A Combined Measurement and Modelling Approach to Assess the Sustainability of Whole-Tree Harvesting—A Swedish Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Measurements
2.2. Estimation of ANC in Soil Solution
2.3. Estimation of Exceedance of Critical Biomass Harvesting (CBH)
2.3.1. Concept
- Only N that leaches is affecting acidity: NO3-N leaching is acidifying, one equivalent, and NH4-N leaching counteracts acidification, one equivalent, based on theories in [25].
- N leaching is not affected by whole-tree harvesting.
- N accumulating in soil organic matter will not contribute to acidification in the future.
2.3.2. The Weathering Model PROFILE
2.3.3. Input Data
2.4. Risk Classification
3. Results
3.1. Present Soil Status: ANC in Soil Water (Factor 1)
3.2. Effect of Whole-Tree Harvesting: Exceedance of CBH (Factor 2)
3.3. Risk Classification Based on the Two Factors
4. Discussion
4.1. Geographical Distribution of Risk Classes
4.2. Policy Implications
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Site | Climate | Deposition, kg ha−1 y−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T, °C | Prec, mm | Q, mm | S | NO3-N | NH4-N | Cl | Ca | Mg | K | Na | |
| BD06 | 1.9 | 694 | 289 | 0.65 | 0.69 | 1.10 | 1.84 | 0.59 | 0.15 | 0.44 | 1.02 |
| AC34 | 0.3 | 687 | 555 | 0.50 | 0.59 | 0.93 | 4.27 | 0.57 | 0.17 | 0.78 | 2.38 |
| AC04 | 1.5 | 635 | 411 | 0.52 | 0.57 | 0.90 | 2.08 | 0.50 | 0.16 | 0.85 | 1.16 |
| AC35 | 4.0 | 709 | 346 | 1.91 | 1.15 | 0.99 | 3.36 | 0.79 | 0.30 | 0.74 | 1.87 |
| Y07 | 2.9 | 769 | 439 | 0.77 | 1.30 | 1.11 | 3.99 | 0.76 | 0.20 | 0.49 | 2.22 |
| U06 | 6.9 | 599 | 217 | 0.88 | 2.17 | 2.31 | 4.68 | 1.29 | 0.44 | 1.56 | 2.61 |
| S22 | 5.6 | 834 | 455 | 2.00 | 3.47 | 3.50 | 13.57 | 0.93 | 0.40 | 0.99 | 7.55 |
| S05 | 8.0 | 706 | 304 | 2.00 | 3.83 | 3.82 | 22.99 | 1.64 | 0.75 | 1.60 | 12.79 |
| R09 | 8.4 | 626 | 224 | 1.63 | 3.19 | 4.58 | 22.01 | 1.65 | 1.04 | 1.68 | 12.25 |
| O35 | 7.9 | 1102 | 619 | 5.24 | 5.41 | 4.45 | 70.83 | 2.12 | 2.30 | 1.49 | 39.41 |
| E21 | 7.5 | 653 | 191 | 1.10 | 2.96 | 4.30 | 9.75 | 1.75 | 0.93 | 2.12 | 5.43 |
| P95 | 7.4 | 1047 | 577 | 4.63 | 5.58 | 4.57 | 63.03 | 1.94 | 2.07 | 1.55 | 35.07 |
| F22 | 7.0 | 671 | 236 | 1.41 | 3.28 | 4.76 | 12.64 | 1.79 | 0.93 | 2.24 | 7.03 |
| F12 | 7.0 | 698 | 249 | 1.48 | 3.49 | 5.04 | 19.20 | 1.88 | 1.24 | 2.15 | 10.69 |
| F23 | 7.3 | 681 | 223 | 1.80 | 3.30 | 4.82 | 15.16 | 1.66 | 0.62 | 2.44 | 8.43 |
| F18 | 7.7 | 929 | 477 | 2.62 | 5.63 | 5.32 | 41.02 | 2.24 | 2.04 | 2.26 | 22.83 |
| G22 | 7.7 | 754 | 348 | 1.91 | 3.93 | 5.63 | 20.21 | 1.75 | 1.13 | 2.67 | 11.25 |
| N12 | 8.0 | 1169 | 734 | 6.95 | 6.55 | 6.19 | 107.88 | 2.70 | 3.05 | 2.20 | 60.03 |
| H03 | 8.6 | 564 | 180 | 1.85 | 4.23 | 3.89 | 15.87 | 2.72 | 0.85 | 2.09 | 8.83 |
| N13 | 8.3 | 1097 | 753 | 5.27 | 6.69 | 6.33 | 66.52 | 3.03 | 3.85 | 2.27 | 37.01 |
| H22 | 8.1 | 696 | 231 | 2.00 | 4.56 | 4.25 | 14.11 | 2.31 | 1.12 | 2.13 | 7.85 |
| N19 | 8.2 | 1208 | 568 | 5.34 | 5.73 | 8.67 | 57.48 | 2.63 | 2.39 | 2.37 | 31.98 |
| K13 | 8.8 | 720 | 247 | 3.12 | 5.01 | 4.70 | 24.40 | 2.34 | 1.35 | 2.11 | 13.58 |
| L18 | 8.6 | 873 | 487 | 5.03 | 5.70 | 8.68 | 55.40 | 2.44 | 1.77 | 2.39 | 30.83 |
| L15 | 8.7 | 886 | 371 | 5.93 | 6.50 | 9.41 | 38.55 | 2.76 | 1.65 | 2.75 | 21.45 |
| M16 | 8.7 | 810 | 277 | 8.16 | 7.32 | 10.58 | 54.33 | 2.90 | 1.49 | 2.90 | 30.23 |
Appendix B
| Site | Minerals 1 |
|---|---|
| BD06 | Apa Bt Chl2 Ept Hbl Ill2 Or90 Ab100 An100 CPx Vrm1 |
| AC34 | Apa Cc Chl1 Chl2 Ept Hbl Ill2 Or100 Mus Ab100 An100 Vrm2 |
| AC04 | Apa Bt Chl2 Ept Hbl Ill2 Or90 Ab100 An100 CPx Vrm1 |
| AC35 | Apa Bt Chl2 Ept Hbl Ill2 Or90 Ab100 An100 CPx Vrm1 |
| Y07 | Apa Chl1 Chl2 Ept Hbl Ill2 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| U06 | Apa Chl1 Chl2 Ept Hbl Ill2 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| S22 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| S05 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| R09 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| O35 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| E21 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| P95 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| F22 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| F12 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| F23 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| F18 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| G22 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| N12 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| H03 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| N13 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| H22 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| N19 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| K13 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| L18 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| L15 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| M16 | Apa Chl1 Chl2 Ept Hbl Ill1 Or100 Mus Ab100 An100 Vrm1 Vrm2 |
