Endemic Plants Can Be Resources for Mountain Agro-Ecosystems: The Case of Sanguisorba dodecandra Moretti
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Areas
2.2. Vegetation Survey and Synecology
2.3. Functional Strategy Analysis
2.4. Bromatological Analysis
2.5. Melissopalynological Analysis
3. Results
3.1. Plant Community and Synecology
3.2. Functional Strategy
3.3. Bromatological Features
3.4. Pollen in Honey
4. Discussion
4.1. Floristic and Ecological Features
4.2. Resource for Mountain Agro-Ecosystems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Source of Variance | Mean Square | F6,118 | p | |
|---|---|---|---|---|
| T | 8.95 | 24.22 | <0.01 | ** |
| K | 0.42 | 1.32 | 1.00 | ns |
| L | 2.29 | 6.00 | 0.03 | * |
| F | 1.70 | 4.85 | 0.08 | ns |
| R | 0.73 | 1.17 | 1.00 | ns |
| N | 4.40 | 9.28 | <0.01 | ** |
| H | 4.92 | 5.27 | 0.05 | ns |
| D | 5.38 | 3.72 | 0.22 | ns |
| Sampling Area | Source of Variance | Mean Square | F2,57 | p | |
|---|---|---|---|---|---|
| A | C | 124.41 | 27.40 | <0.01 | ** |
| S | 53.06 | 14.30 | <0.01 | ** | |
| R | 44.94 | 8.88 | <0.01 | ** | |
| B | C | 200.43 | 47.92 | <0.01 | ** |
| S | 103.52 | 50.38 | <0.01 | ** | |
| R | 127.79 | 22.28 | <0.01 | ** | |
| C | C | 6.61 | 2.39 | 0.10 | ns |
| S | 13.26 | 7.80 | <0.01 | ** | |
| R | 26.33 | 6.58 | <0.01 | ** |
References
- Ozenda, P. L’endémisme au niveau de l’ensemble du Systéme alpin. Acta Bot. Gall. 1995, 142, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Aeschimann, D.; Lauber, K.; Moser, D.M.; Theurillat, J.-P. Flora Alpina. Ein Atlas Sämtlicher 4500 Gefässpflanzen der Alpen; Paul Haupt: Bern, Switzerland; Stuttgart, Germany; Vienna, Austria, 2004. [Google Scholar]
- Andreis, C.; Armiraglio, S.; Caccianiga, M.; Cerabolini, B.; Ravazzi, C. Alpi Orobie e Prealpi Carbonatiche: Un hot spot di endemicità. In La Flora in Italia; Blasi, C., Biondi, E., Eds.; Sapienza Università Editrice: Roma, Italy, 2017; pp. 100–103. [Google Scholar]
- Pawlowski, B. Remarques sur l’endémisme dans la flore des Alpes et des Carpates. Vegetatio 1970, 21, 181–243. [Google Scholar] [CrossRef]
- Martini, F.; Bona, E.; Federici, G.; Fenaroli, F.; Perico, G.; Danieli, S.; Fantini, G.; Mangili, L.; Tagliaferri, F.; Zanotti, E. Flora Vascolare Della Lombardia Centro-Orientale; LINT: Trieste, Italy, 2012. [Google Scholar]
- Pignatti, S. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Corti, A. Botanica valtellinese. Atti Soc. Ital. Sc. Nat. 1959, 98, 1–83. [Google Scholar]
- Andreis, C.; Ravazzi, C.; Salvetti, A. Approccio palinologico al problema dell’endemismo orobico: Dati preliminari su Sanguisorba dodecandra Moretti (Rosaceae). Giorn. Bot. Ital. 1994, 128, 243. [Google Scholar] [CrossRef]
- Gentili, R.; Parolo, G.; Rossi, G.; Abeli, T. Sanguisorba Dodecandra. In The IUCN Red List of Threatened Species 2010; IUCN: Gland, Switzerland, 2010; p. e.T170361A6769027. [Google Scholar]
- Pirola, A. Ricerche su Sanguisorba dodecandra Moretti. Atti Ist. Bot. Labor. Crittog. Univ. Pavia 1964, 21, 69–104. [Google Scholar]
- Ferlinghetti, R. Sanguisorba Dodecandra Fiore Esclusivo Delle Orobie; Castelletti Grafica Immagine: Ponte Nossa, BG, Italy, 2011. [Google Scholar]
- Parolo, G. I consorzi a Sanguisorba dodecandra Moretti della Val di Togno (Alpi Retiche, Sondrio). Webbia 2004, 59, 177–188. [Google Scholar] [CrossRef]
- Pirola, A.; Credaro, V. Sur la sociologie de Sanguisorba dodecandra Moretti, Espace endemica des Alpes Orobiennes (Sondrio—Italie). Doc. Phytosoc. 1978, 4, 841–846. [Google Scholar]
- Tagliaferri, F. Distribuzione di Sanguisorba dodecandra in Val di Scalve. Nat. Brescia 1992, 27, 57–58. [Google Scholar]
- Fuchs-Eckert, H.P. Il Frasnej, Sanguisorba dodecandra Moretti. Not. Banca Pop. Sondrio 1990, 52, 48–57. [Google Scholar]
- FAB (Flora Alpina Bergamasca). FAB—Atlante Digitale. 2021. Available online: https://app.floralpinabergamasca.net (accessed on 5 December 2021).
