Effect of Electrolysis on Activated Sludge during the Hydrolysis and Acidogenesis Stages in the Anaerobic Digestion of Poultry Manure
Abstract
:1. Introduction
- Microscopy of anaerobic activated sludge during anaerobic digestion in MECs and cells without electrodes for comparison;
- Study of the qualitative and quantitative compositions of the obtained biogas under conventional conditions (cell without electrodes) and with electrolysis (two-chamber MEC), with a focus on the formation of methane in the biogas;
- Study of changes in the values of pH, ORP, and TDS under conventional conditions (cell without electrodes) and with electrolysis (two-chamber MEC);
- Formalization of a generalized model of the effect of electrolysis on the anaerobic digestion process based on the data obtained and previous studies.
2. Materials and Methods
2.1. Characteristics of Substrate—Poultry Manure
2.2. Characteristics of Innoculate—Activated Sludge
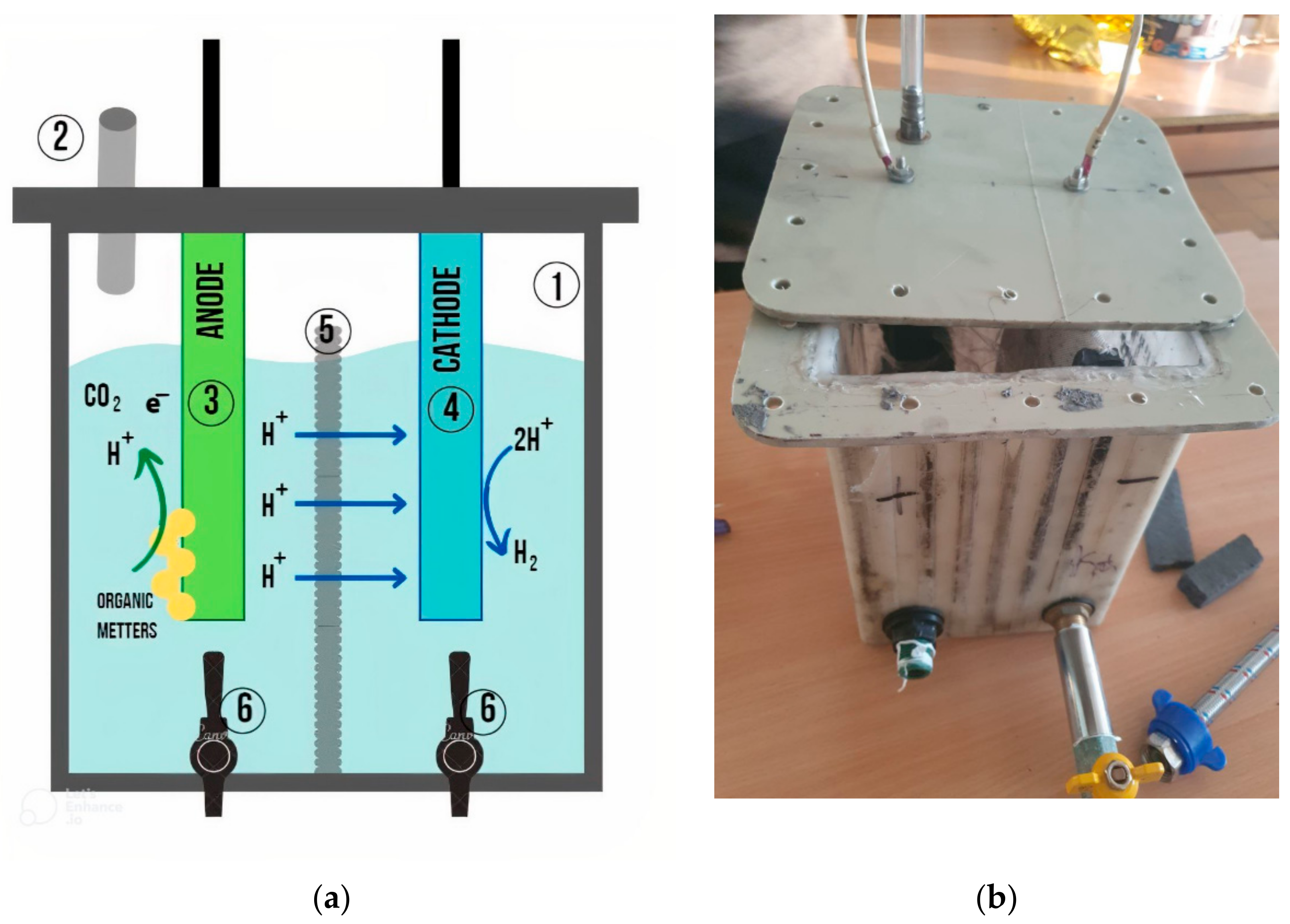
2.3. Laboratory Set-Ups
2.3.1. Two-Chamber Membrane Bioreactor MEC Used in the Study
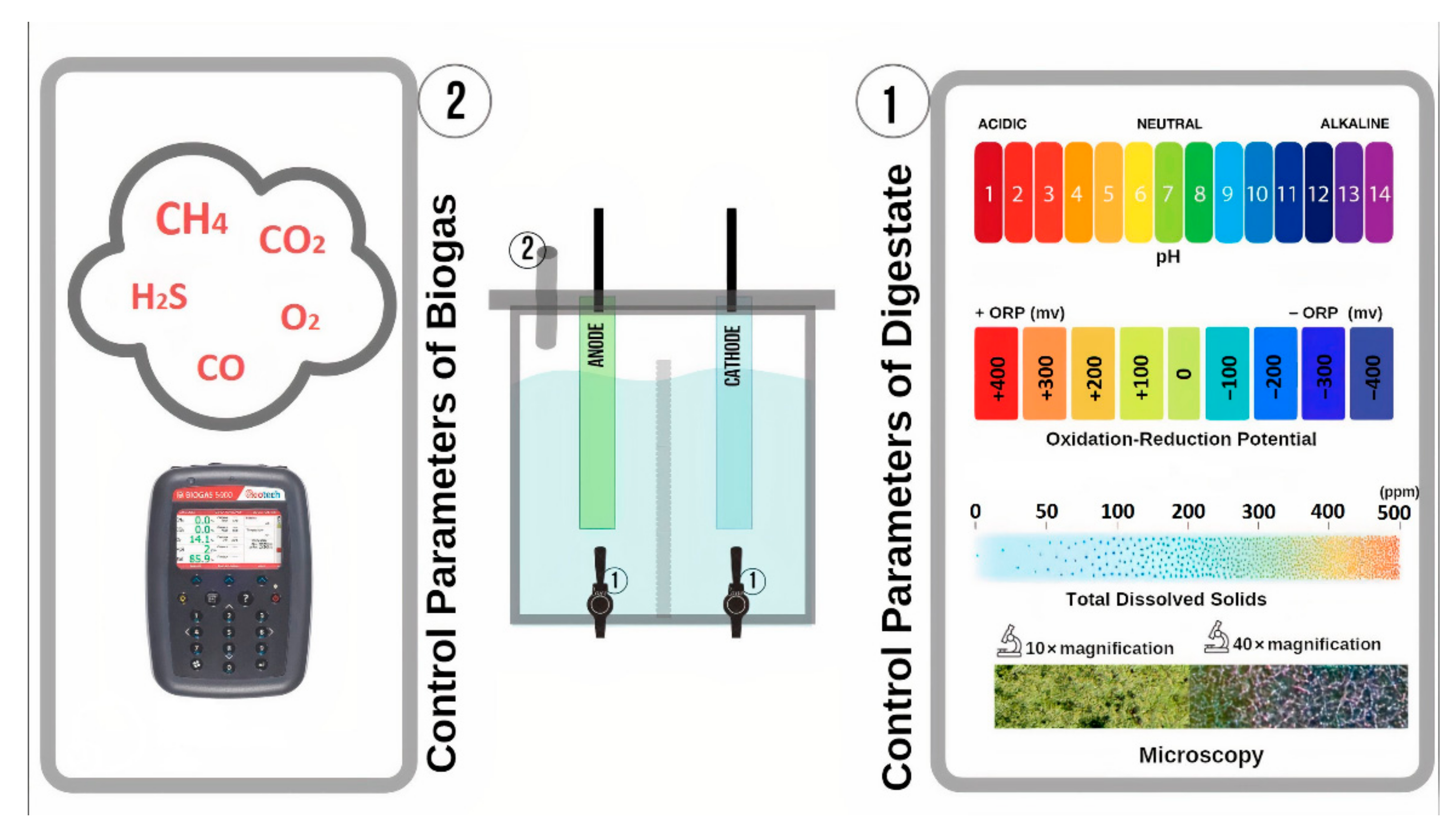
2.3.2. Control Parameters of the Anaerobic Digestion Process
3. Results and Discussion
3.1. Microscopy of Anaerobic Activated Sludge during Anaerobic Digestion in MECs and Cells without Electrodes for Comparison
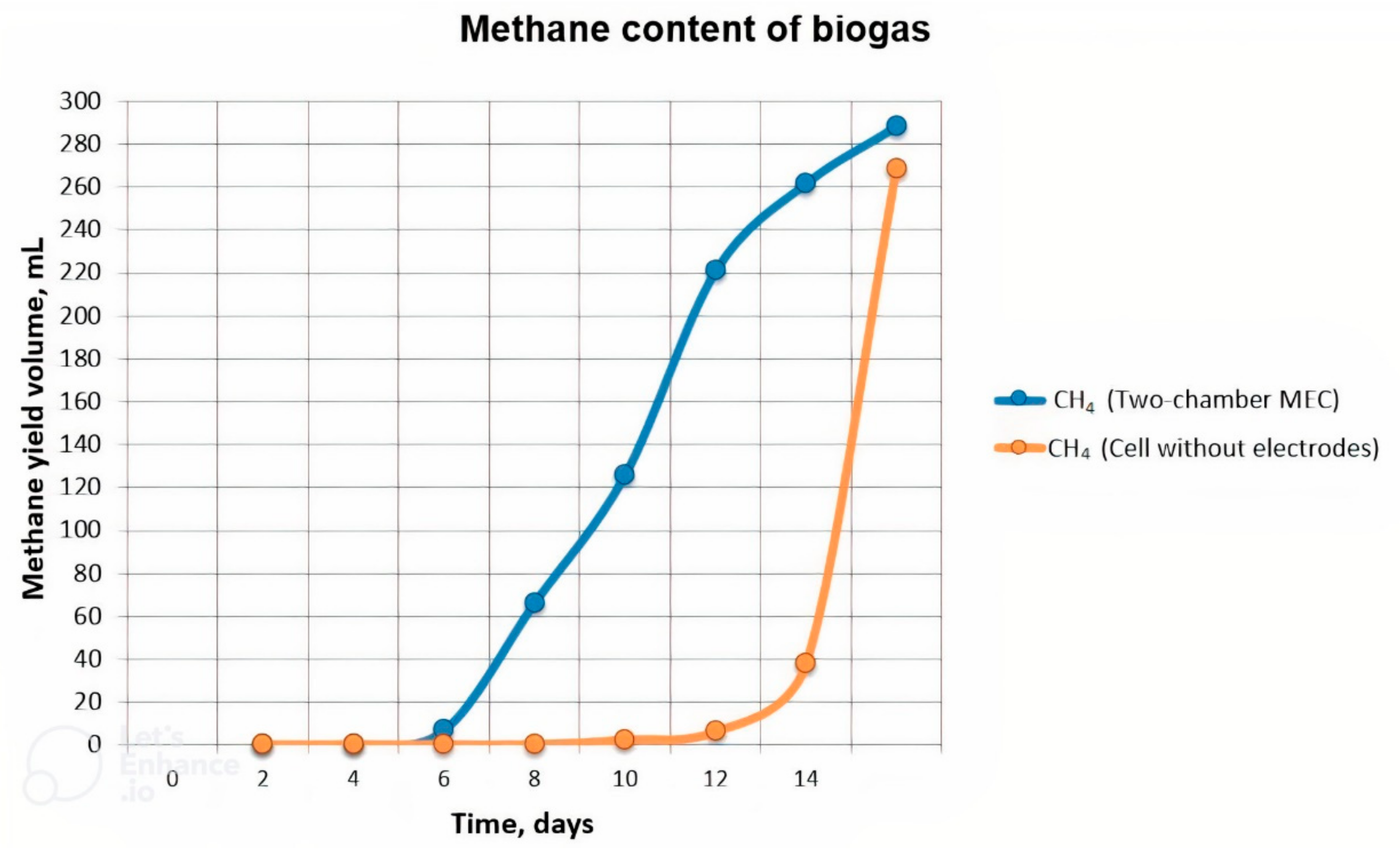
3.2. Study of the Qualitative and Quantitative Composition of the Obtained Biogas under Conventional Conditions and with Electrolysis (Two-Chamber MEC)
