Value-Added White Beer: Influence of Red Grape Skin Extract on the Chemical Composition, Sensory and Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Grape Skin Extract (GSE)
2.3. Phytochemical Characterization of GSE
2.3.1. Total Monomeric Anthocyanin Content
2.3.2. Total Flavonoids Content
2.3.3. Total Phenolic Content
2.3.4. DPPH Radical Scavenging Activity
2.3.5. ABTS Radical Cation Scavenging Activity
2.3.6. High-Performance Liquid Chromatography (HPLC) Analysis of Anthocyanins
2.4. Beer Enrichment with GSE and Physicochemical Characterization
2.5. Statistical Analysis
3. Results
3.1. Phytochemical Characterization of GSE
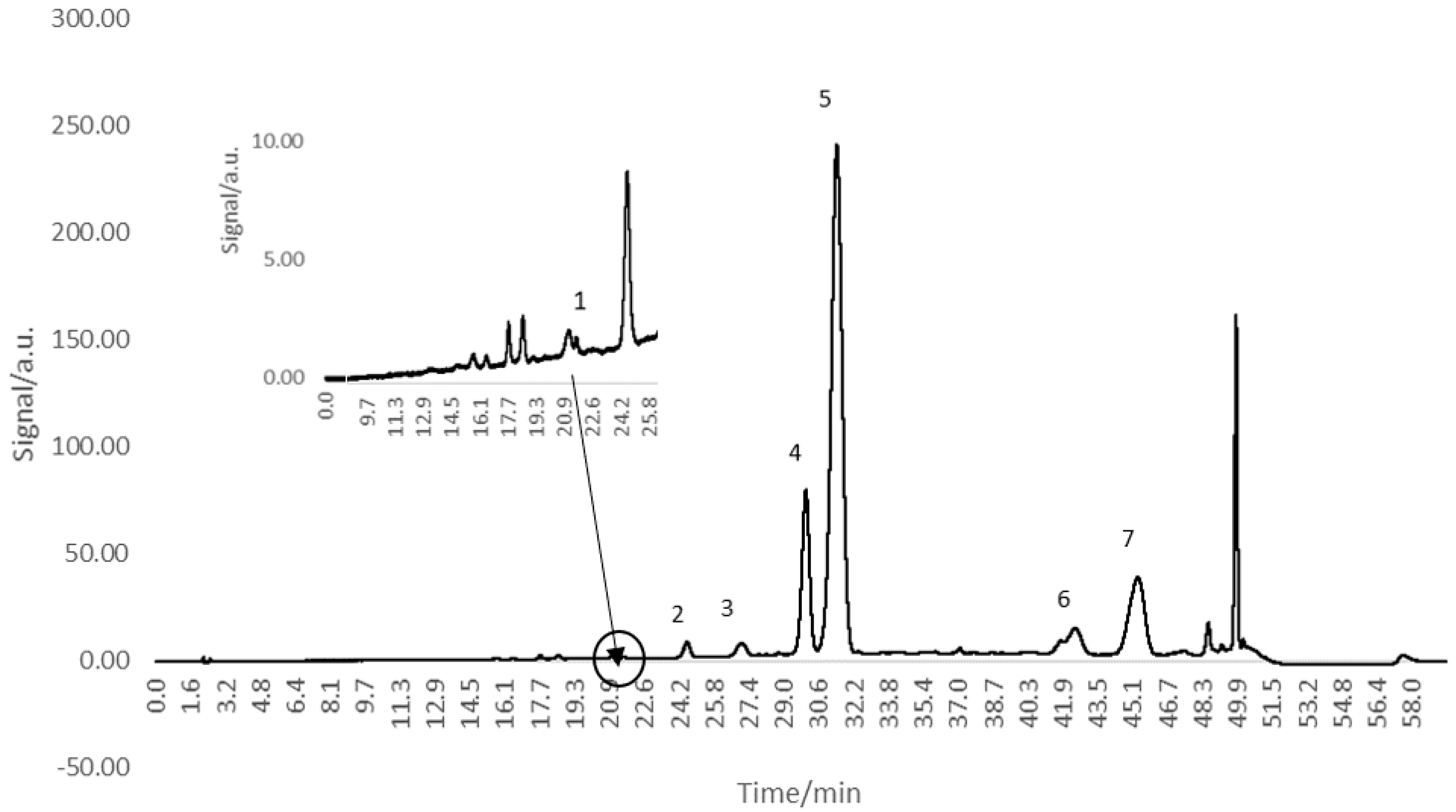
3.2. HPLC Analysis of Anthocyanins
3.3. Phytochemical Characterization of Beer Enriched with GSE
3.4. Antioxidant Activity of Beer Enriched with GSE
3.5. Color of Beer Enriched with GSE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Vidal, J.; Ávila, M.I.; Labbe, M.; Cohen, S.; Salazar, F.N. Effect of the addition of propolis extract on bioactive compounds and antioxidant activity of craft beer. J. Chem. 2017, 2017, 6716053. [Google Scholar] [CrossRef] [Green Version]
- Tafulo, P.A.R.; Queirós, R.B.; Delerue-Matos, C.M.; Sales, M.G.F. Control and comparison of the antioxidant capacity of beers. Food Res. Int. 2010, 43, 1702–1709. [Google Scholar] [CrossRef] [Green Version]
- Bravo, A.; Scherer, E.; Madrid, J.; Herrera, J.; Virtanen, H.; RangelAldao, R. Identification of a-dicarbonylic compounds in aged beers: Their role in beer aging process. Proc. Eur. Brew. Conv. Congr. 2002, 39, 13–23. [Google Scholar]
- Aprodu, I. Effect of Processing on the Antioxidant Activity of Beer. In Beer: From Production to Distribution; Legault, A., Ed.; Nova: New York, NY, USA, 2020; pp. 1–23. ISBN 978-1-53618-414-3. [Google Scholar]