| Mineral | Si | Ti | Al | Fe | Mg | Ca | Na | K | P | H |
|---|---|---|---|---|---|---|---|---|---|---|
| Albite | 3 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Anorthite | 2 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Apatite | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 3 | 1 |
| Biotite | 218 | 9 | 136 | 90 | 90 | 0 | 4 | 68 | 0 | 162 |
| Calcite | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Chlorite1 | 138 | 1 | 124 | 124 | 107 | 3 | 2 | 0 | 0 | 442 |
| Chlorite2 | 87 | 1 | 100 | 58 | 103 | 0 | 0 | 0 | 0 | 302 |
| Clinopyroxene | 943 | 7 | 53 | 107 | 433 | 390 | 20 | 0 | 0 | 0 |
| Epidote | 62 | 0 | 48 | 15 | 0 | 40 | 0 | 0 | 0 | 22 |
| Gibbsite | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hematite | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hornblende | 10,100 | 183 | 3600 | 3000 | 3500 | 2767 | 900 | 300 | 0 | 3133 |
| Illite1 | 68 | 0 | 52 | 0 | 0 | 0 | 0 | 12 | 0 | 40 |
| Illite2 | 66 | 0 | 42 | 8 | 4 | 2 | 0 | 13 | 0 | 40 |
| Muscovite | 60 | 1 | 48 | 6 | 4 | 0 | 1 | 22 | 0 | 47 |
| Orthoclase90 | 30 | 0 | 10 | 0 | 0 | 0 | 1 | 9 | 0 | 0 |
| Orthoclase100 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Quartz | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rutile | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vermiculite1 | 293 | 0 | 162 | 182 | 103 | 20 | 0 | 0 | 0 | 804 |
| Vermiculite2 | 123 | 0 | 68 | 22 | 103 | 10 | 0 | 0 | 0 | 490 |
| Water | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Appendix C
| Database | Number of Sites | Site Names | Average (%) | St dev |
|---|---|---|---|---|
| NORDSOIL1 | 16 | See [41] | 12 | 8 |
| SWETHRO 2 | 9 | L05, LO7, M10, BD06, O35B, M16B, P95B, P95C, P95D | 25 | 15 |
| Whole-tree harvesting experiments 3 | 4 | Tönnersjöheden, Kosta, Lövliden, Lund | 9 | 7 |
| Total | 29 | 15 | 13 |
Appendix D
| Site | Layer Thickness (cm) | Density (kg m−3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| BD06 | 3.9 | 14.0 | 36.0 | 0.0 | 0.0 | 200 | 1378 | 1591 | ||
| AC34 | 8.0 | 10.0 | 11.0 | 29.0 | 0.0 | 130 | 890 | 917 | 1183 | |
| AC04 | 2.3 | 8.0 | 9.0 | 20.0 | 13.0 | 106 | 1024 | 1116 | 1205 | 1519 |
| AC35 | 6.3 | 11.0 | 9.0 | 24.0 | 6.0 | 285 | 1659 | 1659 | 1878 | 1436 |
| Y07 | 1.3 | 4.0 | 11.0 | 25.0 | 10.0 | 213 | 903 | 891 | 915 | 1377 |
| U06 | 3.9 | 7.0 | 4.0 | 25.0 | 14.0 | 113 | 623 | 1212 | 1492 | 1386 |
| S22 | 1.9 | 10.0 | 15.0 | 25.0 | 0.0 | 135 | 834 | 817 | 978 | |
| S05 | 5.5 | 12.0 | 20.0 | 15.0 | 3.0 | 382 | 1194 | 1526 | 1298 | 1340 |
| R09 | 1.8 | 6.0 | 14.0 | 30.0 | 0.0 | 149 | 901 | 1352 | 1513 | |
| O35 | 6.4 | 9.0 | 7.0 | 23.0 | 11.0 | 172 | 1186 | 1202 | 935 | 1575 |
| E21 | 1.6 | 7.0 | 9.0 | 30.0 | 4.0 | 193 | 919 | 986 | 1313 | 1326 |
| P95 | 9.5 | 7.0 | 37.0 | 6.0 | 0.0 | 123 | 1267 | 1063 | 1420 | |
| F22 | 1.8 | 4.0 | 24.0 | 22.0 | 0.0 | 108 | 577 | 867 | 1129 | |
| F12 | 1.5 | 8.0 | 15.0 | 15.0 | 12.0 | 103 | 658 | 1073 | 1538 | 1239 |
| F23 | 2.0 | 8.0 | 10.0 | 25.0 | 7.0 | 131 | 674 | 914 | 1165 | 1246 |
| F18 | 4.3 | 5.0 | 15.0 | 30.0 | 0.0 | 110 | 1107 | 1090 | 1500 | |
| G22 | 2.9 | 5.0 | 9.0 | 26.0 | 10.0 | 163 | 1033 | 1548 | 1609 | 1609 |
| N12 | 4.4 | 5.0 | 9.0 | 29.0 | 7.0 | 143 | 606 | 779 | 1716 | 1716 |
| H03 | 6.4 | 10.0 | 10.0 | 20.0 | 10.0 | 111 | 1219 | 1162 | 1001 | 1676 |
| N13 | 4.6 | 9.0 | 11.0 | 19.0 | 11.0 | 139 | 893 | 910 | 1082 | 939 |
| H22 | 2.6 | 10.0 | 15.0 | 25.0 | 0.0 | 174 | 1053 | 764 | 1408 | |
| N19 | 3.2 | 10.0 | 7.0 | 33.0 | 0.0 | 128 | 713 | 761 | 1066 | |
| K13 | 4.0 | 5.0 | 10.0 | 35.0 | 0.0 | 225 | 515 | 886 | 1382 | |
| L18 | 4.8 | 12.0 | 10.0 | 28.0 | 0.0 | 277 | 802 | 848 | 1144 | |
| L15 | 7.0 | 13.0 | 10.0 | 20.0 | 7.0 | 151 | 1363 | 1035 | 897 | 1171 |
| M16 | 4.1 | 4.0 | 10.0 | 36.0 | 0.0 | 195 | 758 | 1052 | 1068 | |
| Site | Specific Surface Area (m2 m−3) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| BD06 | 28,151 | 1,059,207 | 687,921 | ||
| AC34 | 9598 | 495,895 | 471,949 | 554,705 | |
| AC04 | 4821 | 314,861 | 334,683 | 411,358 | 459,973 |
| AC35 | 6887 | 256,904 | 477,979 | 300,161 | 458,813 |
| Y07 | 28,327 | 288,376 | 197,188 | 241,860 | 671,414 |
| U06 | 4302 | 176,673 | 391,887 | 564,446 | 418,842 |
| S22 | 23,270 | 315,461 | 350,423 | 476,259 | |
| S05 | 12,082 | 545,612 | 845,384 | 643,632 | 773,341 |
| R09 | 10,883 | 110,442 | 224,527 | 261,998 | |
| O35 | 6258 | 326,562 | 293,151 | 124,139 | 216,583 |
| E21 | 45,320 | 410,031 | 480,261 | 553,340 | 474,955 |
