Flame Retardance and Char Analysis of an Eco-Friendly Polyurethane Hyperbranched Hybrid Using the Sol–Gel Method
Abstract
1. Introduction
2. Experimental
2.1. Materials
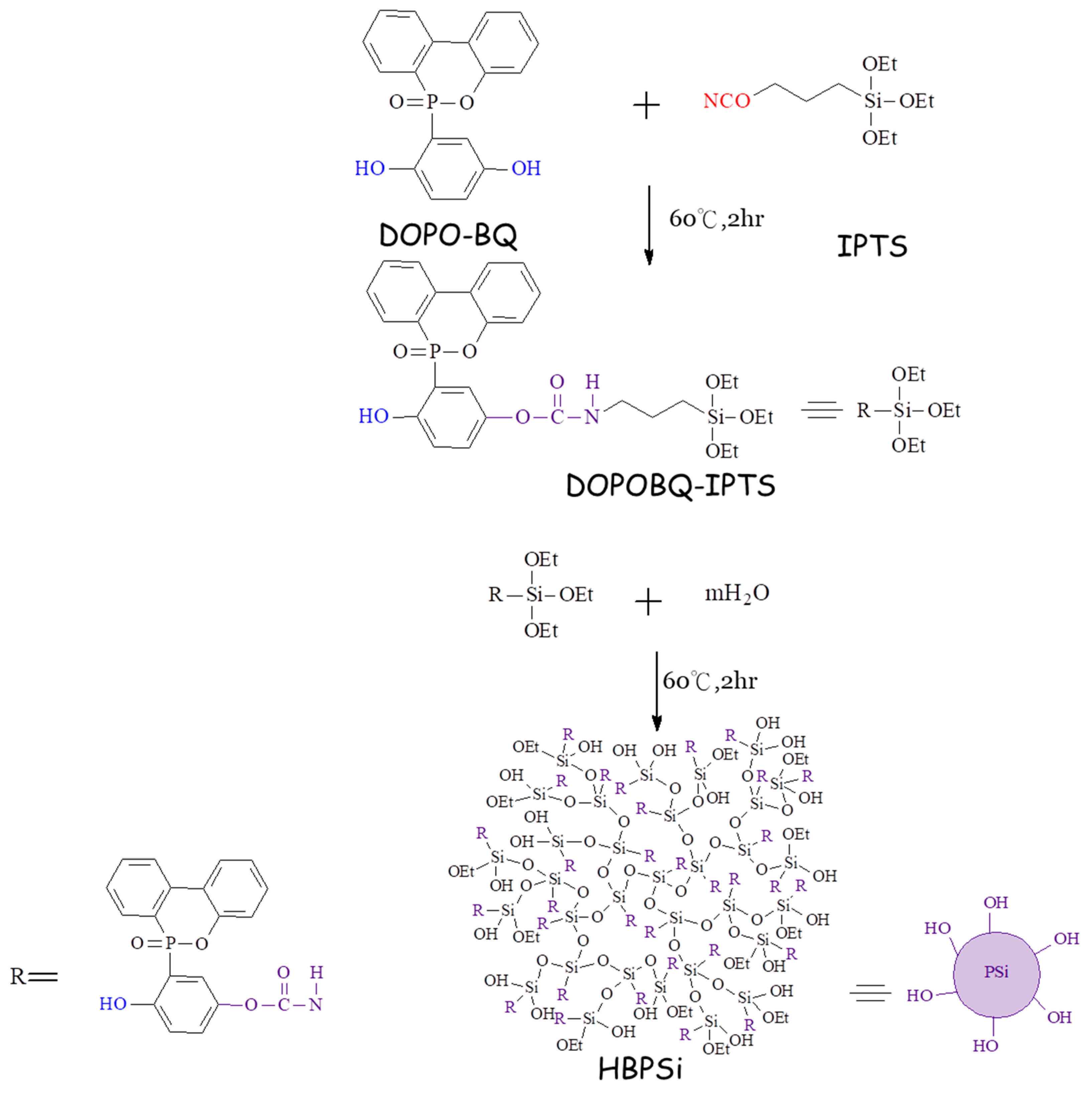
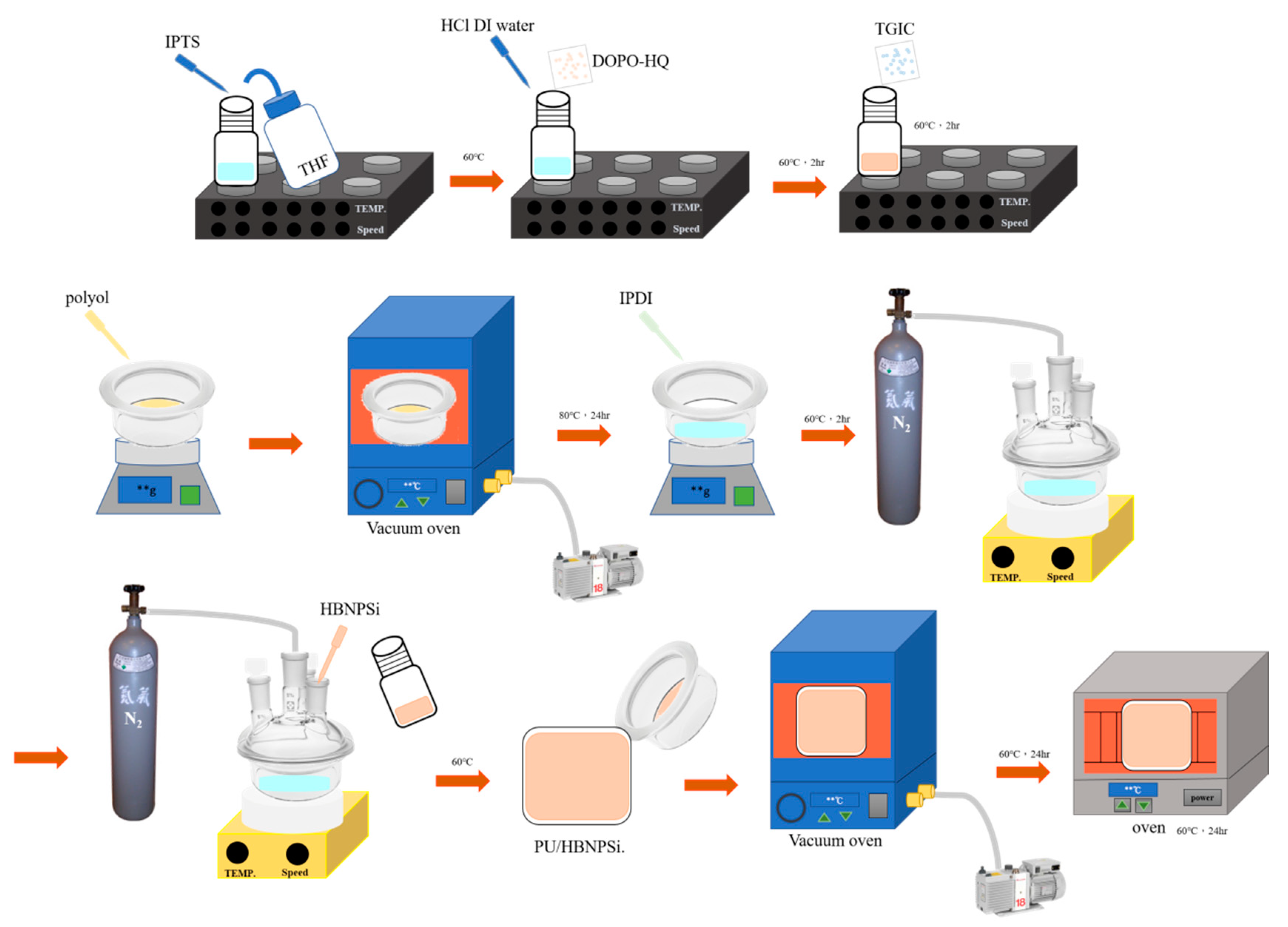
2.2. Preparation of DOPOBQ-IPTS-TGIC
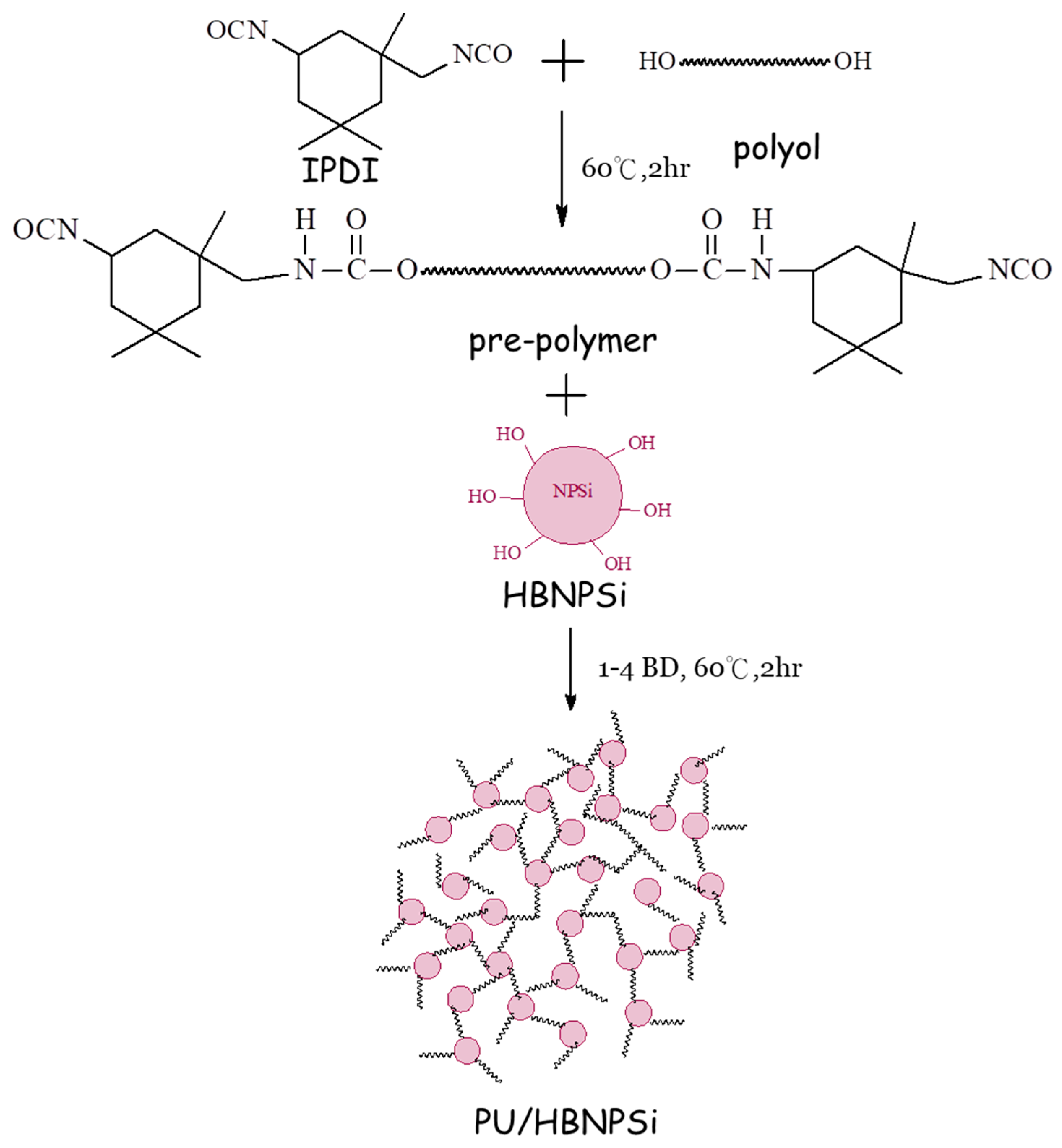
2.3. Preparation of PU/HBNPSi Hybrid
2.4. Measurements
3. Results and Discussion
3.1. 29Si NMR
3.2. P- and Si-Mapping of EDX
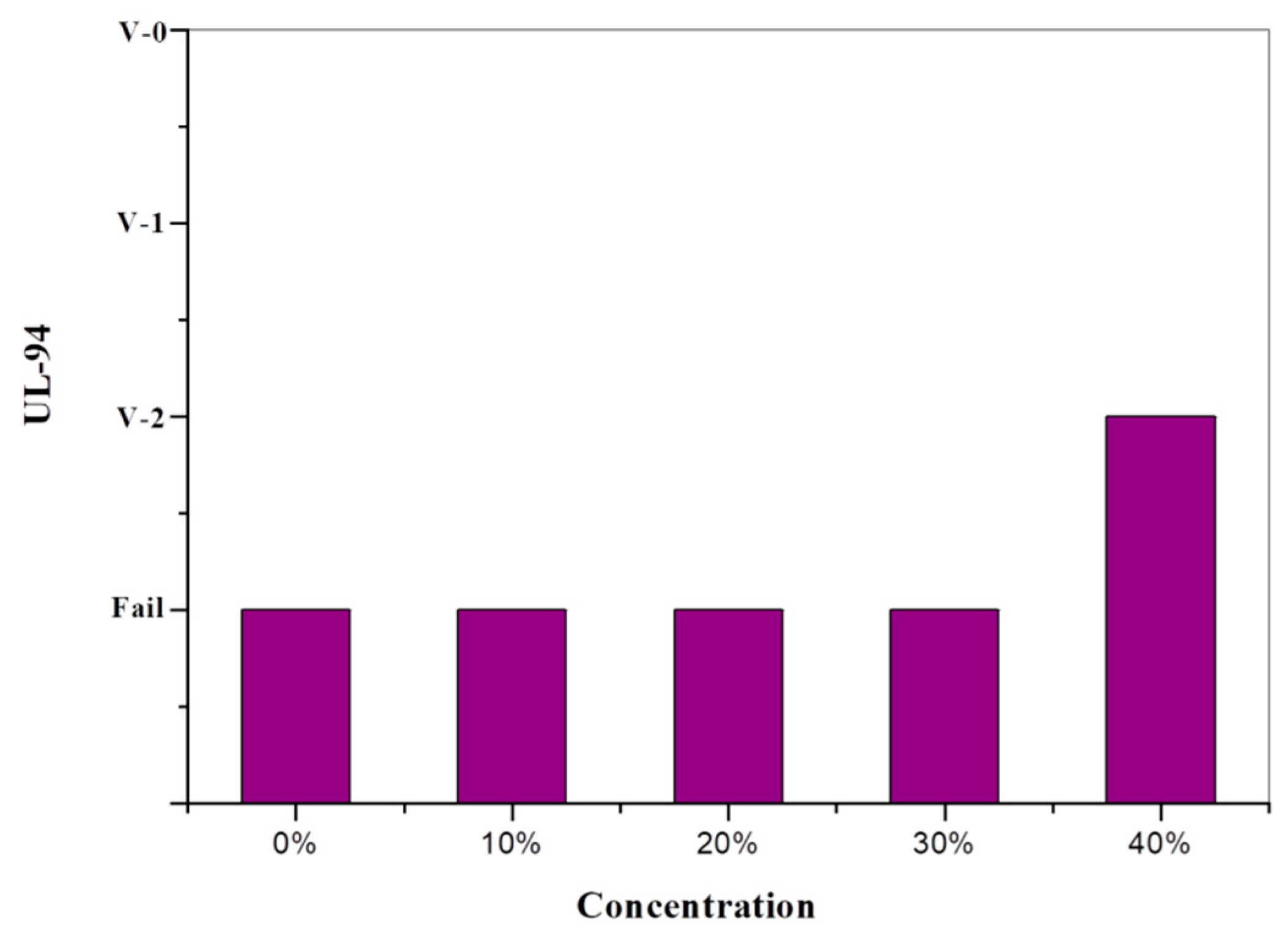
3.3. Flame Retardance Analysis
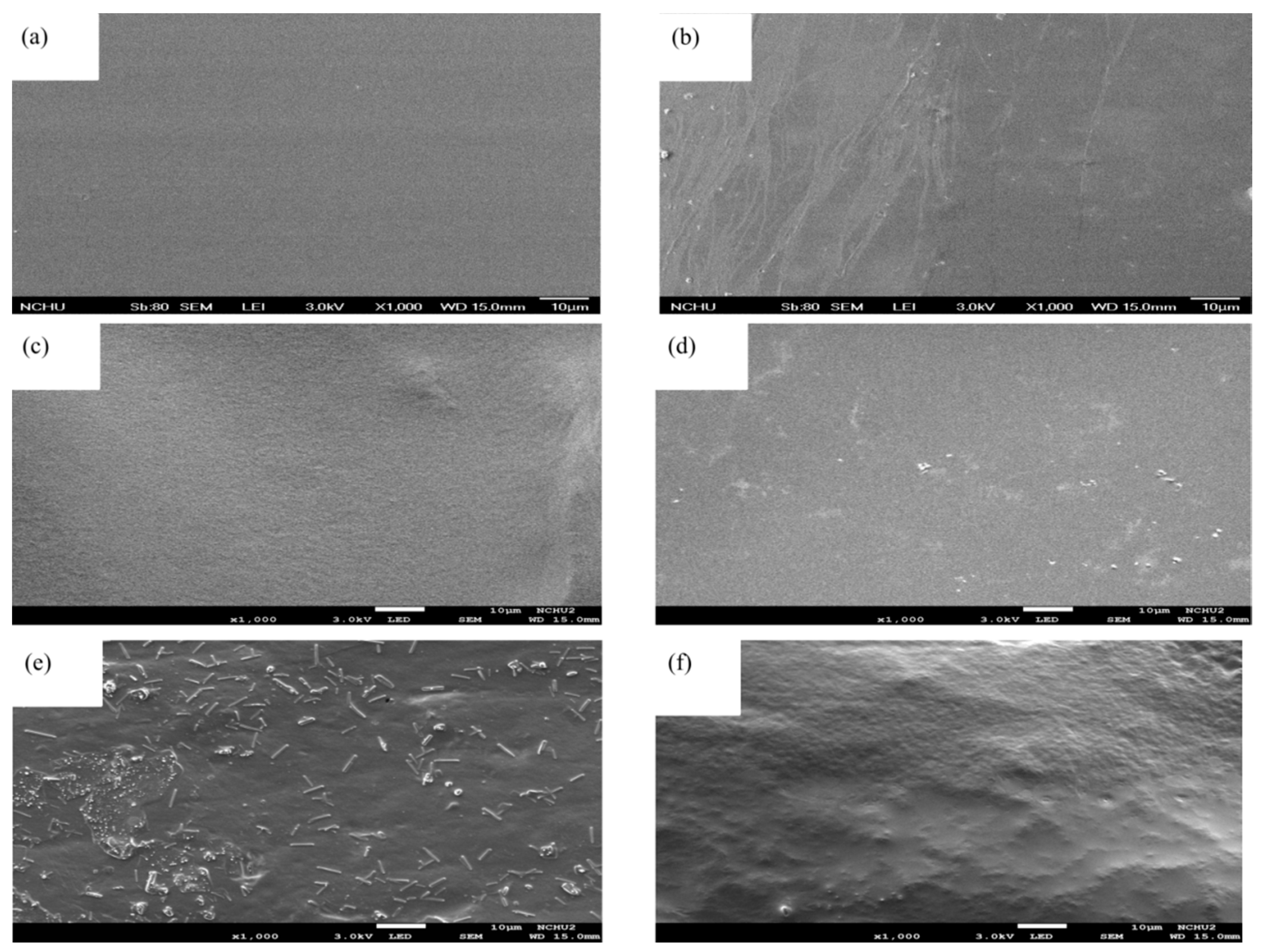
3.4. Morphology Analysis
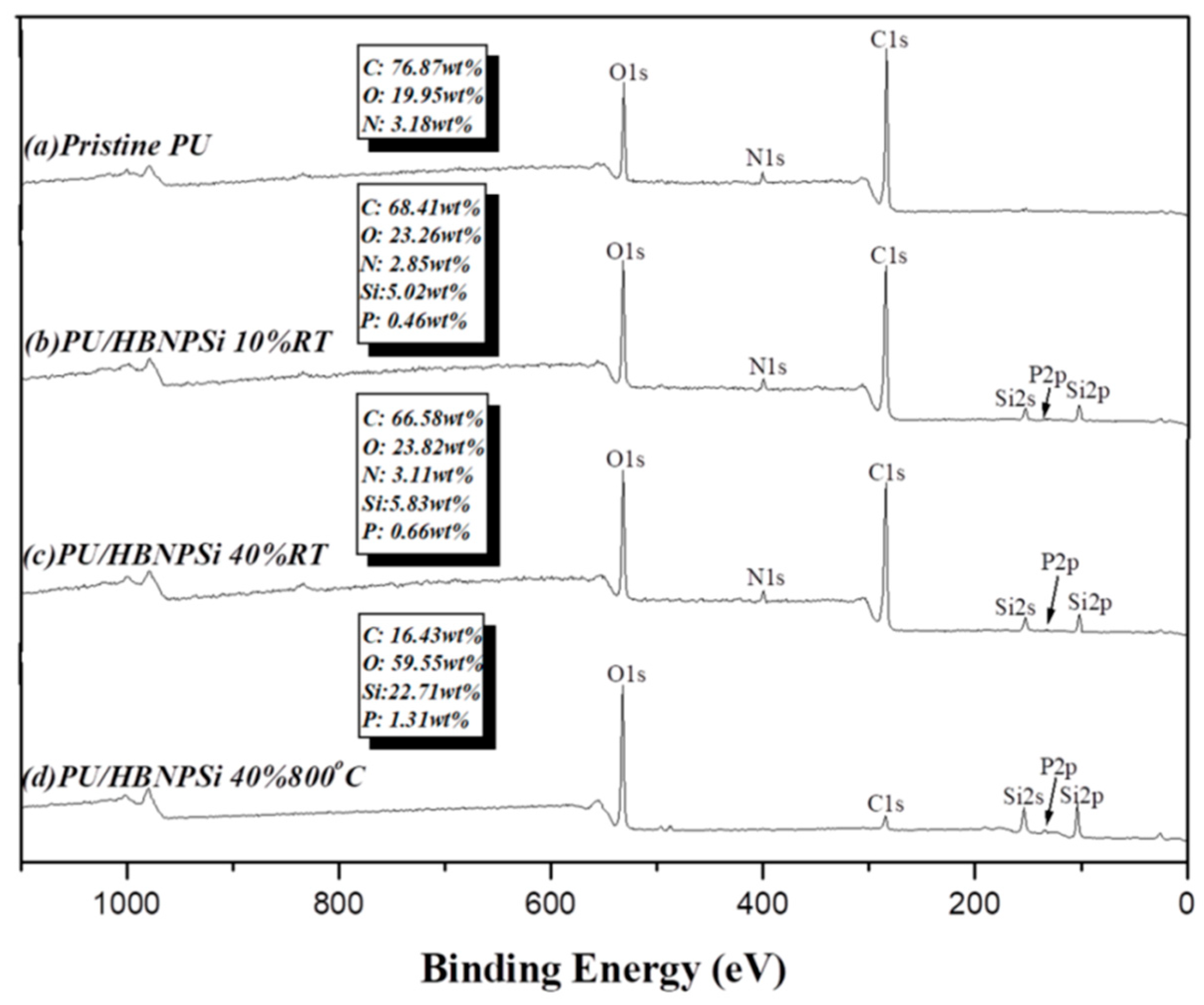
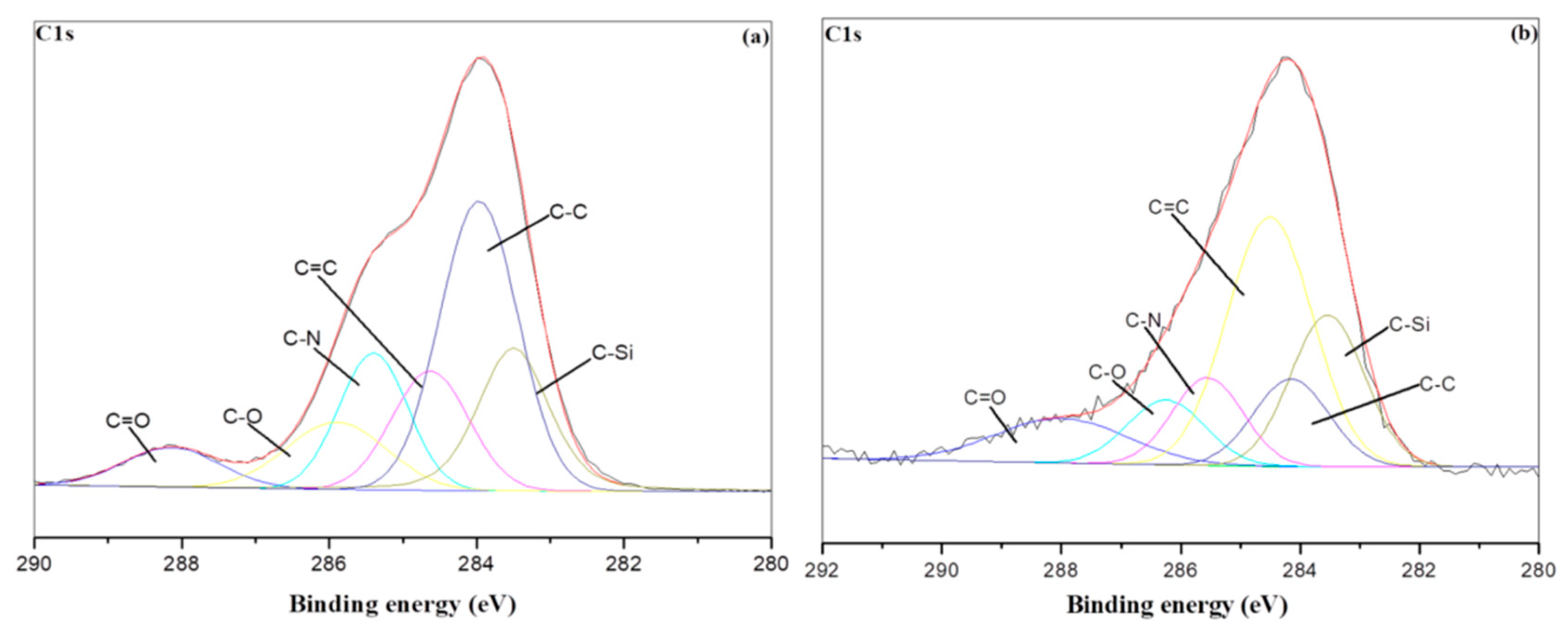
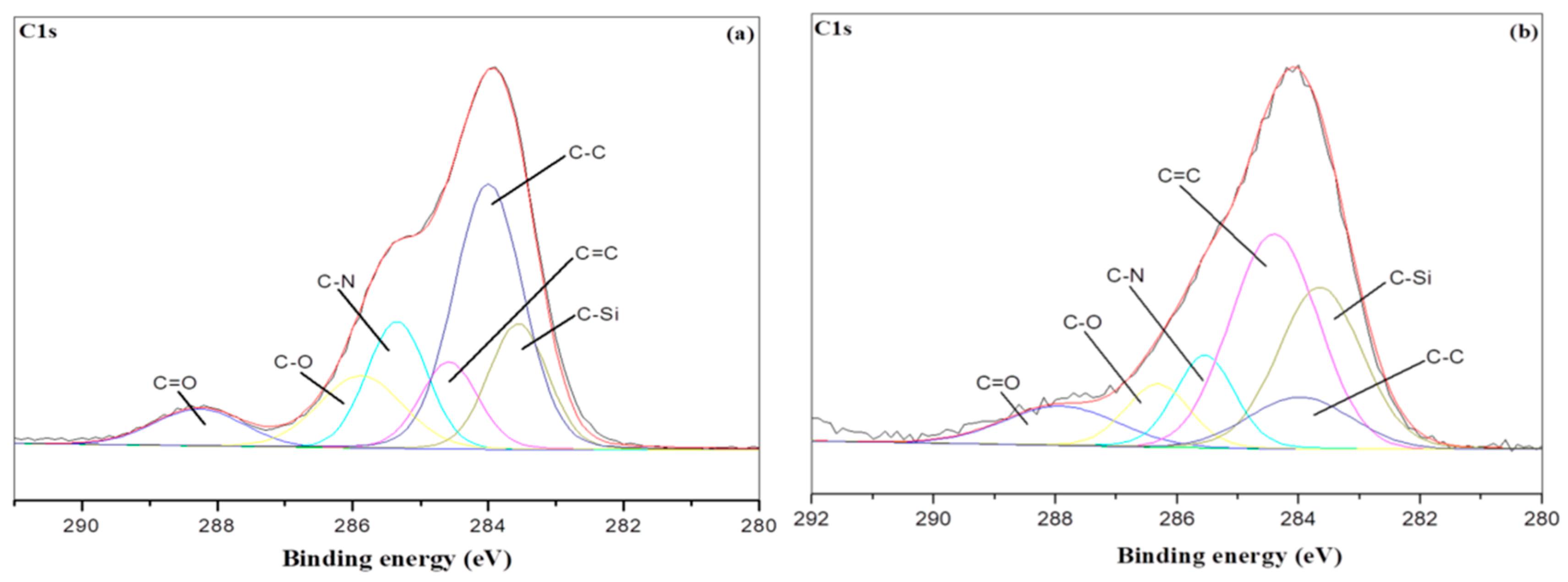
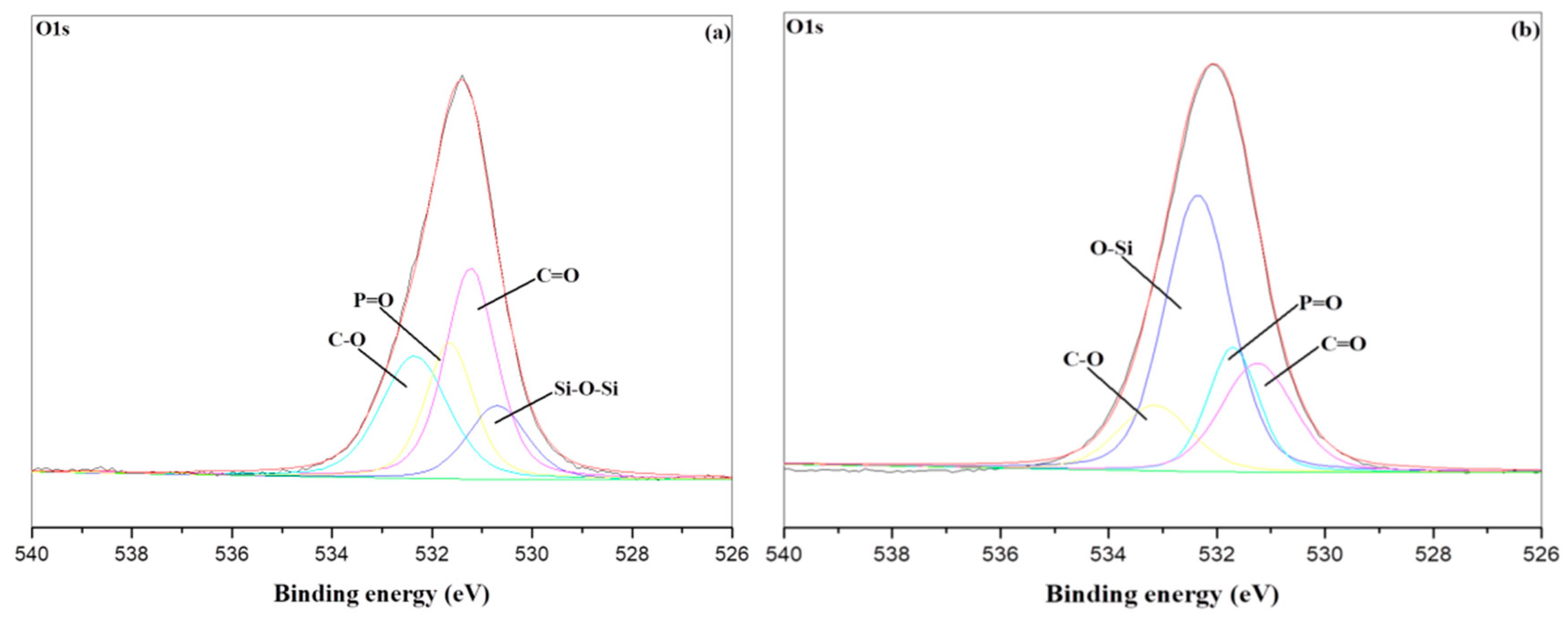
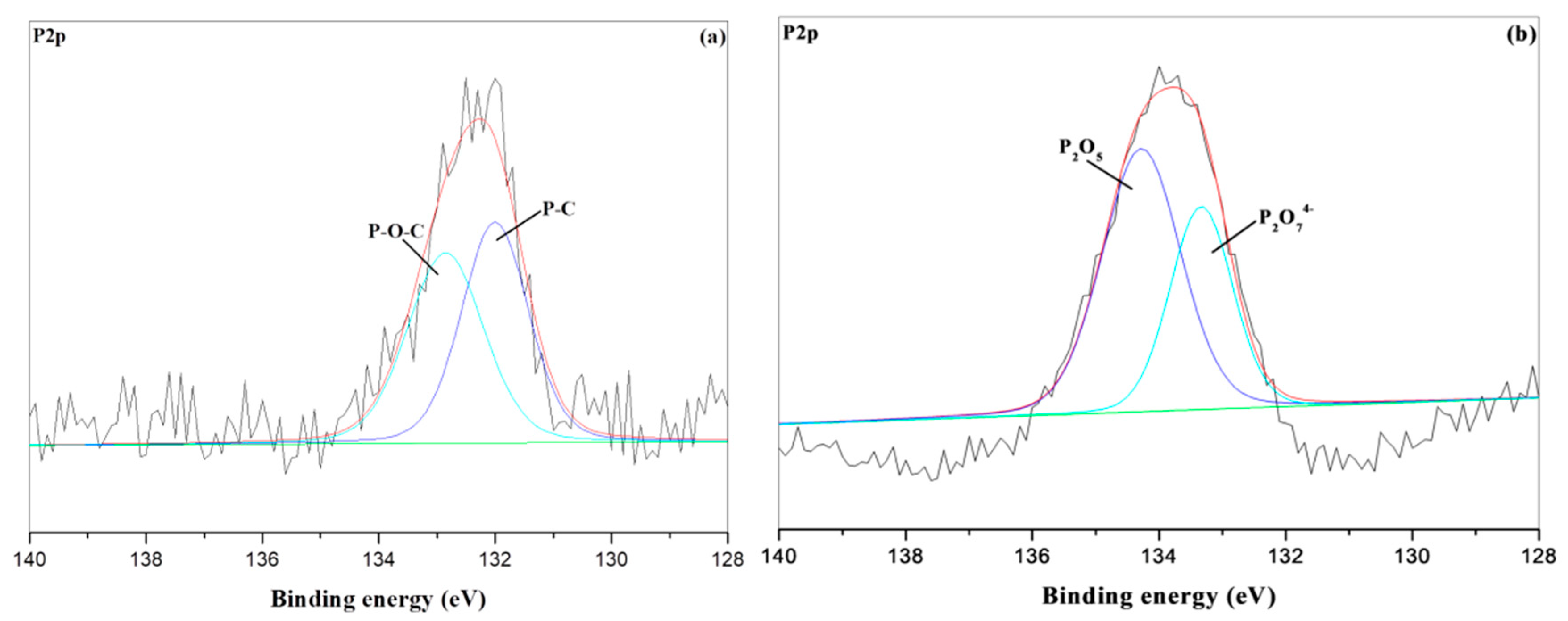
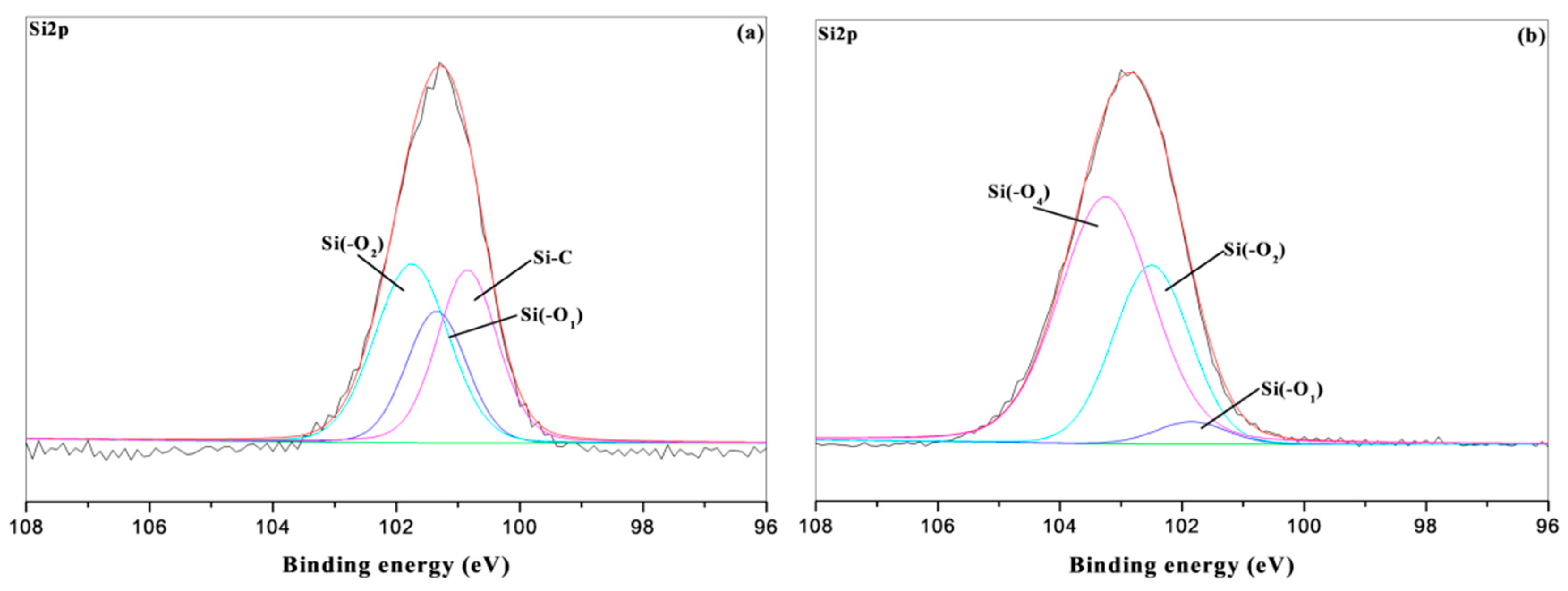
3.5. XPS Char Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.; Yadav, R.; Kolhe, M.A.; Bhosale, R.S.; Narayan, R. 8-Hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) based high fluorescent, pH stimuli waterborne polyurethane coatings. Polymer 2018, 136, 157–165. [Google Scholar] [CrossRef]
- Bedő, D.; Imre, B.; Domján, A.; Schöne, P.; Vancsoe, G.J.; Pukánszky, B. Coupling of poly(lactic acid) with a polyurethane elastomer by reactive processing. Eur. Polym. J. 2017, 97, 409–417. [Google Scholar] [CrossRef]