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes and Ecosystem Properties; Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Giupponi, L.; Giorgi, A. A contribution to the knowledge of Linaria tonzigii Lona, a steno-endemic species of the Orobie Bergamasche Regional Park (Italian Alps). Eco.mont 2019, 11, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Giupponi, L.; Giorgi, A. Effectiveness of modern leaf analysis tools for the morpho-ecological study of plants: The case of Primula albenensis. Nord. J. Bot. 2019, 37, 302386. [Google Scholar]
- Giupponi, L. Intraspecific variation in functional strategy and leaf shape of Campanula elatinoides reveals adaptation to climate. Flora 2020, 268, 151605. [Google Scholar] [CrossRef]
- Giupponi, L.; Borgonovo, G.; Panseri, S.; Giorgi, A. Multidisciplinary study of a little-known landrace of Fagopyrum tataricum Gaertn. of Valtellina (Italian Alps). Genet. Resour. Crop Evol. 2019, 66, 783–796. [Google Scholar] [CrossRef]
- Cerabolini, B.E.L.; Brusa, G.; Ceriani, R.M.; De Andreis, R.; Luzzaro, A.; Pierce, S. Can CSR classification be generally applied outside Britain? Plant Ecol. 2010, 210, 253–261. [Google Scholar] [CrossRef]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Díaz, S.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, V.G. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Landolt, E.; Bäumler, B.; Erhardt, A.; Hegg, O.; Klötzli, F.; Lämmle, R.W.; Nobis, M.; Rudmann-Mayree, K.; Schweingruber, H.F.; Theurillat, J.P.; et al. Flora Indicativa. Ecological Indicator Values and Biological Attributes of the Flora of Switzerland and the Alps; Editions des Conservatoire et Jardin botaniques de la Ville de Genève & Haupt Verlag: Bern, Switzerland; Stuttgart, Germany; Vienna, Austria, 2010. [Google Scholar]
- Blanc, S.; Brun, F.; Di Vita, G.; Mosso, A. Traditional beekeeping in rural areas: Profitability analysis and feasibility of pollination services. Qual. Access Success 2018, 19, 72–79. [Google Scholar]
- Farooq, A.; Surendra, R.J.; Min, B.G. Beekeeping and Rural Development; International Centre for Integrated Mountain Development Khumaltar: Lalitpur, Nepal; Kathmandu, Nepal, 2007. [Google Scholar]
- CODEX STAN 12-1981; Revised Codex Standard for Honey. Revised 1st in 1987, Revised 2nd in 2001; Codex Alimentarius Commission: Rome, Italy, 1981.
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Buchin, S.; Martin, B.; Dupont, D.; Bornard, A.; Achilleos, C. Influence of the composition of Alpine highland pasture on the chemical, rheological and sensory properties of cheese. J. Dairy Res. 1999, 66, 579–588. [Google Scholar] [CrossRef]
- Regione Lombardia. Documento di Azione Regionale per l’Adattamento al Cambiamento Climatico. 2016. Available online: https://www.regione.lombardia.it/wps/wcm/connect/946249ce-87c4-4c39-88f9-5eab3a264f14/Documento+Azione+Adattamento+RL_9dic.pdf?MOD=AJPERES&CACHEID=946249ce-87c4-4c39-88f9-5eab3a264f14 (accessed on 29 May 2022).
