Testing the Applicability of the Safe-by-Design Concept: A Theoretical Case Study Using Polymer Nanoclay Composites for Coffee Capsules
Abstract
:1. Introduction
2. Method—An Integrative Transdisciplinary Approach
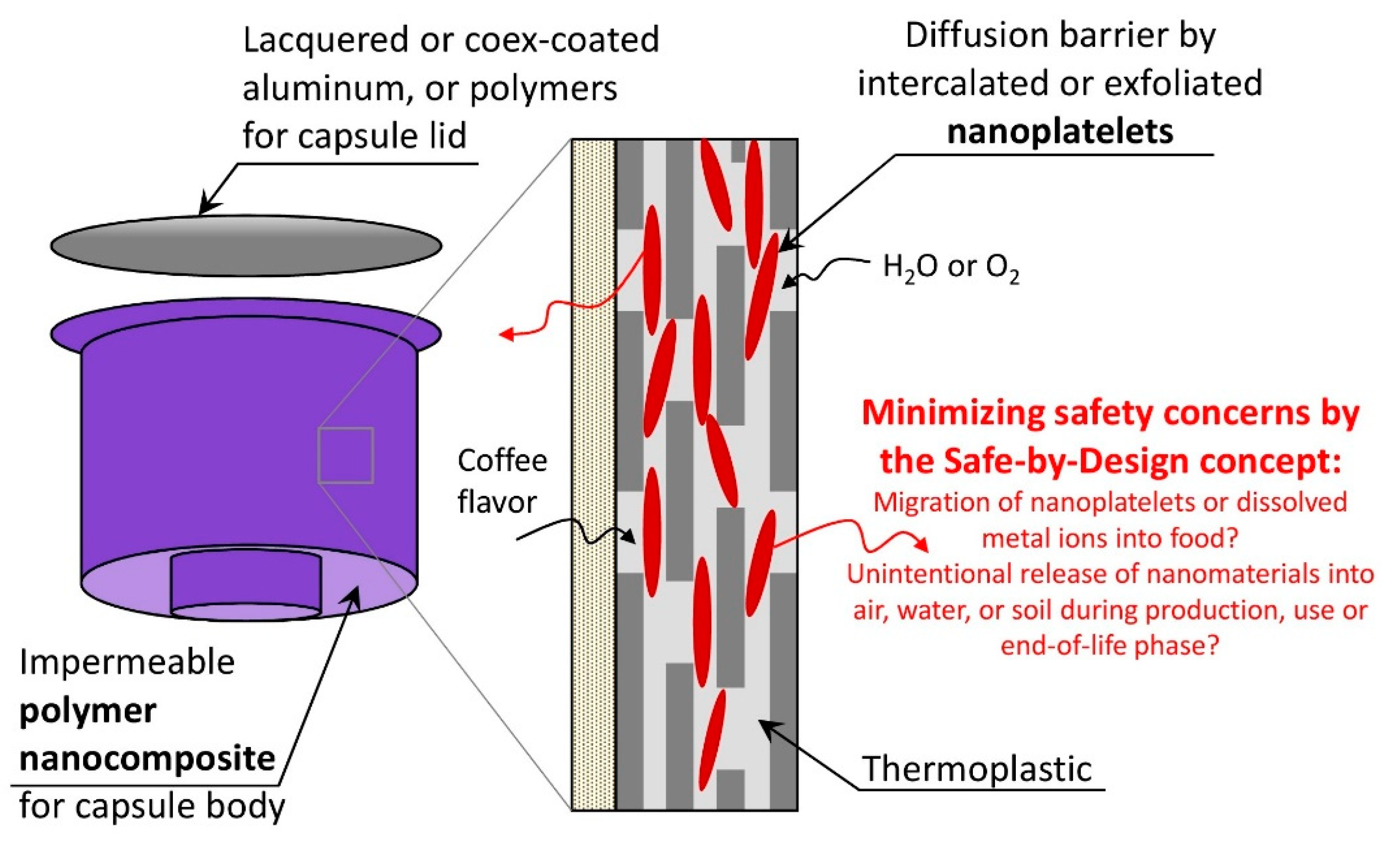
2.1. Description of (Theoretical) Case Study and Material Composition
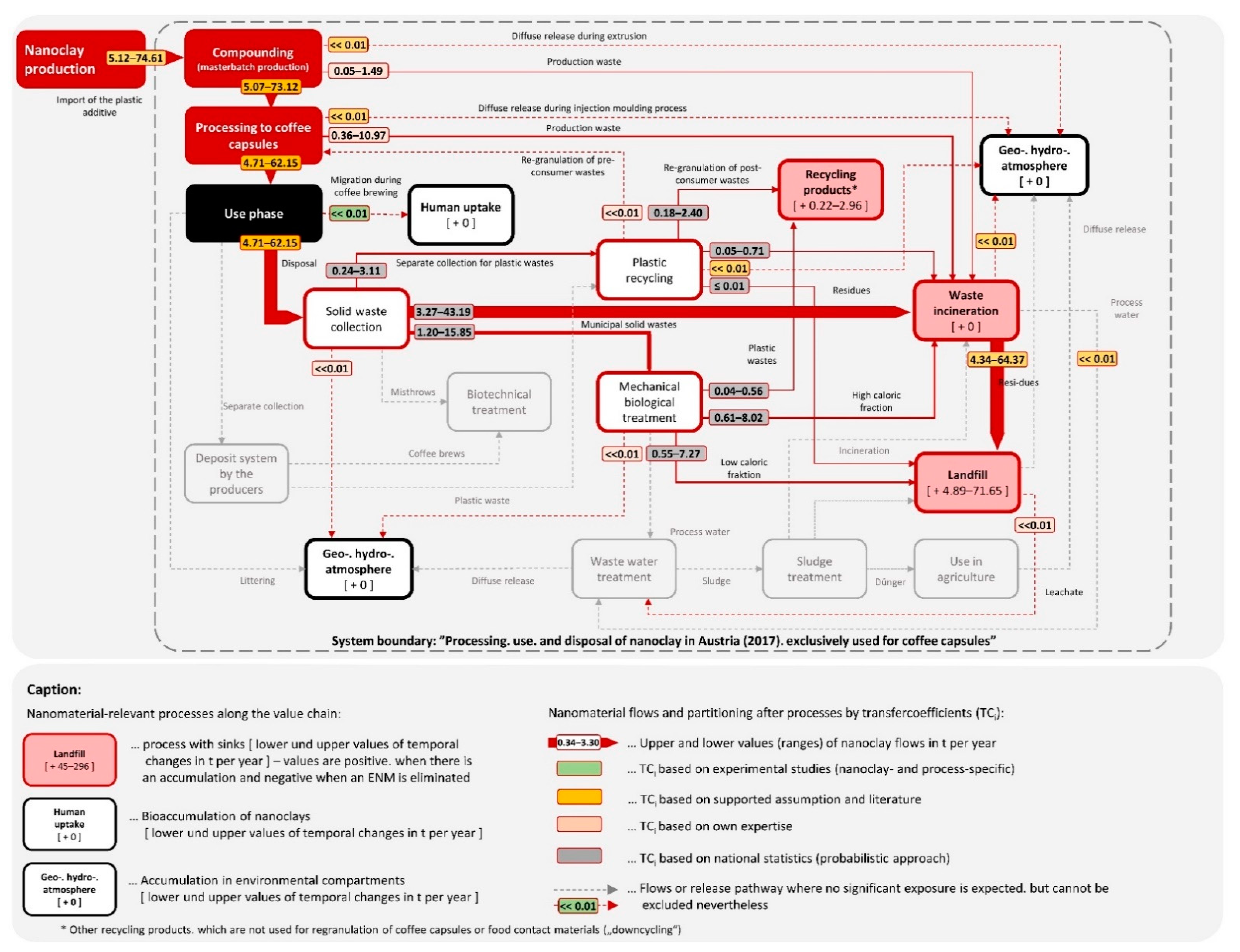
2.2. Material Flow Analysis (MFA)
2.3. Stakeholder Involvement
3. Results
3.1. State of Knowledge about the Risks for Human Health and the Environment
3.2. Exposure Modeling for Nanoclay
Qualitative Description of the Environmental Fate
3.3. Identified Strengths and Weaknesses of the Safe-by-Design Concept
3.3.1. Manufacturer’s Perspective
3.3.2. Stakeholder Opinions Gathered during Stakeholder Workshops
Knowledge and Uncertainty
Innovation and Safety
Resources
Social Framework
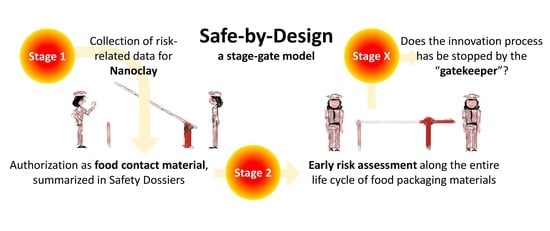
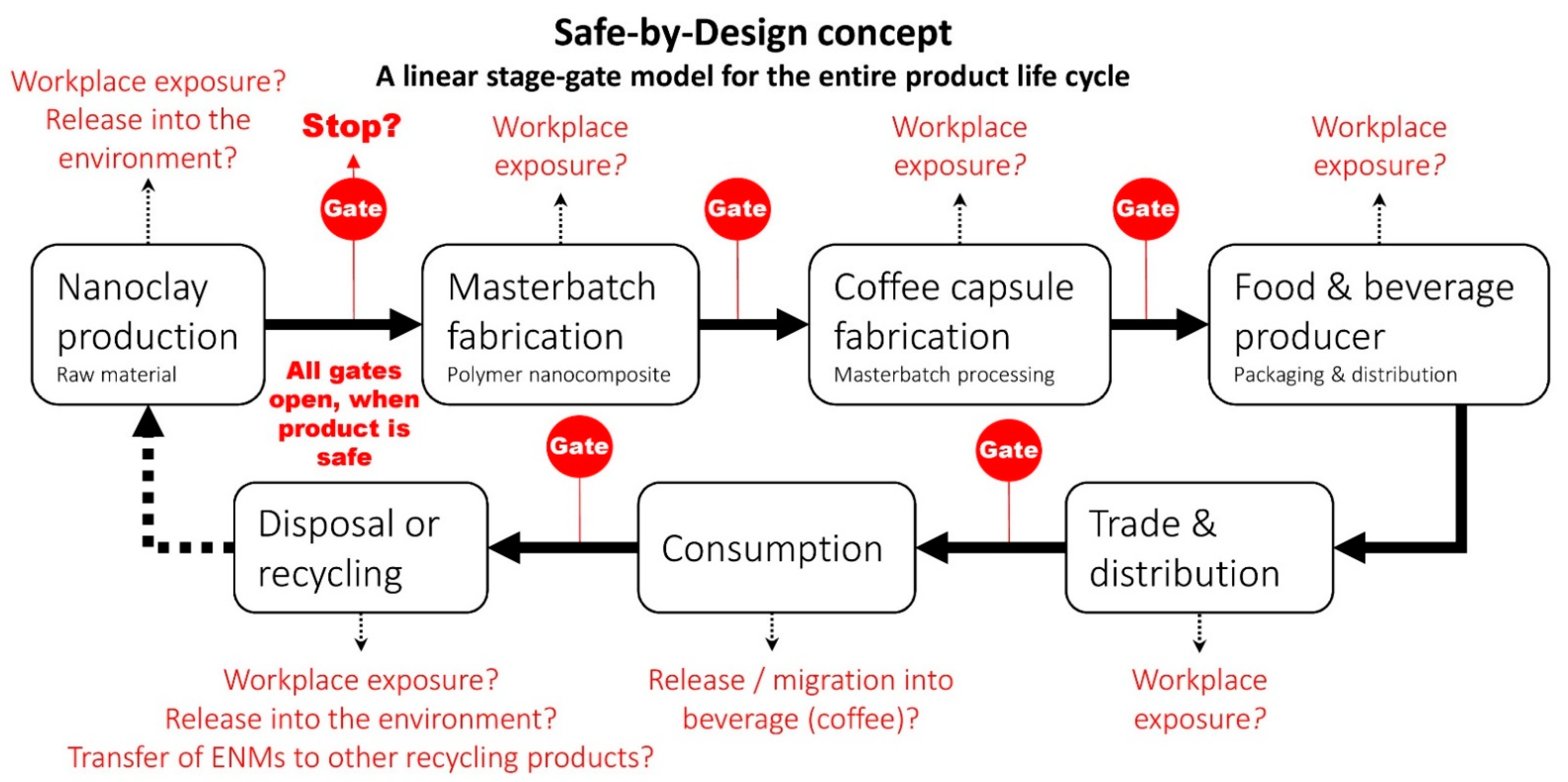
Stage–Gate Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNEP. Frontiers 2017—Emerging Issues of Environmental Concern; United Nations Environment Programme: Nairobi, Kenya, 2017. [Google Scholar]
- Tinkle, S.S. Maximizing Safe Design of Engineered Nanomaterials: The NIH and NIEHS Research Perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, C.; Walker, L.A.; Matzke, M.; Lahive, E.; Harrison, S.; Crossley, A.; Park, B.; Lofts, S.; Lynch, I.; Vázquez-Campos, S.; et al. Key Principles and Operational Practices for Improved Nanotechnology Environmental Exposure Assessment. Nat. Nanotechnol. 2020, 15, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Krystek, P.; Shandilya, N.; Fransman, W. Human Health Risk Assessments and Characterization of Nanomaterials: Are We Ready for the Next (Active) Generations? Ann. Work Expo. Health 2021, 65, 748–759. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental Transformation and Nano-Toxicity of Engineered Nano-Particles (ENPs) in Aquatic and Terrestrial Organisms. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2523–2581. [Google Scholar] [CrossRef]