| P95 | 9760 | 605,526 | 553,612 | 451,694 | |
| F22 | 3574 | 160,078 | 237,043 | 739,259 | |
| F12 | 6175 | 222,081 | 218,479 | 362,367 | 401,651 |
| F23 | 13,343 | 245,158 | 333,636 | 510,119 | 515,197 |
| F18 | 4811 | 383,490 | 649,066 | 999,059 | |
| G22 | 18,670 | 310,687 | 529,126 | 571,219 | 611,470 |
| N12 | 13,878 | 239,824 | 336,315 | 121,409 | 501,299 |
| H03 | 2661 | 395,470 | 355,255 | 378,365 | 495,719 |
| N13 | 7203 | 499,499 | 497,650 | 564,692 | 507,460 |
| H22 | 23,495 | 245,005 | 109,938 | 883,003 | |
| N19 | 10,033 | 325,829 | 335,266 | 609,040 | |
| K13 | 33,601 | 147,733 | 286,103 | 461,641 | |
| L18 | 24,927 | 187,979 | 250,305 | 393,976 | |
| L15 | 2825 | 101,456 | 523,967 | 68,315 | 75,974 |
| M16 | 13,648 | 203,506 | 227,709 | 169,613 | |
References
- Schöpp, W.; Posch, M.; Mylona, S.; Johansson, M. Long-term development of acid deposition (1880–2030) in sensitive freshwater regions in Europe. Hydrol. Earth Syst. Sci. 2003, 7, 436–446. [Google Scholar] [CrossRef]
- Ferm, M.; Granat, L.; Engardt, M.; Karlsson, G.P.; Danielsson, H.; Karlsson, P.E.; Hansen, K. Wet deposition of ammonium, nitrate and non-sea-salt sulphate in Sweden 1955 through 2017. Atmos. Environ. X 2019, 2. [Google Scholar] [CrossRef]
- Grünewald, U.; Reuss, J.O.; Johnson, D.W. Acid Deposition and the Acidification of Soils and Waters. Ecological Studies 59–119 pp. New York, Berlin, Heidelberg, Tokyo: Springer-Verlag 1986. ISBN 3-540-96290-5. 94,-DM. Int. Rev. Der Gesamten Hydrobiol. Und Hydrogr. 1988, 73, 124. [Google Scholar] [CrossRef]
- Johnson, J.; Graf Pannatier, E.; Carnicelli, S.; Cecchini, G.; Clarke, N.; Cools, N.; Hansen, K.; Meesenburg, H.; Nieminen, T.M.; Pihl-Karlsson, G.; et al. The response of soil solution chemistry in European forests to decreasing acid deposition. Glob. Chang. Biol. 2018, 24, 3603–3619. [Google Scholar] [CrossRef]
- Akselsson, C.; Hultberg, H.; Karlsson, P.E.; Pihl Karlsson, G.; Hellsten, S. Acidification trends in south Swedish forest soils 1986–2008: Slow recovery and high sensitivity to sea-salt episodes. Sci. Total Environ. 2013, 444, 271–287. [Google Scholar] [CrossRef]
- Pannatier, E.G.; Thimonier, A.; Schmitt, M.; Walthert, L.; Waldner, P. A decade of monitoring at Swiss Long-Term Forest Ecosystem Research (LWF) sites: Can we observe trends in atmospheric acid deposition and in soil solution acidity? Environ. Monit. Assess. 2011, 174, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Belyazid, S.; Westling, O.; Sverdrup, H. Modelling changes in forest soil chemistry at 16 Swedish coniferous forest sites following deposition reduction. Environ. Pollut. 2006, 144, 596–609. [Google Scholar] [CrossRef]
- Nilsson, S.I.; Miller, H.G.; Miller, J.D. Forest Growth as a Possible Cause of Soil and Water Acidification: An Examination of the Concepts. Oikos 1982, 39, 40–49. [Google Scholar] [CrossRef]
- Van Breemen, N.; Mulder, J.; Driscoll, C.T. Acidification and alkalinization of soils. Plant. Soil 1983, 75, 283–308. [Google Scholar] [CrossRef]
- Riek, W.; Russ, A.; Martin, J. Soil acidification and nutrient sustainability of forest ecosystems in the northeastern German lowlands—Results of the national forest soil inventory. Folia For. Pol. Ser. A 2012, 54, 187–195. [Google Scholar]
- Iwald, J.; Löfgren, S.; Stendahl, J.; Karltun, E. Acidifying effect of removal of tree stumps and logging residues as compared to atmospheric deposition. For. Ecol. Manag. 2013, 290, 49–58. [Google Scholar] [CrossRef]
- Lucas, R.W.; Holmström, H.; Lämås, T. Intensive forest harvesting and pools of base cations in forest ecosystems: A modeling study using the Heureka decision support system. For. Ecol. Manag. 2014, 325, 26–36. [Google Scholar] [CrossRef]
- Akselsson, C.; Belyazid, S. Critical biomass harvesting—Applying a new concept for Swedish forest soils. For. Ecol. Manag. 2018, 409, 67–73. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L. Estimation of nutrient removals in stem-only and whole-tree harvesting of Scots pine, Norway spruce, and birch stands with generalized nutrient equations. Eur. J. For. Res. 2012, 131, 945–964. [Google Scholar] [CrossRef]
- Clarke, N.; Kiær, L.P.; Janne Kjønaas, O.; Bárcena, T.G.; Vesterdal, L.; Stupak, I.; Finér, L.; Jacobson, S.; Armolaitis, K.; Lazdina, D.; et al. Effects of intensive biomass harvesting on forest soils in the Nordic countries and the UK: A meta-analysis. For. Ecol. Manag. 2021, 482, 118877. [Google Scholar] [CrossRef]
- Reid, W.V.; Ali, M.K.; Field, C.B. The future of bioenergy. Glob. Chang. Biol. 2020, 26, 274–286. [Google Scholar] [CrossRef] [PubMed]
- The Swedish Forest Agency’s Statistics Database. Available online: http://pxweb.skogsstyrelsen.se/pxweb/en/ (accessed on 9 February 2021).
