High-Pressure Processing for Sustainable Food Supply
Abstract
1. Introduction
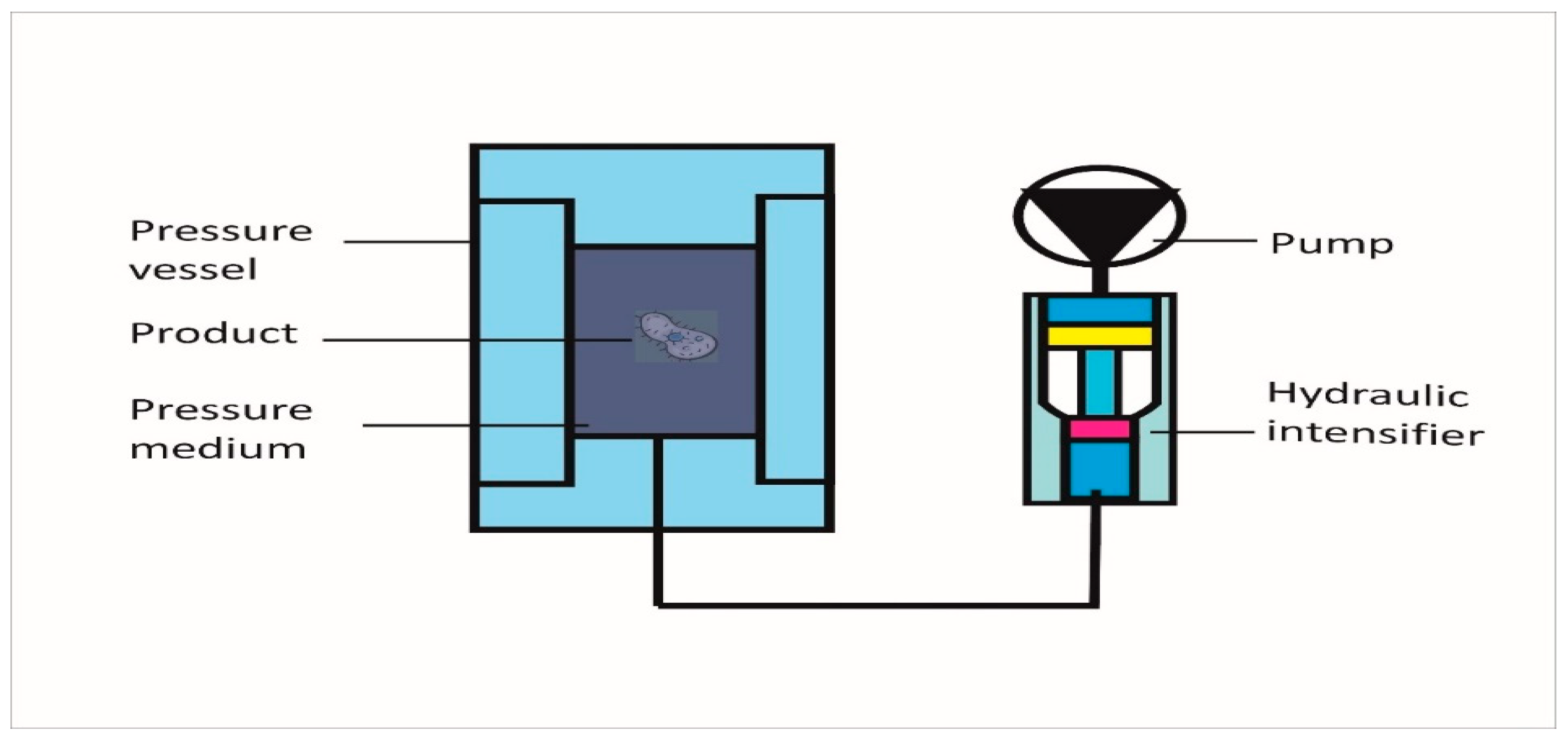
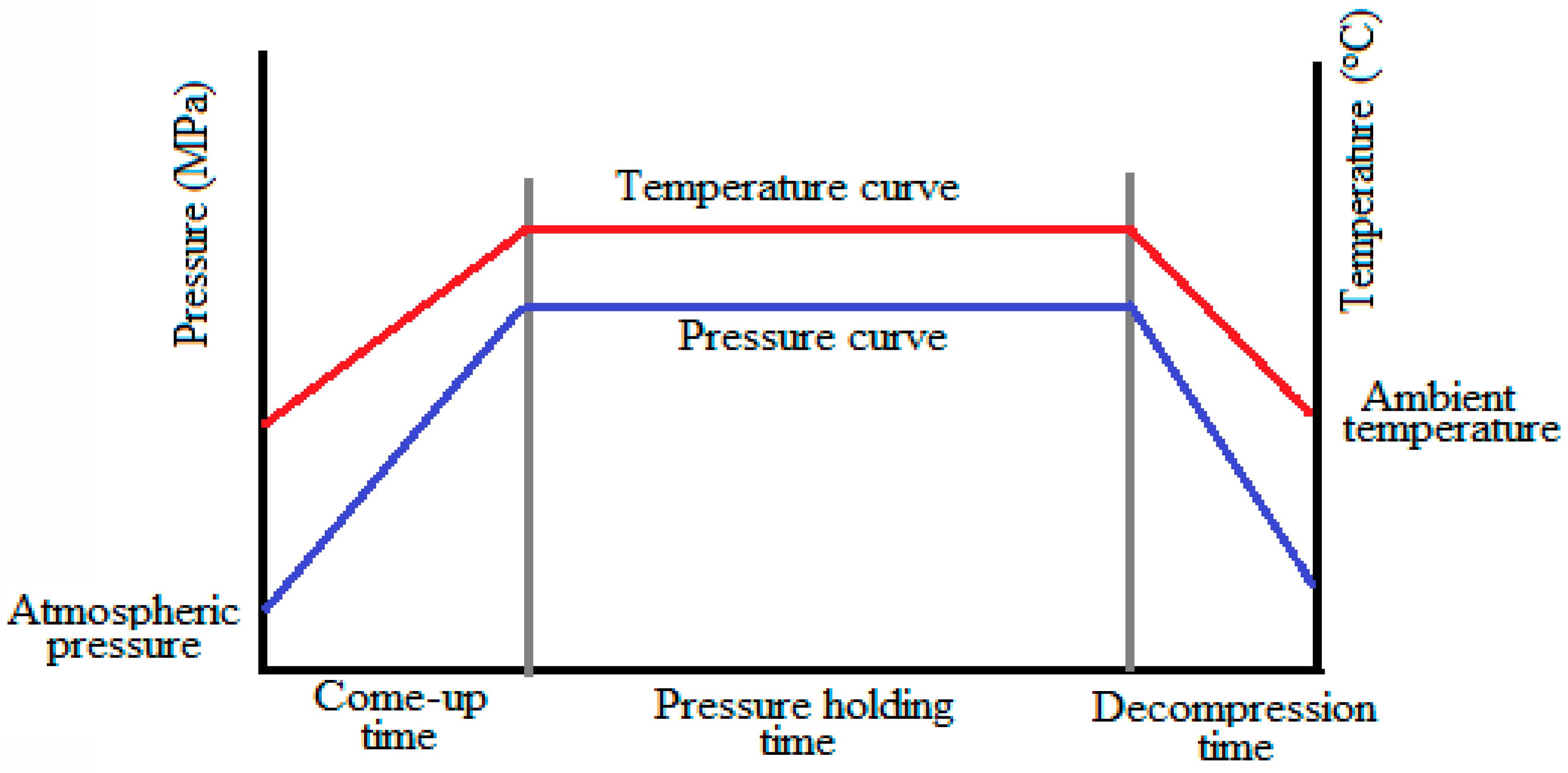
2. Working Mechanism of HPP
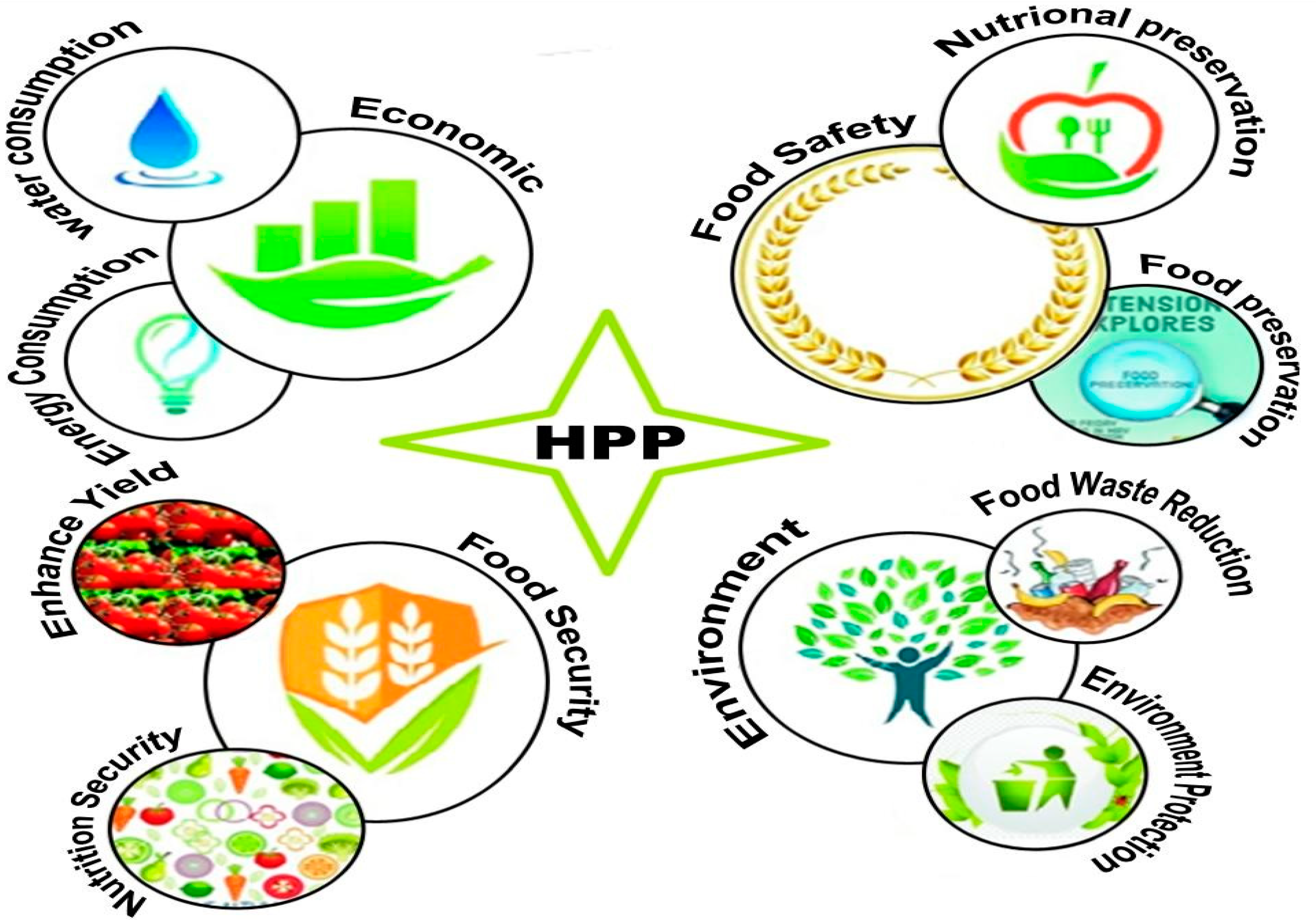
3. HPP Processing towards Sustainability
3.1. Food Safety
3.1.1. Food Preservation
- i.