- Pascoe, H.M.; Ames, J.M.; Chandra, S. Critical stages of the brewing process for changes in antioxidant activity and levels of phenolic compounds in ale. J. Am. Soc. Brew. Chem. 2003, 61, 203–209. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Ng, K.R.; Lyu, X.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 270, 123–129. [Google Scholar] [CrossRef]
- Constantin, O.E.; Skrt, M.; Poklar Ulrih, N.; Râpeanu, G. Anthocyanins profile, total phenolics, and antioxidant activity of two Romanian red grape varieties: Feteasca neagra and Babeasca neagra (Vitis vinifera). Chem. Pap. 2015, 69, 1573–1581. [Google Scholar] [CrossRef]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Effect of thermal treatment on phenolic compounds from plum (Prunus domestica) extracts—A kinetic study. J. Food Eng. 2016, 171, 200–207. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colourants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Dewanto, V.; Wu, X.K.; Adom, K.; Hai Liu, R. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, H.I.; Rodriguez-Varela, L.I.; Ferreira, R.S.; Parada-Alfonso, F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids 2010, 51, 319–324. [Google Scholar] [CrossRef]
- Serea, D.; Râpeanu, G.; Constantin, O.E.; Bahrim, G.E.; Stănciuc, N.; Croitoru, C. Ultrasound and enzymatic assisted extractions of bioactive compounds found in red grape skins Băbească neagră (Vitis vinifera variety). Ann. Univ. Dunarea Jos Galati FascicleVI-Food Technol. 2021, 45, 9–25. [Google Scholar] [CrossRef]
- ASBC Methods of Analysis. In American Society of Brewing Chemists; The Society: St. Paul, MN, USA, 2009.