- Malakar, A.; Kanel, S.R.; Ray, C.; Snow, D.D.; Nadagouda, M.N. Nanomaterials in the Environment, Human Exposure Pathway, and Health Effects: A Review. Sci. Total Environ. 2021, 759, 143470. [Google Scholar] [CrossRef] [PubMed]
- Isigonis, P.; Afantitis, A.; Antunes, D.; Bartonova, A.; Beitollahi, A.; Bohmer, N.; Bouman, E.; Chaudhry, Q.; Cimpan, M.R.; Cimpan, E.; et al. Risk Governance of Emerging Technologies Demonstrated in Terms of Its Applicability to Nanomaterials. Small 2020, 16, e2003303. [Google Scholar] [CrossRef]
- Tavernaro, I.; Dekkers, S.; Soeteman-Hernández, L.G.; Herbeck-Engel, P.; Noorlander, C.; Kraegeloh, A. Safe-by-Design Part II: A Strategy for Balancing Safety and Functionality in the Different Stages of the Innovation Process. NanoImpact 2021, 24, 100354. [Google Scholar] [CrossRef]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Riego Sintes, J.; Rauscher, H. Towards Safe and Sustainable Innovation in Nanotechnology: State-of-Play for Smart Nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2018/1881 of 3 December 2018 Amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Annexes I, III, VI, V. Off. J. Eur. Union 2018, L308, 1–21. [Google Scholar]
- Pavlicek, A.; Part, F.; Rose, G.E.; Praetorius, A.; Miernicki, M.; Gazsó, A.; Huber-Humer, M. A European Nano-Registry as a Reliable Database for Quantitative Risk Assessment of Nanomaterials? A Comparison of National Approaches. NanoImpact 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Cooper, R.G. Stage-Gate Systems: A New Tool for Managing New Products. Bus. Horiz. 1990, 33, 44–54. [Google Scholar] [CrossRef]
- Cooper, R.G. Perspective: The Stage-Gates® Idea-to-Launch Process - Update, What’s New, and NexGen Systems. J. Prod. Innov. Manag. 2008, 25, 213–232. [Google Scholar] [CrossRef]
