Stabilization of a Clay Soil Using Cementing Material from Spent Refractories and Ground-Granulated Blast Furnace Slag
Abstract
:1. Introduction
2. Materials and Methods
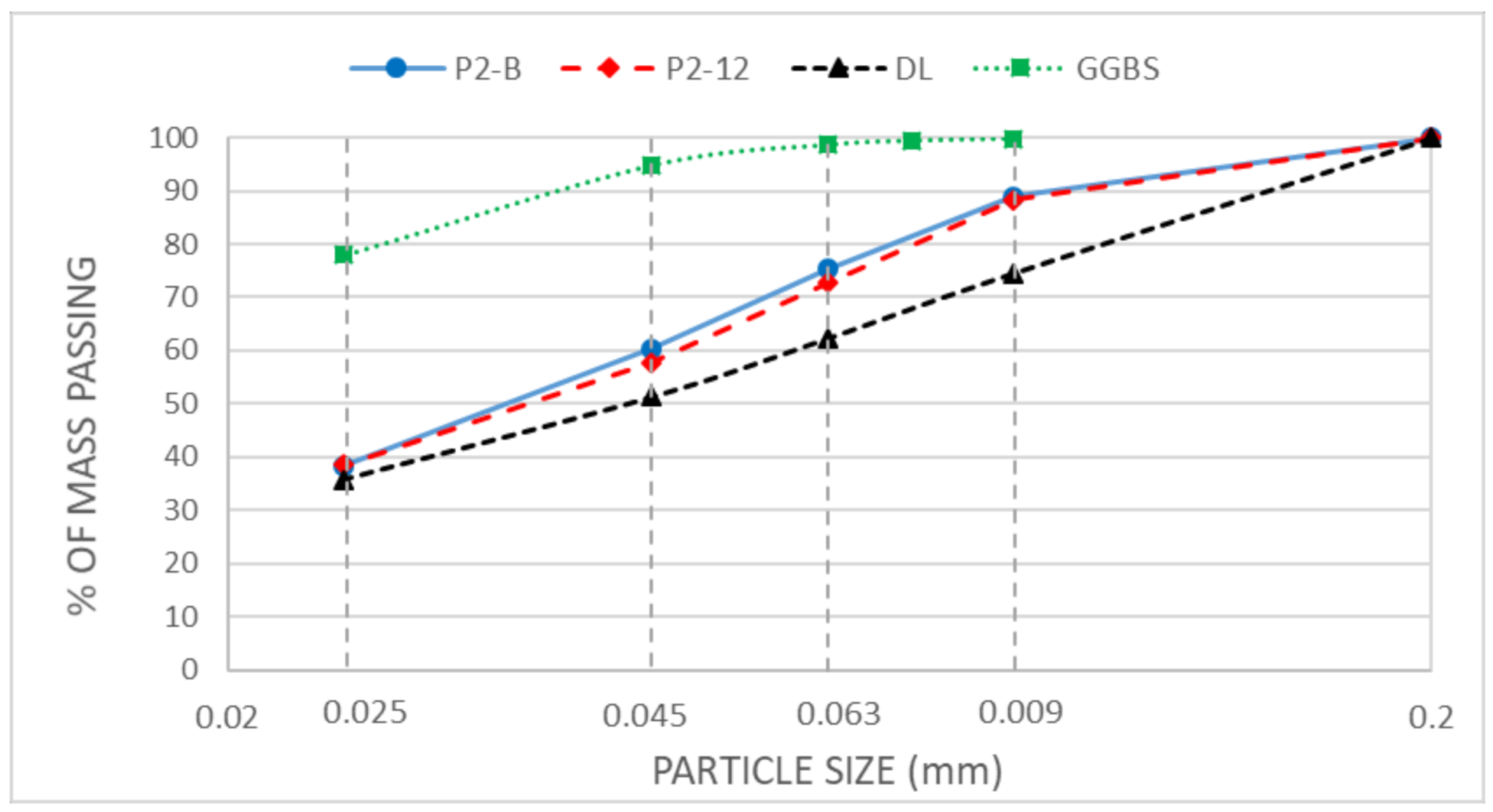
2.1. Materials
2.2. Samples’ Manufacturing and Testing
3. Results and Discussion
3.1. Soil Plasticity
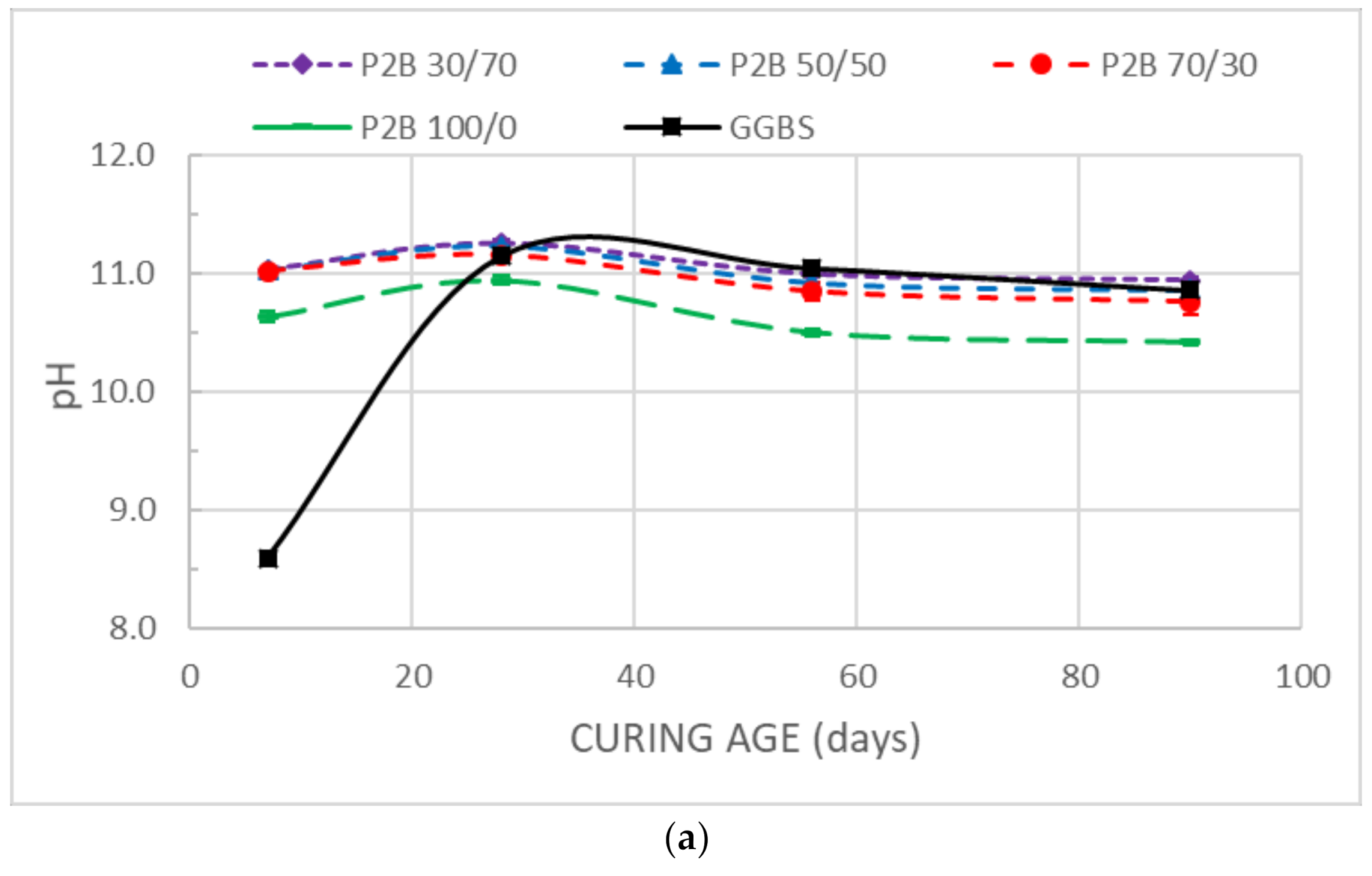
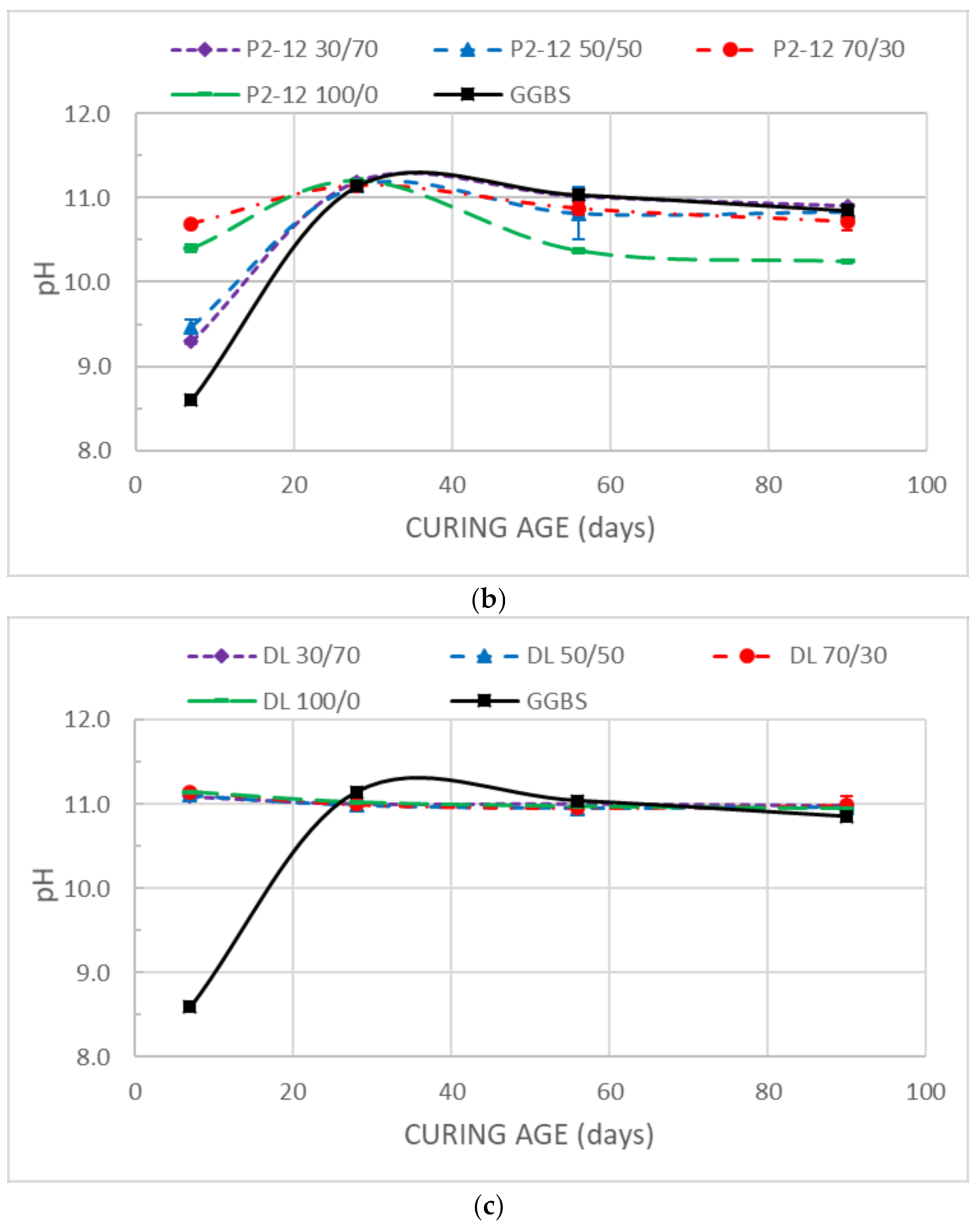
