review and editing, A.M.A.-E., Y.A.E.-A., A.M.E., E.M.H., S.F.A., N.S.Z., A.E.-N.G.E.G., K.M.E. and A.I.E.; writing—original draft preparation, A.M.A.-E. and A.I.E.; funding acquisition, S.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Researchers Supporting Project (RSP-2021/241), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project (RSP-2021/241), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 133. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2020, 10, 36. [Google Scholar] [CrossRef]
- Ammar, N.M.; Hassan, H.; Ahmed, R.; El Gendy, A.E.-N.; Abd-ElGawad, A.; Farrag, A.R.; Farag, A.R.; Elshamy, A.; Afifi, S. Gastro-protective effect of Artemisia sieberi essential oil against ethanol-induced ulcer in rats as revealed via biochemical, histopathological and metabolomics analysis. Biomarkers 2022, 27, 247–257. [Google Scholar] [CrossRef]
- Fayed, E.M.; Abd-EIGawad, A.M.; Elshamy, A.I.; El-Halawany, E.S.F.; EI-Amier, Y.A. Essential oil of Deverra tortuosa aerial parts: Detailed chemical profile, allelopathic, antimicrobial, and antioxidant activities. Chem. Biodivers. 2021, 18, e2000914. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elgamal, A.M.; Ei-Amier, Y.A.; Mohamed, T.A.; El Gendy, A.G.; Elshamy, A.I. Chemical composition, allelopathic, antioxidant, and anti-inflammatory activities of sesquiterpenes rich essential oil of Cleome amblyocarpa Barratte & Murb. Plants 2021, 10, 1294. [Google Scholar]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Nasser El Gendy, A.; Al-Rowaily, S.L.; Assaeed, A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019, 16, e1900278. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Austin, D.F.; Staples, G.W.; Simão-Bianchini, R. A synopsis of Ipomoea (Convolvulaceae) in the Americas: Further corrections, changes, and additions. Taxon 2015, 64, 625–633. [Google Scholar] [CrossRef]
- Bovell-Benjamin, A.C. Sweet potato: A review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 2007, 52, 1–59. [Google Scholar] [PubMed]
- Marie, D.E.; Dejan, B.; Quetin-Leclercq, J. GC-MS Analysis of the leaf essential oil of Ipomea pes-caprae, a traditional herbal medicine in mauritius. Nat. Prod. Commun. 2007, 2, 1225–1228. [Google Scholar] [CrossRef] [Green Version]
- Jenett-Siems, K.; Weigl, R.; Kaloga, M.; Schulz, J.; Eich, E. Ipobscurines C and D: Macrolactam-type indole alkaloids from the seeds of Ipomoea obscura. Phytochemistry 2003, 62, 1257–1263. [Google Scholar] [CrossRef]
- Kourouma, V.; Mu, T.H.; Zhang, M.; Sun, H.N. Comparative study on chemical composition, polyphenols, flavonoids, carotenoids and antioxidant activities of various cultivars of sweet potato. Int. J. Food Sci. Technol. 2020, 55, 369–378. [Google Scholar] [CrossRef]
- Fatima, N.; Rahman, M.M.; Khan, M.A.; Fu, J. A review on Ipomoea carnea: Pharmacology, toxicology and phytochemistry. J. Complement. Integr. Med. 2014, 11, 55–62. [Google Scholar] [CrossRef]
- Wadnerwar, N.; Deogade, M. Future perspectives of therapeutic claims of an ethnopharmacological drug Ipomoea carnea Jacq.-A critical review. Int. J. Ayurvedic Med. 2022, 12, 177–184. [Google Scholar] [CrossRef]
- Padhi, S.; Tayung, K. Antimicrobial activity and molecular characterization of an endophytic fungus, Quambalaria sp. isolated from Ipomoea carnea. Ann. Microbiol. 2013, 63, 793–800. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Zahra, S.S.; Ahmed, M.; Fatima, H.; Mirza, B.; Haq, I.-u.; Khan, S.U. Polyphenolic profiling of Ipomoea carnea Jacq. by HPLC-DAD and its implications in oxidative stress and cancer. Nat. Prod. Res. 2019, 33, 2099–2104. [Google Scholar] [CrossRef]