- Liquid Food Preservation:
- ii.
- Solid Food Preservation
3.1.2. Effect of HPP on Food Contaminants
3.1.3. Reduction of Pesticides
3.1.4. Degradation of Toxins
3.2. Food Security
3.2.1. Sustainable Nutrition Security
3.2.2. Food Yield
3.3. Environmental Sustainability
3.3.1. Reduction of Food Waste
3.3.2. Effects on Food Packaging
3.4. Economic Sustainability
3.4.1. Yield Efficiency
3.4.2. Water Efficiency
3.4.3. Energy Efficiency
4. Limitations of HPP Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naveed, R.; Abdul-Malek, Z.; Roobab, U.; Abdullah, M.; Naderipour, A.; Imran, M.; Bekhit, A.E.; Liu, Z.; Aadil, R.M. Pulsed Electric Field: A Potential Alternative towards a Sustainable Food Processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Loveday, S.M. Food Proteins: Technological, Nutritional, and Sustainability Attributes of Traditional and Emerging Proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef]
- Mak, T.M.W.; Xiong, X.; Tsang, D.C.W.; Yu, I.K.M.; Poon, C.S. Sustainable Food Waste Management towards Circular Bioeconomy: Policy Review, Limitations and Opportunities. Bioresour. Technol. 2020, 297, 122497. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane Technologies Assisting Plant-Based and Agro-Food by-Products Processing: A Comprehensive Review. Trends Food Sci. Technol. 2020, 95, 219–232. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Khan, N.; Ahmad, M.H.; Liu, Z.W.; Aadil, R.M. Effective Valorization of Food Wastes and By-Products through Pulsed Electric Field: A Systematic Review. J. Food Process Eng. 2021, 44, e13629. [Google Scholar] [CrossRef]
- Režek Jambrak, A.; Vukušić, T.; Donsi, F.; Paniwnyk, L.; Djekic, I. Three Pillars of Novel Nonthermal Food Technologies: Food Safety, Quality, and Environment. J. Food Qual. 2018, 2018, 8619707. [Google Scholar] [CrossRef]
- Mújica-Paz, H.; Valdez-Fragoso, A.; Samson, C.T.; Welti-Chanes, J.; Torres, A. High-Pressure Processing Technologies for the Pasteurization and Sterilization of Foods. Food Bioprocess Technol. 2011, 4, 969–985. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; Bekhit, A.E.D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical Systems for Pulsed Electric Field Applications in the Food Industry: An Engineering Perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Aadil, R.M.; Khalil, A.A.; Rehman, A.; Khalid, A.; Inam-ur-Raheem, M.; Karim, A.; Gill, A.A.; Abid, M.; Afraz, M.T. Assessing the Impact of Ultra-Sonication and Thermo-Ultrasound on Antioxidant Indices and Polyphenolic Profile of Apple-Grape Juice Blend. J. Food Process. Preserv. 2020, 44, e14406. [Google Scholar] [CrossRef]
- Liu, Z.W.; Liu, L.J.; Zhou, Y.X.; Tan, Y.C.; Cheng, J.H.; Bekhit, A.E.D.; Inam-Ur-Raheem, M.; Aadil, R.M. Dielectric-Barrier Discharge (DBD) Plasma Treatment Reduces IgG Binding Capacity of β-Lactoglobulin by Inducing Structural Changes. Food Chem. 2021, 358, 129821. [Google Scholar] [CrossRef]
- Nor Hasni, H.; Koh, P.C.; Noranizan, M.A.; Megat Mohd Tahir, P.N.F.; Mohamad, A.; Limpot, N.; Hamid, N.; Aadil, R.M. High-Pressure Processing Treatment for Ready-to-Drink Sabah Snake Grass Juice. J. Food Process. Preserv. 2020, 44, e14508. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Zeng, X.A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined Impact of Pulsed Electric Field and Ultrasound on Bioactive Compounds and FT-IR Analysis of Almond Extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Woldemariam, H.W.; Emire, S.A. High Pressure Processing of Foods for Microbial and Mycotoxins Control: Current Trends and Future Prospects. Cogent Food Agric. 2019, 5, 1622184. [Google Scholar] [CrossRef]
- Jermann, C.; Koutchma, T.; Margas, E.; Leadley, C.; Ros-Polski, V. Mapping Trends in Novel and Emerging Food Processing Technologies around the World. Innov. Food Sci. Emerg. Technol. 2015, 31, 14–27. [Google Scholar] [CrossRef]
- Roobab, U.; Khan, A.W.; Lorenzo, J.M.; Arshad, R.N.; Chen, B.-R.; Zeng, X.-A.; Bekhit, A.E.-D.; Suleman, R.; Aadil, R.M. A Systematic Review of Clean-Label Alternatives to Synthetic Additives in Raw and Processed Meat with a Special Emphasis on High-Pressure Processing (2018–2021). Food Res. Int. 2021, 150, 110792. [Google Scholar] [CrossRef]
- Huang, H.W.; Wu, S.J.; Lu, J.K.; Shyu, Y.T.; Wang, C.Y. Current Status and Future Trends of High-Pressure Processing in Food Industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Raghubeer, E.V.; Phan, B.N.; Onuoha, E.; Diggins, S.; Aguilar, V.; Swanson, S.; Lee, A. The Use of High-Pressure Processing (HPP) to Improve the Safety and Quality of Raw Coconut (Cocos Nucifera L.) Water. Int. J. Food Microbiol. 2020, 331, 108697. [Google Scholar] [CrossRef]
- Sazonova, S.; Galoburda, R.; Gramatina, I. Application of High-Pressure Processing for Safety and Shelf-Life Quality of Meat—A Review. In Proceedings of the 11th Baltic Conference on Food Science and Technology, Jelgava, Latvia, 27–28 April 2017. [Google Scholar] [CrossRef]
- Hite, B.H. The Effect of Pressure in the Preservation of Milk: A Preliminary Report; West Virginia University: Morgantown, WV, USA, 1899. [Google Scholar] [CrossRef]
- Sukmanov, V.; Hanjun, M.; Li, Y. Effect of High Pressure Processing on Meat and Meat Products. A Review. Ukr. Food J. 2019, 8, 448–469. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frígola, A. High Pressure Treatment Effect on Physicochemical and Nutritional Properties of Fluid Foods During Storage: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 307–322. [Google Scholar] [CrossRef]
- Misra, N.N.; Koubaa, M.; Roohinejad, S.; Juliano, P.; Alpas, H.; Inácio, R.S.; Saraiva, J.A.; Barba, F.J. Landmarks in the Historical Development of Twenty First Century Food Processing Technologies. Food Res. Int. 2017, 97, 318–339. [Google Scholar] [CrossRef]
- de Oliveira, T.L.C.; Ramos, A.L.S.; Ramos, E.M.; Piccoli, R.H.; Cristianini, M. Preservation of Foods by High Pressure Processing. Trends Food Sci. Technol. 2015, 45, 60–85. [Google Scholar] [CrossRef]
- Zhang, H.; Tikekar, R.V.; Ding, Q.; Gilbert, A.R.; Wimsatt, S.T. Inactivation of Foodborne Pathogens by the Synergistic Combinations of Food Processing Technologies and Food-Grade Compounds. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2110–2138. [Google Scholar] [CrossRef] [PubMed]
- Roobab, U.; Inam-Ur-Raheem, M.; Khan, A.W.; Arshad, R.N.; Zeng, X.; Aadil, R.M. Innovations in High-Pressure Technologies for the Development of Clean Label Dairy Products: A Review. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Chauhan, O.P. Non-Thermal Processing of Foods; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Daher, D.; Le Gourrierec, S.; Pérez-Lamela, C. Effect of High Pressure Processing on the Microbial Inactivation in Fruit Preparations and Other Vegetable Based Beverages. Agriculture 2017, 7, 72. [Google Scholar] [CrossRef]
- Modi, M.T.K. Emerging Technologies in Food Science. Available online: http://worldfoodscience.com/content/sib-emerging-and-new-technologies-food-science-and-technology (accessed on 8 August 2021).