3.2. pH
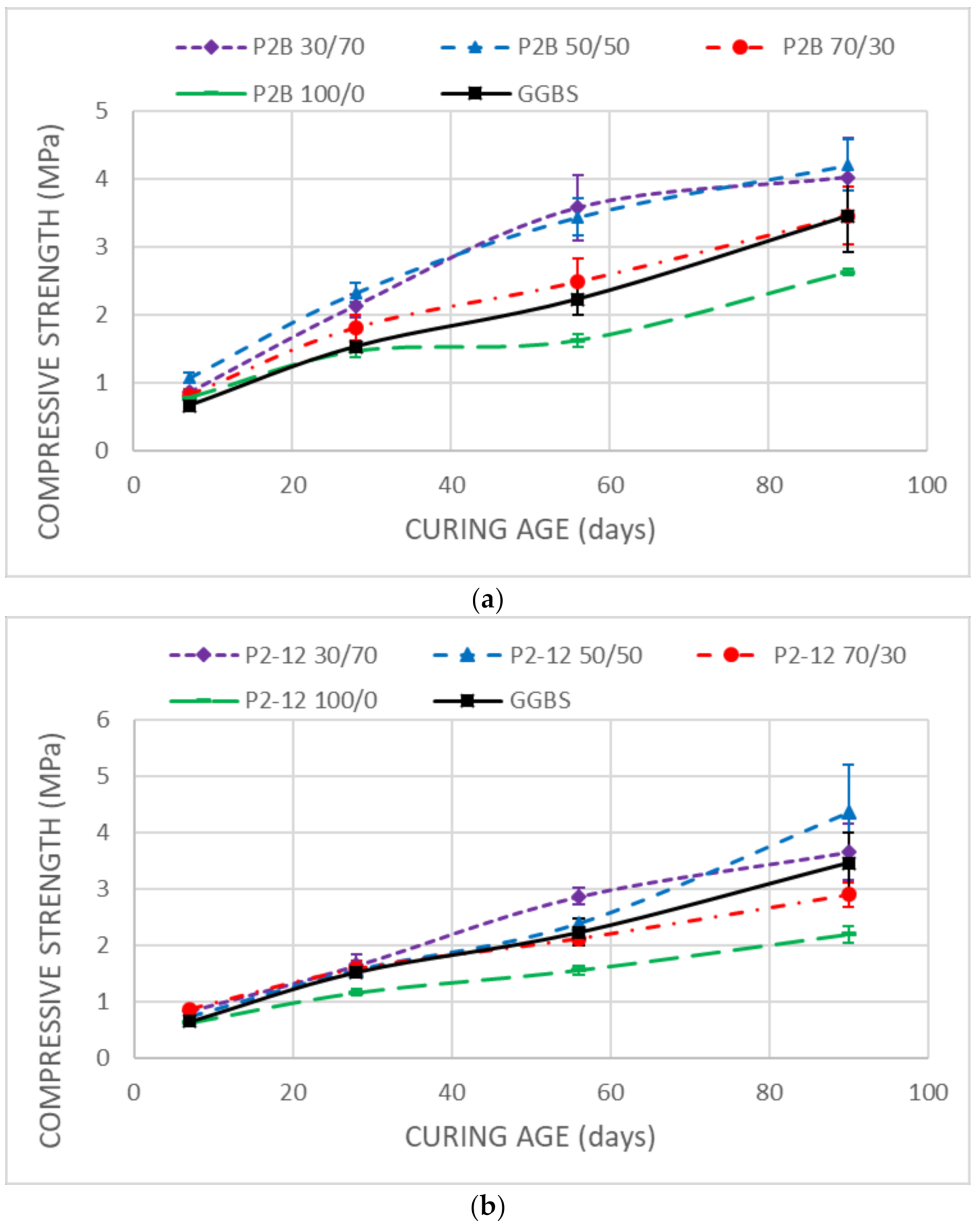
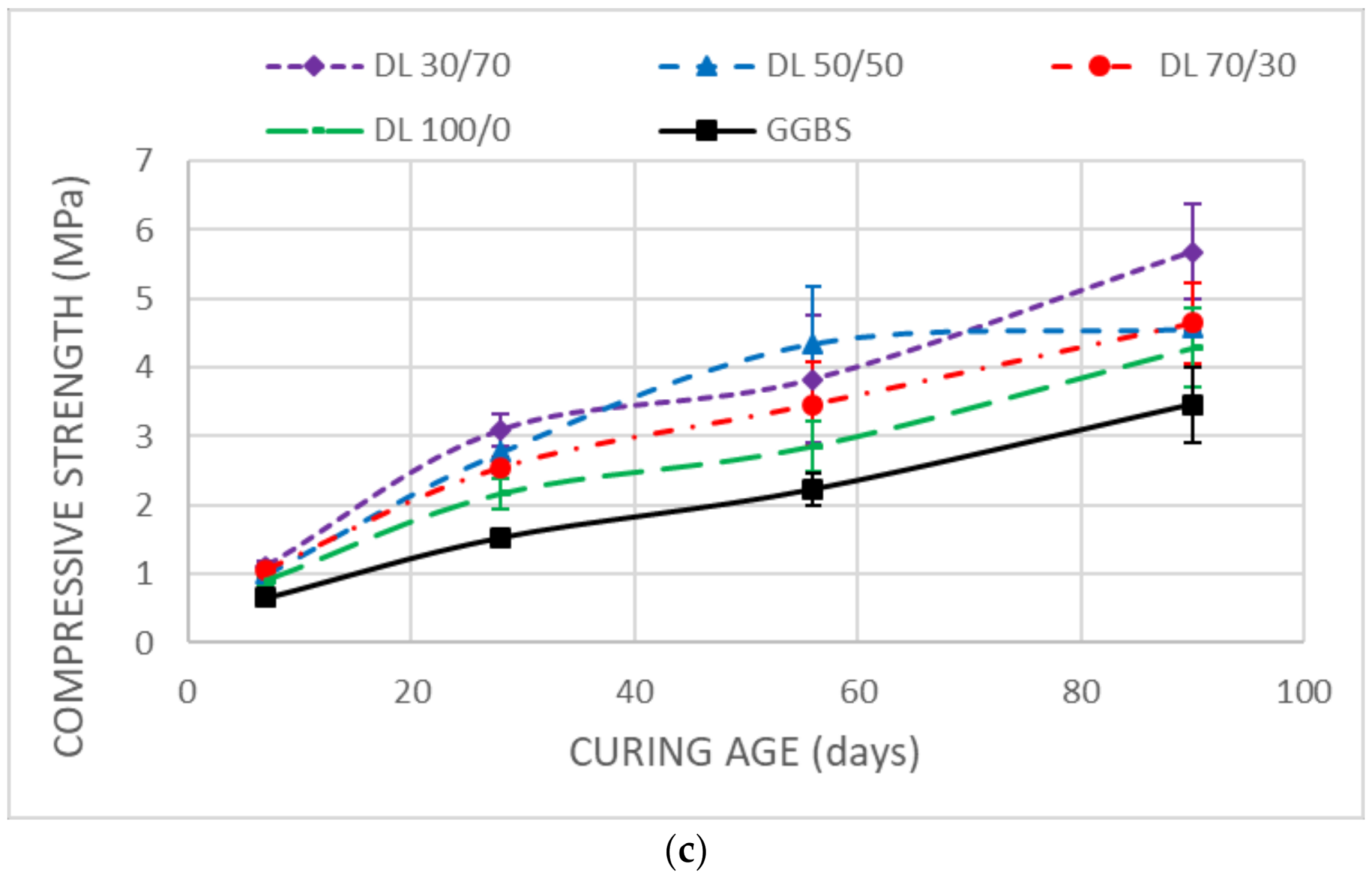
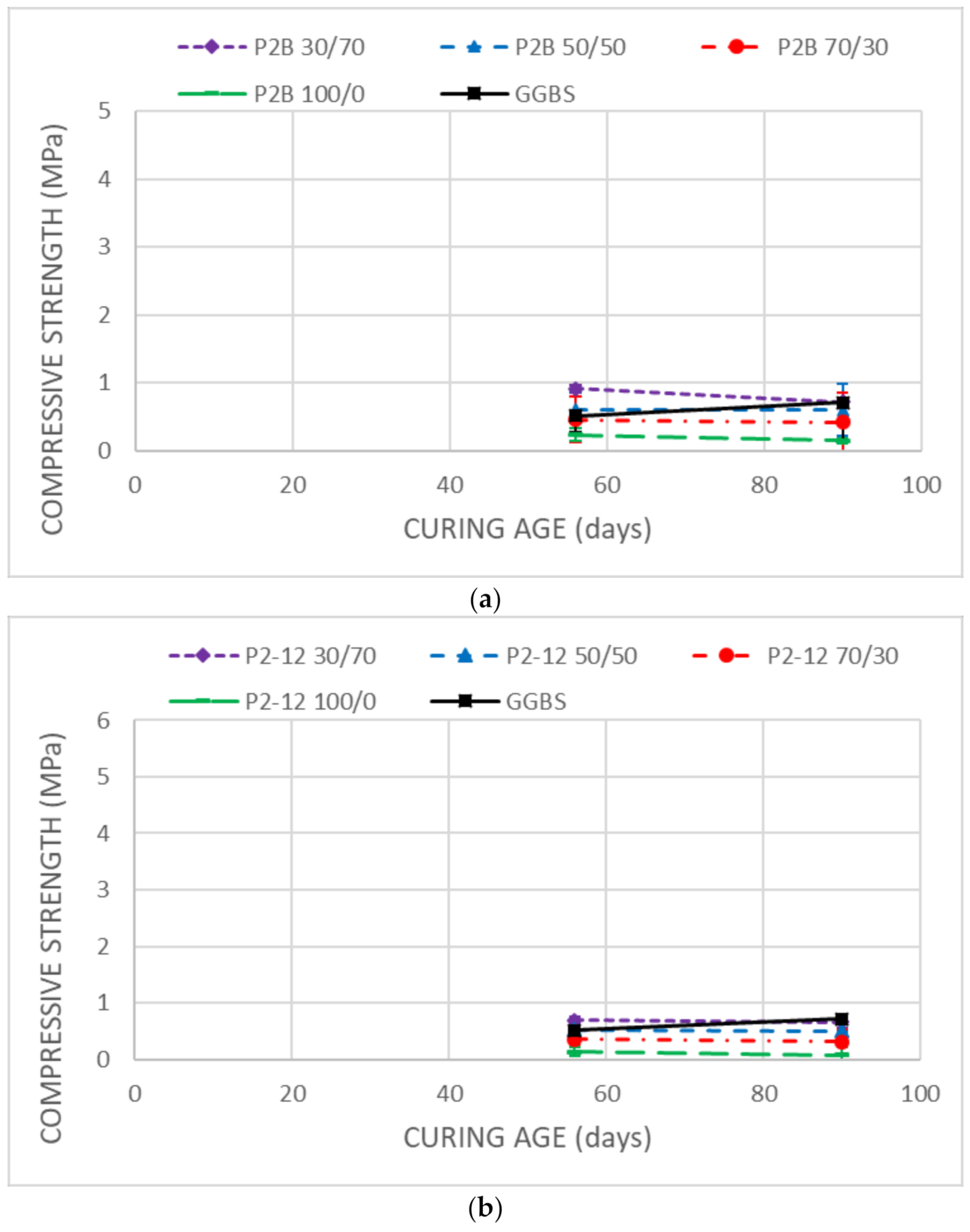
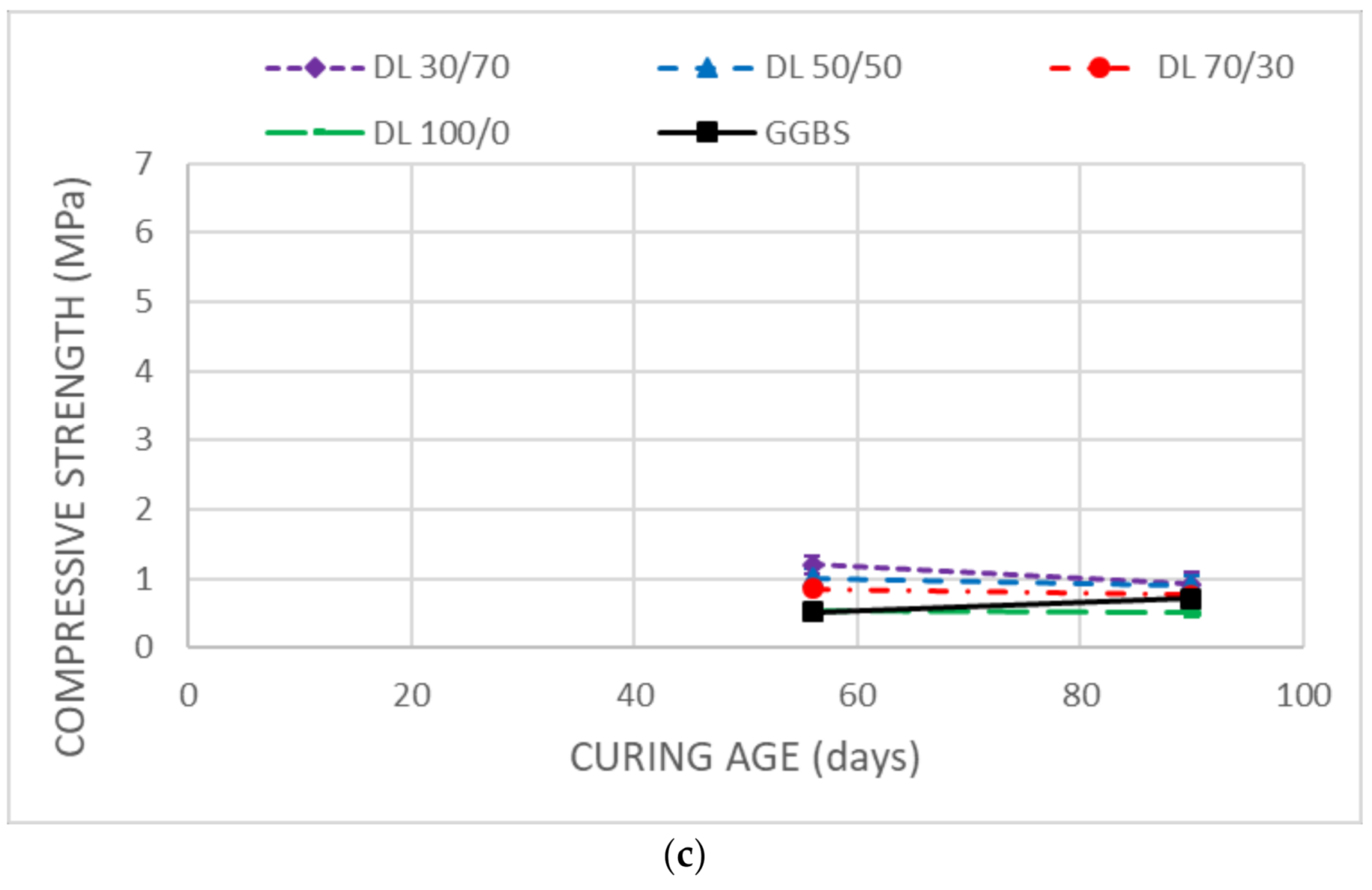
3.3. Mechanical Strength
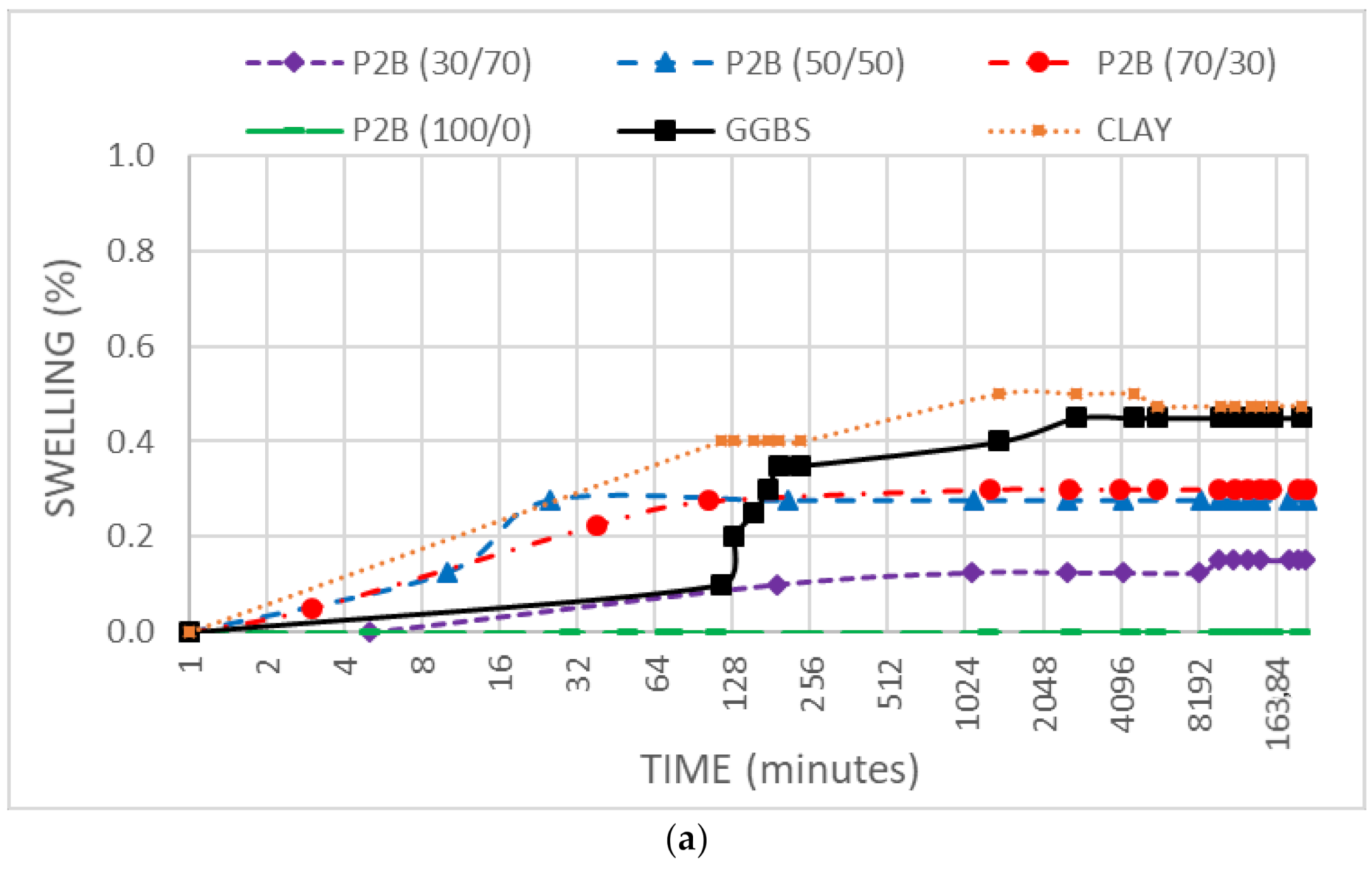
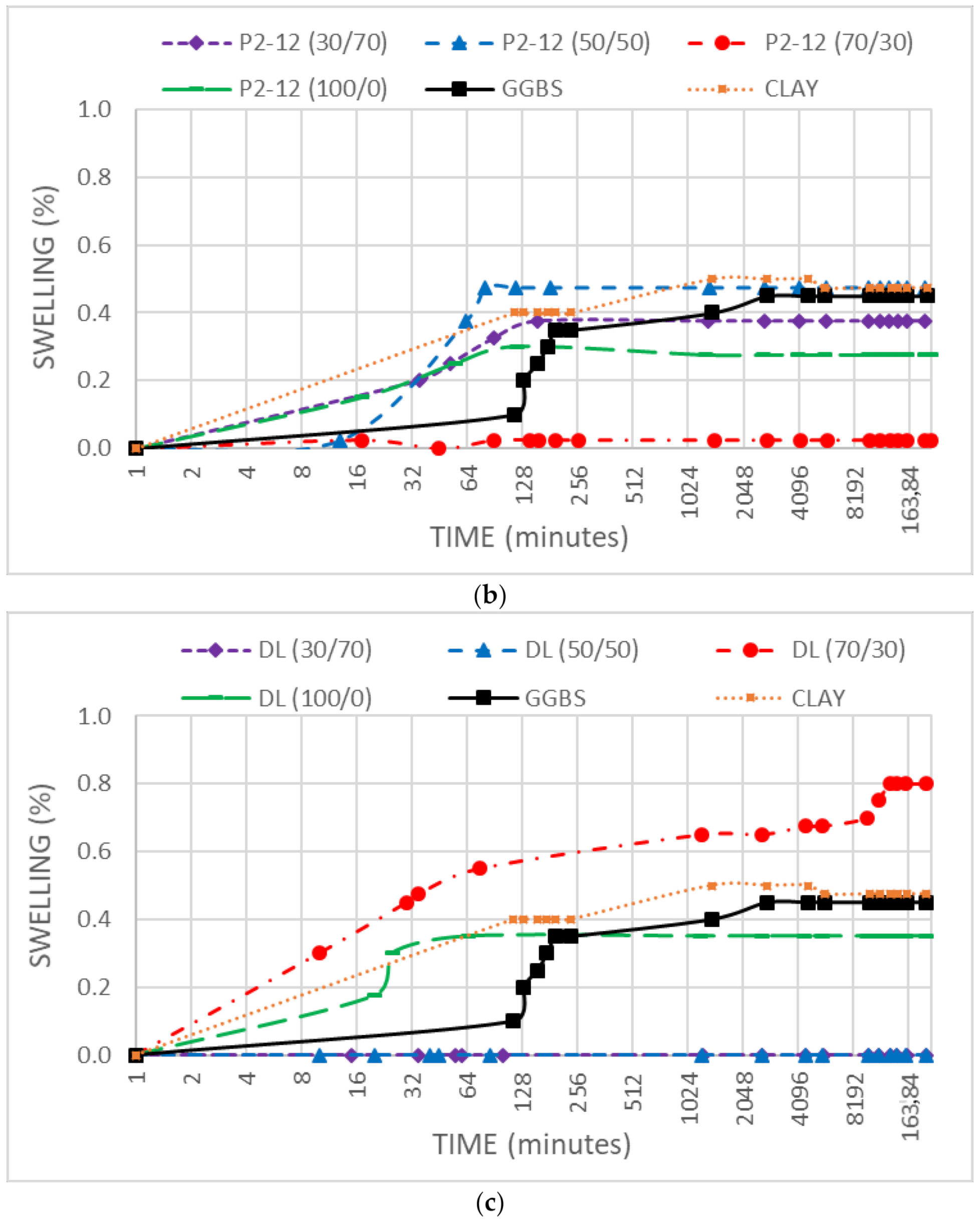
3.4. Swell Stress
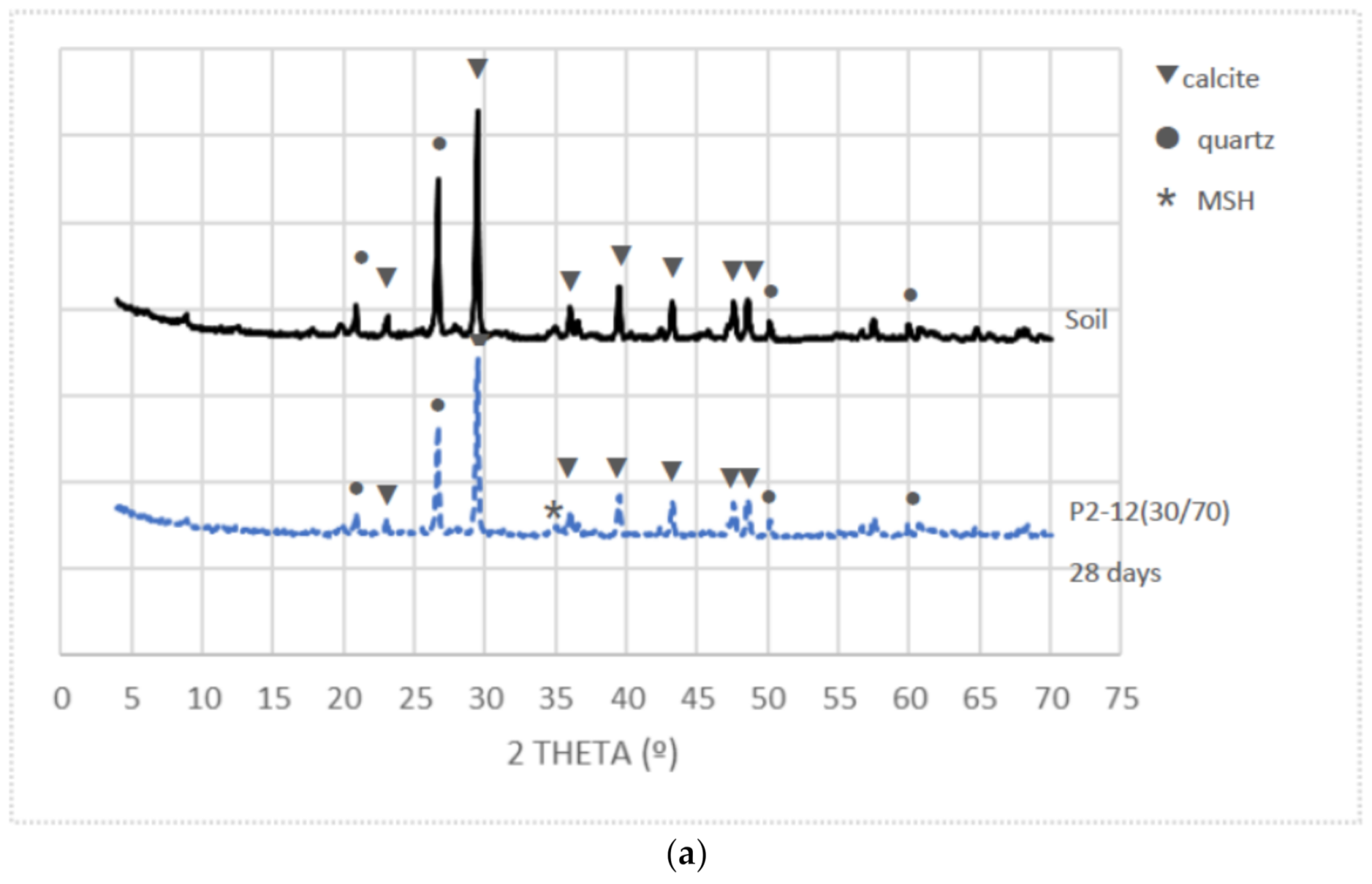