- Börjesson, P.; Hansson, J.; Berndes, G. Future demand for forest-based biomass for energy purposes in Sweden. For. Ecol. Manag. 2017, 383, 17–26. [Google Scholar] [CrossRef]
- Sverdrup, H.; De Vries, W. Calculating critical loads for acidity with the simple mass balance method. WaterAir Soil Pollut. 1994, 72, 20. [Google Scholar] [CrossRef]
- Fölster, J.; Bringmark, L.; Lundin, L. Temporal and Spatial Variations in Soil Water Chemistry at Three Acid Forest Sites. WaterAirSoil Pollut. 2003, 146, 171–195. [Google Scholar] [CrossRef]
- Pihl Karlsson, G.; Akselsson, C.; Hellsten, S.; Karlsson, P.E. Reduced European emissions of S and N—Effects on air concentrations, deposition and soil water chemistry in Swedish forests. Environ. Pollut. 2011, 159, 3571–3582. [Google Scholar] [CrossRef]
- Warfvinge, P.; Sverdrup, H. Critical Load of Acidity to Swedish Forest Soils. Methods, Data and Results; Lund University, Department of Chemical Engineering II: Scania, Sweden, 1995. [Google Scholar]
- Karlsson, P.E.; Pihl Karlsson, G.; Hellsten, S.; Akselsson, C.; Ferm, M.; Hultberg, H. Total deposition of inorganic nitrogen to Norway spruce forests—Applying a surrogate surface method across a deposition gradient in Sweden. Atmos. Environ. 2019, 217. [Google Scholar] [CrossRef]
- Posch, M.; de Smet, P.; Hettelingh, J.-P.; Downing, R. Calculation and Mapping of Critical Thresholds in Europe. Status Report 1995; Coordination Center of Effects, Rijksinstituut voor Volksgezondheid en Milieu RIVM: Bilthoven, The Netherlands, 1995. [Google Scholar]
- Galloway, J.N. Acid deposition: Perspectives in time and space. WaterAirSoil Pollut. 1995, 85, 15–24. [Google Scholar] [CrossRef]
- Sverdrup, H.; Warfvinge, P. Calculating field weathering rates using a mechanistic geochemical model PROFILE. Appl. Geochem. 1993, 8, 273–283. [Google Scholar] [CrossRef]
- Posch, M.; Kurz, D. A2M—A program to compute all possible mineral modes from geochemical analyses. Comput. Geosci. 2007, 33, 563–572. [Google Scholar] [CrossRef]
- Persson, C.; Roos, E.; Lövblad, G. Meso-Scale Modelling of Base Cation Deposition in Sweden; Springer: Boston, MA, USA, 2000; pp. 117–125. [Google Scholar] [CrossRef]
- Lövblad, G.; Persson, C.; Klein, T.; Ruoho-Airola, T.; Hovmand, M.; Tarrason, L.; Tørseth, K.; Moldan, F.; Larssen, T.; Rapp, L. The Deposition of Base Cations in the Nordic Countries; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2004. [Google Scholar]
- Futter, M.N.; Ring, E.; Högbom, L.; Entenmann, S.; Bishop, K.H. Consequences of nitrate leaching following stem-only harvesting of Swedish forests are dependent on spatial scale. Environ. Pollut. 2010, 158, 3552–3559. [Google Scholar] [CrossRef]
- Akselsson, C.; Olsson, J.; Belyazid, S.; Capell, R. Can increased weathering rates due to future warming compensate for base cation losses following whole-tree harvesting in spruce forests? Biogeochemistry 2016, 128, 89–105. [Google Scholar] [CrossRef]