- Shandilya, N.; Marcoulaki, E.; Barruetabeña, L.; Rodríguez Llopis, I.; Noorlander, C.; SánchezJiménez, A.; Oudart, Y.; Puelles, R.C.R.C.; Pérez-Fernández, M.; Falk, A.; et al. Perspective on a Risk-Based Roadmap towards the Implementation of the Safe Innovation Approach for Industry. Nano Impact 2020. [Google Scholar] [CrossRef]
- Hristozov, D.; Gottardo, S.; Semenzin, E.; Oomen, A.; Bos, P.; Peijnenburg, W.; van Tongeren, M.; Nowack, B.; Hunt, N.; Brunelli, A.; et al. Frameworks and Tools for Risk Assessment of Manufactured Nanomaterials. Environ. Int. 2016, 95, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Salieri, B.; Barruetabeña, L.; Rodríguez-Llopis, I.; Jacobsen, N.R.; Manier, N.; Trouiller, B.; Chapon, V.; Hadrup, N.; Jiménez, A.S.; Micheletti, C.; et al. Integrative Approach in a Safe by Design Context Combining Risk, Life Cycle and Socio-Economic Assessment for Safer and Sustainable Nanomaterials. NanoImpact 2021, 23, 100335. [Google Scholar] [CrossRef]
- Gottardo, S.; Alessandrelli, M.; Amenta, V.; Atluri, R.; Barberio, G.; Bekker, C.; Bergonzo, P.; Bleeker, E.; Booth, A.; Borges, T.; et al. NANoREG Framework for the Safety Assessment of Nanomaterials; European Commission Joint Research Centre: Ispra, Italy, 2017. [Google Scholar]
- European Parliament and Council. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliam. Off. J. Eur. Union 2015, L327, 1–22. [Google Scholar]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2011, L12, 1–89. [Google Scholar]
- European Commission. Commission Regulation (EC) No 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2013, L135, 3–11. [Google Scholar]
- European Parliament and Council. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Off. J. Eur. Union 2008, 51, 16–33. [Google Scholar]
