Utilization of Silicon Carbide Sludge as Metakaolin-Based Geopolymer Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Heat Evolution of Geopolymers with SiC Sludge
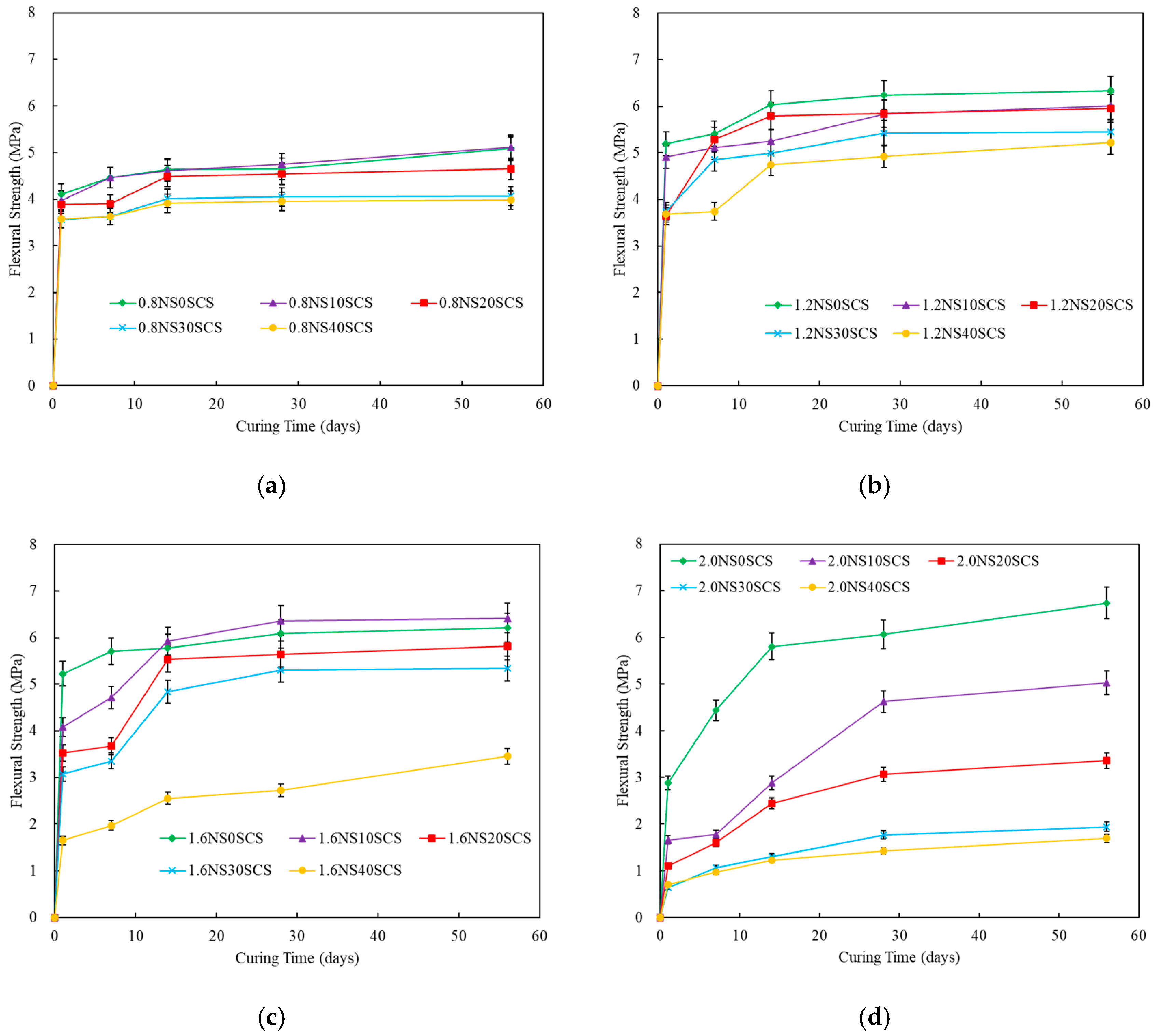
3.2. Mechanical Properties
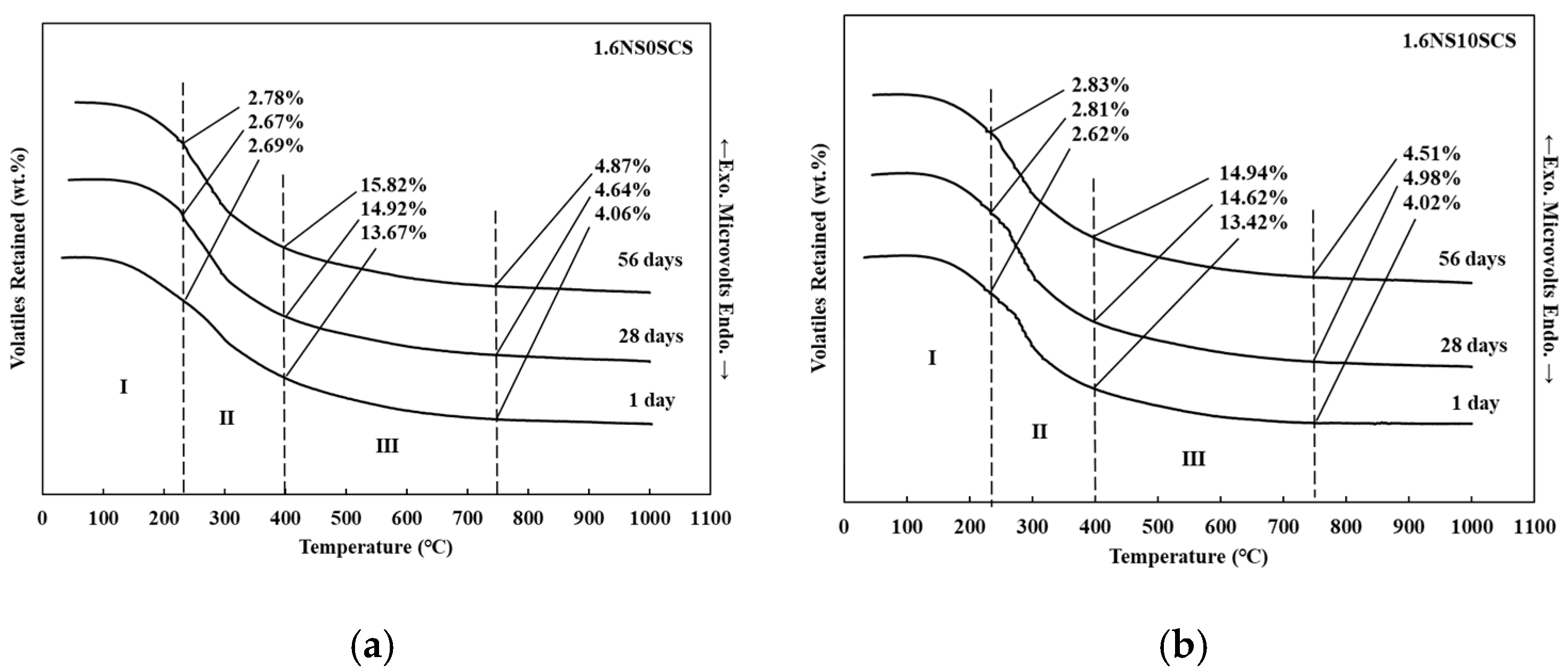
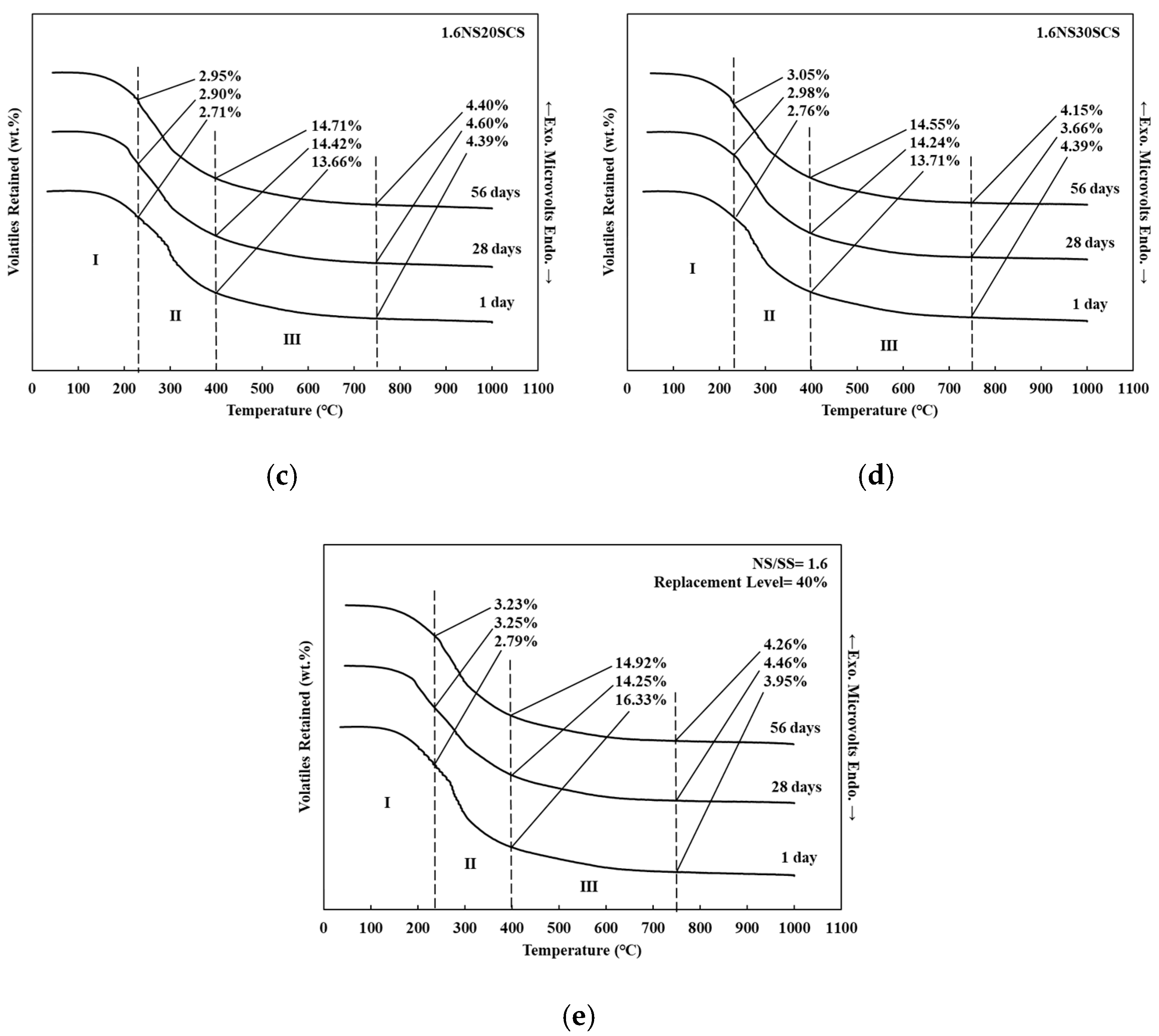
3.3. DTA/TG of Geopolymers with SiC Sludge
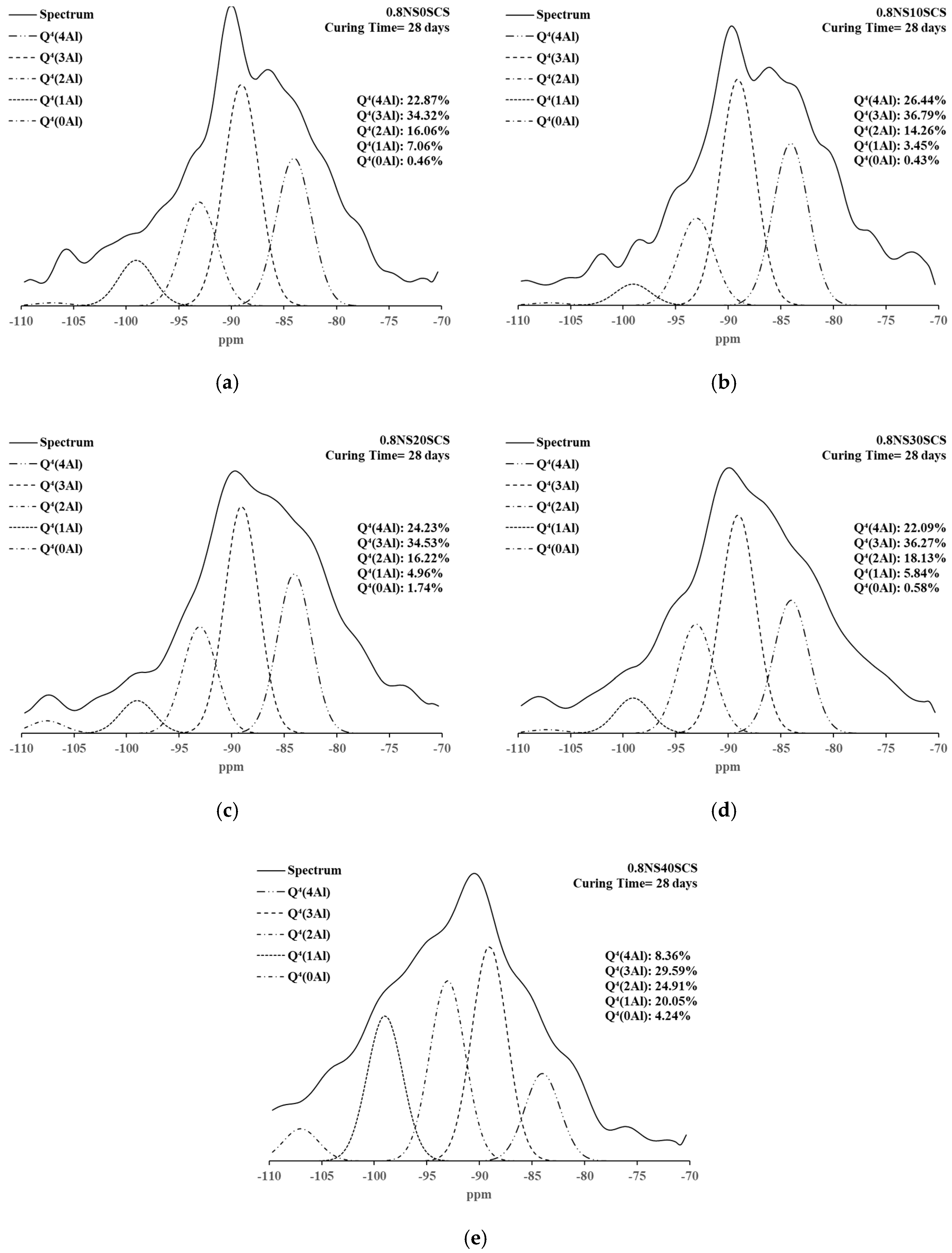
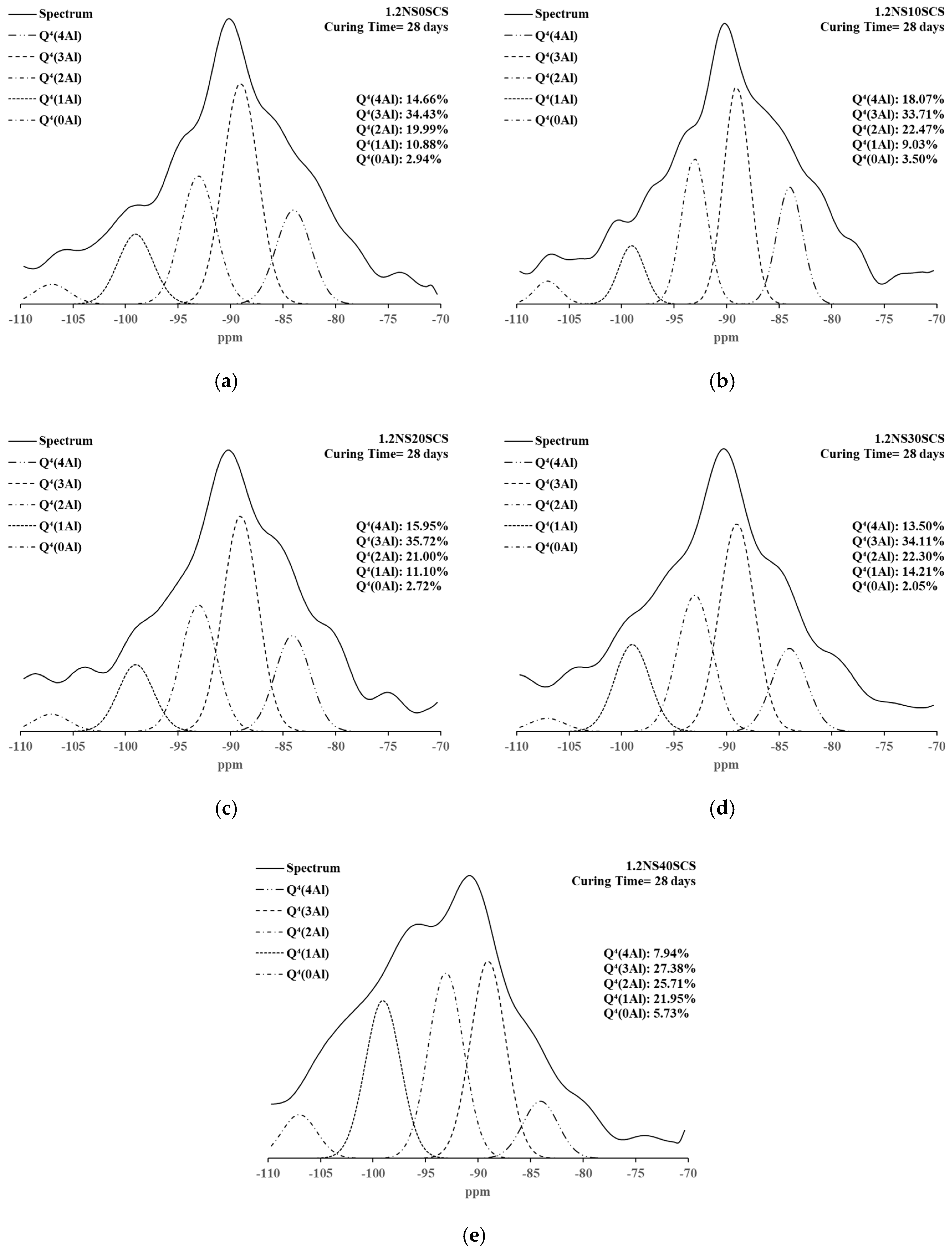
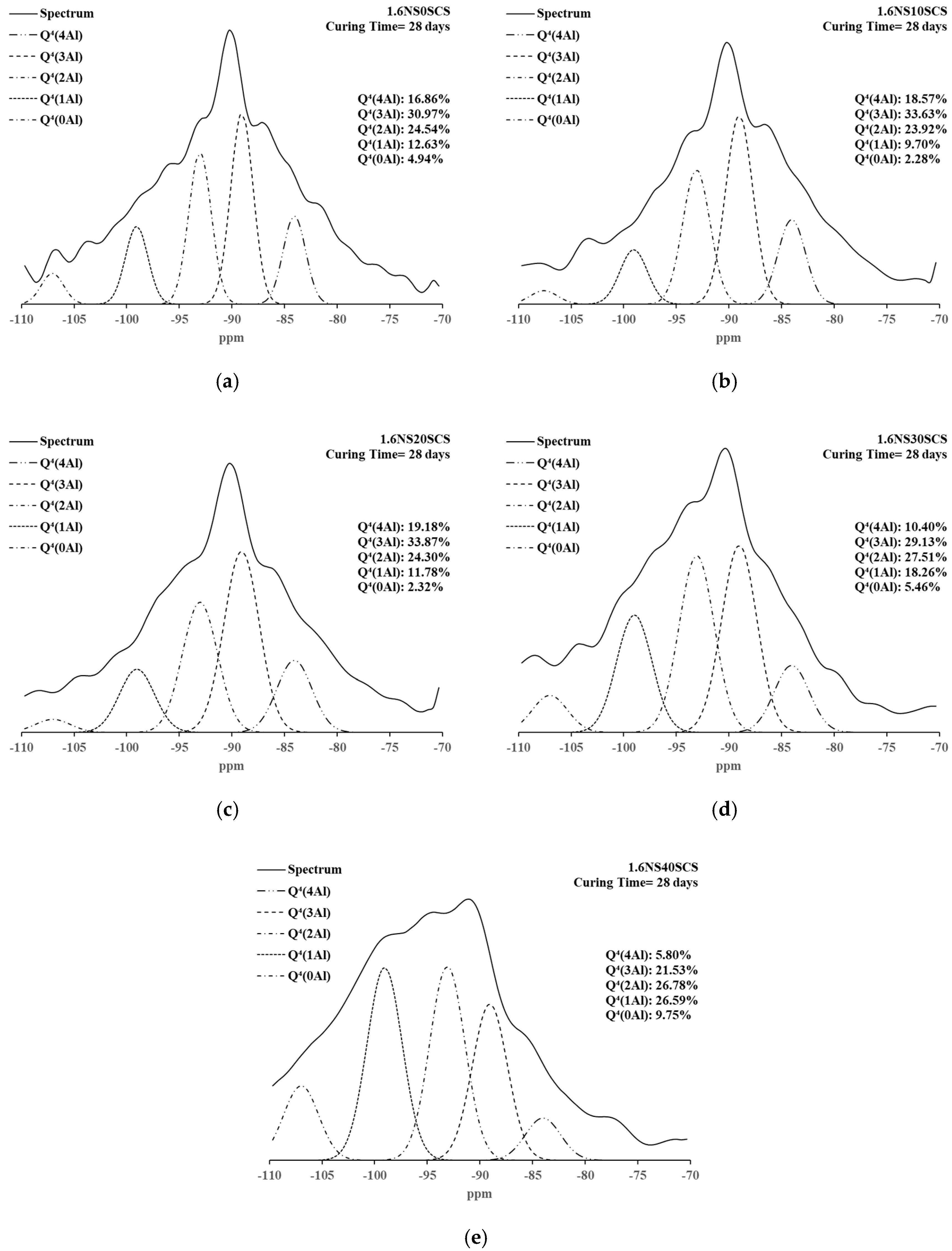