- Swedish Forest Agency. Skogliga Konsekvensanalyser 2008: SKA-VB 08; Swedish Forest Agency: Jönköping, Sweden, 2008. [Google Scholar]
- Sverdrup, H.; Warfvinge, P.; Rabenhorst, M.; Janicki, A.; Morgan, R.; Bowman, M. Critical loads and steady-state chemistry for streams in the state of Maryland. Environ. Pollut. 1992, 77, 195–203. [Google Scholar] [CrossRef]
- Braun, S.; Tresch, S.; Augustin, S. Soil solution in Swiss forest stands: A 20 year’s time series. PLoS ONE 2020, 15, e0227530. [Google Scholar] [CrossRef]
- Pihl Karlsson, G.; Hellsten, S.; Akselsson, C.; Karlsson, P.E. Försurning och Övergödning i Skåne län Resultat från Krondroppsnätet till och med 2018/19; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2020. [Google Scholar]
- Ågren, A.; Löfgren, S. pH sensitivity of Swedish forest streams related to catchment characteristics and geographical location—Implications for forest bioenergy harvest and ash return. For. Ecol. Manag. 2012, 276, 10–23. [Google Scholar] [CrossRef]
- Jonard, M.; Fürst, A.; Verstraeten, A.; Thimonier, A.; Timmermann, V.; Potočić, N.; Waldner, P.; Benham, S.; Hansen, K.; Merilä, P.; et al. Tree mineral nutrition is deteriorating in Europe. Glob. Chang. Biol. 2015, 21, 418–430. [Google Scholar] [CrossRef]
- Jonard, M.; Legout, A.; Nicolas, M.; Dambrine, E.; Nys, C.; Ulrich, E.; van der Perre, R.; Ponette, Q. Deterioration of Norway spruce vitality despite a sharp decline in acid deposition: A long-term integrated perspective. Glob. Chang. Biol. 2012, 18, 711–725. [Google Scholar] [CrossRef]
- Schulze, E.D. Air Pollution and Forest Decline in a Spruce (Picea abies) Forest. Science 1989, 244, 776–783. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.; Akselsson, C.; Egnell, G.; Löfgren, S.; Olsson, B.A. Realizing the energy potential of forest biomass in Sweden—How much is environmentally sustainable? For. Ecol. Manag. 2017, 383, 3–16. [Google Scholar] [CrossRef]
- Stendahl, J.; Akselsson, C.; Melkerud, P.-A.; Belyazid, S. Pedon-scale silicate weathering: Comparison of the PROFILE model and the depletion method at 16 forest sites in Sweden. Geoderma 2013, 211–212, 65–74. [Google Scholar] [CrossRef]
- Brandtberg, P.-O.; Olsson, B.A. Changes in the effects of whole-tree harvesting on soil chemistry during 10years of stand development. For. Ecol. Manag. 2012, 277, 150–162. [Google Scholar] [CrossRef]
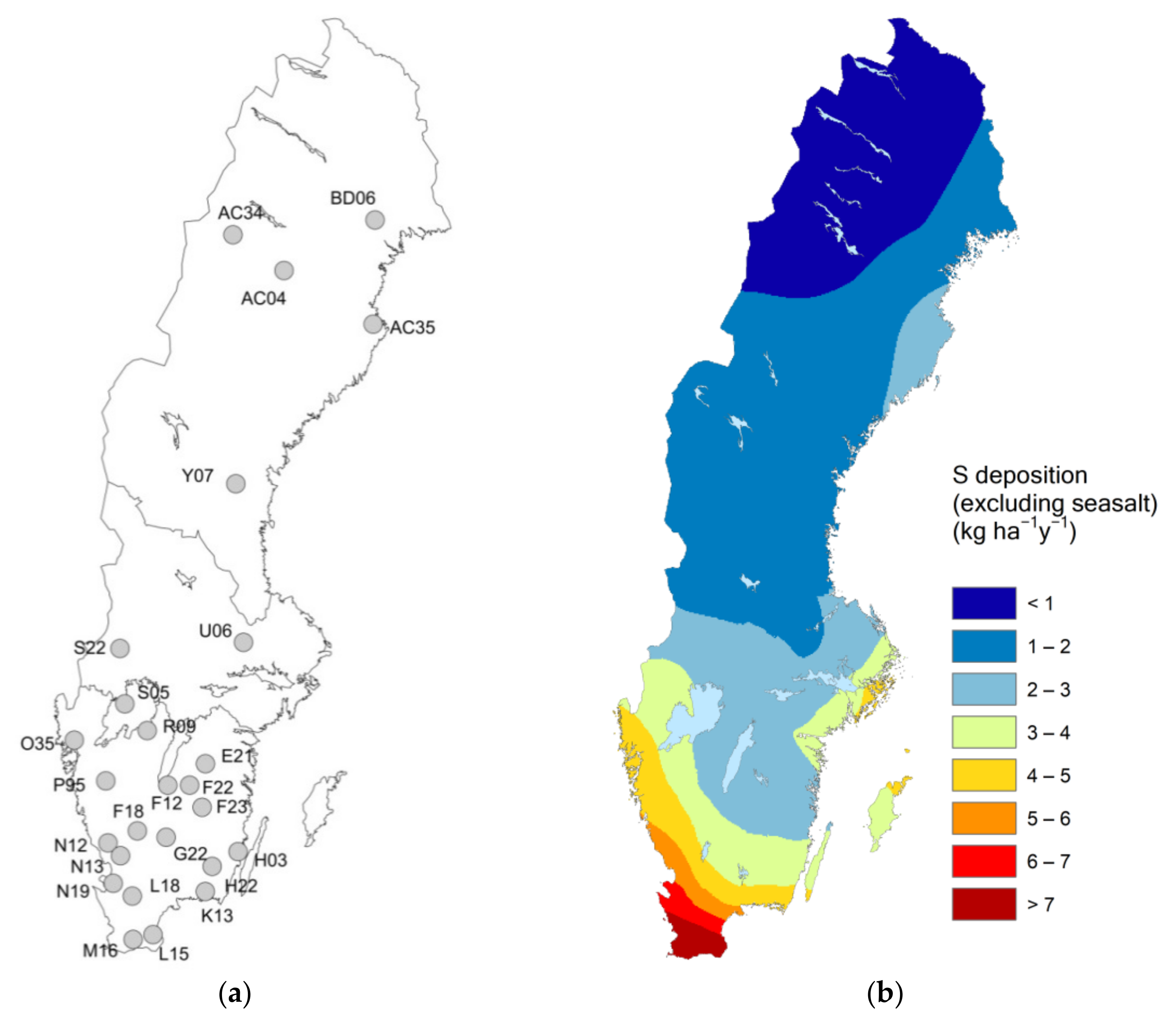
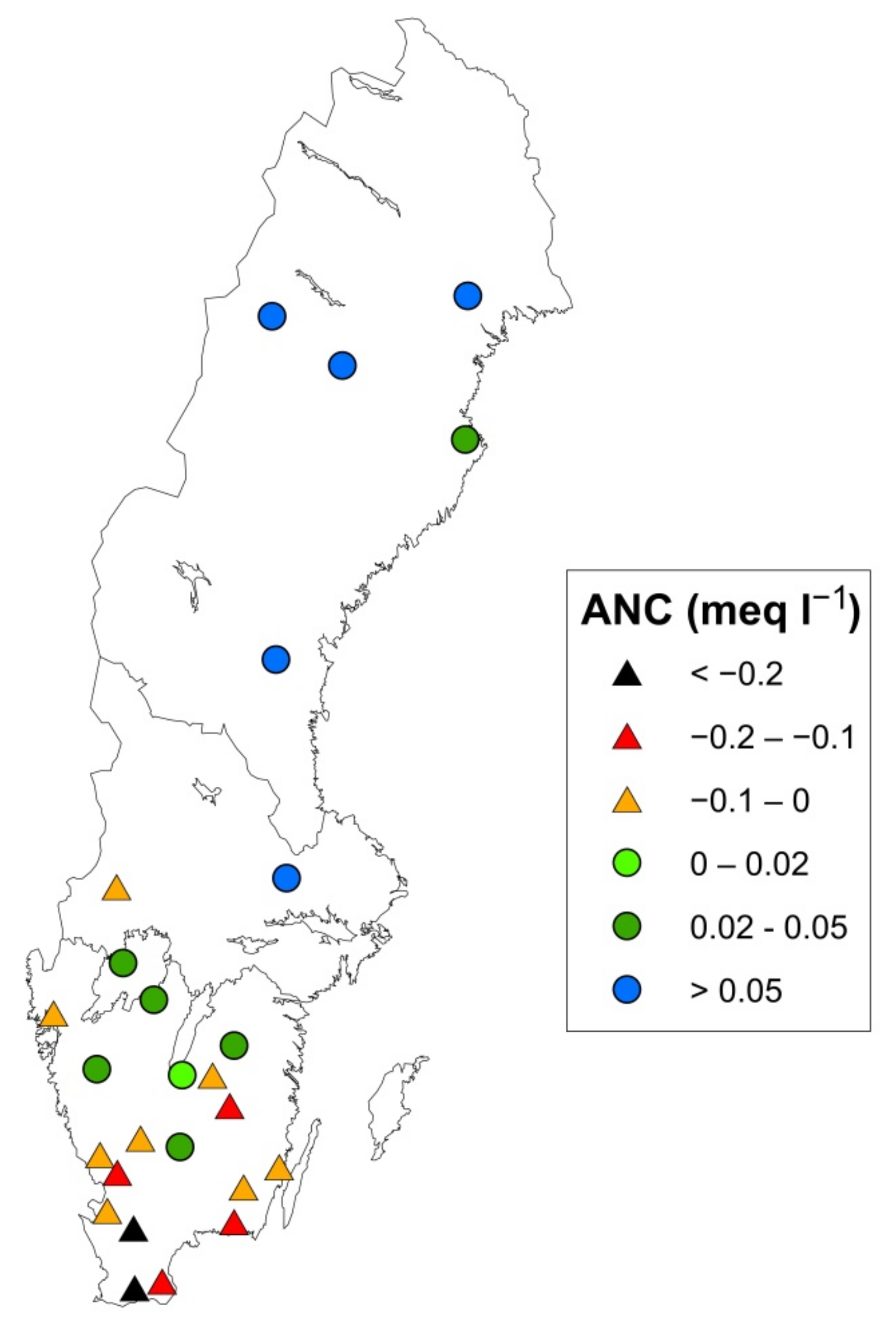
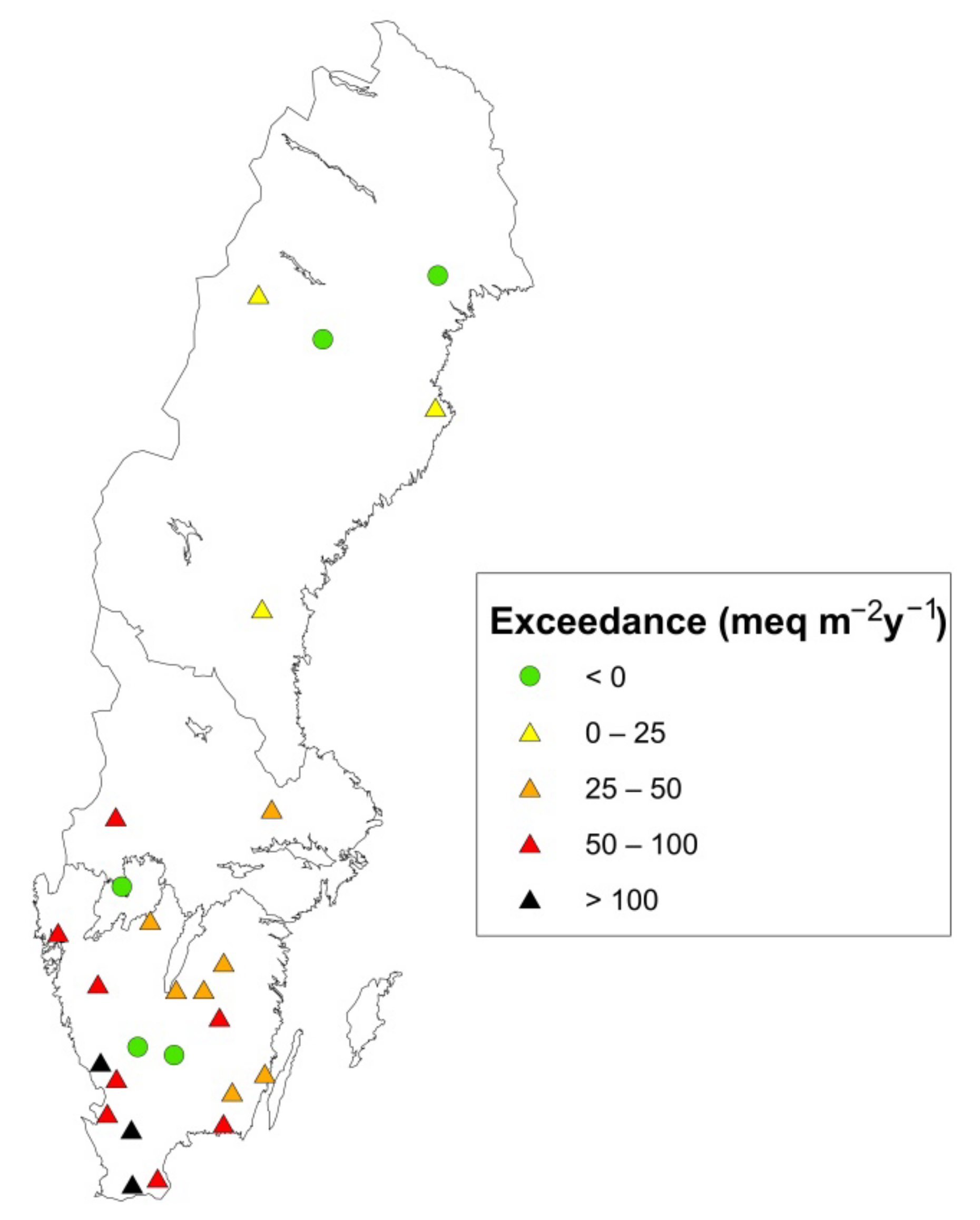
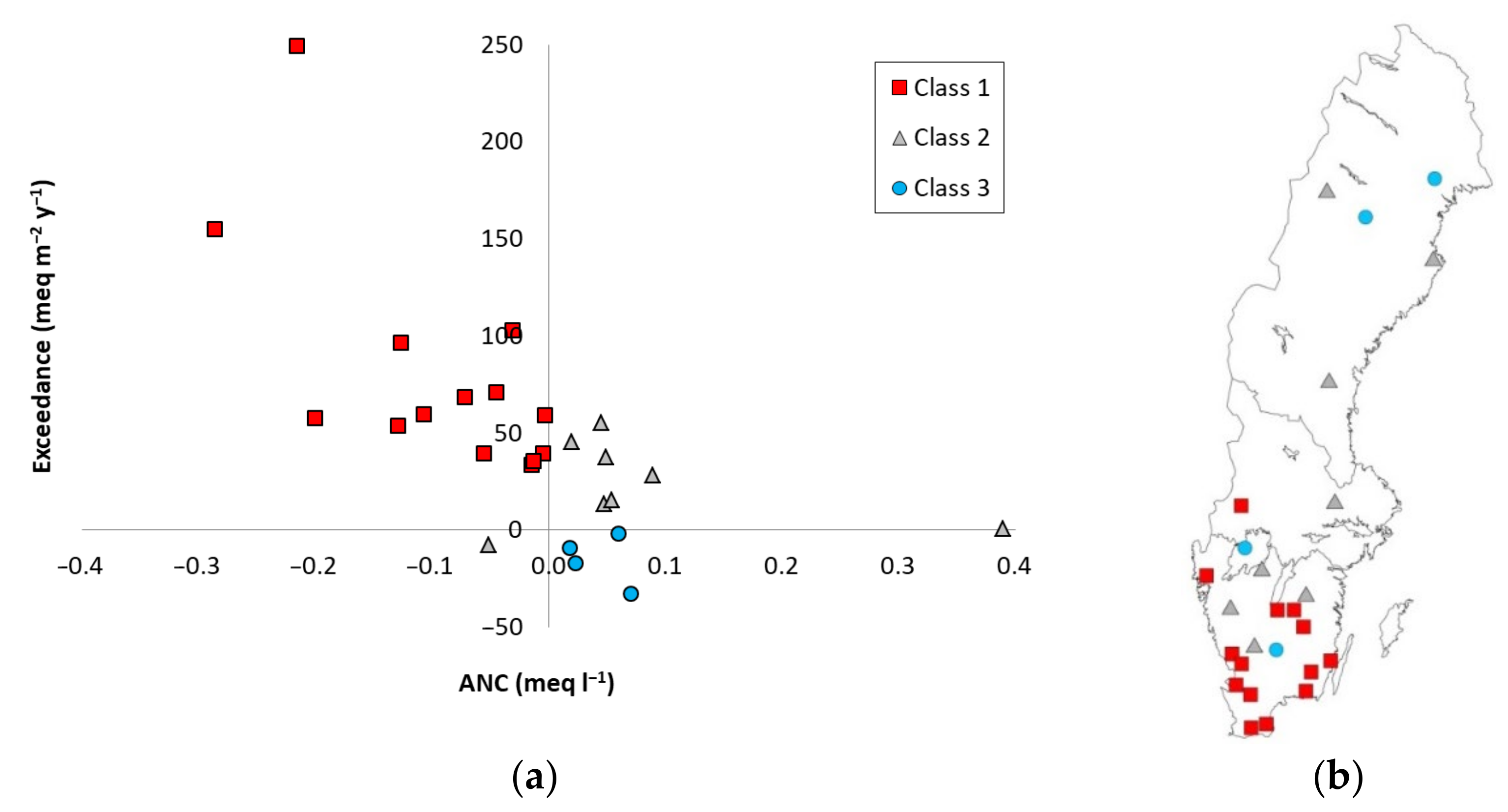
| Site | Lat | Long | Site Index 1 | Moisture Class 6 | Stoni-ness, % | Data Period 7 | BC Dep OF 8 | No of Lysimeters | Region |
|---|---|---|---|---|---|---|---|---|---|
| BD06 | 66.066 | 21.468 | G18 2 | 4 | 20 | 2016–2018 | Yes | 5 | North |
| AC34 | 65.945 | 16.314 | G17 4 | 3 | 20 | 2014–2018 | No | 4–5 | North |