- European Parliament and Council. Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 Establishing a Common Authorisation Procedure for Food Additives, Food Enzymes and Food Flavourings. Off. J. Eur. Union 2008, L354, 1–6. [Google Scholar]
- European Parliament and Council. Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. Off. J. Eur. Union 2013, L181, 35–56. [Google Scholar]
- European Parliament and Council. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Off. J. Eur. Union 2011, L304, 18–63. [Google Scholar]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical Review of the Migration Potential of Nanoparticles in Food Contact Plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Gazsó, A.; Rose, G.E.; Pavlicek, A.; Gressler, S. Regulating Nanotechnological Applications for Food Contact Materials. Eur. J. Risk Regul. 2019, 10, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Feagin, J.R.; Orum, A.M.; Sjoberg, G. A Case for the Case Study; University of North Carolina Press: Chapel Hill, CA, USA, 1991. [Google Scholar]
- Yin, R.K. Case Study Research: Design and Methods, 3rd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2003; ISBN 9780761925521. [Google Scholar]
- European Chemicals Agency (ECHA). Substance Information-Montmorillonite. Available online: https://echa.europa.eu/de/substance-information/-/substanceinfo/100.013.899 (accessed on 24 November 2021).
- European Commission (EC). Reference Substance Database for Food Contact Materials. Available online: https://ec.europa.eu/jrc/en/eurl/food-contact-materials/reference-substance-database (accessed on 24 November 2021).
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for Food Packaging Applications: An Overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Ko, S. Nanoclays in Food and Beverage Packaging. J. Nanomater. 2019. [Google Scholar] [CrossRef] [Green Version]