- Campus, M. High Pressure Processing of Meat, Meat Products and Seafood. Food Eng. Rev. 2010, 2, 256–273. [Google Scholar] [CrossRef]
- Bolumar, T.; Orlien, V.; Bak, K.H.; Aganovic, K.; Sikes, A.; Guyon, C.; Stübler, A.-S.; de Lamballerie, M.; Hertel, C.; Brüggemann, D.A. High-Pressure Processing (HPP) of Meat Products: Impact on Quality and Applications. In Present and Future of High Pressure Processing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–244. [Google Scholar] [CrossRef]
- Morton, J.D.; Pearson, R.G.; Lee, H.Y.Y.; Smithson, S.; Mason, S.L.; Bickerstaffe, R. High Pressure Processing Improves the Tenderness and Quality of Hot-Boned Beef. Meat Sci. 2017, 133, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, S.; Gemmell, C.; Lau, A.T.Y.; Arvaj, L.; Strange, P.; Gao, A.; Barbut, S. High Pressure Processing during Drying of Fermented Sausages Can Enhance Safety and Reduce Time Required to Produce a Dry Fermented Product. Food Control 2020, 113, 107224. [Google Scholar] [CrossRef]
- Usaga, J.; Acosta, Ó.; Churey, J.J.; Padilla-Zakour, O.I.; Worobo, R.W. Evaluation of High Pressure Processing (HPP) Inactivation of Escherichia Coli O157:H7, Salmonella Enterica, and Listeria Monocytogenes in Acid and Acidified Juices and Beverages. Int. J. Food Microbiol. 2021, 339, 109034. [Google Scholar] [CrossRef]
- Kapoor, S.; Singh, M.P.; Vatankhah, H.; Deshwal, G.K.; Ramaswamy, H.S. Production and Quality Improvement of Indian Cottage Cheese (Paneer) Using High Pressure Processing. Innov. Food Sci. Emerg. Technol. 2021, 72, 102746. [Google Scholar] [CrossRef]
- Limsangouan, N.; Charunuch, C.; Sastry, S.K.; Srichamnong, W.; Jittanit, W. High Pressure Processing of Tamarind (Tamarindus Indica) Seed for Xyloglucan Extraction. LWT 2020, 134, 110112. [Google Scholar] [CrossRef]
- Agcam, E.; Akyıldız, A.; Kamat, S.; Balasubramaniam, V.M. Bioactive Compounds Extraction from the Black Carrot Pomace with Assistance of High Pressure Processing: An Optimization Study. Waste Biomass Valorization 2021, 12, 5959–5977. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Y.; Ding, Y.; Wang, D.; Deng, Y. High Hydrostatic Pressure-Assisted Extraction of High-Molecular-Weight Melanoidins from Black Garlic: Composition, Structure, and Bioactive Properties. J. Food Qual. 2019, 2019, 1682749. [Google Scholar] [CrossRef]
- Park, C.Y.; Kim, S.; Lee, D.; Park, D.J.; Imm, J.Y. Enzyme and High Pressure Assisted Extraction of Tricin from Rice Hull and Biological Activities of Rice Hull Extract. Food Sci. Biotechnol. 2016, 25, 159. [Google Scholar] [CrossRef]
- Uribe, E.; Delgadillo, A.; Giovagnoli-Vicunã, C.; Quispe-Fuentes, I.; Zura-Bravo, L. Extraction Techniques for Bioactive Compounds and Antioxidant Capacity Determination of Chilean Papaya (Vasconcellea Pubescens) Fruit. J. Chem. 2015, 2015, 347532. [Google Scholar] [CrossRef]
- Prego, R.; Fidalgo, L.G.; Saraiva, J.A.; Vázquez, M.; Aubourg, S.P. Impact of Prior High-Pressure Processing on Lipid Damage and Volatile Amines Formation in Mackerel Muscle Subjected to Frozen Storage and Canning. LWT 2021, 135, 109957. [Google Scholar] [CrossRef]
- De Ancos, B.; Rodrigo, M.J.; Sánchez-Moreno, C.; Pilar Cano, M.; Zacarías, L. Effect of High-Pressure Processing Applied as Pretreatment on Carotenoids, Flavonoids and Vitamin C in Juice of the Sweet Oranges “Navel” and the Red-Fleshed “Cara Cara”. Food Res. Int. 2020, 132, 109105. [Google Scholar] [CrossRef]
- Albertos, I.; Martin-Diana, A.B.; Sanz, M.A.; Barat, J.M.; Diez, A.M.; Jaime, I.; Rico, D. Effect of High Pressure Processing or Freezing Technologies as Pretreatment in Vacuum Fried Carrot Snacks. Innov. Food Sci. Emerg. Technol. 2016, 33, 115–122. [Google Scholar] [CrossRef]
- Wu, S.; Tong, Y.; Zhang, C.; Zhao, W.; Lyu, X.; Shao, Y.; Yang, R. High Pressure Processing Pretreatment of Chinese Mitten Crab (Eriocheir Sinensis) for Quality Attributes Assessment. Innov. Food Sci. Emerg. Technol. 2021, 73, 102793. [Google Scholar] [CrossRef]
- Mat Yusoff, M.; Gordon, M.H.; Ezeh, O.; Niranjan, K. High Pressure Pre-Treatment of Moringa Oleifera Seed Kernels Prior to Aqueous Enzymatic Oil Extraction. Innov. Food Sci. Emerg. Technol. 2017, 39, 129–136. [Google Scholar] [CrossRef]
- Kaya, E.C.; Oztop, M.H.; Alpas, H. Effect of High-Pressure Processing (HPP) on Production and Characterization of Chia Seed Oil Nanoemulsions. LWT 2021, 141, 110872. [Google Scholar] [CrossRef]
- Razi, S.M.; Motamedzadegan, A.; Matia-Merino, L.; Shahidi, S.A.; Rashidinejad, A. The Effect of PH and High-Pressure Processing (HPP) on the Rheological Properties of Egg White Albumin and Basil Seed Gum Mixtures. Food Hydrocoll. 2019, 94, 399–410. [Google Scholar] [CrossRef]
- Chai, H.E.; Sheen, S. Effect of High Pressure Processing, Allyl Isothiocyanate, and Acetic Acid Stresses on Salmonella Survivals, Storage, and Appearance Color in Raw Ground Chicken Meat. Food Control 2021, 123, 107784. [Google Scholar] [CrossRef]
- Cartagena, L.; Puértolas, E.; Martínez de Marañón, I. Evolution of Quality Parameters of High Pressure Processing (HPP) Pretreated Albacore (Thunnus Alalunga) during Long-Term Frozen Storage. Innov. Food Sci. Emerg. Technol. 2020, 62, 102334. [Google Scholar] [CrossRef]
- Nuñez-Mancilla, Y.; Pérez-Won, M.; Uribe, E.; Vega-Gálvez, A.; Di Scala, K. Osmotic Dehydration under High Hydrostatic Pressure: Effects on Antioxidant Activity, Total Phenolics Compounds, Vitamin C and Colour of Strawberry (Fragaria Vesca). LWT-Food Sci. Technol. 2013, 52, 151–156. [Google Scholar] [CrossRef]
- Balakrishna, A.K.; Wazed, M.A.; Farid, M. A Review on the Effect of High Pressure Processing (HPP) on Gelatinization and Infusion of Nutrients. Molecules 2020, 25, 2369. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Barbosa-Cánovas, G.V.; Lelieveld, H.L.M. High-Pressure Processing Equipment for the Food Industry. In High Pressure Processing of Food; Springer: New York, NY, USA, 2016; pp. 39–65. [Google Scholar] [CrossRef]
- FerstI, C.; FerstI, P. Food for Thought High Pressure Processing. Available online: https://www.academia.edu/30986916/FOOD_FOR_THOUGHT_HIGH_PRESSURE_PROCESSING_INSIGHTS_ON_TECHNOLOGY_AND_REGULATORY_REQUIREMENTS_Introduction_and_Background%22%3Ehttps:/www.academia.edu/30986916/FOOD_FOR_THOUGHT_HIGH_PRESSURE_PROCESSING_INSIGHTS_ON_TECHNOLOGY_AND_REGULATORY_REQUIREMENTS_Introduction_and_Background (accessed on 9 August 2021).
- Tao, Y.; Sun, D.-W.; Hogan, E.; Kelly, A.L. High-Pressure Processing of Foods: An Overview. In Emerging Technologies for Food Processing, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 3–24. [Google Scholar] [CrossRef]
- Cavender, G.A. Continuous High Pressure Processing of Liquid Foods: An Analysis of Physical, Structural and Microbial Effects. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2011. [Google Scholar]
- Chawla, R.; Patil, G.R.; Singh, A.K. High Hydrostatic Pressure Technology in Dairy Processing: A Review. J. Food Sci. Technol. 2011, 48, 260. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Farkas, D.; Turek, E.J. Preserving Foods through High-Pressure Processing. Food Technol. 2008, 62, 32–38. [Google Scholar]
- Gupta, R.; Mikhaylenko, G.; Balasubramaniam, V.M.; Tang, J. Combined Pressure–Temperature Effects on the Chemical Marker (4-Hydroxy-5-Methyl- 3(2H)-Furanone) Formation in Whey Protein Gels. LWT-Food Sci. Technol. 2011, 10, 2141–2146. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Ting, E.Y.; Stewart, C.M.; Robbins, J.A. Recommended Laboratory Practices for Conducting High-Pressure Microbial Inactivation Experiments. Innov. Food Sci. Emerg. Technol. 2004, 5, 299–306. [Google Scholar] [CrossRef]
- Avsaroglu, M.D.; Bozoglu, F.; Alpas, H.; Largeteau, A.; Demazeau, G. Use of Pulsed-High Hydrostatic Pressure Treatment to Decrease Patulin in Apple Juice. High Press. Res. 2015, 35, 214–222. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Martínez-Monteagudo, S.I.; Gupta, R. Principles and Application of High Pressure–Based Technologies in the Food Industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef] [PubMed]
- Kalagatur, N.K.; Kamasani, J.R.; Mudili, V.; Krishna, K.; Chauhan, O.P.; Sreepathi, M.H. Effect of High Pressure Processing on Growth and Mycotoxin Production of Fusarium Graminearum in Maize. Food Biosci. 2018, 21, 53–59. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Resistance of Byssochlamys Nivea and Neosartorya Fischeri Mould Spores of Different Age to High Pressure Thermal Processing and Thermosonication. J. Food Eng. 2017, 201, 9–16. [Google Scholar] [CrossRef]
- Terefe, N.S.; Buckow, R.; Versteeg, C. Quality-Related Enzymes in Fruit and Vegetable Products: Effects of Novel Food Processing Technologies, Part 1: High-Pressure Processing. Crit. Rev. Food Sci. Nutr. 2014, 54, 24–63. [Google Scholar] [CrossRef] [PubMed]
- Milani, E.; Silva, F.V. Comparing High Pressure Thermal Processing and Thermosonication with Thermal Processing for the Inactivation of Bacteria, Moulds, and Yeasts Spores in Foods. J. Food Eng. 2017, 214, 90–96. [Google Scholar] [CrossRef]
- FAO. Sustainable Healthy Diets Guiding Principles; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Petrescu, D.C.; Vermeir, I.; Petrescu-Mag, R.M. Consumer Understanding of Food Quality, Healthiness, and Environmental Impact: A Cross-National Perspective. Int. J. Environ. Res. Public Health 2020, 17, 169. [Google Scholar] [CrossRef]
- Muntean, M.-V.; Marian, O.; Barbieru, V.; Cătunescu, G.M.; Ranta, O.; Drocas, I.; Terhes, S. High Pressure Processing in Food Industry—Characteristics and Applications. Agric. Agric. Sci. Procedia 2016, 10, 377–383. [Google Scholar] [CrossRef]
- Roobab, U.; Shabbir, M.A.; Khan, A.W.; Arshad, R.N.; Bekhit, A.E.D.; Zeng, X.A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-Pressure Treatments for Better Quality Clean-Label Juices and Beverages: Overview and Advances. LWT 2021, 149, 111828. [Google Scholar] [CrossRef]
- Akhmazillah, M.F.N.; Farid, M.M.; Silva, F.V.M. High Pressure Processing (HPP) of Honey for the Improvement of Nutritional Value. Innov. Food Sci. Emerg. Technol. 2013, 20, 59–63. [Google Scholar] [CrossRef]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G.V. Identification of Equivalent Processing Conditions for Pasteurization of Strawberry Juice by High Pressure, Ultrasound, and Pulsed Electric Fields Processing. Innov. Food Sci. Emerg. Technol. 2019, 57, 102195. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Córdoba, M.G.; Teixeira, P. Evaluation of the Effect of High Pressure on Total Phenolic Content, Antioxidant and Antimicrobial Activity of Citrus Peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Dobiáš, J.; Voldřich, M.; Marek, M.; Chudáčková, K. Changes of Properties of Polymer Packaging Films during High Pressure Treatment. J. Food Eng. 2004, 61, 545–549. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Araújo, P.; Duarte, M.F.; de Freitas, V.; Pintado, M.; Saraiva, J.A. Experimental Design, Modeling, and Optimization of High-Pressure-Assisted Extraction of Bioactive Compounds from Pomegranate Peel. Food Bioprocess Technol. 2017, 10, 886–900. [Google Scholar] [CrossRef]