3.4. 29Si NMR Spectra and Deconvolutions of Geopolymers with SiC Sludge
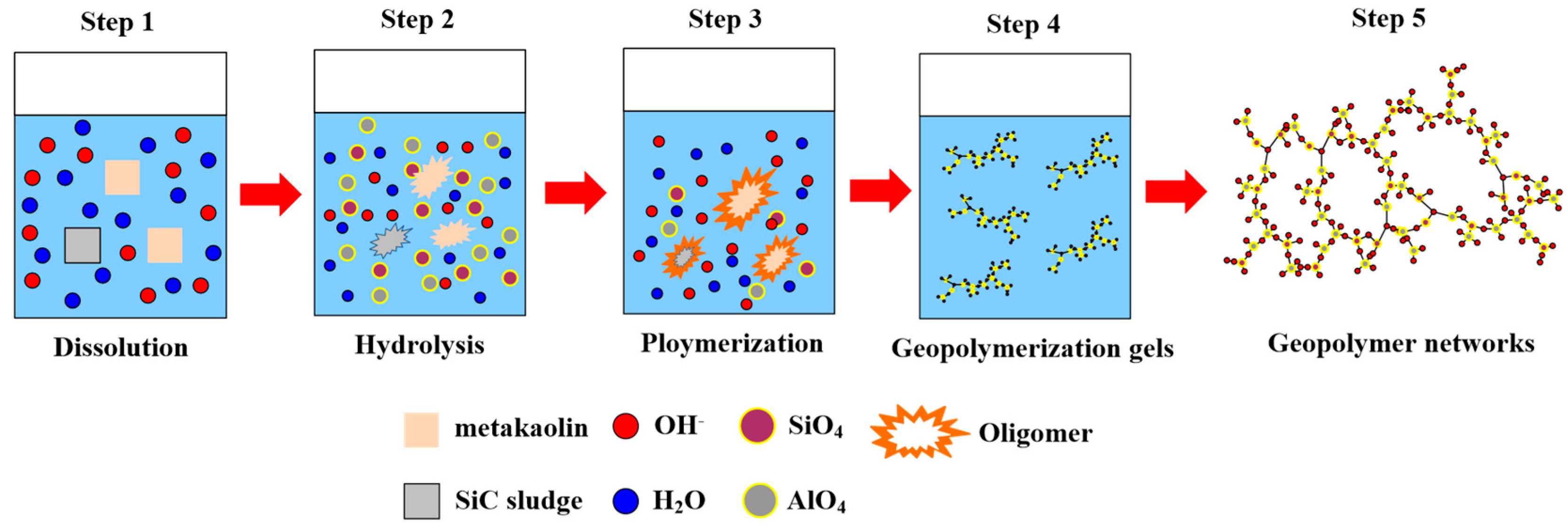
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chowdhury, M.S.; Rahman, K.S.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, M.; Tiong, S.K.; Sopian, K.; Amin, N. An overview of solar photovoltaic panels’ end-of-life material recycling. Energy Strategy Rev. 2020, 27, 100431. [Google Scholar] [CrossRef]