- Duncan, T.V.; Pillai, K. Release of Engineered Nanomaterials from Polymer Nanocomposites: Diffusion, Dissolution, and Desorption. ACS Appl. Mater. Interfaces 2015, 7, 2–19. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Rodríguez, S.; Nerín, C. Nanoclay Migration from Food Packaging Materials. Food Addit. Contam. Part A 2016, 33, 530–539. [Google Scholar] [CrossRef]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on Risk Assessment of the Application of Nanoscience and Nanotechnologies in the Food and Feed Chain: Part 1, Human and Animal Health. EFSA J. 2018, 16, e05327. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Agency (EFSA). EFSA CEF Panel (EFSA Panel on Food Contact Materials Enzymes Flavourings and Processing Aids) Safety Assessment of the Substance Montmorillonite Clay Modified by Dimethyldialkyl(C16–C18)Ammonium Chloride for Use in Food Contact Materials. EFSA J. 2015, 13, 4285. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Inventory of Effective Food Contact Substance (FCS) Notifications. Available online: https://www.fda.gov/food/packaging-food-contact-substances-fcs/inventory-effective-food-contact-substance-fcs-notifications (accessed on 26 October 2021).
- Suzuki, S.; Part, F.; Matsufuji, Y.; Huber-Humer, M. Modeling the Fate and End-of-Life Phase of Engineered Nanomaterials in the Japanese Construction Sector. Waste Manag. 2018, 72, 389–398. [Google Scholar] [CrossRef]
- Part, F.; Berge, N.; Baran, P.; Stringfellow, A.; Sun, W.; Bartelt-Hunt, S.; Mitrano, D.; Li, L.; Hennebert, P.; Quicker, P.; et al. A Review of the Fate of Engineered Nanomaterials in Municipal Solid Waste Streams. Waste Manag. 2018, 75, 427–449. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administartion (FDA). 21 Code of Federal Regulations (CFR), Chapter I Subchapter B Vol. 3. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=170.3 (accessed on 26 October 2021).