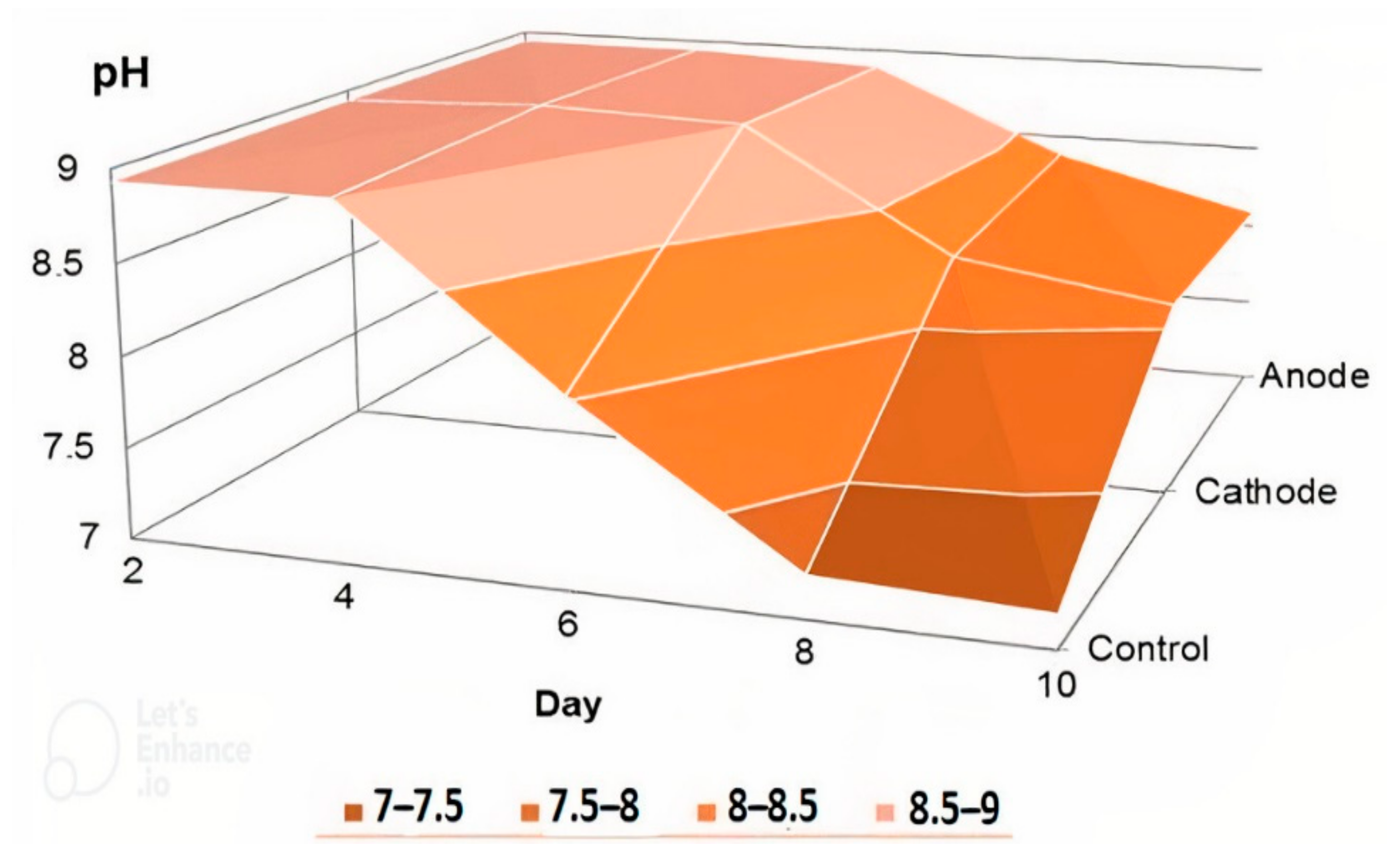
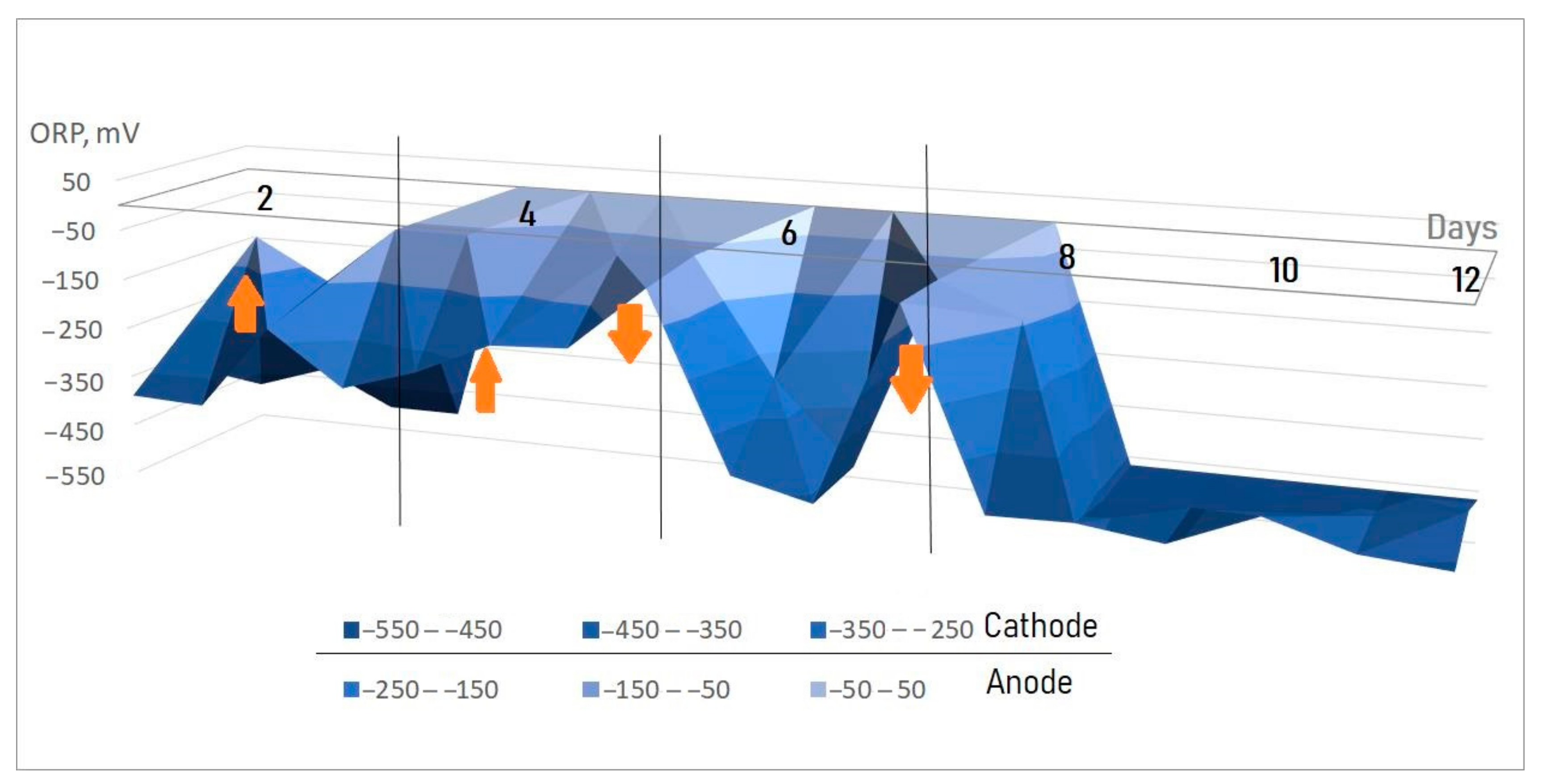
3.3. Study of Changes in Values of pH, ORP, and TDS in MECs and Cells without Electrodes for Comparison
3.4. Formalization of a Generalized Model of the Effect of Electrolysis on the Anaerobic Digestion Process Based on the Data Obtained and Previous Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feki, E.; Khoufi, S.; Loukil, S.; Sayadi, S. Improvement of anaerobic digestion of waste-activated sludge by using H2O2 oxidation, electrolysis, electro-oxidation and thermo-alkaline pretreatments. Environ. Sci. Pollut. Res. 2015, 22, 14717–14726. [Google Scholar] [CrossRef] [PubMed]
- Barrios, J.A.; Cano, A.; Rivera, F.F.; Cisneros, M.E.; Durán, U. Efficiency of integrated electrooxidation and anaerobic digestion of waste activated sludge. Biotechnol. Biofuels 2021, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Mehta, P.; Bourque, J.-S.; Guiot, S.R. Electrolysis-enhanced anaerobic digestion of wastewater. Bioresour. Technol. 2011, 102, 5685–5691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, G.; Banu, J.R.; Kumar, G.; Yogalakshmi, K. Statistical optimization of operating parameters of microbial electrolysis cell treating dairy industry wastewater using quadratic model to enhance energy generation. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Rader, G.K.; Logan, B.E. Multi-electrode continuous flow microbial electrolysis cell for biogas production from acetate. Int. J. Hydrogen Energy 2010, 35, 8848–8854. [Google Scholar] [CrossRef]