| AC04 | 65.408 | 18.112 | G16 3 | 4 | 20 | 2014–2018 | Yes | 5 | North |
| AC35 | 64.542 | 21.086 | G20 2 | 3 | 20 | 2014–2018 | Yes | 5 | North |
| Y07 | 62.284 | 16.348 | G20 3 | 3 | 20 | 2014–2018 | Yes | 5–6 | North |
| U06 | 59.941 | 16.524 | G22 3 | 3 | 60 | 2014–2018 | No | 5–6 | Central |
| S22 | 59.821 | 12.902 | G32 3 | 3 | 5 | 2014–2018 | Yes | 5 | Central |
| S05 | 59.008 | 13.110 | G22 5 | 4 | 5 | 2014–2016 | Yes | 5 | Central |
| R09 | 58.625 | 13.777 | G30 3 | 3 | 15 | 2014–2018 | No | 5 | South |
| O35 | 58.441 | 11.731 | G26 3 | 4 | 5 | 2014–2017 | Yes | 3–5 | South |
| E21 | 58.156 | 15.435 | G32 3 | 3 | 5 | 2014–2018 | No | 3–5 | South |
| P95 | 57.866 | 12.673 | G26 5 | 3 | 15 | 2014–2018 | Yes | 5 | South |
| F22 | 57.839 | 15.000 | G28 3 | 3 | 35 | 2014–2018 | No | 5 | South |
| F12 | 57.825 | 14.394 | G28 3 | 3 | 5 | 2014–2018 | No | 5 | South |
| F23 | 57.509 | 15.341 | G32 3 | 2 | 10 | 2014–2018 | Yes | 4 | South |
| F18 | 57.149 | 13.594 | G26 3 | 3 | 10 | 2014–2018 | No | 5 | South |
| G22 | 57.061 | 14.374 | G28 3 | 4 | 5 | 2014–2018 | Yes | 5 | South |
| N12 | 56.953 | 12.816 | G30 3 | 2 | 50 | 2014–2018 | No | 4–5 | South |
| H03 | 56.853 | 16.316 | G28 3 | 3 | 30 | 2014–2018 | Yes | 5 | South |
| N13 | 56.772 | 13.158 | G30 5 | 3 | 20 | 2014–2018 | Yes | 5 | South |
| H22 | 56.638 | 15.629 | G32 3 | 3 | 20 | 2014–2018 | No | 4 | South |
| N19 | 56.355 | 12.993 | G32 5 | 3 | 10 | 2014–2018 | No | 5 | South |
| K13 | 56.270 | 15.453 | G34 3 | 3 | 10 | 2014–2016 | No | 3–4 | South |
| L18 | 56.177 | 13.521 | G30 5 | 3 | 10 | 2014–2018 | Yes | 5 | South |
| L15 | 55.620 | 14.094 | G34 3 | 3 | 20 | 2014–2018 | No | 5 | South |
| M16 | 66.066 | 21.468 | G36 5 | 3 | 5 | 2014–2018 | Yes | 5 | South |
| Parameter | Use | Source |
|---|---|---|
| Deposition | ||
| Deposition S, Cl | Weath, CBH | Field measurements |
| Deposition of N | Weath | Based on field measurements |
| Deposition of Ca, Mg, K | Weath, CBH | Based on field measurements and modelling |
| Deposition of Na | Weath, CBH | Based on Cl deposition |
| Leaching | ||
| NH4-Nleach | CBH | Based on field measurements and runoff |
| NO3-Nleach | CBH | Based on field measurements, runoff, and an empirical function |
| Climate | ||
| Temperature | Weath | Estimated by SMHI 1, based on measurements |
| Precipitation | Weath | Estimated by SMHI 1, based on measurements |
| Runoff | Weath, CBH | Estimated by SMHI 1, based on measurements |
| Soil properties | ||
| Mineral composition | Weath | Modelled with A 2M 2, based on field measurements |
| Specific surface area | Weath | Estimated based on field measurements |
| Stoniness | Weath | Field observations |
| Moisture | Weath | Field observations |
| Soil bulk density | Weath | Estimated based on field measurements |
| Forest data | ||