- Pruchniewicz, D. Abandonment of traditionally managed mesic mountain meadows affects plant species composition and diversity. Basic Appl. Ecol. 2017, 20, 10–18. [Google Scholar] [CrossRef]
- Tarolli, P.; Straffelini, E. Agriculture in hilly and mountainous landscapes: Threats monitoring and sustainable management. Geogr. Sustain. 2020, 1, 70–76. [Google Scholar] [CrossRef]
- Cislaghi, A.; Giupponi, L.; Tamburini, A.; Giorgi, A.; Bischetti, G.B. The effects of mountain grazing abandonment on plant community, forage value and soil properties: Observations and field measurements in an alpine area. CATENA 2019, 181, 104086. [Google Scholar] [CrossRef]
- Massara, G.F. Prodromo Della Flora Valtellinese; Parco delle Orobie Bergamasche: Sondrio, Italy, 1834. [Google Scholar]
- Leoni, V.; Giupponi, L.; Pavlovic, R.; Gianoncelli, C.; Cecati, F.; Ranzato, E.; Martinotti, S.; Pedrali, D.; Giorgi, A.; Panseri, S. Multidisciplinary analysis of Italian Alpine wildflower honey reveals criticalities, diversity and value. Sci. Rep. 2021, 11, 19316. [Google Scholar] [CrossRef]
- Andreis, C. I distretti geobotanici. In I Tipi Forestali della Lombardia; Del Favero, R., Ed.; Cierre: Verona, Italy, 2002; pp. 36–40. [Google Scholar]
- Blasi, C.; Capotorti, G.; Copiz, R.; Guida, D.; Mollo, B.; Smiraglia, D.; Zavattero, L. Classification and mapping of the ecoregions of Italy. Plant Biosyst. 2014, 148, 1255–1345. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Rivas-Sáenz, S. Sistema de Clasificación Bioclimática Mundial. Centro de Investigaciones Fitosociológicas, España. 2009. Available online: http://pendientedemigracion.ucm.es/info/cif/ (accessed on 1 June 2021).
- Braun-Blanquet, J. Pflanzensoziologie; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1964. [Google Scholar]