- Yoko, A.; Oshima, Y.S. Recovery of silicon from silicon sludge using supercritical water. J. Supercrit. Fluids 2013, 75, 1–5. [Google Scholar] [CrossRef]
- Lan, A.; Liu, C.E.; Yan, H.L.; Yua, H.T.; Li, I.T.; Hsua, H.P.; Lan, C.W. Silicon ingot casting using reusable silicon nitride crucibles made from diamond wire sawing kerf-loss silicon. J. Cryst. Growth 2019, 525, 125184. [Google Scholar] [CrossRef]
- Eblagon, F.; Ehrle, B.; Graule, T.; Kuebler, J. Development of siliconnitride/silicon carbide composites for wood-cutting tools. J. Eur. Ceram. Soc. 2007, 27, 419–428. [Google Scholar] [CrossRef]
- Tsai, T.H. Silicon sawing waste treatment by electrophoresis and gravitational settling. J. Hazard. Mater. 2011, 189, 526–530. [Google Scholar] [CrossRef]
- He, P.G.; Wang, M.R.; Fu, S.; Jia, D.C.; Yan, S.; Yuan, J.K.; Xu, J.H.; Wang, P.F.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Elyamany, H.R.; Elmoaty, A.E.M.A.; Elshaboury, A.M. Magnesium sulfate resistance of geopolymer mortar. Constr. Build. Mater. 2018, 184, 111–127. [Google Scholar] [CrossRef]