| Net harvest loss of BC 3 | Weath, Exc | Estimated based on site index from sites (Table 1) |
| Net harvest loss of N 3 | Weath | Estimated based on site index from sites (Table 1) |
| Site | S | Cl | NO3-N | NH4-N | Ca | Mg | Na | K | ANC | n |
|---|---|---|---|---|---|---|---|---|---|---|
| BD06 | 0.03 | 0.02 | 0.00 | 0.00 | 0.04 | 0.03 | 0.05 | 0.01 | 0.07 | 6 |
| AC34 | 0.09 | 0.05 | 0.00 | 0.00 | 0.42 | 0.03 | 0.07 | 0.01 | 0.39 | 15 |
| AC04 | 0.03 | 0.02 | 0.00 | 0.00 | 0.03 | 0.02 | 0.05 | 0.01 | 0.06 | 15 |
| AC35 | 0.12 | 0.02 | 0.00 | 0.00 | 0.04 | 0.05 | 0.06 | 0.02 | 0.05 | 10 |
| Y07 | 0.02 | 0.02 | 0.00 | 0.00 | 0.03 | 0.02 | 0.06 | 0.00 | 0.05 | 13 |
| U06 | 0.05 | 0.07 | 0.00 | 0.00 | 0.05 | 0.04 | 0.10 | 0.01 | 0.09 | 2 |
| S22 | 0.08 | 0.12 | 0.00 | 0.00 | 0.07 | 0.02 | 0.12 | 0.00 | −0.003 | 14 |
| S05 | 0.07 | 0.15 | 0.00 | 0.00 | 0.03 | 0.03 | 0.19 | 0.00 | 0.02 | 9 |
| R09 | 0.28 | 0.31 | 0.00 | 0.00 | 0.04 | 0.11 | 0.43 | 0.01 | 0.02 | 14 |
| O35 | 0.07 | 0.45 | 0.00 | 0.00 | 0.01 | 0.07 | 0.37 | 0.01 | −0.07 | 11 |
| E21 | 0.15 | 0.02 | 0.00 | 0.00 | 0.10 | 0.04 | 0.04 | 0.04 | 0.05 | 9 |
| P95 | 0.11 | 0.34 | 0.00 | 0.00 | 0.03 | 0.08 | 0.40 | 0.01 | 0.04 | 35 |
| F22 | 0.10 | 0.26 | 0.00 | 0.00 | 0.04 | 0.03 | 0.22 | 0.01 | −0.06 | 8 |
| F12 | 0.13 | 0.23 | 0.00 | 0.00 | 0.10 | 0.03 | 0.21 | 0.00 | −0.005 | 9 |
| F23 | 0.28 | 0.48 | 0.00 | 0.00 | 0.07 | 0.08 | 0.39 | 0.01 | −0.20 | 14 |
| F18 | 0.09 | 0.26 | 0.00 | 0.00 | 0.02 | 0.02 | 0.22 | 0.00 | −0.05 | 12 |
| G22 | 0.17 | 0.40 | 0.00 | 0.00 | 0.07 | 0.10 | 0.41 | 0.02 | 0.02 | 12 |
| N12 | 0.10 | 0.37 | 0.00 | 0.00 | 0.02 | 0.07 | 0.46 | 0.02 | −0.03 | 14 |
| H03 | 0.59 | 0.88 | 0.00 | 0.00 | 0.23 | 0.24 | 0.85 | 0.01 | −0.01 | 8 |
| N13 | 0.14 | 0.52 | 0.01 | 0.00 | 0.01 | 0.04 | 0.53 | 0.00 | −0.13 | 12 |
| H22 | 0.12 | 0.13 | 0.00 | 0.00 | 0.04 | 0.04 | 0.14 | 0.00 | −0.01 | 11 |
| N19 | 0.09 | 0.59 | 0.00 | 0.00 | 0.01 | 0.04 | 0.40 | 0.01 | −0.04 | 12 |
| K13 | 0.26 | 0.46 | 0.00 | 0.00 | 0.03 | 0.06 | 0.51 | 0.01 | −0.11 | 18 |
| L18 | 0.19 | 0.78 | 0.17 | 0.00 | 0.02 | 0.08 | 0.76 | 0.01 | −0.29 | 16 |
| L15 | 0.12 | 0.41 | 0.00 | 0.00 | 0.01 | 0.02 | 0.39 | 0.00 | −0.13 | 11 |
| M16 | 0.15 | 0.74 | 0.45 | 0.00 | 0.13 | 0.18 | 0.35 | 0.02 | −0.22 | 11 |
| Class | Outcome for the Two Risk Factors | Risk Related to Acidification from Whole-Tree Harvesting |
|---|---|---|
| 1 | ANC < 0 and ExcCBH > 0 | High |
| 2 | ANC < 0 and ExcCBH < 0 or ANC > 0 and ExcCBH > 0 | Medium |
| 3 | ANC > 0 and ExcCBH < 0 | Low |
| Site | Weath-ering | Ca + Mg + K dep. | S dep. | NO3-N Leaching | NH4-N Leaching | BC Removal at Harvesting | CBH | Exc. |
|---|---|---|---|---|---|---|---|---|
| BD06 | 52.6 | 5.3 | 4.1 | 1.5 | 0.31 | 19.1 | 51.9 | −32.7 |
| AC34 | 16.5 | 6.2 | 3.1 | 1.4 | 0.59 | 17.7 | 17.1 | 0.6 |
| AC04 | 16.9 | 6.0 | 3.3 | 1.2 | 0.44 | 16.2 | 18.0 | -1.8 |
| AC35 | 15.2 | 8.2 | 11.9 | 1.9 | 0.37 | 22.0 | 8.7 | 13.4 |