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. A new upgraded biogas production process: Coupling microbial electrolysis cell and anaerobic digestion in single-chamber, barrel-shape stainless steel reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Startup of Electromethanogenic Microbial Electrolysis Cells with Two Different Biomass Inocula for Biogas Upgrading. ACS Sustain. Chem. Eng. 2017, 5, 8852–8859. [Google Scholar] [CrossRef] [Green Version]
- Shulipa, Y.O.; Chernysh, Y.Y.; Plyatsuk, L.D.; Fukui, M. Ontological Tools in Anaerobic Fermentation Technologies: Bioinformation Database Applications. J. Eng. Sci. 2020, 7, H1–H8. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, B.; Dong, R.; Lu, H.; Zhou, H.; Cao, W. Pretreatment of poultry manure anaerobic-digested effluents by electrolysis, centrifugation and autoclaving process for Chlorella vulgaris growth and pollutants removal. Environ. Technol. 2015, 36, 837–843. [Google Scholar] [CrossRef]
- Wang, X.; Lu, H.; Zhang, L.; Wang, M.; Zhao, Y.; Li, B. Combination of Electrolysis and Microalgae Cultivation to Treat Effluent from Anaerobic Digestion of Poultry Manure. In Proceedings of the International Symposium Animal Environment and Welfare, Chongqing, China, Leeds, UK, 23–26 October 2015; pp. 179–186. [Google Scholar]