- Giupponi, L.; Leoni, V. VegeT: An easy tool to classify and facilitate the management of seminatural grasslands and dynamically connected vegetation of the Alps. Land 2021, 9, 473. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment or Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2019. Available online: http://www.r-project.org (accessed on 1 June 2021).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Von Der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Barth, O.M. Microscopical analysis of honey samples. Anais da Academia Brasileira de Ciencias 1970, 42, 351–366. [Google Scholar]
- Biondi, E.; Blasi, C. Prodromo Della Vegetazione d’Italia. 2015. Available online: http://www.prodromo-vegetazione-italia.org (accessed on 5 September 2021).
- Mucina, L.; Bültmann, H.; Dierßen, K. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19 (Suppl. S1), 3–264. [Google Scholar] [CrossRef]
- Richardson, F.; Brodribb, T.J.; Jordan, G.J. Amphistomatic leaf surfaces independently regulate gas exchange in response to variations in evaporative demand. Tree Physiol. 2017, 37, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Mariani, L.; Paolillo, P.L.; Rasio, R. Climi e Suoli Lombardi; Rubattino: Catanzaro, Italy, 2001. [Google Scholar]
- Andreis, C. Tipificazione Floristico-Vegetazionale del Cotico Erboso Delle Malghe Che Producono “Bagoss”; Comunità Montana della Valsabbia: Nozza, Italy, 1997. [Google Scholar]
- Fratini, A. Digeribilità. Come fare per aumentarla. Inf. Zoot. 2013, 2, 38–42. [Google Scholar]
- Martin, B.; Coulon, J.B. Milk production and cheese characteristics. 1. Influence of milk production conditions on herd milk clotting ability. Lait 1995, 75, 61–80. [Google Scholar] [CrossRef]