| Y07 | 7.7 | 6.7 | 4.8 | 1.9 | 0.47 | 22.0 | 6.5 | 15.5 |
| U06 | 3.9 | 14.0 | 5.5 | 4.2 | 0.23 | 34.8 | 6.6 | 28.2 |
| S22 | 15.9 | 10.4 | 12.4 | 8.0 | 0.49 | 60.2 | 0.9 | 59.3 |
| S05 | 54.1 | 18.4 | 12.5 | 3.6 | 0.33 | 30.7 | 47.5 | −16.8 |
| R09 | 18.4 | 21.1 | 10.2 | 7.7 | 0.24 | 58.5 | 13.0 | 45.6 |
| O35 | 12.9 | 33.3 | 32.7 | 8.4 | 0.66 | 45.8 | −22.7 | 68.5 |
| E21 | 25.7 | 21.8 | 6.9 | 8.9 | 0.20 | 65.5 | 28.1 | 37.4 |
| P95 | 19.3 | 30.7 | 28.9 | 5.9 | 0.62 | 45.8 | −9.5 | 55.2 |
| F22 | 10.8 | 22.3 | 8.8 | 6.8 | 0.25 | 52.1 | 12.8 | 39.4 |
| F12 | 10.8 | 25.0 | 9.2 | 6.8 | 0.27 | 52.1 | 12.4 | 39.7 |
| F23 | 13.9 | 19.6 | 11.2 | 8.7 | 0.24 | 65.5 | 7.7 | 57.7 |
| F18 | 58.1 | 33.7 | 16.4 | 5.9 | 0.51 | 45.8 | 53.6 | −7.8 |
| G22 | 63.0 | 24.9 | 11.9 | 6.8 | 0.37 | 52.1 | 61.5 | −9.3 |
| N12 | 5.3 | 44.2 | 43.4 | 7.8 | 0.79 | 58.5 | −44.2 | 102.7 |
| H03 | 17.0 | 25.9 | 11.5 | 6.8 | 0.19 | 52.1 | 18.4 | 33.7 |
| N13 | 22.2 | 52.6 | 32.9 | 11.5 | 0.81 | 58.5 | 4.6 | 53.9 |
| H22 | 30.2 | 26.1 | 12.5 | 8.7 | 0.25 | 65.5 | 29.7 | 35.7 |
| N19 | 20.2 | 38.8 | 33.3 | 8.8 | 0.61 | 65.5 | −5.6 | 71.0 |
| K13 | 24.0 | 28.1 | 19.4 | 9.9 | 0.26 | 73.0 | 13.3 | 59.7 |
| L18 | 12.2 | 32.9 | 31.4 | 88.6 | 0.52 | 58.5 | −96.6 | 155.1 |
| L15 | 4.1 | 34.4 | 37.0 | 9.9 | 0.40 | 73.0 | −23.4 | 96.4 |
| M16 | 4.9 | 34.1 | 50.9 | 135.6 | 0.30 | 80.5 | −169.0 | 249.5 |
| Region | Number of Sites | ||
|---|---|---|---|
| Class 1 | Class 2 | Class 3 | |
| North | 0 | 3 | 2 |
| Central | 1 | 1 | 1 |
| South | 13 | 4 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akselsson, C.; Kronnäs, V.; Stadlinger, N.; Zanchi, G.; Belyazid, S.; Karlsson, P.E.; Hellsten, S.; Karlsson, G.P. A Combined Measurement and Modelling Approach to Assess the Sustainability of Whole-Tree Harvesting—A Swedish Case Study. Sustainability 2021, 13, 2395. https://doi.org/10.3390/su13042395
Akselsson C, Kronnäs V, Stadlinger N, Zanchi G, Belyazid S, Karlsson PE, Hellsten S, Karlsson GP. A Combined Measurement and Modelling Approach to Assess the Sustainability of Whole-Tree Harvesting—A Swedish Case Study. Sustainability. 2021; 13(4):2395. https://doi.org/10.3390/su13042395
Chicago/Turabian StyleAkselsson, Cecilia, Veronika Kronnäs, Nadja Stadlinger, Giuliana Zanchi, Salim Belyazid, Per Erik Karlsson, Sofie Hellsten, and Gunilla Pihl Karlsson. 2021. "A Combined Measurement and Modelling Approach to Assess the Sustainability of Whole-Tree Harvesting—A Swedish Case Study" Sustainability 13, no. 4: 2395. https://doi.org/10.3390/su13042395
APA StyleAkselsson, C., Kronnäs, V., Stadlinger, N., Zanchi, G., Belyazid, S., Karlsson, P. E., Hellsten, S., & Karlsson, G. P. (2021). A Combined Measurement and Modelling Approach to Assess the Sustainability of Whole-Tree Harvesting—A Swedish Case Study. Sustainability, 13(4), 2395. https://doi.org/10.3390/su13042395