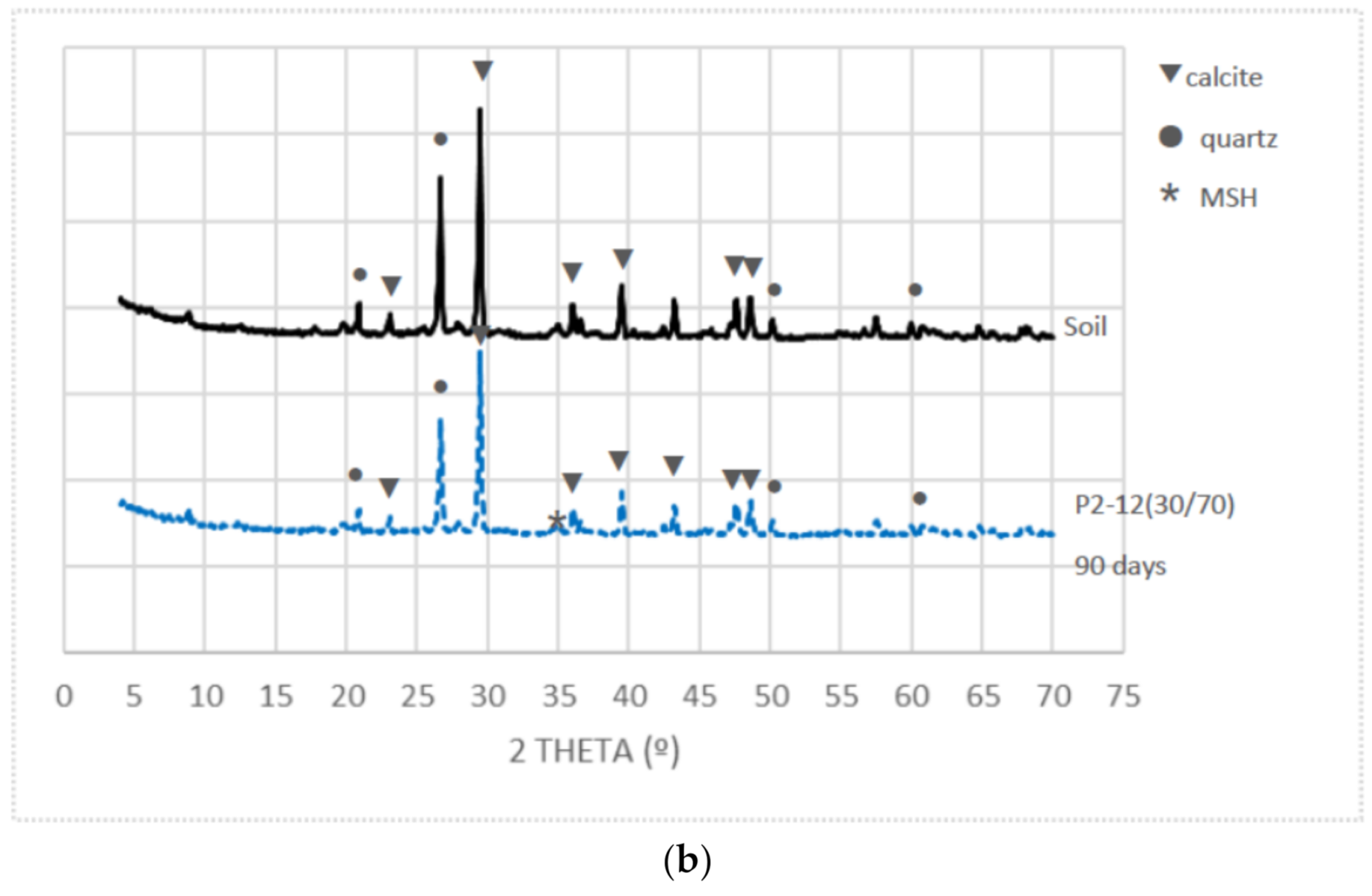
3.5. X-ray Diffraction
4. Conclusions
- DMRR and GGBS showed a low effect against the soil’s swelling because of their low free CaO and free MgO. The modification of the plasticity observed was attributed to the soil particles’ substitution and to a lesser extent to flocculation and cementation processes because of the low reactivity of the additives.
- The pH values at earlier ages of the stabilized samples are related to the free CaO and MgO provided by DMRR and GGBS, reaching values adequate for the occurrence of pozzolanic reactions. The pH evolution is consistent with hydration and cementation processes.
- Unsoaked samples increased the UCS results during all the curing time. DMRR demonstrated their ability to stabilize the soil as well as to activate GGBS. The optimum DMRR:GGBS ratio oscillates between 30:70 and 50:50. Based on its chemical composition, DL was the more effective DMRR, as expected.
- The UCS decreases observed in the stabilized samples after soaking demonstrated that the soil, after the treatment, keeps high affinity and water-holding capacity that diminishes its bearing capacity.
- The binders considered have demonstrated a low effect against the soil swelling, depending mainly on the DMRR free CaO content. No swelling processes related to delayed MgO hydration were observed.
- XRD showed that the mineralogy changes in the stabilized samples were mainly due to the particles’ soil substitution and the MSH cementitious products’ formation.