- Leiber, F.; Kreuzer, M.; Nigg, D.; Wettstein, H.R.; Scheeder, M.R.L. A study on the causes for the elevated n − 3 fatty acids in cow’s milk of alpine origin. Lipids 2005, 40, 191–202. [Google Scholar] [CrossRef]
- Abilleira, E.; Virto, M.; Najera, A.I.; Salmeron, J.; Albisu, M.; Perez-Elortondo, F.J.; de Gordoa, J.C.R.; de Renobales, M.; Barron, L.J.R. Effects of seasonal changes in feeding management under part-time grazing on the evolution of the composition and coagulation properties of raw milk from ewes. J. Dairy Sci. 2010, 93, 3902–3909. [Google Scholar] [CrossRef]
- Ikonen, T.; Ruottinen, O.; Syväoja, E.L.; Saarinen, K.; Pahkala, E.; Ojala, M. Effect of milk coagulation properties of herd bulk milks on yield and composition of Emmental cheese. Agric. Food Sci. Finl. 1999, 8, 411–422. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Shingfield, K.J.; Lee, M.R.F.; Scollan, N.D. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Anim. Feed Sci. Technol. 2006, 131, 168–206. [Google Scholar] [CrossRef]
- Petersen, M.B.; Søegaard, K.; Jensen, S.K. Herb feeding increases n − 3 and n − 6 fatty acids in cow milk. Livest. Sci. 2011, 141, 90–94. [Google Scholar] [CrossRef]
- Cabiddu, A.; Salis, L.; Tweed, J.K.S.; Molle, G.; Decandia, M.; Lee, M.R.F. The influence of plant polyphenols on lipolysis and biohydrogenation in dried forages at different phenological stages: In vitro study. J. Sci. Food Agric. 2010, 90, 829–835. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Rapak, A.; Ochmian, I. Profile and Content of Phenolic Compounds in Leaves, Flowers, Roots, and Stalks of Sanguisorba officinalis L. Determined with the LC-DAD-ESI-QTOF-MS/MS Analysis and Their In Vitro Antioxidant, Antidiabetic, Antiproliferative Potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef]