- Zhu, S.W.; Shi, W.F. Flame retardant mechanism of hyperbranched polyurethane acrylates used for UV curable flame retardant coatings. Polym. Degrad. Stab. 2002, 75, 543–547. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Zhu, T.; Hua, Z.; Chen, Q.; Yu, X. Blends of thermoplastic polyurethane and polyether–polyimide: Preparation and properties. Polymer 2001, 42, 1493–1500. [Google Scholar] [CrossRef]
- Chen, X.; Ma, C.; Jiao, C. Enhancement of flame-retardant performance of thermoplastic polyurethane with the incorporation of aluminum hypophosphite and iron-graphene. Polym. Degrad. Stab. 2016, 129, 275–285. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Modesti, M.; Besco, S.; Hrelja, D.; Donadi, S. Influence of phosphorus valency on thermal behaviour of flame retarded polyurethane foams. Polym. Degrad. Stab. 2011, 96, 1455–1461. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, Q.; Zhang, N.; Guo, X.; Yan, H. Preparation of a novel polysiloxane and its synergistic effect with ammonium polyphosphate on the flame retardancy of polypropylene. Polym. Degrad. Stab. 2018, 150, 73–85. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, G.; Huang, X.; Bu, J.; Sun, X.; Jiang, P. Molecular structures of (3-aminopropyl)trialkoxysilane on hydroxylated barium titanate nanoparticle surfaces induced by different solvents and their effect on electrical properties of barium titanate based polymer nanocomposites. Appl. Surf. Sci. 2016, 364, 798–807. [Google Scholar] [CrossRef]
- Luo, H.; Rao, W.; Zhao, P.; Wang, L.; Liu, Y.; Yu, C. An efficient organic/inorganic phosphorus-nitrogen-silicon flame retardant towards low-flammability epoxy resin. Polym. Degrad. Stab. 2020, 178, 109195. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Jiang, Y.; He, H.; Liu, H.; Huang, H.; Wan, C. Facile synthesis of a novel transparent hyperbranched phosphorous/ nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 379, 120793. [Google Scholar] [CrossRef]
- Duan, L.; Yang, H.; Song, L.; Hou, Y.; Wang, W.; Gui, Z.; Hu, Y. Hyperbranched phosphorus/nitrogen-containing polymer in combination with ammonium polyphosphate as a novel flame retardant system for polypropylene. Polym. Degrad. Stab. 2016, 134, 179–185. [Google Scholar] [CrossRef]
- Jin, Q.F.; Liao, G.X.; Jian, X.G. Synthesis and characterization of trimethoxysilyl-functionalized poly(phthalazinone ether ketone). Chin. Chem. Lett. 2007, 8, 1137–1140. [Google Scholar] [CrossRef]