- Masanet, E.; Masanet, E.; Worrell, E.; Graus, W.; Galitsky, C. Energy Efficiency Improvement and Cost Saving Opportunities for the Fruit and Vegetable Processing Industry. An ENERGY STAR Guide for Energy and Plant Managers; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2008. [CrossRef]
- Barba, F.J.; Terefe, N.S.; Buckow, R.; Knorr, D.; Orlien, V. New Opportunities and Perspectives of High Pressure Treatment to Improve Health and Safety Attributes of Foods. A Review. Food Res. Int. 2015, 77, 725–742. [Google Scholar] [CrossRef]
- Jung, S.; Mahfuz, A.A. Low Temperature Dry Extrusion and High-Pressure Processing Prior to Enzyme-Assisted Aqueous Extraction of Full Fat Soybean Flakes. Food Chem. 2009, 114, 947–954. [Google Scholar] [CrossRef]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High Pressure Processing of Carrot Juice: Effect of Static and Multi-Pulsed Pressure on the Polyphenolic Profile, Oxidoreductases Activity and Colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Jambrak, A.R.; Granato, D.; Montesano, D.; Kovačević, D.B. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Luo, S. The Mechanism for Enhancing Extraction of Ferulic Acid from Radix Angelica Sinensis by High Hydrostatic Pressure. Sep. Purif. Technol. 2016, 165, 208–213. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuña, C.; Jamett, F. High Hydrostatic Pressure and Ultrasound Extractions of Antioxidant Compounds, Sulforaphane and Fatty Acids from Chilean Papaya (Vasconcellea Pubescens) Seeds: Effects of Extraction Conditions and Methods. LWT-Food Sci. Technol. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Ramaswamy, H.S.; Yu, Y.; Zhu, S.; Wang, J.; Li, H. High Pressure Extraction of Astaxanthin from Shrimp Waste (Penaeus Vannamei Boone): Effect on Yield and Antioxidant Activity. J. Food Process Eng. 2017, 40, e12353. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Poojary, M.M.; Barba, F.J.; Koubaa, M.; Lorenzo, J.M.; Mañes, J.; Moltó, J.C. Thermal and Non-Thermal Preservation Techniques of Tiger Nuts’ Beverage “Horchata de Chufa”. Implications for Food Safety, Nutritional and Quality Properties. Food Res. Int. 2018, 105, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E.D. Impact of nonthermal technologies on microbiological quality of juices: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 437–457. [Google Scholar] [CrossRef]
- Woolf, A.B.; Wibisono, R.; Farr, J.; Hallett, I.; Richter, L.; Oey, I.; Wohlers, M.; Zhou, J.; Fletcher, G.C.; Requejo-Jackman, C. Effect of High Pressure Processing on Avocado Slices. Innov. Food Sci. Emerg. Technol. 2013, 18, 65–73. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, S.; Yang, H.; Kamboh, A.A.; Ahmad, Z.; Tume, R.K.; Zhou, G. Improvement of Color, Texture and Food Safety of Ready-to-Eat High Pressure-Heat Treated Duck Breast. Food Chem. 2018, 277, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Codex Alimentarius Commission; Food and Agriculture Organization of the United Nations; World Health Organization. Codex Alimentarius: Food Hygiene Basic Texts; FAO: Rome, Italy, 2001. [Google Scholar]
- Kök, M.S. Application of Food Safety Management Systems (ISO 22000/HACCP) in the Turkish Poultry Industry: A Comparison Based on Enterprise Size. J. Food Prot. 2009, 72, 2221–2225. [Google Scholar] [CrossRef] [PubMed]
- Simonin, H.; Duranton, F.; de Lamballerie, M. New Insights into the High-Pressure Processing of Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2012, 11, 285–306. [Google Scholar] [CrossRef]
- Teixeira, P.; Kolomeytseva, M.; Silva, J.; Castro, S.M. High Hydrostatic Pressure Applied to Ready-to-Eat Meat Products Focus on Listeria Monocytogenes Inactivation. Available online: https://www.researchgate.net/publication/266386629_High_Hydrostatic_Pressure_Applied_to_Ready-to-eat_Meat_Products_Focus_on_Listeria_monocytogenes_inactivation (accessed on 20 September 2021).
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food Preservation Techniques and Nanotechnology for Increased Shelf Life of Fruits, Vegetables, Beverages and Spices: A Review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef] [PubMed]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stübler, A.-S.; de Lamballerie, M.; Hertel, C.; Brüggemann, D.A. High-Pressure Processing of Meat: Molecular Impacts and Industrial Applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 332–368. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Almeida, A.; Delgadillo, I.; Saraiva, J.; Cunha, Â. Susceptibility of Listeria Monocytogenes to High Pressure Processing: A Review. Food Rev. Int. 2016, 32, 377–399. [Google Scholar] [CrossRef]
- Chan, J.T.Y.; Omana, D.A.; Betti, M. Application of High Pressure Processing to Improve the Functional Properties of Pale, Soft, and Exudative (PSE)-like Turkey Meat. Innov. Food Sci. Emerg. Technol. 2011, 12, 216–225. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; Jäger, H.; Knorr, D.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Impact of Pulsed Electric Fields, High Hydrostatic Pressure, and Thermal Pasteurization Selected Characteristics of Opuntia Dillenii Cactus Juice. LWT-Food Sci. Technol. 2017, 79, 534–542. [Google Scholar] [CrossRef]
- González-Angulo, M.; Clauwers, C.; Harastani, R.; Tonello, C.; Jaime, I.; Rovira, J.; Michiels, C.W. Evaluation of Factors Influencing the Growth of Non-Toxigenic Clostridium Botulinum Type E and Clostridium Sp. in High-Pressure Processed and Conditioned Tender Coconut Water from Thailand. Food Res. Int. 2020, 134, 109278. [Google Scholar] [CrossRef] [PubMed]
- Gouvea, F.S.; Padilla-Zakour, O.I.; Worobo, R.W.; Xavier, B.M.; Walter, E.H.M.; Rosenthal, A. Effect of High-Pressure Processing on Bacterial Inactivation in Açaí Juices with Varying PH and Soluble Solids Content. Innov. Food Sci. Emerg. Technol. 2020, 66, 102490. [Google Scholar] [CrossRef]
- Jiao, R.; Gao, J.; Li, Y.; Zhang, X.; Zhang, M.; Ye, Y.; Wu, Q.; Fan, H. Short Communication: Effects of High-Pressure Processing on the Inactivity of Cronobacter Sakazakii in Whole Milk and Skim Milk Samples. J. Dairy Sci. 2016, 99, 7881–7885. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Zhao, L.; Wang, Y.; Liao, X. Potential of High-Pressure Processing and High-Temperature/Short-Time Thermal Processing on Microbial, Physicochemical and Sensory Assurance of Clear Cucumber Juice. Innov. Food Sci. Emerg. Technol. 2016, 34, 51–58. [Google Scholar] [CrossRef]
- Nasiłowska, J.; Sokołowska, B.; Fonberg-Broczek, M. Long-Term Storage of Vegetable Juices Treated by High Hydrostatic Pressure: Assurance of the Microbial Safety. Biomed. Res. Int. 2018, 2018, 7389381. [Google Scholar] [CrossRef] [PubMed]
- Stratakos, A.C.; Inguglia, E.S.; Linton, M.; Tollerton, J.; Murphy, L.; Corcionivoschi, N.; Koidis, A.; Tiwari, B.K. Effect of High Pressure Processing on the Safety, Shelf Life and Quality of Raw Milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 325–333. [Google Scholar] [CrossRef]
- Van Luong, T.S.; Moir, C.; Chandry, P.S.; Pinfold, T.; Olivier, S.; Broussolle, V.; Bowman, J.P. Combined High Pressure and Heat Treatment Effectively Disintegrates Spore Membranes and Inactivates Alicyclobacillus Acidoterrestris Spores in Acidic Fruit Juice Beverage. Innov. Food Sci. Emerg. Technol. 2020, 66, 102523. [Google Scholar] [CrossRef]
- van Wyk, S.; Silva, F.V.M. High Pressure Processing Inactivation of Brettanomyces Bruxellensis in Seven Different Table Wines. Food Control 2017, 81, 1–8. [Google Scholar] [CrossRef]
- Hartyáni, P.; Dalmadi, I.; Knorr, D. Electronic Nose Investigation of Alicyclobacillus Acidoterrestris Inoculated Apple and Orange Juice Treated by High Hydrostatic Pressure. Food Control 2013, 32, 262–269. [Google Scholar] [CrossRef]
- Hiremath, N.D.; Ramaswamy, H.S. High-Pressure Destruction Kinetics of Spoilage and Pathogenic Microorganisms in Mango Juice. J. Food Process. Preserv. 2012, 36, 113–125. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, M.; Chen, H. Inactivation of Escherichia Coli O157: H7 and Salmonella Spp. in Strawberry Puree by High Hydrostatic Pressure with/without Subsequent Frozen Storage. Int. J. Food Microbiol. 2013, 160, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, H.M.; Yoo, S.; Seo, B.; Ghafoor, K.; Kim, J.U.; Lee, D.-U.; Park, J. Combination of TiO2-UV Photocatalysis and High Hydrostatic Pressure to Inactivate Bacterial Pathogens and Yeast in Commercial Apple Juice. Food Bioprocess Technol. 2016, 9, 182–190. [Google Scholar] [CrossRef]
- Syed, Q.A.; Buffa, M.; Guamis, B.; Saldo, J. Effect of Compression and Decompression Rates of High Hydrostatic Pressure on Inactivation of Staphylococcus Aureus in Different Matrices. Food Bioprocess Technol. 2014, 7, 1202–1207. [Google Scholar] [CrossRef]
- Sehrawat, R.; Kaur, B.P.; Nema, P.K.; Tewari, S.; Kumar, L. Microbial Inactivation by High Pressure Processing: Principle, Mechanism and Factors Responsible. Food Sci. Biotechnol. 2021, 30, 19–35. [Google Scholar] [CrossRef]
- Rendueles, E.; Omer, M.K.; Alvseike, O.; Alonso-Calleja, C.; Capita, R.; Prieto, M. Microbiological Food Safety Assessment of High Hydrostatic Pressure Processing: A Review. LWT-Food Sci. Technol. 2011, 44, 1251–1260. [Google Scholar] [CrossRef]
- García-Gimeno, R.M.; Izquierdo, G.D.P. High Hydrostatic Pressure Treatment of Meat Products. In Food Processing; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sheen, S.; Sommers, C.; Sheen, L.Y. Modeling the Survival of Escherichia Coli O157:H7 under Hydrostatic Pressure, Process Temperature, Time and Allyl Isothiocyanate Stresses in Ground Chicken Meat. Front. Microbiol. 2018, 9, 1871. [Google Scholar] [CrossRef]
- Koutchma, T. Adapting High Hydrostatic Pressure (HPP) for Food Processing Operations, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; Available online: https://www.elsevier.com/books/adapting-high-hydrostatic-pressure-hpp-for-food-processing-operations/koutchma/978-0-12-420091-3 (accessed on 3 July 2021).