- Bunse, M.; Lorenz, P.; Stintzing, F.C.; Kammerer, D.R. Characterization of secondary metabolites in flowers of Sanguisorba officinalis L. by HPLC-DAD-MSn and GC/MS. Chem. Biodivers. 2020, 17, e1900724. [Google Scholar] [CrossRef]
- Werner, W.; Paulissen, D. Program VEGBASE database of indicator values of vascular plants after Ellenberg and their evaluation to the personal computer. In Indicator Values of Plants in Central Europe. Scripta Geobotanica, 1st ed.; Ellenberg, H., Weber, H.E., Duell, R., Wirth, V., Werner, W., Paulissen, D., Eds.; Goltze Publisher: Gottingen, Germany, 1992; pp. 238–248. [Google Scholar]
- Roggero, P.P.; Bagella, S.; Farina, R. Un Archivio dati di Indici specifici per la valutazione integrata del valore pastorale. Riv. Agron. 2002, 36, 149–156. [Google Scholar]
- Gusmeroli, F. Prati, Pascoli e Paesaggio Alpino; SoZooAlp: San Michele all’Adige, Italy, 2012. [Google Scholar]
- Gusmeroli, F.; Della Marianna, G.; Puccio, C.; Corti, M.; Maggioni, L. Indici foraggeri di specie legnose ed erbacee alpine per il bestiame caprino. Quad. SoZooAlp 2007, 4, 73–82. [Google Scholar]
- Pierik, M.E.; Gusmeroli, F.; Marianna, G.D.; Tamburini, A.; Bocchi, S. Meadows species composition, biodiversity and forage value in an alpine district: Relationships with environmental and dairy farm management variables. Agric. Ecosyst. Environ. 2017, 244, 14–21. [Google Scholar] [CrossRef]
- Delpech, R. Criteres de jugement de la valeur agronomique des praires. Fourrages 1960, 4, 83–98. [Google Scholar]
- Hebda, R.J.; Chinnappa, C.C. Studies on pollen morphology of Rosaceae. Acta Bot. Gall. 1994, 141, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Heo, K.; Cho, J.; Kim, S.-C.; Lee, C.; Chen, W. New insights into pollen morphology and its implications in the phylogeny of Sanguisorba L. (Rosaceae; Sanguisorbeae). Plant Syst. Evol. 2011, 291, 227–242. [Google Scholar] [CrossRef]