- Gottschalk, F.; Scholz, R.W.; Nowack, B. Probabilistic Material Flow Modeling for Assessing the Environmental Exposure to Compounds: Methodology and an Application to Engineered Nano-TiO2 Particles. Environ. Model. Softw. 2010, 25, 320–332. [Google Scholar] [CrossRef]
- Noorlander, C.; Sips, A.; Höck, J.; Höhener, K.; Lehmann, H.C. NANoREG Safe Design (SbD) Concept. 2016. Available online: https://www.rivm.nl/sites/default/files/2018-11/NANoREG%20WP6%20Task%206.2%20Safe%20by%20Design%20concept.pdf (accessed on 26 October 2021).
- Bessa, M.J.; Brandão, F.; Viana, M.; Gomes, J.F.; Monfort, E.; Cassee, F.R.; Fraga, S.; Teixeira, J.P. Nanoparticle Exposure and Hazard in the Ceramic Industry: An Overview of Potential Sources, Toxicity and Health Effects. Environ. Res. 2020, 184, 109297. [Google Scholar] [CrossRef] [PubMed]
- Maisanaba, S.; Pichardo, S.; Puerto, M.; Gutiérrez-Praena, D.; Cameán, A.M.; Jos, A. Toxicological Evaluation of Clay Minerals and Derived Nanocomposites: A Review. Environ. Res. 2015, 138, 233–254. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Yu, Z.G.; Zhu, M.Y.; Shen, L.Q. Quaternary Ammonium Compounds (QACs): A Review on Occurrence, Fate and Toxicity in the Environment. Sci. Total Environ. 2015, 518, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Cooperation and Development (OECD) Working Party on Resource Productivity and Waste. Recycling of Waste Containing Nanomaterials; Organisation for Economic Cooperation and Development: Paris, France, 2015. [Google Scholar]
- Federal Ministry for Sustainability and Tourism. Federal Waste Management Plan 2017 Part 1; Federal Ministry of Sustainability and Tourism: Vienna, Austria, 2017.
- Deutsche Forschungsgemeinschaft (DFG). List of MAK and BAT Values 2012 (Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area); Wiley-VCH Verlag GmbH & Co. KGaA: Bonn, Germany, 2012; ISBN 9783527323081. [Google Scholar]
- Miller, M.R.; Poland, C.A. Nanotoxicology: The Need for a Human Touch? Small 2020, 16. [Google Scholar] [CrossRef]
- Miller, M.R.; Raftis, J.B.; Langrish, J.P.; McLean, S.G.; Samutrtai, P.; Connell, S.P.; Wilson, S.; Vesey, A.T.; Fokkens, P.H.B.; Boere, A.J.F.; et al. Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano 2017, 11, 4542–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonruksa, P.; Bello, D.; Zhang, J.; Isaacs, J.A.; Mead, J.L.; Woskie, S.R. Characterization of Potential Exposures to Nanoparticles and Fibers during Manufacturing and Recycling of Carbon Nanotube Reinforced Polypropylene Composites. Ann. Occup. Hyg. 2015, 60, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Boonruksa, P.; Bello, D.; Zhang, J.; Isaacs, J.A.; Mead, J.L.; Woskie, S.R. Exposures to Nanoparticles and Fibers during Injection Molding and Recycling of Carbon Nanotube Reinforced Polycarbonate Composites. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Wohlleben, W.; Meyer, J.; Muller, J.; Müller, P.; Vilsmeier, K.; Stahlmecke, B.; Kuhlbusch, T.A.J. Release from Nanomaterials during Their Use Phase: Combined Mechanical and Chemical Stresses Applied to Simple and Multi-Filler Nanocomposites Mimicking Wear of Nano-Reinforced Tires. Environ. Sci. Nano 2016, 3, 1036–1051. [Google Scholar] [CrossRef]
- Golanski, L.; Guiot, A.; Pras, M.; Malarde, M.; Tardif, F. Release-Ability of Nano Fillers from Different Nanomaterials (toward the Acceptability of Nanoproduct). J. Nanoparticle Res. 2012, 14, 962. [Google Scholar] [CrossRef]
- Schlagenhauf, L.; Nüesch, F.; Wang, J. Release of Carbon Nanotubes from Polymer Nanocomposites. Fibers 2014, 2, 108–127. [Google Scholar] [CrossRef]
- Wohlleben, W.; Kuhlbusch, T.A.J.; Schnekenburger, J.; Lehr, C.M. Safety of Nanomaterials along Their Lifecycle: Release, Exposure, and Human Hazards; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781466567887. [Google Scholar]