- Pérez-Santaescolástica, C.; Carballo, J.; Fulladosa, E.; Munekata, P.E.S.; Bastianello Campagnol, P.C.; Gómez, B.; Lorenzo, J.M. Influence of High-Pressure Processing at Different Temperatures on Free Amino Acid and Volatile Compound Profiles of Dry-Cured Ham. Food Res. Int. 2019, 116, 49–56. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (aw) on Microbial Stability as a Hurdle in Food Preservation. In Water Activity in Foods; Wiley: Hoboken, NJ, USA, 2020; pp. 323–355. [Google Scholar] [CrossRef]
- Dash, K.K.; Balasubramaniam, V.M.; Kamat, S. High Pressure Assisted Osmotic Dehydrated Ginger Slices. J. Food Eng. 2019, 247, 19–29. [Google Scholar] [CrossRef]
- Cheng, L.; Zhu, Z.; Sun, D.W. Impacts of High Pressure Assisted Freezing on the Denaturation of Polyphenol Oxidase. Food Chem. 2021, 335, 127485. [Google Scholar] [CrossRef]
- Otero, L.; Martino, M.; Zaritzky, N.; Solas, M.; Sanz, P.D. Preservation of Microstructure in Peach and Mango during High-Pressure-Shift Freezing. J. Food Sci. 2000, 65, 466–470. [Google Scholar] [CrossRef]
- Cui, Y.; Xuan, X.; Ling, J.; Liao, X.; Zhang, H.; Shang, H.; Lin, X. Effects of High Hydrostatic Pressure-Assisted Thawing on the Physicohemical Characteristics of Silver Pomfret (Pampus Argenteus). Food Sci. Nutr. 2019, 7, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Préstamo, G.; Palomares, L.; Sanz, P. Broccoli (Brasica Oleracea) Treated under Pressure-Shift Freezing Process. Eur. Food Res. Technol. 2004, 219, 598–604. [Google Scholar] [CrossRef][Green Version]
- Mastovska, K. Modern Analysis of Chemical Contaminants in Food. Available online: https://www.food-safety.com/articles/4460-modern-analysis-of-chemical-contaminants-in-food (accessed on 3 July 2021).
- Nerín, C.; Aznar, M.; Carrizo, D. Food Contamination during Food Process. Trends Food Sci. Technol. 2016, 48, 63–68. [Google Scholar] [CrossRef]
- Martin, A.; Beutin, L. Characteristics of Shiga Toxin-Producing Escherichia Coli from Meat and Milk Products of Different Origins and Association with Food Producing Animals as Main Contamination Sources. Int. J. Food Microbiol. 2011, 146, 99–104. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Jabbar, S.; Nazir, A.; Mann, A.A.; Khan, M.K.I.; Abdullah, A.; Ramzan, A. Quality Evaluation of Grapefruit Juice by Thermal and High Pressure Processing Treatment. Pak. J. Agric. Res. 2017, 30, 209–309. [Google Scholar] [CrossRef]
- Evert-Arriagada, K.; Hernández-Herrero, M.M.; Guamis, B.; Trujillo, A.J. Commercial Application of High-Pressure Processing for Increasing Starter-Free Fresh Cheese Shelf-Life. LWT-Food Sci. Technol. 2014, 55, 498–505. [Google Scholar] [CrossRef]
- Georget, E.; Sevenich, R.; Reineke, K.; Mathys, A.; Heinz, V.; Callanan, M.; Rauh, C.; Knorr, D. Inactivation of Microorganisms by High Isostatic Pressure Processing in Complex Matrices: A Review. Innov. Food Sci. Emerg. Technol. 2015, 27, 1–14. [Google Scholar] [CrossRef]
- Sevenich, R.; Kleinstueck, E.; Crews, C.; Anderson, W.; Pye, C.; Riddellova, K.; Hradecky, J.; Moravcova, E.; Reineke, K.; Knorr, D. High-Pressure Thermal Sterilization: Food Safety and Food Quality of Baby Food Puree. J. Food Sci. 2014, 79, M230–M237. [Google Scholar] [CrossRef]
- Kultur, G.; Misra, N.N.; Barba, F.J.; Koubaa, M.; Gökmen, V.; Alpas, H. Microbial Inactivation and Evaluation of Furan Formation in High Hydrostatic Pressure (HHP) Treated Vegetable-Based Infant Food. Food Res. Int. 2017, 101, 17–23. [Google Scholar] [CrossRef]
- Iizuka, T.; Shimizu, A. Removal of Pesticide Residue from Brussels Sprouts by Hydrostatic Pressure. Innov. Food Sci. Emerg. Technol. 2014, 22, 70–75. [Google Scholar] [CrossRef]
- Ionel, B. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part Two: Regulations. Universul Jurid. 2018, 16–19. Available online: https://www.ceeol.com/search/article-detail?id=624697 (accessed on 22 November 2021).
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A Review of the Global Pesticide Legislation and the Scale of Challenge in Reaching the Global Harmonization of Food Safety Standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef]
- Ionel, B. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part One: The Role of European Institutions in Laying down and Passing Laws Specific to the Veterinary Sanitary and Food Safety Area. Available online: https://www.researchgate.net/publication/316716657_EUROPEAN_REGULATION_IN_THE_VETERINARY_SANITARY_AND_FOOD_SAFETY_AREA_A_COMPONENT_OF_THE_EUROPEAN_POLICIES_ON_THE_SAFETY_OF_FOOD_PRODUCTS_AND_THE_PROTECTION_OF_CONSUMER_INTERESTS_A_2007_RETROSPECTIVE_PA (accessed on 22 November 2021).
- Gopal, K.R. High Pressure Processing of Fruits and Vegetable Products: A Review. Int. J. Pure Appl. Biosci. 2017, 5, 680–692. [Google Scholar] [CrossRef]
- Pereira, R.N.; Vicente, A.A. Environmental Impact of Novel Thermal and Non-Thermal Technologies in Food Processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef]
- Bhilwadikar, T.; Pounraj, S.; Manivannan, S.; Rastogi, N.K.; Negi, P.S. Decontamination of Microorganisms and Pesticides from Fresh Fruits and Vegetables: A Comprehensive Review from Common Household Processes to Modern Techniques. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1003–1038. [Google Scholar] [CrossRef]
- Cámara, M.A.; Cermeño, S.; Martínez, G.; Oliva, J. Removal Residues of Pesticides in Apricot, Peach and Orange Processed and Dietary Exposure Assessment. Food Chem. 2020, 325, 126936. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Occurrence of Environmental Pollutants in Foodstuffs: A Review of Organic vs. Conventional Food. Food Chem. Toxicol. 2019, 125, 370–375. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of Pesticide Residue Analysis in Fruits and Vegetables. Pre-Treatment, Extraction and Detection Techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, R.; Hayrapetyan, H.; Vollebregt, M.; Dijksterhuis, J. Comparing Thermal Inactivation to a Combined Process of Moderate Heat and High Pressure: Effect on Ascospores in Strawberry Puree. Int. J. Food Microbiol. 2020, 325, 108629. [Google Scholar] [CrossRef] [PubMed]
- None, E.; FVM, S. Inactivation of Byssochlamys Nivea Ascospores in Strawberry Puree by High Pressure, Power Ultrasound and Thermal Processing. Int. J. Food Microbiol. 2015, 214, 129–136. [Google Scholar] [CrossRef]
- Evelyn; Kim, H.J.; Silva, F.V.M. Modeling the Inactivation of Neosartorya Fischeri Ascospores in Apple Juice by High Pressure, Power Ultrasound and Thermal Processing. Food Control 2016, 59, 530–537. [Google Scholar] [CrossRef]
- Huang, H.W.; Yang, B.B.; Wang, C.Y. Effects of High Pressure Processing on Immunoreactivity and Microbiological Safety of Crushed Peanuts. Food Control 2014, 42, 290–295. [Google Scholar] [CrossRef]
- Tokuşoǧlu, Ö.; Alpas, H.; Bozoǧlu, F. High Hydrostatic Pressure Effects on Mold Flora, Citrinin Mycotoxin, Hydroxytyrosol, Oleuropein Phenolics and Antioxidant Activity of Black Table Olives. Innov. Food Sci. Emerg. Technol. 2010, 11, 250–258. [Google Scholar] [CrossRef]
- Xie, H.; Wen, Y.; Choi, Y.; Zhang, X. Global Trends on Food Security Research: A Bibliometric Analysis. Land 2021, 10, 119. [Google Scholar] [CrossRef]
- FAO; IFAD; WFP. The State of Food Insecurity in the World 2015. Meeting the 2015 International Hunger Targets: Taking Stock of Uneven Progress. Policy Support and Governance. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/policy-support/tools-and-publications/resources-details/en/c/469455/ (accessed on 13 July 2021).