- Wang, X.-T.; Zhao, L.; Chen, C.; Chen, K.-Y.; Yang, H.; Xu, X.-J.; Zhou, X.; Liu, W.-Z.; Xing, D.-F.; Ren, N.-Q.; et al. Microbial electrolysis cells (MEC) accelerated methane production from the enhanced hydrolysis and acidogenesis of raw waste activated sludge. Chem. Eng. J. 2021, 413, 127472. [Google Scholar] [CrossRef]
- Heng, G.C.; Isa, M.H.; Lock, S.S.M.; Ng, C.A. Process Optimization of Waste Activated Sludge in Anaerobic Digestion and Biogas Production by Electrochemical Pre-Treatment Using Ruthenium Oxide Coated Titanium Electrodes. Sustainability 2021, 13, 4874. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, W.; Shi, Y.; Wang, B.; Huang, C.; Liu, C.; Wang, A. Microbial electrolysis enhanced bioconversion of waste sludge lysate for hydrogen production compared with anaerobic digestion. Sci. Total Environ. 2021, 767, 144344. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, W.; Cui, M.; Zhu, Y.; Wang, L.; Wang, A.; Huang, C. Enhanced methane production in an up-flow microbial electrolysis assisted reactors: Hydrodynamics characteristics and electron balance under different spatial distributions of bioelectrodes. Water Res. 2021, 191, 116813. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cai, C.; Chen, Y.; Liu, H.; Liu, R.; Yang, D.; Dong, B.; Dai, X. Secondary acidogenic fermentation of waste activated sludge via voltage supplementation: Insights from sludge structure and enzymes activity. Sci. Total Environ. 2021, 797, 149161. [Google Scholar] [CrossRef]