- Goupy, P.; Hugues, M.; Boivin, P.; Amiot, M.J. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J. Sci. Food Agri. 1999, 79, 1625–1634. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stănciuc, N.; Yan, Y.; Ghinea, I.O.; Ungureanu, C.; Cîrciumaru, A.; Wang, D.; Poklar Ulrih, N.; Râpeanu, G. Polymers and protein-associated vesicles for the microencapsulation of anthocyanins from grape skins used for food applications. J. Sci. Food Agric. 2021, 101, 2676–2686. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef] [PubMed]
- Guaita, M.; Bosso, A. Polyphenolic Characterization of Grape Skins and Seeds of Four Italian Red Cultivars at Harvest and after Fermentative Maceration. Foods 2019, 8, 395. [Google Scholar] [CrossRef] [Green Version]
- Yammine, S.; Delsart, C.; Vitrac, X.; Ghidoss, R. Characterisation of polyphenols and antioxidant potential of red and white pomace by-product extracts using subcritical water extraction. OENO One 2020, 54, 263–278. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonalves, A.E.D.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Santos, L.P.; Morais, D.R.; Souza, N.E.; Cottica, S.M.; Boroski, M.; Visentainer, J.V. Phenolic compounds and fatty acids in different parts of Vitis labrusca and V. vinifera grapes. Int. Food Res. J. 2011, 44, 1414–1418. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Budić-Leto, I.; Mucalo, A.; Ljubenkov, I.; Zdunić, G. Anthocyanin profile of wild grape Vitis vinifera in the eastern Adriatic region. Sci. Hortic. 2018, 238, 32–37. [Google Scholar] [CrossRef]
- Benmeziane, F.; Cadot, Y.; Djamai, R.; Djermoun, L. Determination of major anthocyanin pigments and flavonols in red grape skin of some table grape varieties (Vitis vinifera sp.) by highperformance liquid chromatography—Photodiode array detection (HPLC-DAD). OENO One 2016, 50, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Kharadze, M.; Japaridze, I.; Kalandia, A.; Vanidze, M. Anthocyanins and antioxidant activity of red wines made from endemic grape varieties. Ann. Agrar. Sci. 2018, 16, 181–184. [Google Scholar] [CrossRef]
- Silva, L.R.; Queiroz, M. Bioactive compounds of red grapes from Dão region (Portugal): Evaluation of phenolic and organic profile. Asian Pac. J. Trop. Biomed. 2016, 6, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Horincar, G.; Enachi, E.; Bolea, C.; Râpeanu, G.; Aprodu, I. Value-Added Lager Beer Enriched with Eggplant (Solanum melongena L.) Peel Extract. Molecules 2020, 25, 731. [Google Scholar] [CrossRef] [Green Version]
- Veljović, M.; Despotović, S.; Stojanović, M.; Pecić, S.; Vukosavljević, P.; Belović, M.; Leskošek-Čukalović, I. The fermentation kinetics and physicochemical properties of special beer with addition of prokupac grape variety. Chem. Ind. Chem. Eng. Q. 2015, 21, 391–397. [Google Scholar] [CrossRef]