- Ambiga, S.; Jeyaraj, M. Evaluation of in vitro antioxidant activity of Ipomoea carnea Jacq. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 327–338. [Google Scholar]
- Khalid, M.S.; Singh, R.K.; Kumar, S.J.; Suresh, D.; Rao, S.K.; Reddy, N.I. Antidiabetic activity of aqueous extract of Ipomoea carnea leaves in streptozotocin induced diabetic rats. Int. J. Pharmacol. Biol. Sci. 2011, 5, 45–54. [Google Scholar]
- Hueza, I.M.; Górniak, S.L. The immunomodulatory effects of Ipomoea carnea in rats vary depending on life stage. Hum. Exp. Toxicol. 2011, 30, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Gupta, G.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Wound healing effect of ethanolic extract from morning glory (Ipomoea carnea Jacq.) leaves by using different models in rats. Pak. J. Pharm. Sci. 2018, 31, 1355–1361. [Google Scholar] [PubMed]
- Khatiwora, E.; Adsul, V.B.; Kulkarni, M.M.; Deshpande, N.; Kashalkar, R. Spectroscopic determination of total phenol and flavonoid contents of Ipomoea carnea. Int. J. ChemTech Res. 2010, 2, 1698–1701. [Google Scholar]
- Haraguchi, M.; Gorniak, S.L.; Ikeda, K.; Minami, Y.; Kato, A.; Watson, A.A.; Nash, R.J.; Molyneux, R.J.; Asano, N. Alkaloidal components in the poisonous plant, Ipomoea carnea (Convolvulaceae). J. Agric. Food Chem. 2003, 51, 4995–5000. [Google Scholar] [CrossRef]
- Tenório, T.M.; Moraes, M.M.; Camara, C.A.; Araujo, C.A.; Silva, M.M.; Rodrigues, L.V. Scents from the Brazilian Atlantic Forest Biome: Chemical composition of essential oils from the leaves and flowers of seven species of Ipomoea (Convolvulaceae). J. Essent. Oil Res. 2021, 33, 567–583. [Google Scholar] [CrossRef]
- Kameoka, H.; Kubo, K.; Miyazawa, M. Essential oil components of water-convolvulus (Ipomoea aquatica Forsk.). J. Essent. Oil Res. 1992, 4, 219–222. [Google Scholar] [CrossRef]
- Joshi, R.K. Sesquiterpene-rich volatile constituents of Ipomoea obscura (L.) Ker-Gawl. Nat. Prod. Res. 2015, 29, 1935–1937. [Google Scholar] [CrossRef]
- da Silva Júnior, O.S.; Franco, C.d.J.P.; de Moraes, A.A.B.; Cruz, J.N.; da Costa, K.S.; do Nascimento, L.D.; de Aguiar Andrade, E.H. In silico analyses of toxicity of the major constituents of essential oils from two Ipomoea L. species. Toxicon 2021, 195, 111–118. [Google Scholar] [CrossRef]
- Ogunmoye, A.; Adebayo, M.A.; Inikpi, E.; Ogunwande, I.A. Chemical constituents of essential oil from the leaves of Ipomoea batatas L. (Lam.). Int. Res. J. Pure Appl. Chem. 2015, 7, 42–48. [Google Scholar] [CrossRef]
- Tackholm, V. Students’ Flora of Egypt, 2nd ed.; Cairo University Press: Cairo, Egypt, 1974. [Google Scholar]
- Boulos, L. Flora of Egypt; All Hadara Publishing: Cairo, Egypt, 1995; Volume 1. [Google Scholar]