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting Non-Thermal Food Processing and Preservation Methods—Action Mechanisms, Pros and Cons: A Technological Update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Avellaneda, Z.; Pateiro-Moure, M.; Chotyakul, N.; Torres, J.A.; Welti-Chanes, J.; Pérez-Lamela, C. Benefits and Limitations of Food Processing by High-Pressure Technologies: Effects on Functional Compounds and Abiotic Contaminants. CYTA-J. Food 2011, 9, 351–364. [Google Scholar] [CrossRef]
- García, A.F.; Butz, P.; Bognàr, A.; Tauscher, B. Antioxidative Capacity, Nutrient Content and Sensory Quality of Orange Juice and an Orange-Lemon-Carrot Juice Product after High Pressure Treatment and Storage in Different Packaging. Eur. Food Res. Technol. 2001, 213, 290–296. [Google Scholar] [CrossRef]
- Aaby, K.; Grimsbo, I.H.; Hovda, M.B.; Rode, T.M. Effect of High Pressure and Thermal Processing on Shelf Life and Quality of Strawberry Purée and Juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Ascorbic Acid Is the Only Bioactive That Is Better Preserved by High Hydrostatic Pressure than by Thermal Treatment of a Vegetable Beverage. J. Agric. Food Chem. 2010, 58, 10070–10075. [Google Scholar] [CrossRef]
- Kiełczewska, K.; Jankowska, A.; Dąbrowska, A.; Wachowska, M.; Ziajka, J. The Effect of High Pressure Treatment on the Dispersion of Fat Globules and the Fatty Acid Profile of Caprine Milk. Int. Dairy J. 2020, 102, 104607. [Google Scholar] [CrossRef]
- Rivas-Cañedo, A.; Martínez-Onandi, N.; Gaya, P.; Nuñez, M.; Picon, A. Effect of High-Pressure Processing and Chemical Composition Lipid Oxidation, Aminopeptidase Activity and Free Amino Acids of Serrano Dry-Cured Ham. Meat Sci. 2021, 172, 108349. [Google Scholar] [CrossRef] [PubMed]
- Cadesky, L.; Walkling-Ribeiro, M.; Kriner, K.T.; Karwe, M.V.; Moraru, C.I. Structural Changes Induced by High-Pressure Processing in Micellar Casein and Milk Protein Concentrates. J. Dairy Sci. 2017, 100, 7055–7070. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of Boiling, Ultra-High Temperature and High Hydrostatic Pressure on Free Amino Acids, Flavor Characteristics and Sensory Profiles in Chinese Rice Wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef]
- Stinco, C.M.; Szczepańska, J.; Marszałek, K.; Pinto, C.A.; Inácio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.A.; Meléndez-Martínez, A.J. Effect of High-Pressure Processing on Carotenoids Profile, Colour, Microbial and Enzymatic Stability of Cloudy Carrot Juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef] [PubMed]
- Błaszczak, W.; Latocha, P.; Jeż, M.; Wiczkowski, W. The Impact of High-Pressure Processing on the Polyphenol Profile and Anti-Glycaemic, Anti-Hypertensive and Anti-Cholinergic Activities of Extracts Obtained from Kiwiberry (Actinidia Arguta) Fruits. Food Chem. 2021, 343, 128421. [Google Scholar] [CrossRef]
- da Silveira, T.F.F.; Cristianini, M.; Kuhnle, G.G.; Ribeiro, A.B.; Filho, J.T.; Godoy, H.T. Anthocyanins, Non-Anthocyanin Phenolics, Tocopherols and Antioxidant Capacity of Açaí Juice (Euterpe Oleracea) as Affected by High Pressure Processing and Thermal Pasteurization. Innov. Food Sci. Emerg. Technol. 2019, 55, 88–96. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.; Li, Z.; Xie, X.; Gong, E.S.; Tian, J.; Si, X.; Wang, Y.; Gao, N.; Shu, C.; et al. Effects of High Hydrostatic Pressure and Thermal Processing on Anthocyanin Content, Polyphenol Oxidase and β-Glucosidase Activities, Color, and Antioxidant Activities of Blueberry (Vaccinium Spp.) Puree. Food Chem. 2021, 342, 128564. [Google Scholar] [CrossRef]
- Marszałek, K.; Doesburg, P.; Starzonek, S.; Szczepańska, J.; Woźniak, Ł.; Lorenzo, J.M.; Skaopska, S.; Rzoska, S.; Barba, F.J. Comparative Effect of Supercritical Carbon Dioxide and High Pressure Processing on Structural Changes and Activity Loss of Oxidoreductive Enzymes. J. CO2 Util. 2019, 29, 46–56. [Google Scholar] [CrossRef]
- Lu, S.Y.; Chu, Y.L.; Sridhar, K.; Tsai, P.J. Effect of Ultrasound, High-Pressure Processing, and Enzymatic Hydrolysis on Carbohydrate Hydrolyzing Enzymes and Antioxidant Activity of Lemon (Citrus Limon) Flavedo. LWT 2021, 138, 110511. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, S.; Brannan, R.G. Effect of High Pressure Processing, Browning Treatments, and Refrigerated Storage on Sensory Analysis, Color, and Polyphenol Oxidase Activity in Pawpaw (Asimina Triloba L.) Pulp. LWT-Food Sci. Technol. 2017, 86, 49–54. [Google Scholar] [CrossRef]
- Cao, B.; Fang, L.; Liu, C.; Min, W.; Liu, J. Effects of High Hydrostatic Pressure on the Functional and Rheological Properties of the Protein Fraction Extracted from Pine Nuts. Food Sci. Technol. Int. 2018, 24, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, L.; Li, Y. Exploring High Hydrostatic Pressure-Mediated Germination to Enhance Functionality and Quality Attributes of Wholegrain Brown Rice. Food Chem. 2018, 249, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Popović, V.; Koutchma, T.; Warriner, K.; Zhu, Y. Effect of Thermal, High Hydrostatic Pressure, and Ultraviolet-C Processing on the Microbial Inactivation, Vitamins, Chlorophyll, Antioxidants, Enzyme Activity, and Color of Wheatgrass Juice. J. Food Process Eng. 2020, 43, e13036. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy Expectations of High Hydrostatic Pressure Treatment in Food Processing Industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Moltó-Puigmartí, C.; Permanyer, M.; Castellote, A.I.; López-Sabater, M.C. Effects of Pasteurisation and High-Pressure Processing on Vitamin C, Tocopherols and Fatty Acids in Mature Human Milk. Food Chem. 2011, 124, 697–702. [Google Scholar] [CrossRef]
- Nayak, P.K.; Rayaguru, K.; Radha Krishnan, K. Quality Comparison of Elephant Apple Juices after High-Pressure Processing and Thermal Treatment. J. Sci. Food Agric. 2017, 97, 1404–1411. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barnaba, C.; Villani, F.; Barbosa-Cánovas, G.V. Effects of Thermal and High Pressure Treatments in Color and Chemical Attributes of an Oil-Based Spinach Sauce. LWT-Food Sci. Technol. 2015, 60, 86–94. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of High Pressure Modification Conformation and Gelation Properties of Myofibrillar Protein. Food Chem. 2017, 217, 678–686. [Google Scholar] [CrossRef]
- Chan, J.T.Y.; Omana, D.A.; Betti, M. Effect of Ultimate PH and Freezing on the Biochemical Properties of Proteins in Turkey Breast Meat. Food Chem. 2011, 127, 109–117. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Kumar, S.; Nagraj, R.; Narasimhamurthy, R.; Raju, P.S. Effect of High Pressure Processing on Yield, Quality and Storage Stability of Peanut Paneer. Int. J. Food Sci. Technol. 2015, 50, 1515–1521. [Google Scholar] [CrossRef]
- Lowder, A.C.; Waite-Cusic, J.G.; Mireles Dewitt, C.A. High Pressure–Low Temperature Processing of Beef: Effects on Survival of Internalized E. Coli O157:H7 and Quality Characteristics. Innov. Food Sci. Emerg. Technol. 2014, 26, 18–25. [Google Scholar] [CrossRef]
- Gao, H.; Zeng, J.; Ma, H.; Wang, Z.; Pan, R. Improving Tenderness of Goose Breast by Ultra-High Pressure. Int. J. Food Prop. 2015, 18, 1693–1701. [Google Scholar] [CrossRef]
- Ma, H.; Ledward, D.A. High Pressure Processing of Fresh Meat—Is It Worth It? Meat Sci. 2013, 95, 897–903. [Google Scholar] [CrossRef]
- Zhang, H.; Tchabo, W.; Ma, Y. Quality of Extracts from Blueberry Pomace by High Hydrostatic Pressure, Ultrasonic, Microwave and Heating Extraction: A Comparison Study. Emir. J. Food Agric. 2017, 29, 815–819. [Google Scholar] [CrossRef]
- Volkov, A.Y.; Kruglikov, N.A.; Alexandrov, A.V.; Kotkova, V.V. Use of High Hydrostatic Pressure for Food Sterilization and Seed Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2020, 1008, 012012. [Google Scholar] [CrossRef]
- Irna, C.; Jaswir, I.; Othman, R.; Jimat, D.N. Comparison between High-Pressure Processing and Chemical Extraction: Astaxanthin Yield From Six Species of Shrimp Carapace. J. Diet. Suppl. 2018, 15, 805–813. [Google Scholar] [CrossRef]
- Huppertz, T.; Hinz, K.; Zobrist, M.R.; Uniacke, T.; Kelly, A.L.; Fox, P.F. Effects of High Pressure Treatment on the Rennet Coagulation and Cheese-Making Properties of Heated Milk. Innov. Food Sci. Emerg. Technol. 2005, 6, 279–285. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Skripnuk, D.F.; Davydenko, V.A.; Romashkina, G.F.; Khuziakhmetov, R.R. Consumer Trust in Quality and Safety of Food Products in Western Siberia. Agronomy 2021, 11, 257. [Google Scholar] [CrossRef]
- De Oliveira, C.F.; Gurak, P.D.; Marczak, L.D.; Karwe, M. Extraction of Carotenoids from Passion Fruit Peel Assisted by High Pressure. X CIGR Sect. IV Int. Tech. Symp. 2016, 51, 2108–3111. [Google Scholar]
- George, J.M.; Sowbhagya, H.B.; Rastogi, N.K. Effect of High Pressure Pretreatment on Drying Kinetics and Oleoresin Extraction from Ginger. Dry. Technol. 2017, 36, 1107–1116. [Google Scholar] [CrossRef]
- Xi, J.; Yan, L. Optimization of Pressure-Enhanced Solid-Liquid Extraction of Flavonoids from Flos Sophorae and Evaluation of Their Antioxidant Activity. Sep. Purif. Technol. 2017, 175, 170–176. [Google Scholar] [CrossRef]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable Extraction of Valuable Components from Spirulina Assisted by Pulsed Electric Fields Technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- Cacace, F.; Bottani, E.; Rizzi, A.; Vignali, G. Evaluation of the Economic and Environmental Sustainability of High Pressure Processing of Foods. Innov. Food Sci. Emerg. Technol. 2020, 60, 102281. [Google Scholar] [CrossRef]
- Yordanov, D.G.; Angelova, G.V. High Pressure Processing for Foods Preserving. Biotechnol. Biotechnol. Equip. 2010, 24, 1940–1945. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Cheela, V.R.S.; D’Adamo, I.; Iacovidou, E.; Islam, M.R.; Johnson, M.; Miller, T.R.; Parajuly, K.; Parchomenko, A.; Radhakrishan, L.; et al. Zero Waste Approach towards a Sustainable Waste Management. Resour. Environ. Sustain. 2021, 3, 100014. [Google Scholar] [CrossRef]
- Atuonwu, J.C.; Leadley, C.; Bosman, A.; Tassou, S.A. High-Pressure Processing, Microwave, Ohmic, and Conventional Thermal Pasteurization: Quality Aspects and Energy Economics. J. Food Process Eng. 2020, 43, e13328. [Google Scholar] [CrossRef]
- FAO. Food Loss and Food Waste. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/food-loss-and-food-waste/flw-data (accessed on 13 July 2021).