- Dordevic, S.; Popovic, D.; Despotovic, S.; Veljovic, M.; Atanackovic, M.; Cvejic, J.; Nedovic, V.; Leskosek-Cukalovik, I. Extracts of medicinal plants as functional beer additives. Chem. Ind. Chem. Eng. Q. 2016, 22, 301–308. [Google Scholar]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Gąsior, J.; Głowacki, A. Assessment of Volatiles and Polyphenol Content, Physicochemical Parameters and Antioxidant Activity in Beers with Dotted Hawthorn (Crataegus punctata). Foods 2020, 9, 775. [Google Scholar] [CrossRef]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef]
- Pai, T.V.; Sawant, S.Y.; Ghatak, A.A.; Chaturvedi, P.A.; Gupte, A.M.; Desai, N.S. Characterization of Indian beers: Chemical composition and antioxidant potential. J. Food Sci. Technol. 2015, 52, 1414–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, D.E.; Brookes, P.A.; Stevens, R.B.; Boulton, C.A. Brewing: Science and Practice; Woodhead Publishing: Sawston, UK, 2004; Volume 108. [Google Scholar]
- Bamforth, C.; Russell, I.; Stewart, G. (Eds.) Shellhammer TH Beer color. In Beer: A Quality Perspective; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 213–227. [Google Scholar]
- Castaneda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]


| Phytochemical Characteristics | ||
| TMA | mg C3G/g DW GSE | 6.26 ± 1.39 |
| TFC | mg CE/g DW GSE | 22.65 ± 0.36 |
| TPC | mg GAE/g DW GSE | 42.44 ± 1.50 |
| Antioxidant Activity | ||
| DPPH | Inhibition, % | 63.05 ± 4.37 |
| mmol TE/mL | 16.50 ± 1.44 | |
| ABTS | Inhibition, % | 80.16 ± 0.67 |
| mmol TE/mL | 1.09 ± 0.01 | |
| Physico-Chemical Characteristics | Beer Control |
|---|---|
| Alcohol, % mass | 4.29 ± 0.01 |
| Alcohol, % vol | 5.39 ± 0.02 |
| Real extract, °P | 4.25 ± 0.01 |
| Original extract, °P | 12.48 ± 0.02 |
| Apparent extract, °P | 2.05 ± 0.01 |
| CO2, g/100 mL | 0.68 ± 0.02 |
| pH | 4.88 ± 0.02 |
| Sample | Bioactive Compounds | Storage Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| BC | TMA, mgC3G/mL | Nd. * | Nd. * | Nd. * | Nd. * |
| TFC, mg CE/mL | 0.841 ± 0.004 aD | 0.808 ± 0.010 aC | 0.786 ± 0.077 aC | 0.788 ± 0.058 aB | |
| TPC, mg GAE/mL | 3.167 ± 0.059 aC | 3.118 ± 0.228 aC | 3.080 ± 0.066 aC | 2.975 ± 0.064 aC | |
| B/GSE1 | TMA, mgC3G/mL | 0.005 ± 0.000 aC | 0.005 ± 0.000 aC | 0.005 ± 0.000 aC | 0.004 ± 0.001 aC |
| TFC, mg CE/mL | 0.964 ± 0.047 aC | 0.961 ± 0.105 aBC | 0.961 ± 0.105 aBC | 0.919 ± 0.118 aAB | |
| TPC, mg GAE/mL | 3.640 ± 0.299 aB | 3.640 ± 0.061 aB | 3.530 ± 0.233 aBC | 3.375 ± 0.171 aB | |
| B/GSE5 | TMA, mgC3G/mL | 0.019 ± 0.000 aB | 0.017 ± 0.000 bB | 0.016 ± 0.001 cB | 0.016 ± 0.001 cB |
| TFC, mg CE/mL | 1.096 ± 0.039 aB | 1.071 ± 0.091 aAB | 1.063 ± 0.085 aAB | 1.009 ± 0.020 aAB | |
| TPC, mg GAE/mL | 3.995 ± 0.096 aA | 3.981 ± 0.188 aB | 3.804 ± 0.435 aAB | 3.608 ± 0.145 aB | |
| B/GSE10 | TMA, mgC3G/mL | 0.027 ± 0.001 aA | 0.027 ± 0.001 aA | 0.023 ± 0.001 BA | 0.022 ± 0.001 bA |