- Mosqueiro, T.; Cook, C.; Huerta, R.; Gadau, J.; Smith, B.; Pinter-Wollman, N. Task allocation and site fidelity jointly influence foraging regulation in honeybee colonies. R. Soc. Open Sci. 2017, 4, 170344. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, J.; de Vere, N.; Griffith, A.; Ford, C.R.; Allainguillaume, J.; Hegarty, M.J.; Baillie, L.; Adams-Groom, B. Using DNA Metabarcoding to Identify the Floral Composition of Honey: A New Tool for Investigating Honey Bee Foraging Preferences. PLoS ONE 2015, 10, e0134735. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Canale, A.; Romano, D.; Flamini, G.; Tavarini, S.; Martini, A.; Ascrizzi, R.; Conte, G.; Mele, M.; Angelini, L.G. Flower scent bouquet variation and bee pollinator visits in Stevia rebaudiana Bertoni (Asteraceae), a source of natural sweeteners. Arthropod Plant Interact. 2017, 11, 381–388. [Google Scholar] [CrossRef]
- Persano Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
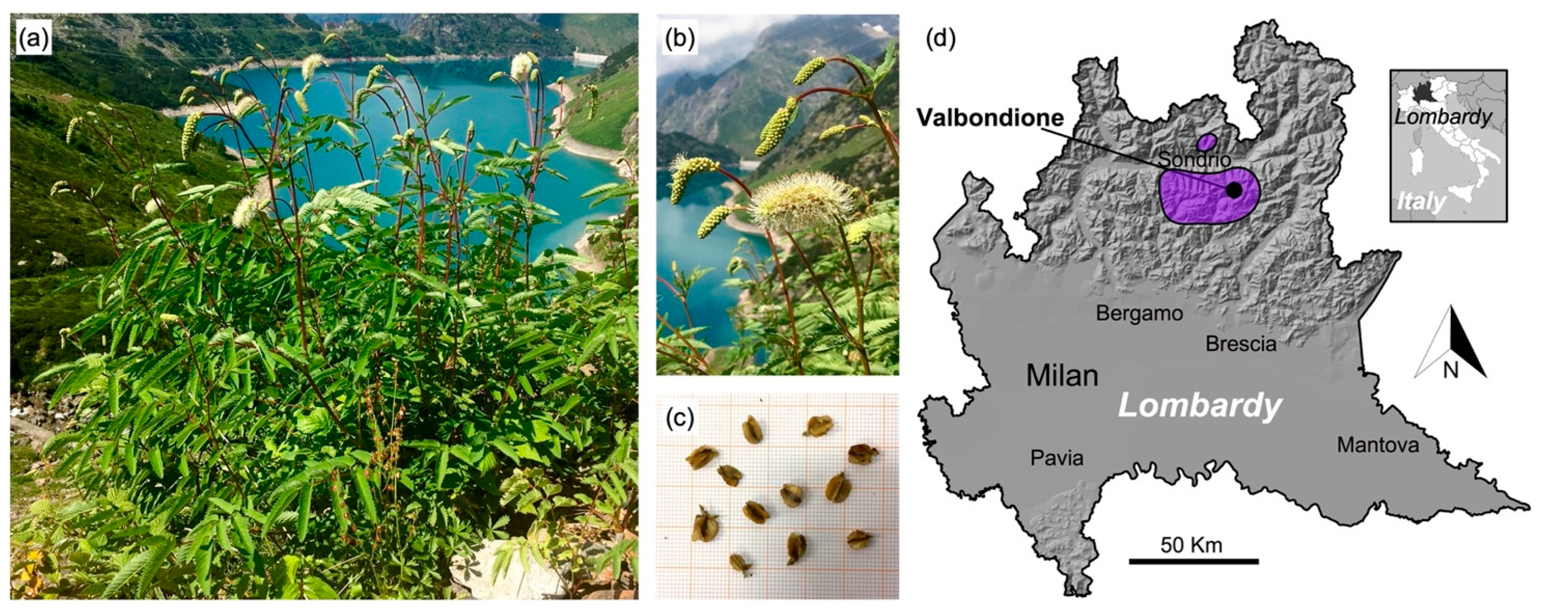
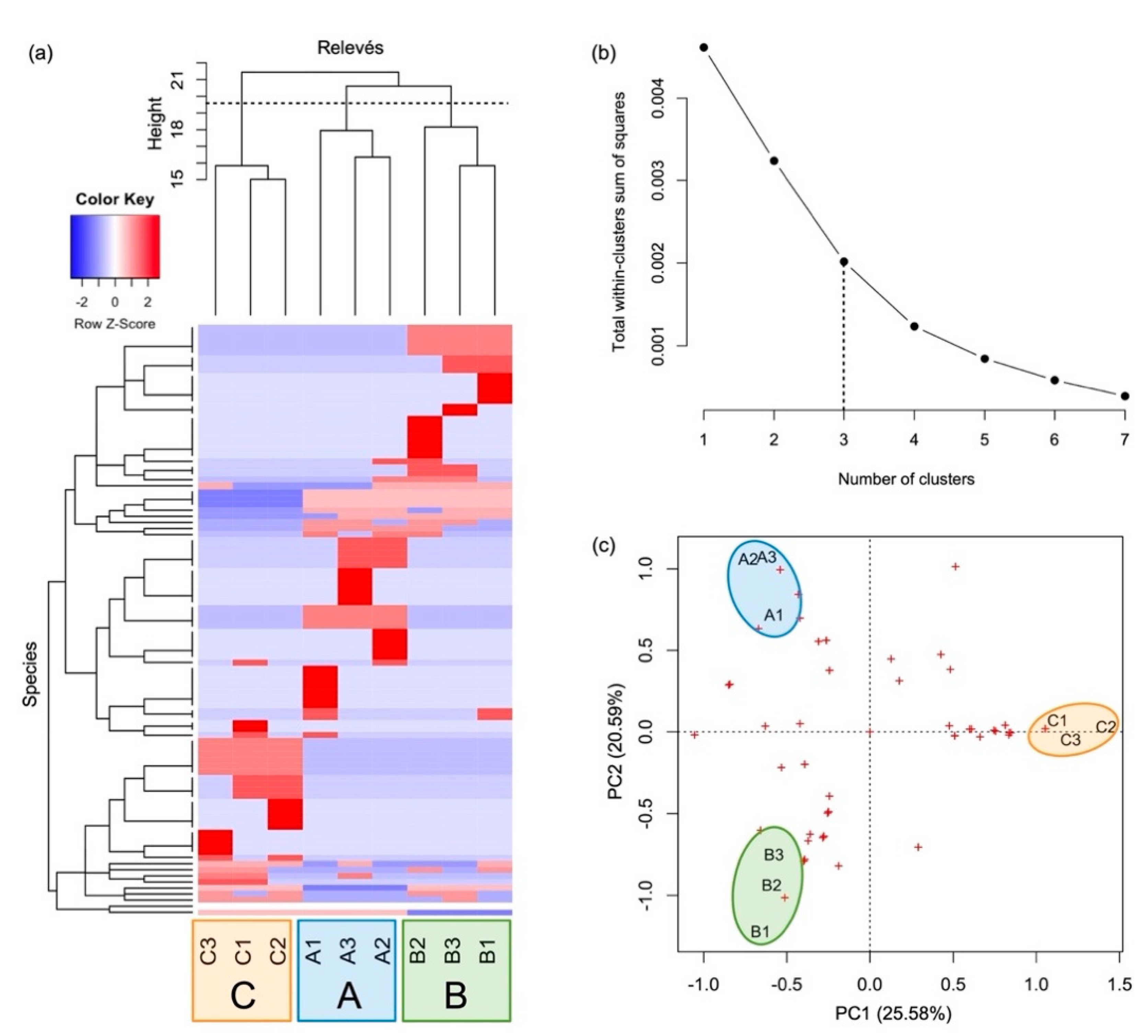
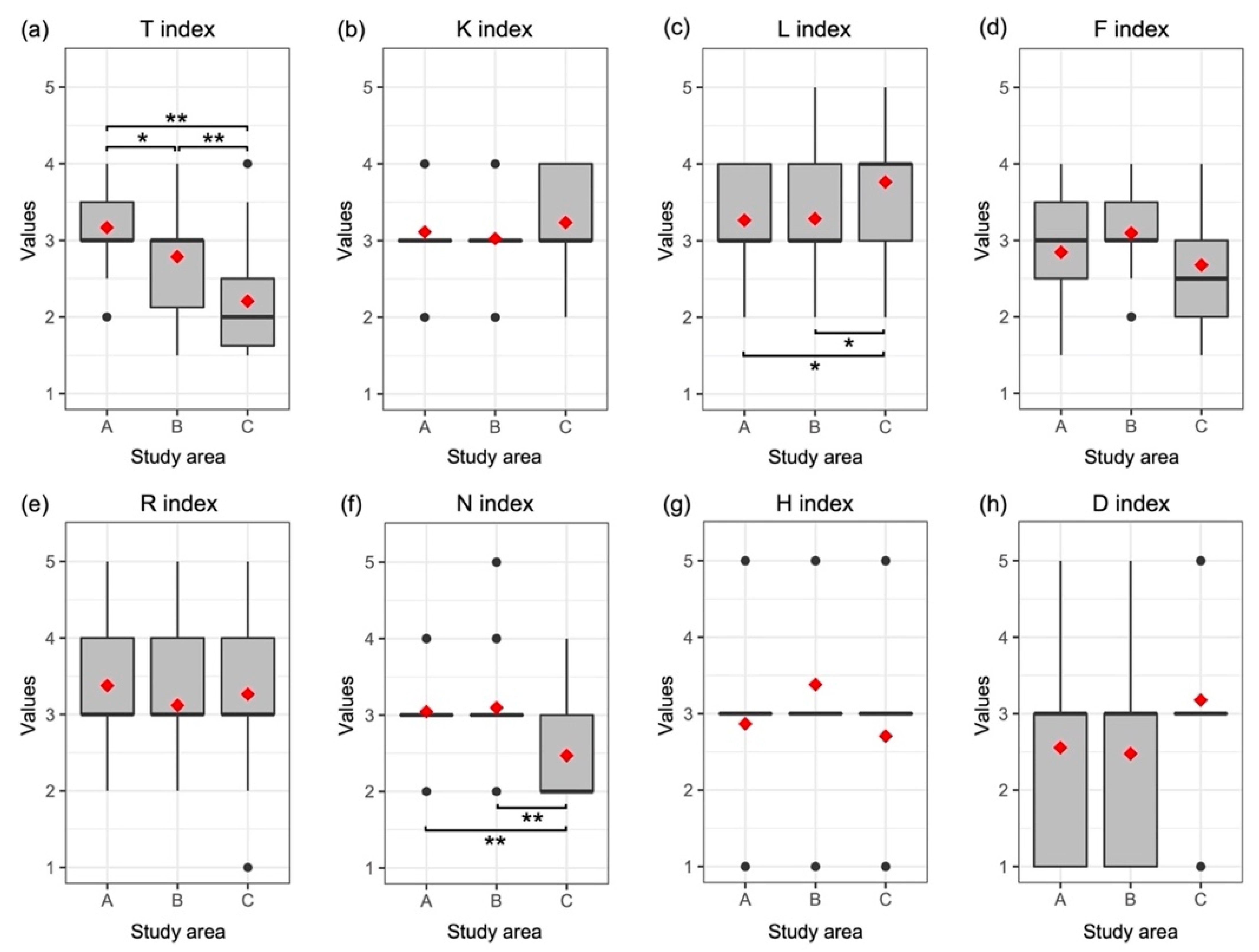

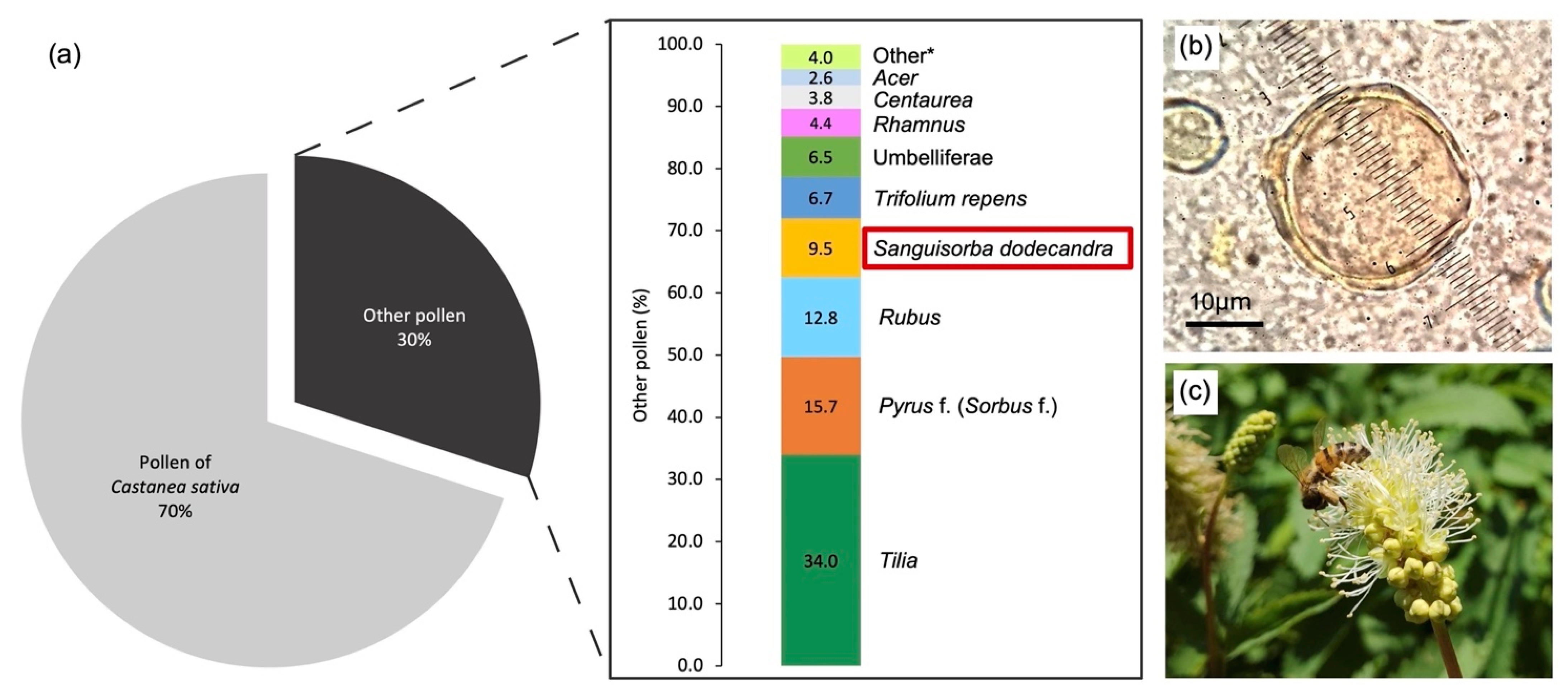
| Sampling Area | Municipality | Latitude N | Longitude E | Elevation (m a.s.l.) | Slope (°) | Aspect (°) |
|---|---|---|---|---|---|---|
| A | Valbondione (BG) | 46°03′02″ | 10°01′40″ | 1215 | 36 | 250 |
| B | Valbondione (BG) | 46°03′08″ | 10°00′45″ | 1610 | 32 | 186 |
| C | Valbondione (BG) | 46°04′06″ | 10°03′51″ | 2000 | 41 | 165 |
| Parameter | Phenological Stage | One-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| I | II | III | Mean Square | F2,6 | p | ||