- Bao, H.; Yang, H.; Zhang, H.; Liu, Y.; Su, H.; Shen, M. Improving methane productivity of waste activated sludge by ultrasound and alkali pretreatment in microbial electrolysis cell and anaerobic digestion coupled system. Environ. Res. 2019, 180, 108863. [Google Scholar] [CrossRef]
- Shtepa, V.; Balintova, M.; Chernysh, Y.; Chubur, V.; Demcak, S. Rationale for the Combined Use of Biological Processes and AOPs in Wastewater Treatment Tasks. Appl. Sci. 2021, 11, 7551. [Google Scholar] [CrossRef]
- Onursal, E.; Oechsner, H.; Ekinci, K. Biogas Production Potential of Rose Oil Processing Wastes and Quail Manure in Turkiye: Assessment by Hohenheim Batch Test. Tarım Makinaları Bilimi Derg. 2011, 7, 393–398. [Google Scholar]
- Sousa, M.S.; Tinôco, I.F.F.; Souza, F.C.; Inoue, K.R.A.; Matos, F.T. Chemical and Microbiological Characterization of Quail Wastes. In Proceedings of the American Society of Agricultural and Biological Engineers, Dallas, TX, USA, 29 July–1 August 2012. [Google Scholar] [CrossRef]
- Jijai, S.; Muleng, S.; Noynoo, L.; Siripatana, C. Kinetic model of biogas production from co-digestion of Thai rice noodle wastewater with rice husk and different type of manure with ash supplement. IOP Conf. Series Earth Environ. Sci. 2020, 463, 12008. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.H.L.; dos Santos, L.A.; Oliveira, C.R.d.M.; Porto, T.S.; Jucá, J.F.T.; Santos, A.F.D.M.S. Determination of methane generation potential and evaluation of kinetic models in poultry wastes. Biocatal. Agric. Biotechnol. 2021, 32, 101936. [Google Scholar] [CrossRef]