- Abd-ELGawad, A.M.; Al-Rowaily, S.L.; Assaeed, A.M.; Ei-Amier, Y.A.; El Gendy, A.E.-N.G.; Omer, E.; Al-Dosari, D.H.; Bonanomi, G.; Kassem, H.S.; Elshamy, A.I. Comparative chemical profiles and phytotoxic activity of essential oils of two ecospecies of Pulicaria undulata (L.) CA Mey. Plants 2021, 10, 2366. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. Flavour Fragr. J. 2010, 25, 219–312. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lorian, V. Antibiotics in Laboratory Medicine; Lippincott Williams & Wilkins: Baltimore, PA, USA, 2005. [Google Scholar]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Assaeed, A.M.; Al-Rowaily, S.L. Interspecific variations in the habitats of Reichardia tingitana (L.) Roth leading to changes in its bioactive constituents and allelopathic activity. Saudi J. Biol. Sci. 2020, 27, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Assaeed, A.M.; Elgamal, A.M.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Dar, B.A.; Mohamed, T.K.; Elshamy, A.I. Essential oil of Calotropis procera: Comparative chemical profiles, antimicrobial activity, and allelopathic potential on weeds. Molecules 2020, 25, 5203. [Google Scholar] [CrossRef]
- Saleh, I.; Abd-ElGawad, A.; El Gendy, A.E.-N.; Abd El Aty, A.; Mohamed, T.; Kassem, H.; Aldosri, F.; Elshamy, A.; Hegazy, M.-E.F. Phytotoxic and antimicrobial activities of Teucrium polium and Thymus decussatus essential oils extracted using hydrodistillation and microwave-assisted techniques. Plants 2020, 9, 716. [Google Scholar] [CrossRef]
- da Silva, W.M.F.; Kringel, D.H.; de Souza, E.J.D.; da Rosa Zavareze, E.; Dias, A.R.G. Basil essential oil: Methods of extraction, chemical composition, biological activities, and food applications. Food Bioprocess Technol. 2021, 15, 1–27. [Google Scholar] [CrossRef]
- Elshamy, A.; Abd-ElGawad, A.; Mohamed, T.; El Gendy, A.E.N.; Abd El Aty, A.A.; Saleh, I.; Moustafa, M.F.; Hussien, T.A.; Pare, P.W.; Hegazy, M.E.F. Extraction development for antimicrobial and phytotoxic essential oils from Asteraceae species: Achillea fragrantissima, Artemisia judaica and Tanacetum sinaicum. Flavour Fragr. J. 2021, 36, 352–364. [Google Scholar] [CrossRef]
- Alam, I.; Forid, S.; Roney, M.; Aluwi, F.F.M.; Huq, M. Antioxidant and antibacterial activity of Ipomoea mauritiana Jacq.: A traditionally used medicinal plant in Bangladesh. Clin. Phytosci. 2020, 6, 35. [Google Scholar] [CrossRef]
- Qasim, M.; Abideen, Z.; Adnan, M.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. S. Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Bonanomi, G.; Gendy, A.E.-N.G.E.; Elgorban, A.M.; Alamery, S.F.; Elshamy, A.I. Chemical composition of Kickxia aegyptiaca essential oil and its potential antioxidant and antimicrobial activities. Plants 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.M.; Matar, S.A.; Litescu, S.-C.; Abuhamdah, S.; Al-Jaber, H.I.; Afifi, F.U. Biological activities of the hydro-alcoholic and aqueous extracts of Achillea fragrantissima (Forssk.) grown in Jordan. Nat. Sci. 2014, 6, 23–30. [Google Scholar]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Omer, E.A.; Dar, B.A.; Al-Taisan, W.a.A.; Elshamy, A.I. Essential oil enriched with oxygenated constituents from invasive plant Argemone ochroleuca exhibited potent phytotoxic effects. Plants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016, 80, 36–41. [Google Scholar] [CrossRef]
- Maizi, Y.; Meddah, B.; Tir Touil Meddah, A.; Gabaldon Hernandez, J.A. Seasonal variation in essential oil content, chemical composition and antioxidant activity of Teucrium polium L. growing in Mascara (North West of Algeria). J. Appl. Biotechnol. Rep. 2019, 6, 151–157. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Gursoy, N.; Tepe, B.; Akpulat, H.A. Chemical composition and antioxidant activity of the essential oils of Salvia palaestina (Bentham) and S. ceratophylla (L.). Rec. Nat. Prod. 2012, 6, 278–287. [Google Scholar]