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef]
- Timmermans, A.J.M.; Ambuko, J.; Belik, W.; Huang, J. Food Losses and Waste in the Context of Sustainable Food Systems; CFS Committee on World Food Security HLPE: Rome, Italy, 2014. [Google Scholar]
- Shafiee-Jood, M.; Cai, X. Reducing Food Loss and Waste to Enhance Food Security and Environmental Sustainability. Environ. Sci. Technol. 2016, 50, 8432–8443. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Calvo, M.M.; Sánchez-Faure, A.; Montero, P.; Gómez-Guillén, M.C. Development, Properties, and Stability of Antioxidant Shrimp Muscle Protein Films Incorporating Carotenoid-Containing Extracts from Food by-Products. LWT-Food Sci. Technol. 2015, 64, 189–196. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Wang, M.; Lu, R. Effects of Pulsed Electric Fields on Bioactive Components, Colour and Flavour of Green Tea Infusions. Int. J. Food Sci. Technol. 2009, 44, 312–321. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L. Effect of Ultrasounds and High Pressure Homogenization the Extraction of Antioxidant Polyphenols from Lettuce Waste. Innov. Food Sci. Emerg. Technol. 2018, 50, 11–19. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Giovagnoli-Vicuña, C.; Cañas-Sarazúa, R. Optimization of Extraction Yield, Flavonoids and Lycopene from Tomato Pulp by High. Hydrostatic Pressure-Assisted Extraction; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 278. [Google Scholar] [CrossRef]
- Torres-Ossandón, M.J.; Vega-Gálvez, A.; López, J.; Stucken, K.; Romero, J.; Di Scala, K. Effects of High Hydrostatic Pressure Processing and Supercritical Fluid Extraction Bioactive Compounds and Antioxidant Capacity of Cape Gooseberry Pulp (Physalis Peruviana L.). J. Supercrit. Fluids 2018, 138, 215–220. [Google Scholar] [CrossRef]
- Cascaes Teles, A.S.; Hidalgo Chávez, D.W.; Zarur Coelho, M.A.; Rosenthal, A.; Fortes Gottschalk, L.M.; Tonon, R.V. Combination of Enzyme-Assisted Extraction and High Hydrostatic Pressure for Phenolic Compounds Recovery from Grape Pomace. J. Food Eng. 2020, 288, 110128. [Google Scholar] [CrossRef]
- García-Parra, J.; González-Cebrino, F.; Delgado, J.; Cava, R.; Ramírez, R. High Pressure Assisted Thermal Processing of Pumpkin Purée: Effect on Microbial Counts, Color, Bioactive Compounds and Polyphenoloxidase Enzyme. Food Bioprod. Process. 2016, 98, 124–132. [Google Scholar] [CrossRef]
- García-Parra, J.; González-Cebrino, F.; Delgado-Adámez, J.; Cava, R.; Martín-Belloso, O.; Elez-Martínez, P.; Ramírez, R. Application of Innovative Technologies, Moderate-Intensity Pulsed Electric Fields and High-Pressure Thermal Treatment, to Preserve and/or Improve the Bioactive Compounds Content of Pumpkin. Innov. Food Sci. Emerg. Technol. 2018, 45, 53–61. [Google Scholar] [CrossRef]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Kovačević, D.B. Pulsed Electric Fields as an Alternative to Thermal Processing for Preservation of Nutritive and Physicochemical Properties of Beverages: A Review. J. Food Process Eng. 2018, 41, e12638. [Google Scholar] [CrossRef]
- Caili, F.U.; Huan, S.; Quanhong, L.I. A Review on Pharmacological Activities and Utilization Technologies of Pumpkin. Plant. Foods Hum. Nutr. 2006, 61, 73–80. [Google Scholar] [CrossRef]
- Garvey, M. Food Pollution: A Comprehensive Review of Chemical and Biological Sources of Food Contamination and Impact on Human Health. Nutrire 2019, 44, 1. [Google Scholar] [CrossRef]
- Dalsgaard, H.; Food, A.A.-E. Improving Energy Efficiency. In Environmentally-Friendly Food Processing; Woodhead Publishing Ltd.: Sawston, UK, 2003. [Google Scholar]
- Ayvaz, H.; Balasubramaniam, V.M.; Koutchma, T. High Pressure Effects on Packaging Materials. In High Pressure Processing of Food; Springer: New York, NY, USA, 2016; pp. 73–93. [Google Scholar] [CrossRef]
- Ayvaz, H.; Schirmer, S.; Parulekar, Y.; Balasubramaniam, V.M.; Somerville, J.A.; Daryaei, H. Influence of Selected Packaging Materials on Some Quality Aspects of Pressure-Assisted Thermally Processed Carrots during Storage. LWT-Food Sci. Technol. 2012, 46, 437–447. [Google Scholar] [CrossRef]
- Dhawan, S.; Barbosa-Cànovas, G.V.; Tang, J.; Sablani, S.S. Oxygen Barrier and Enthalpy of Melting of Multilayer EVOH Films after Pressure-Assisted Thermal Processing and during Storage. J. Appl. Polym. Sci. 2011, 122, 1538–1545. [Google Scholar] [CrossRef]
- Dhawan, S.; Varney, C.; Barbosa-Cánovas, G.V.; Tang, J.; Selim, F.; Sablani, S.S. Pressure-Assisted Thermal Sterilization Effects on Gas Barrier, Morphological, and Free Volume Properties of Multilayer EVOH Films. J. Food Eng. 2014, 128, 40–45. [Google Scholar] [CrossRef]
- Fleckenstein, B.S.; Sterr, J.; Langowski, H.-C. The Influence of High Pressure Treatment and Thermal Pasteurization the Surface of Polymeric Packaging Films. Packag. Technol. Sci. 2016, 29, 323–336. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A. Application of High-Pressure Processing and Polylactide/Cinnamon Oil Packaging on Chicken Sample for Inactivation and Inhibition of Listeria Monocytogenes and Salmonella Typhimurium, and Post-Processing Film Properties. Food Control 2017, 78, 160–168. [Google Scholar] [CrossRef]
- Yoo, S.; Holloman, C.; Tomasko, D.; Koelling, K.; Pascall, M.A. Effect of High Pressure Processing on the Thermal and Mechanical Properties of Polyethylene Films Measured by Dynamical Mechanical and Tensile Analyses. Packag. Technol. Sci. 2014, 27, 169–178. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; de Paiva Alves, J. High Pressure Technology Improves the Quality and Yield in the Seafood Industry. Available online: https://www.researchgate.net/publication/267509875_High_pressure_technology_improves_the_quality_and_yield_in_the_seafood_industry (accessed on 21 September 2021).
- Torres, J.A.; Velazquez, G. Commercial Opportunities and Research Challenges in the High Pressure Processing of Foods. J. Food Eng. 2005, 67, 95–112. [Google Scholar] [CrossRef]
- Abenoza, M.; Benito, M.; Saldaña, G.; Álvarez, I.; Raso, J.; Sánchez-Gimeno, A.C. Effects of Pulsed Electric Field on Yield Extraction and Quality of Olive Oil. Food Bioprocess Technol. 2013, 6, 1367–1373. [Google Scholar] [CrossRef]
- Arshad, R.N.; Buntat, Z.B.; Dastgheib, A.M.; Jusoh, Y.M.M.; Munir, A.; Aadil, R.M.; Ahmad, M.H. Continuous Flow Treatment Chamber for Liquid Food Processing through Pulsed Electric Field. J. Comput. Theor. Nanosci. 2020, 17, 1492–1498. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of High Hydrostatic Pressure and Ultrasound-Assisted Extractions as a Novel Approach for Pectin and Polyphenols Recovery from Tomato Peel Waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barros, L.; Carvalho, A.M.; Oliveira, M.B.P.P.; Saraiva, J.A.; Ferreira, I.C.F.R. Cold Extraction of Phenolic Compounds from Watercress by High Hydrostatic Pressure: Process Modelling and Optimization. Sep. Purif. Technol. 2018, 192, 501–512. [Google Scholar] [CrossRef]
- Duffuler, P.; Giarratano, M.; Naderi, N.; Suwal, S.; Marciniak, A.; Perreault, V.; Offret, C.; Brisson, G.; House, J.D.; Pouliot, Y.; et al. High Hydrostatic Pressure Induced Extraction and Selective Transfer of β-Phosvitin from the Egg Yolk Granule to Plasma Fractions. Food Chem. 2020, 321, 126696. [Google Scholar] [CrossRef]
- Huang, Y.L.; Tsai, Y.H. Extraction of Chitosan from Squid Pen Waste by High Hydrostatic Pressure: Effects on Physicochemical Properties and Antioxidant Activities of Chitosan. Int. J. Biol. Macromol. 2020, 160, 677–687. [Google Scholar] [CrossRef]
- Jun, X. Caffeine Extraction from Green Tea Leaves Assisted by High Pressure Processing. J. Food Eng. 2009, 94, 105–109. [Google Scholar] [CrossRef]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and High Pressure Assisted Extraction of Carotenoids from Tomato Waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Naderi, N.; Pouliot, Y.; House, J.D.; Doyen, A. High Hydrostatic Pressure Effect in Extraction of 5-Methyltetrahydrofolate (5-MTHF) from Egg Yolk and Granule Fractions. Innov. Food Sci. Emerg. Technol. 2017, 43, 191–200. [Google Scholar] [CrossRef]
- Pagan, R.J.; Prasad, P. Eco-Efficiency, Water Conservation and Food Processing in Australia—UQ eSpace. 2005. Available online: https://espace.library.uq.edu.au/view/UQ:102827 (accessed on 13 July 2021).
- Atuonwu, J.C.; Tassou, S.A. Energy Issues in Microwave Food Processing: A Review of Developments and the Enabling Potentials of Solid-State Power Delivery. Crit. Rev. Food Sci. Nutr. 2018, 59, 1392–1407. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.K.; Zerdin, K.; Howe, E.; Goicoechea, D.; Paramanandhan, P.; Stockman, R.; Sellahewa, J.; Szabo, E.A.; Johnson, R.L.; Stewart, C.M. The Effect of High Pressure Processing on the Microbial, Physical and Chemical Properties of Valencia and Navel Orange Juice. Innov. Food Sci. Emerg. Technol. 2004, 5, 135–149. [Google Scholar] [CrossRef]
- Landl, A.; Abadias, M.; Sárraga, C.; Viñas, I.; Picouet, P.A. Effect of High Pressure Processing on the Quality of Acidified Granny Smith Apple Purée Product. Innov. Food Sci. Emerg. Technol. 2010, 11, 557–564. [Google Scholar] [CrossRef]
- Kowalczyk, W.; Hartmann, C.; Delgado, A. Freezing and Thawing at the High Hydrostatic Pressure Conditions—Modelling and Numerical Simulation. PAMM 2003, 3, 388–389. [Google Scholar] [CrossRef]
- Pham, Q.T. Advances in Food Freezing/Thawing/Freeze Concentration Modelling and Techniques. Jpn. J. Food Eng. 2008, 9, 21–32. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Zhang, H.Q.; Geveke, D.J. Cost Analysis of Commercial Pasteurization of Orange Juice by Pulsed Electric Fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 72–78. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Geveke, D.J. Cost Analysis and Environmental Impact of Pulsed Electric Fields and High Pressure Processing in Comparison with Thermal Pasteurization. Food Bioprocess Technol. 2014, 7, 1928–1937. [Google Scholar] [CrossRef]
- Huang, Y.; Gan, Y.; Li, F.; Yan, C.; Li, H.; Feng, Q. Effects of High Pressure in Combination with Thermal Treatment on Lipid Hydrolysis and Oxidation in Pork. LWT-Food Sci. Technol. 2015, 63, 136–143. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and Challenges in High Pressure Processing of Foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, R.; Balasubramaniam, V.; Kaletun, G. High Pressure Processing: Fact Sheet for Food Processors. Available online: https://blog.ideasinfood.com/files/hppfactsheet.pdf (accessed on 8 August 2021).