- Borowski, S.; Domański, J.; Weatherley, L. Anaerobic co-digestion of swine and poultry manure with municipal sewage sludge. Waste Manag. 2014, 34, 513–521. [Google Scholar] [CrossRef]
- Borowski, S.; Weatherley, L. Co-digestion of solid poultry manure with municipal sewage sludge. Bioresour. Technol. 2013, 142, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Borowski, S.; Kucner, M. The use of sugar beet pulp stillage for co-digestion with sewage sludge and poultry manure. Waste Manag. Res. J. Sustain. Circ. Econ. 2019, 37, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Sillero, L.; Solera, R.; Perez, M. Anaerobic co-digestion of sewage sludge, wine vinasse and poultry manure for bio-hydrogen production. Int. J. Hydrogen Energy 2022, 47, 3667–3678. [Google Scholar] [CrossRef]
- Fierro, J.; Martínez, J.E.; Rosas, J.G.; Blanco, D.; Gómez, X. Anaerobic codigestion of poultry manure and sewage sludge under solid-phase configuration. Environ. Prog. Sustain. Energy 2014, 33, 866–872. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Suthar, S. Anaerobic digestion of activated sludge, anaerobic granular sludge and cow dung with food waste for enhanced methane production. J. Clean. Prod. 2017, 164, 557–566. [Google Scholar] [CrossRef]