- Paquet, O.; Salon, M.C.B.; Zeno, E.; Belgacem, M.N. Hydrolysis-condensation kinetics of 3-(2-amino-ethylamino)propyl-trimethoxysilane. Mater. Sci. Eng. C 2012, 32, 487–493. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Ramos, J.M.; Klein, R.; Lucas, A.D.; Rodriguez, F.J. Thermal degradation and fire behaviour of novel polyurethanes based on phosphate polyols. Polym. Degrad. Stab. 2014, 101, 40–51. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G. The novel silicon-containing epoxy/PEPA phosphate flame retardantfor transparent intumescent fire resistant coating. Appl. Surf. Sci. 2016, 385, 453–463. [Google Scholar] [CrossRef]
- Wu, C.S.; Liu, Y.L.; Chiu, Y.S. Epoxy resins possessing flame retardant elements from silicon incorporated epoxy compounds cured with phosphorus or nitrogen containing curing agents. Polymer 2002, 43, 4277–4284. [Google Scholar] [CrossRef]
- Liu, W.; Chen, D.Q.; Wang, Y.Z.; Wang, D.Y.; Qu, M.H. Char-forming mechanism of a novel polymeric flame retardant with char agent. Polym. Degrad. Stab. 2007, 92, 1046–1052. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Jiao, C.; Song, L. Preparation and thermal properties of a novel flame-retardant coating. Polym. Degrad. Stab. 2007, 92, 1141–1150. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, P.; Qian, Y.; Liu, J. The synthesis of a novel graphene-based inorganic–organic hybrid flame retardant and its application in epoxy resin. Composites B 2014, 60, 341–349. [Google Scholar] [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Lu, C.H.; Yang, S.Y.; Lee, S.H.; Hsiao, M.C.; Yen, M.Y.; Chiou, K.C.; Lee, T.M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Zhou, S.; Song, L.; Wang, Z.; Hu, Y.; Xing, W. Flame retardation and char formation mechanism of intumescent flame retarded polypropylene composites containing melamine phosphate and pentaerythritol phosphate. Polym. Degrad. Stab. 2008, 93, 1799–1806. [Google Scholar] [CrossRef]
- Dutkiewicz, M.; Przybylak, M.; Januszewski, R.; Maciejewski, H. Synthesis and flame retardant efficacy of hexakis(3-(triethoxysilyl)propyloxy)cyclotriphosphazene/silica coatings for cotton fabrics. Polym. Degrad. Stab. 2018, 148, 10–18. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.J. Enhanced hydrolysis resistance of biodegradable polymers and bio-composites. Polym. Degrad. Stab. 2008, 93, 1544–1553. [Google Scholar] [CrossRef]
- Kannan, G.A.; Choudhury, N.R.; Dutta, K.N. Synthesis and characterization of methacrylate phospho-silicate hybrid for thin film applications. Polymer 2007, 48, 7078–7086. [Google Scholar] [CrossRef]
- Chana, M.L.; Lau, K.T.; Wong, T.T.; Cardona, F. Interfacial bonding characteristic of nanoclay/polymer composites. Appl. Surf. Sci. 2011, 258, 860–864. [Google Scholar] [CrossRef]
- Chai, B.; Yan, J.; Wang, C.; Ren, Z.; Zhu, Y. Enhanced visible light photocatalytic degradation of Rhodamine B over phosphorus doped graphitic carbon nitride. Appl. Surf. Sci. 2017, 391, 376–383. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Fan, H.; Yang, R. Study on mechanism of phosphorus–silicon synergistic flame retardancy on epoxy resins. Polym. Degrad. Stab. 2012, 97, 2241–2248. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, Y.P.; He, M.X.; Wang, X.L.; Chen, L.; Wang, Y.Z. Phosphorus-containing copolyesters: The effect of ionic group and its analogous phosphorus heterocycles on their flame-retardant and anti-dripping performances. Polymer 2015, 60, 50–61. [Google Scholar] [CrossRef]
- Huang, C.; Puziy, M.A.; Sun, T.; Poddubnaya, I.O.; Fabián, S.G.; Juan, M.D.T.; Denisa, H.J. Capacitive behaviours of phosphorus-rich carbons derived from lignocelluloses. Electrochim. Acta 2014, 137, 219–227. [Google Scholar] [CrossRef]