- Spent refractories showed low effect against soil plasticity and swelling
- Spent refractories show some reactivity due to their pH and free lime and MgO contents
- Spent refractories stabilized the soil and activated GGBS
- Stabilized soil keeps high affinity and water-holding capacity
- No delayed swelling processes related to MgO hydration were observed
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behnood, A. Soil and clay stabilization with calcium- and non-calcium-based additives: A state-of-the-art review of challenges, approaches and techniques. Transp. Geotech. 2018, 17, 14–32. [Google Scholar] [CrossRef]
- Khazaei, J.; Moayedi, H. Soft Expansive Soil Improvement by Eco-Friendly Waste and Quick Lime. Arab. J. Sci. Eng. 2019, 44, 8337–8346. [Google Scholar] [CrossRef]
- Okeke, C.A.U. Engineering behaviour of lime- and waste ceramic dust-stabilized expansive soil under continuous leaching. Bull. Eng. Geol. Environ. 2020, 79, 2169–2185. [Google Scholar] [CrossRef]
- Hozatlıoğlu, D.T.; Yılmaz, I. Shallow mixing and column performances of lime, fly ash and gypsum on the stabilization of swelling soils. Eng. Geol. 2021, 280, 105931. [Google Scholar] [CrossRef]
- Baldovino, J.J.A.; Izzo, R.L.S.; Rose, J.L.; Domingos, M.D.I. Strength, durability, and microstructure of geopolymers based on recycled-glass powder waste and dolomitic lime for soil stabilization. Constr. Build. Mater. 2021, 271, 121874. [Google Scholar] [CrossRef]
- Chenarboni, H.A.; Lajevardi, S.H.; MolaAbasi, H.; Zeighami, E. The effect of zeolite and cement stabilization on the mechanical behavior of expansive soils. Constr. Build. Mater. 2021, 272, 121630. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Tang, Z.; Wang, X.; Sheng, D. Sustainable regenerated binding materials (RBM) utilizing industrial solid wastes for soil and aggregate stabilization. J. Clean. Prod. 2020, 275, 122991. [Google Scholar] [CrossRef]
- Yi, Y.; Zheng, X.; Liu, S.; Al-Tabbaa, A. Comparison of reactive magnesia- and carbide slag-activated ground granulated blastfurnace slag and Portland cement for stabilisation of a natural soil. Appl. Clay Sci. 2015, 111, 21–26. [Google Scholar] [CrossRef]
- Seco, A.; Miqueleiz, L.; Prieto, E.; Marcelino, S.; García, B.; Urmeneta, P. Sulfate soils stabilization with magnesium-based binders. Appl. Clay Sci. 2017, 135, 457–464. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y.; Puppala, A.J. Suppressing Ettringite-Inducen Swelling of Gypseous Soil Using Magnesia-Activated Ground Granulated Blast-Furnace Slag. J. Geotech. Geoenviron. Eng. 2020, 146, 6020008. [Google Scholar] [CrossRef]
- Zhang, F. Magnesium Oxide Based Binders as Low-Carbon Cements; Imperial College: London, UK, 2012. [Google Scholar]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B.; Liu, C.; Gao, L. Incorporation of reactive magnesia and quicklime in sustainable binders for soil stabilisation. Eng. Geol. 2015, 195, 53–62. [Google Scholar] [CrossRef]
- Conejo, A.N.; Lule, R.G.; Lopéz, F.; Rodriguez, R. Recycling MgO-C refractory in electric arc furnaces. Resour. Conserv. Recycl. 2006, 49, 14–31. [Google Scholar] [CrossRef]
- Arianpour, F.; Kazemi, F.; Fard, F.G. Characterization, microstructure and corrosion behavior of magnesia refractories produced from recycled refractory aggregates. Miner. Eng. 2010, 23, 273–276. [Google Scholar] [CrossRef]
- Silva, A.P.; Segadães, A.M.; Lopes, R.A. Castable systems designed with powders reclaimed from dismantled steel induction furnace refractory linings. Ceram. Int. 2017, 43, 5020–5031. [Google Scholar] [CrossRef]
- Horckmans, L.; Nielsen, P.; Dierckx, P.; Ducastel, A. Recycling of refractory bricks used in basic steelmaking: A review. Resour. Conserv. Recycl. 2019, 140, 297–304. [Google Scholar] [CrossRef]
- Fang, H.; Smith, J.D.; Peaslee, K.D. Study of spent refractory waste recycling from metal manufacturers in Missouri. Resour. Conserv. Recycl. 1999, 25, 111–124. [Google Scholar] [CrossRef]
- AENOR. UNE-EN ISO 17892-12:2019. Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 12: Determination of Liquid and Plastic Limits; ISO: Geneva, Swithzerland, 2019. [Google Scholar]
- AENOR. UNE 103204:2019. Organic Matter Content of a Soil by the Potassium Permanganate Method; UNE: Madrid, Spain, 2019. [Google Scholar]
- AENOR. UNE-EN 1744-1:2010+A1:2013. Tests for Chemical Properties of Aggregates—Part 1: Chemical Analysis; UNE: Madrid, Spain, 2013. [Google Scholar]
- AENOR. UNE 103500:1994. Geotechnic. Compactation Standard Test. Standard Proctor; UNE: Madrid, Spain, 1994. [Google Scholar]
- AENOR. UNE-EN ISO 17892-7:2019. Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 7: Unconfined Compression Test; ISO: Geneva, Swithzerland, 2019. [Google Scholar]
- AENOR. UNE 103601:1996. Test for Free Swelling of Soils in Oedometer Device; UNE: Madrid, Spain, 1996. [Google Scholar]
- AENOR. UNE 103502:1995. Test Laboratory Method for Determining in a Soil the C.B.R Index; UNE: Madrid, Spain, 1995. [Google Scholar]
- Subbarao, G.V.R.; Siddartha, D.; Muralikrishna, T.; Sailaja, K.S.; Sowmya, T. Industrial Wastes in Soil Improvement. ISRN Civ. Eng. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Deepak, M.S.; Rohini, S.; Harini, B.S.; Ananthi, G.B.G. Influence of fly-ash on the engineering characteristics of stabilised clay soil. Mater. Today Proc. 2020, 37, 2014–2018. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, S. Modeling for the use of waste materials (Bottom ash and fly ash) in soil stabilization. Mater. Today Proc. 2020, 33, 1610–1614. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Hu, J.; Tang, Y.; Niu, Y.; Wei, J.; Yu, Q. Characterization of reaction products and reaction process of MgO-SiO2-H2O system at room temperature. Constr. Build. Mater. 2014, 61, 252–259. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, X.; Guo, L.; Zhang, S.; Chen, J. Strength and hydration products of cemented paste backfill from sulphide-rich tailings using reactive MgO-activated slag as a binder. Constr. Build. Mater. 2019, 203, 111–119. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Strength and hydration products of reactive MgO-silica pastes. Cem. Concr. Compos. 2014, 52, 27–33. [Google Scholar] [CrossRef]