- Antonella Marone, A.; Ayala-Campos, O.; Trably, E.; Carmona-Martínez, A.A.; Moscoviz, R.; Eric Latrille, E.; Jean-Philippe Steyer, J.-P.H.; Alcaraz-Gonzalez, V.; Bernet, N. Coupling Dark Fermentation and Microbial Electrolysis to Enhance Bio-Hydrogen Production from Agro-Industrial Wastewaters and By-Products in a Bio-Refinery Framework. J. Appl. Electrochem. 2017, 42, 1609–1621. [Google Scholar]
- Yanuka-Golub, K.; Baransi-Karkaby, K.; Szczupak, A.; Reshef, L.; Rishpon, J.; Shechter, R.; Gophna, U.; Sabbah, I. An electrode-assisted anaerobic digestion process for the production of high-quality biogas. Water Sci. Technol. 2019, 79, 2145–2155. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, A.; Jia, J.; Liang, Q.; Liu, Q.; Xing, D.; Ren, N. Characterization of methane production and microbial community shifts during waste activated sludge degradation in microbial electrolysis cells. Bioresour. Technol. 2015, 175, 68–74. [Google Scholar] [CrossRef]
- Sevillano, C.B.A.; Chiappero, M.; Gomez, X.; Fiore, S.; Martínez, E.J. Improving the Anaerobic Digestion of Wine-Industry Liquid Wastes: Treatment by Electro-Oxidation and Use of Biochar as an Additive. Energies 2020, 13, 5971. [Google Scholar] [CrossRef]
- Dalkilic, K.; Ugurlu, F. Enhancement of Biogas Production from Cattle Manure in a Combined Microbial Electrolysis Cell and Anaerobic Digestion System at Short Hydraulic Retention Time. 2022. Available online: https://assets.researchsquare.com/files/rs-1501290/v1/c0eb57e1-7216-43a9-9233-0b3384f15e3f.pdf?c=1652369073 (accessed on 20 May 2022).