- Kingston, C.; Zepp, R.; Andrady, A.; Boverhof, D.; Fehir, R.; Hawkins, D.; Roberts, J.; Sayre, P.; Shelton, B.; Sultan, Y.; et al. Release Characteristics of Selected Carbon Nanotube Polymer Composites. Carbon 2014, 68, 33–57. [Google Scholar] [CrossRef]
- Amin, M.F.M.; Heijman, S.G.J.; Rietveld, L.C. Nanoclay for Micropollutant Removal in Wastewater-Effective Alternative? In Advancement of Materials and Nanotechnology III; Trans Tech Publications Ltd.: Baech, Switzerland, 2014; Volume 1024, pp. 11–14. [Google Scholar]
- Stagnaro, S.M.; Volzone, C.; Huck, L. Nanoclay as Adsorbent: Evaluation for Removing Dyes Used in the Textile Industry. Procedia Mater. Sci. 2015, 8, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Bitinis, N.; Fortunati, E.; Verdejo, R.; Bras, J.; Kenny, J.M.; Torre, L.; López-Manchado, M.A. Poly(Lactic Acid)/Natural Rubber/Cellulose Nanocrystal Bionanocomposites. Part II: Properties Evaluation. Carbohydr. Polym. 2013, 96, 621–627. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Dominici, F.; Santulli, C.; Kenny, J.M.; Torre, L. Investigation of Thermo-Mechanical, Chemical and Degradative Properties of PLA-Limonene Films Reinforced with Cellulose Nanocrystals Extracted from Phormium Tenax Leaves. Eur. Polym. J. 2014, 56, 77–91. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of Poly(Lactic Acid) and Its Nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Gitipour, A.; El Badawy, A.; Arambewela, M.; Miller, B.; Scheckel, K.; Elk, M.; Ryu, H.; Gomez-Alvarez, V.; Santo Domingo, J.; Thiel, S.; et al. The Impact of Silver Nanoparticles on the Composting of Municipal Solid Waste. Environ. Sci. Technol. 2013, 47, 14385–14393. [Google Scholar] [CrossRef] [PubMed]
- Colman, B.P.; Arnaout, C.L.; Anciaux, S.; Gunsch, C.K.; Hochella, M.F., Jr.; Kim, B.; Lowry, G.V.; McGill, B.M.; Reinsch, B.C.; Richardson, C.J.; et al. Low Concentrations of Silver Nanoparticles in Biosolids Cause Adverse Ecosystem Responses under Realistic Field Scenario. PLoS ONE 2013, 8, e57189. [Google Scholar]
- Stamou, I.; Antizar-Ladislao, B. A Life Cycle Assessment of the Use of Compost from Contaminated Biodegradable Municipal Solid Waste with Silver and Titanium Dioxide Nanoparticles. J. Clean. Prod. 2016, 135, 884–891. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Westerhoff, P.; Hristovski, K.; Jin, V.L.; Johnson, M.V.V.; Arnold, J.G. Metal and Nanoparticle Occurrence in Biosolid-Amended Soils. Sci. Total Environ. 2014, 485–486, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Börner, R.; Meiller, M.; Oischinger, J.; Daschner, R. Untersuchung möglicher Umweltauswirkungen bei der Entsorgung nanomaterialhaltiger Abfälle in Abfallbehandlungsanlagen. In Umweltforschungsplan des Bundesministeriums für Umwelt, Naturschutz, Bau und Reaktorsicherheit; Umweltbundesamt: Dessau-Roßlau, Germany, 2016. [Google Scholar]
- Walser, T.; Limbach, L.K.; Brogioli, R.; Erismann, E.; Flamigni, L.; Hattendorf, B.; Juchli, M.; Krumeich, F.; Ludwig, C.; Prikopsky, K.; et al. Persistence of Engineered Nanoparticles in a Municipal Solid-Waste Incineration Plant. Nat. Nanotechnol. 2012, 7, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Baran, P.; Quicker, P. Verbleib Und Verhalten von Nanopartikeln Bei Der Abfallverbrennung. Österreichische Wasser- und Abfallwirtschaft 2017, 69, 51–65. [Google Scholar] [CrossRef]
- Paur, H.R.; Baumann, W.; Hauser, M.; Lang, I.; Teuscher, N.; Seifert, H.; Stapf, D. Thermal Stability and Material Balance of Nanomaterials in Waste Incineration. J. Phys. Conf. Ser. 2017, 838, 012012. [Google Scholar] [CrossRef] [Green Version]
| Main Topic Areas | Subtopics |
|---|---|