| Applications | Treatment Conditions | Food Sample | Treatment Effects | References | |
|---|---|---|---|---|---|
| Meat processing | 175–600 MPa, 3–5 min, | Meat, Meat Products, and Seafood | Showed minimal effect on nutrients and sensorial characters. Slowed down microbial growth and reduced the activity rate of spoilage bacteria | [29,30,31] | |
| Microbial reduction | 300–600 MPa, 5–10 min | Meat, juices, milk products | Caused significant reductions in microbes in food items, i.e., about a 1.6–5-log reduction | [32,33,34] | |
| Extraction | 250–500 MPa, 5–15 min | Seeds, fruits, vegetables, plants, cereals | carbohydrates, bioactive compounds | Extraction yields were enhanced by HPP compared with the conventional method | [35,36,37,38,39] |
| Pretreatment | 200–300 MPa, 2–6 min | Meat, fruits, vegetables | HPP as a pretreatment improved textural, nutritional, and sensorial attributes | [40,41,42,43] | |
| Seed treatment | 200–400 MPa, 10–60 min 20–60 °C | Moringa oleifera kernels, Basil, chia seeds | HPP enhanced the extraction of oil as well as the structure of seeds | [44,45,46] |
| Applications | Target Pressure | Food Samples | References |
|---|---|---|---|
| Pasteurization | (300–600 MPa) | Meat, Milk, fruit juices, cereals | [61,62] |
| Sterilization | (500–900 MPa) | Fruits, macaroni, cheese | [63] |
| Extraction | (250–500 MPa) | Seeds, fruits | [35] |
| Pretreatment | (200–600 MPa) | Meat, vegetables | [40] |
| Contributions | Application | Treatment Conditions | Contributions Toward Sustainability | References |
|---|---|---|---|---|
| Food safety | Nutritional Improvement | 200–600 MPa, 5–15 min | Improved nutritional value by HPP | [69] |
| Food preservation | 200–400 MPa, ˃2 min | More efficiently used for pasteurization of liquid foods and dehydration of solid foods | [70] | |
| Reduction of food contaminants | 30 to 500 MPa, 30–50 °C | Helped to decrease food contaminants and toxins | [59] | |
| Solid food pretreatment | 200 MPa, 0–6 min | Maintained texture and color | [48] | |
| Environmental sustainability | Reduced food waste | 300 MPa, 3 min | Contribution to reducing food waste | [71] |
| Food packaging | Effects on food packaging | 600 a, 60 min; 800 MPa, 10 min, 60 °C | Minimal effect on packaging material | [72] |
| Economic sustainability | Profit attained | 356 and 600 MPa, 30 min | Contributed to the recovery of valuable components from food | [73] |
| Water efficiency | Minimal water consumption | Not only energy saving but also water-saving technology | [74] | |
| Energy efficiency | Minimal energy consumption | 300–700 MPa | Utilised less energy than conventional techniques | [75] |
| Food security | Enhanced yield | 200 and 500 MPa | HPP improved the food yield | [76] |
| Food Sample | Target Product | Treatment Conditions | Log Reduction | References |
|---|---|---|---|---|
| Coconut water | Clostridium botulinum type E and Clostridium sporogenes | 550 MPa, 3 min, 10 °C | 3.0 ± 0.1 | [95] |
| Açaí juice | Bacteria | 600 MPa, 3 min | 5–6 | [96] |
| Whole milk and skim milk | Cronobacter sakazakii | 300 MPa, 400 MPa, 5.0 min | No detection | [97] |
| Cucumber juice | Yeast and mold, total aerobic bacteria | 500 MPa 5 min | 1.35–1.94 | [98] |
| Beetroot and carrot juice | E. coli | 300–500 MPa | No detection | [99] |
| Raw milk | Pathogens | 600 MPa, 3 min | 5 | [100] |
| Acidic fruit juices | Alicyclobacillus acidoterrestris | 600 MPa, 80 °C, 3 min | 2.0–2.5 | [101] |
| Wines | Brettanomyces bruxellensis | 200 MPa, up to 3 min, | 1.1–5.1 | [102] |
| Food Nutrients | Food Sample | Treatment Conditions | Treatment Effects | References |
|---|---|---|---|---|
| Fatty acid | Caprine milk | 200–500 MPa | No marked differences in the fatty acid profile, excluding an increase in branched-chain fatty acids | [151] |
| Free amino acids | Serrano dry-cured ham | 600 MPa, 6 min, 4 °C | No effect of HPP on total FAA levels | [152] |
| Protein | Milk | 150–450 MPa, 15 min | The disintegration of casein micelles at ˃250 MPa; serum proteins were denatured | [153] |
| Free amino acids | Chinese rice wine | 400 MPa or 600 MPa 10 min | HPP increased the free amino acid content | [154] |
| Carotenoids | Cloudy carrot juice | 300, 450, 600 MPa, 5 min, ≈22 °C | The highest carotenoid degradation (41%) occurred at 350 MPa, whereas the lowest (26%) occurred at 600 MPa | [155] |
| Polyphenols | Kiwi berry | 450, 550 or 650 MPa, 5 or 15 min | Caused a significant increase in the individual polyphenol content | [156] |
| Anthocyanins, non-anthocyanin phenolics, tocopherols | Acai juice | 400, 450, 500 and 600 MPa, 5 min, 20 °C | Efficient preservation technique for anthocyanins compared to thermal pasteurization (up to 40%) | [157] |
| Polyphenols, Enzymes | Carrot juice | 450 and 600 MPa, 5 min, ≈ 22 °C | At 300 MPa, maximum inactivation of (PPO) enzymes (57%) was achieved, and at 600 MPa, about 31% inactivation of peroxidase (POD) enzymes was observed. Significant changes in the color parameters and the browning index were observed | [77] |
| Anthocyanin, polyphenol oxidase, and β-glucosidase | Blueberry | 300 MPa | HPP treatment resulted in better puree colour retention than the conventional treatment | [158] |
| Total phenolic compounds, vitamin C | Strawberry | 100 and 500 MPa, 10 min | At 400 MPa, the maximum total phenolic content was obtained; preserved vitamin C content in strawberry | [49] |
| Oxidoreductive enzymes | Mushroom | 200–900 MPa, 5–45 °C, 1–15 min | HPP used in the experiments significantly (p < 0.05) decreased PPO and POD activities, with a greater decrease in the relative activity (RA) of the enzymes observed when the pressure was increased | [159] |
| Carbohydrate hydrolysing enzymes | Lemon flavedo | 400 MPa, 10 min | HPP-treated samples had high levels of carbohydrates Hydrolyzing enzyme inhibition | [160] |
| Polyphenol oxidase | Pawpaw | 600 MPa, 4 °C, 76 s | HPP significantly decreased PPO activity. HPP was proved to be an effective technique for the longer shelf-life of fresh-packaged pawpaw pulp | [161] |
| Components | Sources | Treatment Conditions | Yield | References |
|---|---|---|---|---|
| Polyphenols and anthocyanins | Blueberry pomace | 500 MPa | A marked increase in the yield of polyphenols and anthocyanins was observed, about 70% and 40%, using HPP compared with the conventional heating method (53% and 32%) | [175] |
| Tomatoes | Below 100 MPa | A marked increase in the yield of tomatoes occurred, i.e., >60% | [176] | |
| Astaxanthin | Shrimp Carapace | 210 MPa | Increased the yield from 29.44% to 59.97% by HPP | [177] |
| Cheese | Milk | 250–600 MPa | A 15% increase in the yield of cheese was obtained | [178] |
| M. oleifera oil | M. oleifera (MO) kernels | 50–250 MPa | Free oil (73% w/w) was recovered from ground-sieved kernels by using HPP compared with AEE alone | [44] |
| Food Waste | Treatment Conditions | Recovered Compounds | References |
|---|---|---|---|
| Orange peel | 300 and 500 MPa | Polyphenols | [71] |
| Tomato pulp | 450 MPa | Lycopene, Flavonoids | [196] |
| Cape gooseberry pulp | 300–500 MPa | Bioactive compounds | [197] |
| Grape pomace | 200 MPa | Phenolic compounds | [198] |
| Pumpkin puree | 600 MPa, 70 °C | Bioactive compounds | [199] |
| Components | Sources | Treatment Conditions | Production | References |
|---|---|---|---|---|
| Pectin and polyphenols | Tomato peel waste | 30 and 45 min | Enhanced pectin recovery by about 15% compared with conventional extraction | [216] |
| Phenolic compounds | Watercress | 3.1 min, 600 MPa | 64.68 ± 2.97 mg/g yield recovered | [217] |
| Phosvitin | Egg yolk | 400 and 600 MPa, 5 and 10 min | Phosvitin recovery was maximum at 600 MPa | [218] |
| Chitosan | Squid pen waste | 500 MPa, 10 min | Maximum yield of 81.9% | [219] |
| Xyloglucan | Tamarind seed | 250–500 MPa | Yields were about 51.6–53.0% higher than the conventional yield (46.4%) | [35] |
| Caffeine | Green tea leaves | 500 MPa, 1 min | Maximum yields: 4.0 ± 0.22% | [220] |
| Lycopene | Tomato waste | 700 MPa, 30 min | Maximum yield: 89.4 mg/kg | [221] |
| 5-methyltetrahydrofolate | Egg yolk | 400 MPa, 5 min | Maximum recovery: 93% | [222] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabi, B.G.; Mukhtar, K.; Arshad, R.N.; Radicetti, E.; Tedeschi, P.; Shahbaz, M.U.; Walayat, N.; Nawaz, A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-Pressure Processing for Sustainable Food Supply. Sustainability 2021, 13, 13908. https://doi.org/10.3390/su132413908
Nabi BG, Mukhtar K, Arshad RN, Radicetti E, Tedeschi P, Shahbaz MU, Walayat N, Nawaz A, Inam-Ur-Raheem M, Aadil RM. High-Pressure Processing for Sustainable Food Supply. Sustainability. 2021; 13(24):13908. https://doi.org/10.3390/su132413908
Chicago/Turabian StyleNabi, Brera Ghulam, Kinza Mukhtar, Rai Naveed Arshad, Emanuele Radicetti, Paola Tedeschi, Muhammad Umar Shahbaz, Noman Walayat, Asad Nawaz, Muhammad Inam-Ur-Raheem, and Rana Muhammad Aadil. 2021. "High-Pressure Processing for Sustainable Food Supply" Sustainability 13, no. 24: 13908. https://doi.org/10.3390/su132413908
APA StyleNabi, B. G., Mukhtar, K., Arshad, R. N., Radicetti, E., Tedeschi, P., Shahbaz, M. U., Walayat, N., Nawaz, A., Inam-Ur-Raheem, M., & Aadil, R. M. (2021). High-Pressure Processing for Sustainable Food Supply. Sustainability, 13(24), 13908. https://doi.org/10.3390/su132413908