| PC (% DW) | 12.92 ± 1.89 | 11.36 ± 0.13 | 8.73 ± 0.91 | 13.47 | 9.12 | 0.02 | * |
| NDF (% DW) | 31.33 ± 1.61 | 31.70 ± 3.88 | 38.22 ± 3.09 | 45.06 | 4.98 | 0.05 | ns |
| ADF (% DW) | 25.40 ± 1.64 | 26.52 ± 3.33 | 31.63 ± 3.22 | 33.15 | 4.13 | 0.07 | ns |
| ADL (% DW) | 4.36 ± 0.11 | 5.51 ± 1.52 | 7.123 ± 1.50 | 5.78 | 3.81 | 0.09 | ns |
| Ash (% DW) | 6.55 ± 0.25 | 5.99 ± 0.06 | 5.89 ± 0.26 | 0.39 | 8.69 | 0.02 | * |
| EE (% DW) | 2.08 ± 0.10 | 2.01 ± 0.21 | 2.18 ± 0.26 | 0.02 | 0.52 | 0.62 | ns |
| NFC (% DW) | 47.12 ± 3.62 | 48.94 ± 3.99 | 44.99 ± 3.62 | 11.71 | 0.83 | 0.48 | ns |
| DM (% FW) | 23.38 ± 2.06 | 27.95 ± 0.60 | 33.14 ± 1.73 | 71.64 | 28.40 | <0.01 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giupponi, L.; Leoni, V.; Gianoncelli, C.; Tamburini, A.; Giorgi, A. Endemic Plants Can Be Resources for Mountain Agro-Ecosystems: The Case of Sanguisorba dodecandra Moretti. Sustainability 2022, 14, 6825. https://doi.org/10.3390/su14116825
Giupponi L, Leoni V, Gianoncelli C, Tamburini A, Giorgi A. Endemic Plants Can Be Resources for Mountain Agro-Ecosystems: The Case of Sanguisorba dodecandra Moretti. Sustainability. 2022; 14(11):6825. https://doi.org/10.3390/su14116825
Chicago/Turabian StyleGiupponi, Luca, Valeria Leoni, Carla Gianoncelli, Alberto Tamburini, and Annamaria Giorgi. 2022. "Endemic Plants Can Be Resources for Mountain Agro-Ecosystems: The Case of Sanguisorba dodecandra Moretti" Sustainability 14, no. 11: 6825. https://doi.org/10.3390/su14116825
APA StyleGiupponi, L., Leoni, V., Gianoncelli, C., Tamburini, A., & Giorgi, A. (2022). Endemic Plants Can Be Resources for Mountain Agro-Ecosystems: The Case of Sanguisorba dodecandra Moretti. Sustainability, 14(11), 6825. https://doi.org/10.3390/su14116825