| TFC, mg CE/mL | 1.226 ± 0.029 aA | 1.208 ± 0.038 aA | 1.176 ± 0.048 aA | 1.096 ± 0.141 aA | |
| TPC, mg GAE/mL | 4.477 ± 0.101 aA | 4.469 ± 0.060 aA | 4.210 ± 0.113 aA | 4.057 ± 0.066 bA | |
| Sample | Color Parameters | Storage Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| BC | L* | 66.15 ± 0.11 bA | 66.39 ± 0.27 bA | 66.88 ± 0.36 abA | 67.83 ± 0.81 aA |
| a* | 0.89 ± 0.07 aD | 0.92 ± 0.03 aD | 0.88 ± 0.07 aD | 0.88 ± 0.01 aD | |
| b* | 10.36 ± 0.47 aA | 11.46 ± 0.53 aA | 11.01 ± 0.13 aA | 10.00 ± 1.02 aAB | |
| EBC | 8.28 ± 0.26 aD | 8.29 ± 0.25 aD | 8.28 ± 0.27 aD | 8.28 ± 0.27 aD | |
| B/GSE 1 | L* | 62.93 ± 0.20 aB | 63.65 ± 0.48 aB | 63.35 ± 0.14 aB | 63.15 ± 0.76 aB |
| a* | 4.78 ± 0.09 aC | 4.63 ± 0.33 aC | 4.71 ± 0.20 aC | 4.92 ± 0.39 aC | |
| b* | 10.66 ± 0.24 aA | 9.52 ± 0.32 abB | 9.32 ± 0.49 bC | 9.63 ± 0.74 abAB | |
| EBC | 11.27 ± 0.22 aC | 11.23 ± 0.22 aC | 11.19 ± 0.28 aC | 11.27 ± 0.22 aC | |
| B/GSE 5 | L* | 47.87 ± 0.02 bC | 49.76 ± 0.78 aC | 48.22 ± 0.06 bC | 48.01 ± 0.33 bC |
| a* | 17.41 ± 0.01 aB | 15.67 ± 0.41 cB | 16.13 ± 0.14 bcB | 16.50 ± 0.24 bB | |
| b* | 10.48 ± 0.05 bA | 10.09 ± 0.16 cB | 10.33 ± 0.04 bcB | 11.34 ± 0.17 aA | |
| EBC | 14.44 ± 0.20 aB | 14.42 ± 0.23 aB | 14.42 ± 0.26 aB | 14.44 ± 0.20 aB | |
| B/GSE 10 | L* | 40.28 ± 0.01 bD | 42.53 ± 0.27 bD | 42.94 ± 0.09 abD | 45.89 ± 2.52 aC |
| a* | 25.81 ± 0.05 aA | 23.86 ± 0.07 bA | 23.83 ± 0.11 bA | 21.48 ± 1.27 cA | |
| b* | 8.91 ± 0.03 aB | 8.44 ± 0.04 aC | 8.57 ± 0.04 aD | 8.65 ± 0.53 aB | |
| EBC | 22.65 ± 0.20 aA | 22.66 ± 0.18 aA | 22.66 ± 0.16 aA | 22.65 ± 0.20 aA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serea, D.; Horincar, G.; Constantin, O.E.; Aprodu, I.; Stănciuc, N.; Bahrim, G.E.; Stanciu, S.; Rapeanu, G. Value-Added White Beer: Influence of Red Grape Skin Extract on the Chemical Composition, Sensory and Antioxidant Properties. Sustainability 2022, 14, 9040. https://doi.org/10.3390/su14159040
Serea D, Horincar G, Constantin OE, Aprodu I, Stănciuc N, Bahrim GE, Stanciu S, Rapeanu G. Value-Added White Beer: Influence of Red Grape Skin Extract on the Chemical Composition, Sensory and Antioxidant Properties. Sustainability. 2022; 14(15):9040. https://doi.org/10.3390/su14159040
Chicago/Turabian StyleSerea, Daniela, Georgiana Horincar, Oana Emilia Constantin, Iuliana Aprodu, Nicoleta Stănciuc, Gabriela Elena Bahrim, Silvius Stanciu, and Gabriela Rapeanu. 2022. "Value-Added White Beer: Influence of Red Grape Skin Extract on the Chemical Composition, Sensory and Antioxidant Properties" Sustainability 14, no. 15: 9040. https://doi.org/10.3390/su14159040
APA StyleSerea, D., Horincar, G., Constantin, O. E., Aprodu, I., Stănciuc, N., Bahrim, G. E., Stanciu, S., & Rapeanu, G. (2022). Value-Added White Beer: Influence of Red Grape Skin Extract on the Chemical Composition, Sensory and Antioxidant Properties. Sustainability, 14(15), 9040. https://doi.org/10.3390/su14159040