- Zhang, T.; Cheeseman, C.R.; Vandeperre, L.J. Development of low pH cement systems forming magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2011, 41, 439–442. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Thermogravimetric study on the hydration of reactive magnesia and silica mixture at room temperature. Thermochim. Acta 2013, 566, 162–168. [Google Scholar] [CrossRef]
- Yi, Y.; Liska, M.; Al-Tabbaa, A. Properties of Two Model Soils Stabilized with Different Blends and Contents of GGBS. Mgo, Lime, and PC. J. Mater. Civ. Eng. 2014, 26, 267–274. [Google Scholar] [CrossRef]
- Seco, A.; Ramírez, F.; Miqueleiz, L.; Garci, B.; Prieto, E. The use of non-conventional additives in Marls stabilization. Appl. Clay Sci. 2011, 51, 419–423. [Google Scholar] [CrossRef]
- Miqueleiz, L.; Ramírez, F.; Seco, A.; Nidzam, R.M.; Kinuthia, J.M.; Tair, A.A.; Garcia, R. The use of stabilised Spanish clay soil for sustainable construction materials. Eng. Geol. 2012, 133–134, 9–15. [Google Scholar] [CrossRef]
- Chakraborty, S.; Nair, S. Impact of curing time on moisture-induced damage in lime-treated soils. Int. J. Pavement Eng. 2020, 21, 215–227. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, C.; Xi, P.; Chen, K.; Zhang, F.; Wang, S. Study on flexural properties of active magnesia carbonation concrete with fly ash content. Constr. Build. Mater. 2018, 187, 884–891. [Google Scholar] [CrossRef]
- Gomes, C.M.; de Oliveira, A.D.S. Chemical phases and microstructural analysis of pastes based on magnesia cement. Constr. Build. Mater. 2018, 188, 615–620. [Google Scholar] [CrossRef]
| Extraction Coordinates | ||
|---|---|---|
| X: 612.110 Y: 4.740.296 (UTM 30 ETRS89): | ||
| Soil Mechanic Properties | Chemical Properties | |
| Atterberg limits (UNE 17892-12) [19]: Liquid Limit: 38.4% Plastic Limit: 22.8% Plasticity Index: 15.6% | Organic matter (UNE 103204) [20]: 0.32% | |
| Soluble sulfates (EN 1744-1) [21]: 0.05% | ||
| pH in water: 7.88 | ||
| Mineralogy (X-ray diffraction): Calcite Quartz Halloysite Albite | ||
| Standard Proctor (UNE 103500) [22]: Maximum density: 1.76 g/cm3 Optimum moisture content: 18.5% | ||
| Unified Soil Classification: CL | Chemical composition (X-ray fluorescence): | |
| Unconfined Compression Strength (UNE 17892-7) [23]: Before soaking: 0.619 MPa After soaking: 0.000 MPa | Element: Ca Si Al Fe K Mg S | % 50.41 23.92 10.81 7.78 3.39 1.92 0.09 |
| Free Swelling (UNE 103601) [24]: 0.5% | ||
| Califorfia Bearing Ratio index (UNE 103502) [25]: 2.44 | ||
| Color (based on the Munsell Soil Color Chart): | ||
10YR 7/4 | ||
| Chemical Composition (X-ray Fluorescence) | ||||
|---|---|---|---|---|
| % | P2B | P2-12 | DL | GGBS |
| SiO2 | 12.45 | 10.53 | 12.38 | 32.18 |
| CaO | 10.89 | 9.8 | 29.37 | 43.94 |
| Fe2O3 | 12.13 | 3.31 | 2.54 | 0.33 |
| Al2O3 | 6.51 | 5.32 | 5.53 | 10.40 |
| SO3 | 0.36 | 0.32 | 1.17 | 2.00 |
| Cr2O3 | 0.42 | 0.1 | 0.08 | - |
| P2O5 | 0.11 | 0.06 | 0.13 | - |
| MnO | 0.26 | - | 0.00 | - |
| MgO | 53.75 | 63.55 | 42.88 | 0.25 |
| Reactivity | ||||
| Loss of ignition at 1050 °C | 6.02 | 4.26 | 5.91 | 0.46 |
| Free lime (%) | 0.60 | 0.71 | 3.69 | 0.28 |
| Reactivity (min) | >240 min | >240 min | >240 min | >480 min |
| pH in water | 11.62 | 11.73 | 12.07 | 9.54 |
| COMBINATION | CODE | SOIL | P2-B | P2-12 | DL | GGBS |
|---|---|---|---|---|---|---|
| SOIL | SOIL | 100 | ||||
| SOIL + P2B | P2-B (100/0) | 95 | 5 | |||
| SOIL + P2-12 | P2-12 (100/0) | 95 | 5 | |||
| SOIL + DL | DL (100/0) | 95 | 5 | |||
| SOIL + GGBS | GGBS | 95 | 5 | |||
| SOIL + P2B + GGBS (30/70) | P2-B (30/70) | 95 | 1.5 | 3.5 | ||
| SOIL + P2B + GGBS (50/50) | P2-B (50/50) | 95 | 2.5 | 2.5 | ||
| SOIL + P2B + GGBS (70/30) | P2-B (70/30) | 95 | 3.5 | 1.5 | ||
| SOIL + P2-12 + GGBS (30/70) | P2-12 (30/70) | 95 | 1.5 | 3.5 | ||
| SOIL + P2-12 + GGBS (50/50) | P2-12 (50/50) | 95 | 2.5 | 2.5 | ||
| SOIL + P2-12 + GGBS (70/30) | P2-12 (70/30) | 95 | 3.5 | 1.5 | ||
| SOIL + DL + GGBS (30/70) | DL (30/70) | 95 | 1.5 | 3.5 | ||
| SOIL + DL + GGBS (50/50) | DL (50/50) | 95 | 2.5 | 2.5 | ||
| SOIL + DL + GGBS (70/30) | DL (70/30) | 95 | 3.5 | 1.5 |
| COMBINATION | LL | PL | PI |
|---|---|---|---|
| SOIL | 38.4 | 22.8 | 15.6 |
| P2B (100/0) | 34.2 | 20.0 | 14.2 |
| P2-12 (100/0) | 36.1 | 20.8 | 15.3 |
| DL (100/0) | 38.3 | 21.7 | 16.6 |
| GGBS | 32.8 | 19.9 | 12.9 |
| P2B (30/70) | 33.1 | 20.4 | 12.7 |
| P2B (50/50) | 33.1 | 19.4 | 13.7 |
| P2B (70/30) | 35.3 | 19.5 | 15.8 |
| P2-12 (30/70) | 34.1 | 20.3 | 13.8 |
| P2-12 (50/50) | 35.2 | 20.7 | 14.5 |
| P2-12 (70/30) | 35.8 | 20.0 | 15.8 |
| DL (30/70) | 37.2 | 22.1 | 15.1 |
| DL (50/50) | 38.0 | 22.0 | 16.0 |
| DL (70/30) | 38.5 | 23.0 | 15.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seco, A.; del Castillo, J.M.; Espuelas, S.; Marcelino-Sadaba, S.; Garcia, B. Stabilization of a Clay Soil Using Cementing Material from Spent Refractories and Ground-Granulated Blast Furnace Slag. Sustainability 2021, 13, 3015. https://doi.org/10.3390/su13063015
Seco A, del Castillo JM, Espuelas S, Marcelino-Sadaba S, Garcia B. Stabilization of a Clay Soil Using Cementing Material from Spent Refractories and Ground-Granulated Blast Furnace Slag. Sustainability. 2021; 13(6):3015. https://doi.org/10.3390/su13063015
Chicago/Turabian StyleSeco, Andres, Jesus María del Castillo, Sandra Espuelas, Sara Marcelino-Sadaba, and Benat Garcia. 2021. "Stabilization of a Clay Soil Using Cementing Material from Spent Refractories and Ground-Granulated Blast Furnace Slag" Sustainability 13, no. 6: 3015. https://doi.org/10.3390/su13063015