| Knowledge and Uncertainty | Analytical methods |
| Uncertainty in the innovation process | |
| Risks to the innovation process | |
| Protection of in-house knowledge and expertise | |
| Standardization | |
| Differentiation between materials, products, and processes | |
| Variety of application areas | |
| Risk management | |
| Innovation and Safety | Relation between safety and innovation |
| Innovation and safety as a social process | |
| Integration of external knowledge | |
| Governance | |
| Resources | Time, personnel, and financial resources |
| Social Framework | Risk acceptance |
| Risk perception and communication | |
| Stage–Gate Model | Gatekeeper |
| All-or-nothing principle | |
| Communication | |
| Redundancies |
| Strengths | Weaknesses |
|---|---|
| Generation of nano-specific information and data sources along the entire value chain | Focus on the manufacturing phase (from raw material extraction to market launch of a nanomaterial or nanoproduct) |
| Involvement of external expertise | Unknown role of external experts (intellectual property rights); costly and time-consuming |
| Harmonization and documentation of possible project risks | Focus only on process risks and not on product risks |
| Encouraging harmonization of process flows or project management | Lack of international standardization of the SbD concept |
| Engaging in uncertainty management where risk management is not possible due to lack of data | Significant cost and time investment with no discernible benefit or added value |
| Approach for a structured handling of knowledge gaps and uncertainties | Lack of assessment options |
| Assembly/collection of safety-related documents | Unspecified “safety dossiers” without safety assessments |
| Implementation of the “precautionary matrix” (PCM) and “life cycle mapping” (LCM) planned | Insufficient description of practical implementation of methods; implementation on a voluntary basis |
| Strict sequence of a process (linearity) | Low practicability; lack of correction opportunities |
| Availability of tools for web-based safety assessment or consolidation of relevant nano-specific data | Lack of availability of information (especially on practical suitability) on tools for companies, especially SMEs |
| Documented evidence: taking relevant measures to the best of knowledge | Exclusively based on the processing of existing information already given by EU regulations and standards |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlicek, A.; Part, F.; Gressler, S.; Rose, G.; Gazsó, A.; Ehmoser, E.-K.; Huber-Humer, M. Testing the Applicability of the Safe-by-Design Concept: A Theoretical Case Study Using Polymer Nanoclay Composites for Coffee Capsules. Sustainability 2021, 13, 13951. https://doi.org/10.3390/su132413951
Pavlicek A, Part F, Gressler S, Rose G, Gazsó A, Ehmoser E-K, Huber-Humer M. Testing the Applicability of the Safe-by-Design Concept: A Theoretical Case Study Using Polymer Nanoclay Composites for Coffee Capsules. Sustainability. 2021; 13(24):13951. https://doi.org/10.3390/su132413951
Chicago/Turabian StylePavlicek, Anna, Florian Part, Sabine Gressler, Gloria Rose, André Gazsó, Eva-Kathrin Ehmoser, and Marion Huber-Humer. 2021. "Testing the Applicability of the Safe-by-Design Concept: A Theoretical Case Study Using Polymer Nanoclay Composites for Coffee Capsules" Sustainability 13, no. 24: 13951. https://doi.org/10.3390/su132413951
APA StylePavlicek, A., Part, F., Gressler, S., Rose, G., Gazsó, A., Ehmoser, E.-K., & Huber-Humer, M. (2021). Testing the Applicability of the Safe-by-Design Concept: A Theoretical Case Study Using Polymer Nanoclay Composites for Coffee Capsules. Sustainability, 13(24), 13951. https://doi.org/10.3390/su132413951