- Abd-ElGawad, A.M.; Elshamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef] [Green Version]
- Boligon, A.A.; Schwanz, T.G.; Piana, M.; Bandeira, R.V.; Frohlich, J.K.; Brum, T.F.d.; Zadra, M.; Athayde, M.L. Chemical composition and antioxidant activity of the essential oil of Tabernaemontana catharinensis A. DC. leaves. Nat. Prod. Res. 2013, 27, 68–71. [Google Scholar] [CrossRef]
- González, A.M.; Tracanna, M.I.; Amani, S.M.; Schuff, C.; Poch, M.J.; Bach, H.; Catalán, C.A. Chemical composition, antimicrobial and antioxidant properties of the volatile oil and methanol extract of Xenophyllum poposum. Nat. Prod. Commun. 2012, 7, 1663–1666. [Google Scholar] [CrossRef] [Green Version]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Rao, U. Pharmacological potential of Ipomea pes-caprae (L.) R. Br. whole plant extracts. Pelagia Res. Lib. 2015, 6, 52–60. [Google Scholar]
- Pongprayoon, U.; Baeckström, P.; Jacobsson, U.; Lindström, M.; Bohlin, L. Compounds inhibiting prostaglandin synthesis isolated from Ipomoea pes-caprae. Plant. Med. 1991, 57, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Ghaneian, M.T.; Ehrampoush, M.H.; Jebali, A.; Hekmatimoghaddam, S.; Mahmoudi, M. Antimicrobial activity, toxicity and stability of phytol as a novel surface disinfectant. Environ. Health Eng. Manag. J. 2015, 2, 13–16. [Google Scholar]
- Yuan, B.; Xue, L.-W.; Zhang, Q.-Y.; Kong, W.-W.; Peng, J.; Kou, M.; Jiang, J.-H. Essential oil from sweet potato vines, a potential new natural preservative, and an antioxidant on sweet potato tubers: Assessment of the activity and the constitution. J. Agric. Food Chem. 2016, 64, 7481–7491. [Google Scholar] [CrossRef]
- Stefanello, M.É.A.; Cervi, A.C.; Ito, I.Y.; Salvador, M.J.; Wisniewski Jr, A.; Simionatto, E.L. Chemical composition and antimicrobial activity of essential oils of Eugenia chlorophylla (Myrtaceae). J. Essent. Oil Res. 2008, 20, 75–78. [Google Scholar] [CrossRef]
- Ho, C.-L.; Liao, P.-C.; Wang, E.I.-C.; Su, Y.-C. Composition and antimicrobial activity of the leaf and twig oils of Litsea acutivena from Taiwan. Nat. Prod. Commun. 2011, 6, 1755–1758. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.-C.; Hsu, K.-P.; Wang, E.I.-C.; Ho, C.-L. Composition, in vitro cytotoxic, and antimicrobial activities of the flower essential oil of Diospyros discolor from Taiwan. Nat. Prod. Commun. 2015, 10, 1311–1314. [Google Scholar] [CrossRef] [Green Version]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghavam, M.; Manca, M.L.; Manconi, M.; Bacchetta, G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth. Sci. Rep. 2020, 10, 15647. [Google Scholar] [CrossRef]
- Azaz, D.; Demirci, F.; Satıl, F.; Kürkçüoğlu, M.; Hüsnü, K.; Bașerb, C. Antimicrobial activity of some Satureja essential oils. Z. Naturforsch. C 2002, 57, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial activities of single aroma compounds. Nat. Prod. Commun. 2010, 5, 1365–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claeson, P.; Rådström, P.; Sköld, O.; Nilsson, Å.; Höglund, S. Bactericidal effect of the sesquiterpene T-cadinol on Staphylococcus aureus. Phytother. Res. 1992, 6, 94–98. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Chinou, I.B.; Bougatsos, C.; Perdetzoglou, D. Chemical composition and antimicrobial activities of Helichrysum amorginum cultivated in Greece. J. Essent. Oil Res. 2004, 16, 243–245. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).