- Brookes, N.P.; Fraser, S.; Short, D.R.; Hanley, L.; Fuoco, E.; Roberts, A.; Hutton, S. The effect of ion energy on the chemistry of air-aged polymer films grown from the hyperthermal polyatomic ion Si2OMe5+. J. Electron. Spectrosc. Relat. Phenom. 2001, 121, 281–297. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Li, J. Synergistic flame retarded poly(methyl methacrylate) by nano-ZrO2 and triphenylphosphate. J. Therm. Anal. Calorim. 2011, 103, 741–746. [Google Scholar] [CrossRef]

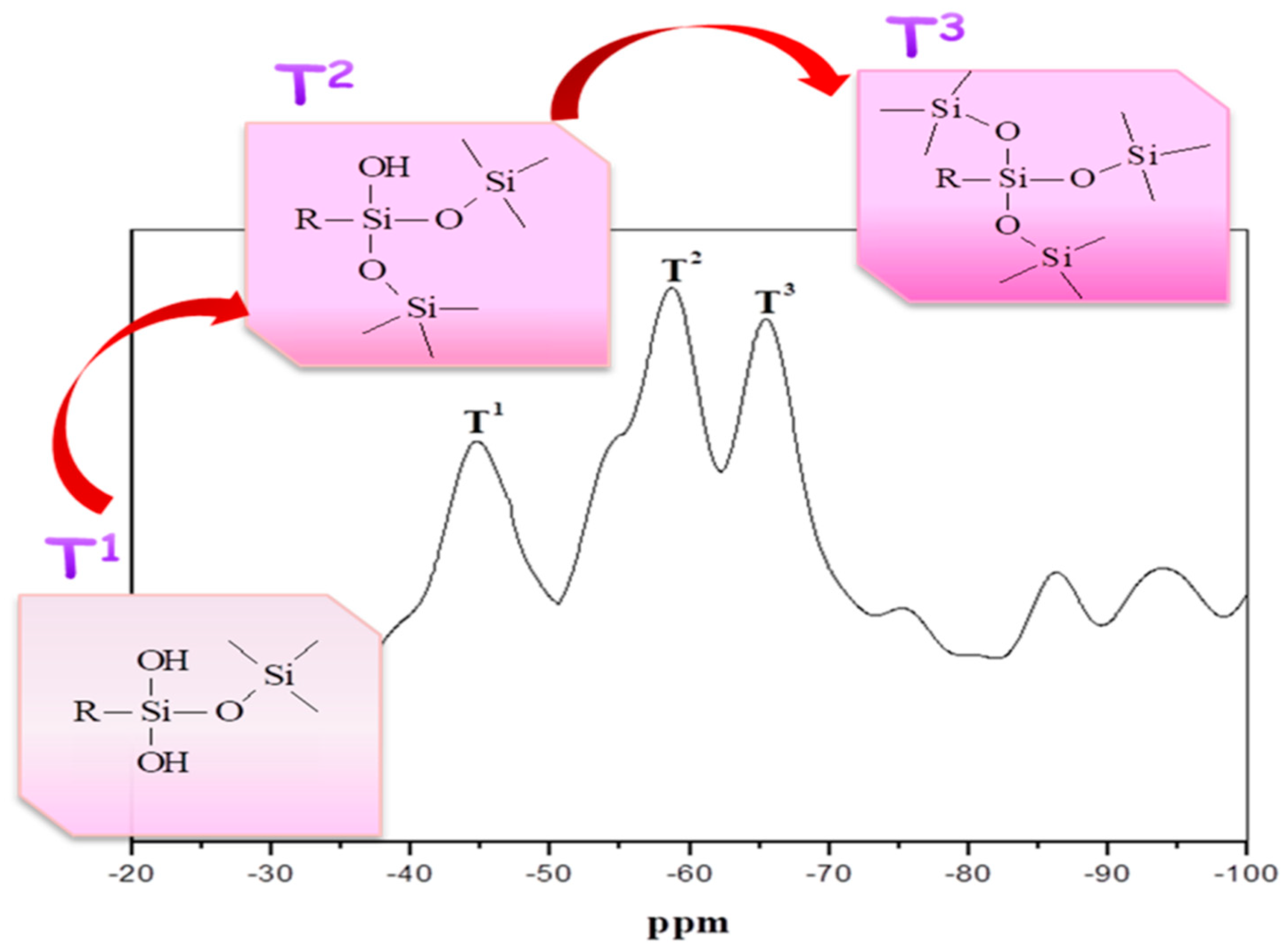
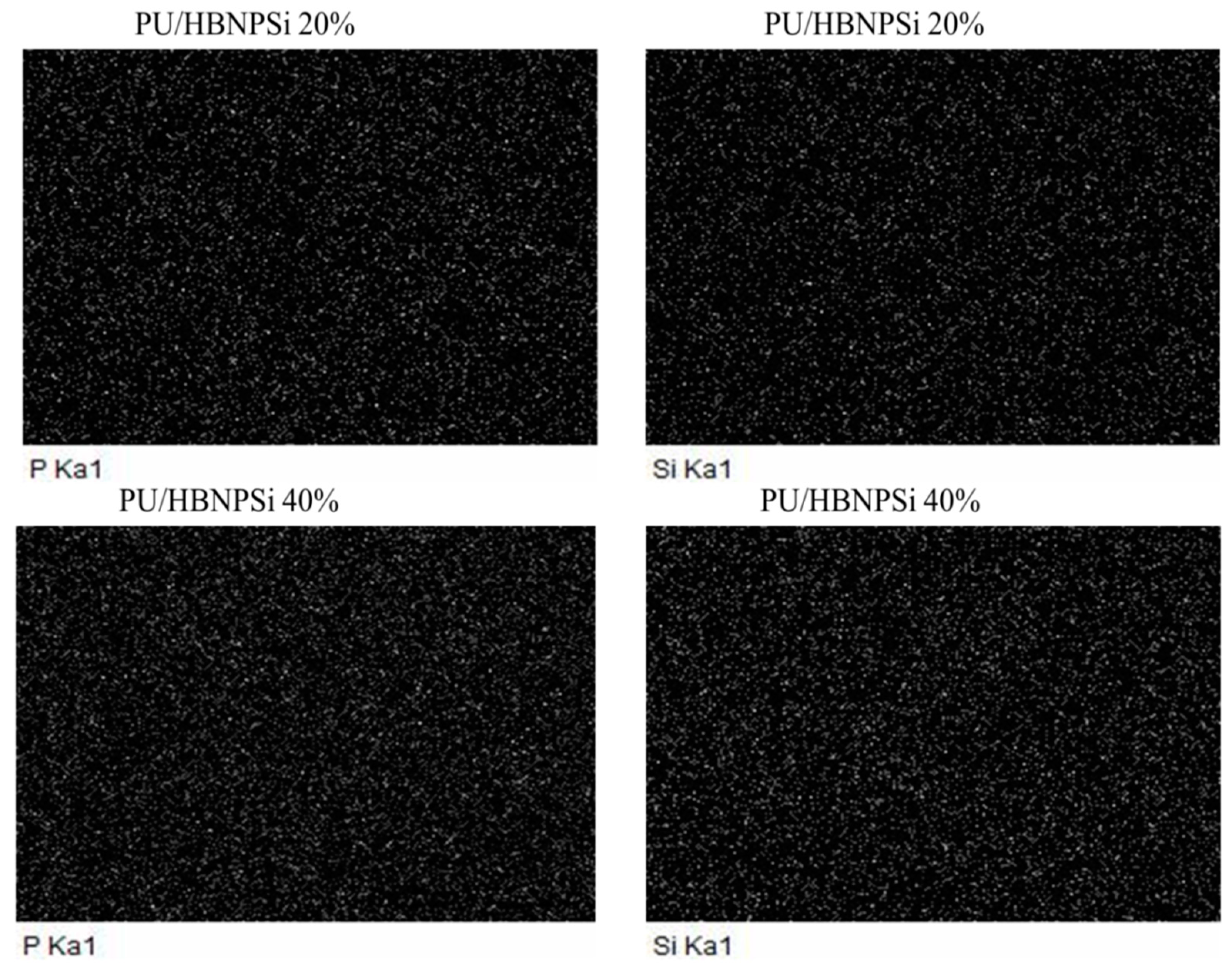
| Sample NO. | Area (%) | ||
|---|---|---|---|
| T1 | T2 | T3 | |
| PU/HBNPSi 40% | 24.3 | 44.3 | 36.8 |
| Sample | UL-94 | ||
|---|---|---|---|
| t1 + t2 (s) | Ranking | Dripping | |
| Pristine PU | >30 | Fail | Yes |
| PU/HBNPSi 10% | >30 | Fail | Yes |
| PU/HBNPSi 20% | >30 | Fail | Yes |
| PU/HBNPSi 30% | >30 | Fail | Yes |
| PU/HBNPSi 40% | 16.8 | V2 | Yes |
| Sample NO. | C1s | |||||
|---|---|---|---|---|---|---|
| C–C/C–H | C-Si | C=C | C–N | C–O | C=O | |
| PU/HBNPSi 10%—RT | 0.31 | 0.16 | 0.11 | 0.12 | 0.15 | 0.08 |
| PU/HBNPSi 10%—800 °C | 0.11 | 0.20 | 0.37 | 0.11 | 0.09 | 0.10 |
| PU/HBNPSi 40%—RT | 0.38 | 0.15 | 0.10 | 0.15 | 0.13 | 0.08 |