- Bragança, I.; Sánchez-Soberón, F.; Pantuzza, G.F.; Alves, A.; Ratola, N. Impurities in biogas: Analytical strategies, occurrence, effects and removal technologies. Biomass Bioenergy 2020, 143, 105878. [Google Scholar] [CrossRef]
- Capri, M.G.; Marais, G. pH adjustment in anaerobic digestion. Water Res. 1975, 9, 307–313. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Manassa, P.; Dawson, M.; Fitzgerald, S.K. Oxidation reduction potential as a parameter to regulate micro-oxygen injection into anaerobic digester for reducing hydrogen sulphide concentration in biogas. Bioresour. Technol. 2014, 173, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vongvichiankul, C.; Deebao, J.; Khongnakorn, W. Relationship between pH, Oxidation Reduction Potential (ORP) and Biogas Production in Mesophilic Screw Anaerobic Digester. Energy Procedia 2017, 138, 877–882. [Google Scholar] [CrossRef]
- Liu, S.Y.; Charles, W.; Ho, G.; Cord-Ruwisch, R.; Cheng, K.Y. Bioelectrochemical enhancement of anaerobic digestion: Comparing single- and two-chamber reactor configurations at thermophilic conditions. Bioresour. Technol. 2017, 245, 1168–1175. [Google Scholar] [CrossRef] [Green Version]
- Jafari, M.; Botte, G.G. Electrochemical treatment of sewage sludge and pathogen inactivation. J. Appl. Electrochem. 2020, 51, 119–130. [Google Scholar] [CrossRef]
- Kuppam, C.; Pandit, S.; Kadier, A.; Dasagrandhi, C.; Velpuri, J. Biohydrogen Production: Integrated Approaches to Improve the Process Efficiency. In Microbial Applications; Kalia, V., Kumar, P., Eds.; Springer: Cham, Switzerland, 2017; Volume 1, pp. 189–210. [Google Scholar] [CrossRef]




| Parameter | Measurement |
|---|---|
| Appearance, color, and odor | Dark brown, crumbly mass, with a specific odor, and with sand inclusions |
| Mass fraction of water, % | 50–65 |
| Mass fraction of macro elements, %: | |
| Nitrogen | 0.7 |
| Phosphorus | 0.5 |
| Potassium | 0.85 |
| Acidity, pH | 7.5–8.0 |
| Gas Cells | Range | Typical Accuracy (Range: Accuracy) | Typical Accuracy (Range: Accuracy) |
|---|---|---|---|
| CH4 | 0–100% | 0–70%: ±0.5% (vol) | 70–100%: ±1.5% (vol) |
| CO2 | 0–100% | 0–60%: ±0.5% (vol) | 60–100%: ±1.5% (vol) |
| O2 | 0–25% | 0–25%: ±1.0% (vol) | |
| H2S | 0–5000 ppm | ±2.0% FS * | |
| CO | 0–2000 ppm | ±2.0%FS * |
| Parameter | Two-Chamber MEC | Cell without Electrodes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 6 | Day 8 | Day 10 | Day 12 | Day 14 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 | |
| V biogas, mL | 800 | 825 | 812 | 810 | 821 | 12 | 18 | 25 | 150 | 850 |
| Gas | Parameter | Two-Chamber MEC | Cell without Electrodes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 6 | Day 8 | Day 10 | Day 12 | Day 14 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 | ||
| Quality | CH4, % | 8.30 | 15.30 | 27.20 | 32.30 | 35.10 | 4.70 | 11.50 | 24.30 | 25.10 | 31.60 |
| CO2, % | 27.30 | 45.20 | 49.90 | 50.10 | 53.50 | 24.00 | 41.00 | 40.20 | 39.60 | 37.70 | |
| O2, % | 1.80 | 1.50 | 1.30 | 1.10 | 0.50 | 3.40 | 2.40 | 2.10 | 1.80 | 1.50 | |
| CO, ppm | 5000+> | 5000+> | 565 | 431 | 236 | 5000+> | 5000+> | 5000+> | 5000+> | 5000+> | |
| H2S, ppm | 5000+> | 3382 | 1251 | 693 | 487 | 5000+> | 5000+> | 5000+> | 5000+> | 5000+> | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernysh, Y.; Balintova, M.; Shtepa, V.; Chubur, V.; Junakova, N. Effect of Electrolysis on Activated Sludge during the Hydrolysis and Acidogenesis Stages in the Anaerobic Digestion of Poultry Manure. Sustainability 2022, 14, 6826. https://doi.org/10.3390/su14116826
Chernysh Y, Balintova M, Shtepa V, Chubur V, Junakova N. Effect of Electrolysis on Activated Sludge during the Hydrolysis and Acidogenesis Stages in the Anaerobic Digestion of Poultry Manure. Sustainability. 2022; 14(11):6826. https://doi.org/10.3390/su14116826
Chicago/Turabian StyleChernysh, Yelizaveta, Magdalena Balintova, Vladimir Shtepa, Viktoriia Chubur, and Natalia Junakova. 2022. "Effect of Electrolysis on Activated Sludge during the Hydrolysis and Acidogenesis Stages in the Anaerobic Digestion of Poultry Manure" Sustainability 14, no. 11: 6826. https://doi.org/10.3390/su14116826
APA StyleChernysh, Y., Balintova, M., Shtepa, V., Chubur, V., & Junakova, N. (2022). Effect of Electrolysis on Activated Sludge during the Hydrolysis and Acidogenesis Stages in the Anaerobic Digestion of Poultry Manure. Sustainability, 14(11), 6826. https://doi.org/10.3390/su14116826