| PU/HBNPSi 40%—800 °C | 0.14 | 0.38 | 0.48 | 0.12 | 0.05 | 0.07 |
| Sample NO. | Temperature | |
|---|---|---|
| RT | 800 °C | |
| PU/HBNPSi 10% | 0.33 | 0.24 |
| PU/HBNPSi 40% | 0.27 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, M.-Y.; Kuan, C.-F.; Kuan, H.-C.; Ke, C.-Y.; Chiang, C.-L. Flame Retardance and Char Analysis of an Eco-Friendly Polyurethane Hyperbranched Hybrid Using the Sol–Gel Method. Sustainability 2021, 13, 486. https://doi.org/10.3390/su13020486
Shen M-Y, Kuan C-F, Kuan H-C, Ke C-Y, Chiang C-L. Flame Retardance and Char Analysis of an Eco-Friendly Polyurethane Hyperbranched Hybrid Using the Sol–Gel Method. Sustainability. 2021; 13(2):486. https://doi.org/10.3390/su13020486
Chicago/Turabian StyleShen, Ming-Yuan, Chen-Feng Kuan, Hsu-Chiang Kuan, Cing-Yu Ke, and Chin-Lung Chiang. 2021. "Flame Retardance and Char Analysis of an Eco-Friendly Polyurethane Hyperbranched Hybrid Using the Sol–Gel Method" Sustainability 13, no. 2: 486. https://doi.org/10.3390/su13020486
APA StyleShen, M.-Y., Kuan, C.-F., Kuan, H.-C., Ke, C.-Y., & Chiang, C.-L. (2021). Flame Retardance and Char Analysis of an Eco-Friendly Polyurethane Hyperbranched Hybrid Using the Sol–Gel Method. Sustainability, 13(2), 486. https://doi.org/10.3390/su13020486