- Leay, L.; Potts, A.; Donoclift, T. Geopolymers from fly ash and their gamma irradiation. Mater. Lett. 2018, 227, 240–242. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.; Wang, Y.; Sun, R.; Rui, Y. Effects of the n(H2O: Na2Oeq) ratio on the geopolymerization process and microstructures of fly ash-based geopolymers. J. Non Cryst. Solids 2019, 511, 19–28. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Zuo, Y.; Chen, W.; Ye, G. Chemical deformation of metakaolin based geopolymer. Cem. Concr. Res. 2019, 120, 108–118. [Google Scholar] [CrossRef]
- Kolezyn’ski, A.; Kro´l, M.; Zychowicz, M. The structure of geopolymers-Theoretical studies. J. Mol. Struct. 2018, 1163, 465–471. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Effects of H2O/Na2O molar ratio on the strength of alkaline activated ground blast furnace slag-ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 56, 158–164. [Google Scholar] [CrossRef]
- Medri, V.; Ruffini, A. Alkali-bonded SiC based foams. J. Eur. Ceram. Soc. 2012, 32, 1907–1913. [Google Scholar] [CrossRef]
- Rahman, A.S.; Shah, C.; Gupta, N. Simultaneous effects of rice husk silica and silicon carbide whiskers on the mechanical properties and morphology of sodium geopolymer. J. Compos. Mater. 2020, 0021998320928579. [Google Scholar] [CrossRef]
- Medri, V.; Fabbri, S.; Ruffini, A.; Dedecek, J.; Vaccari, A. SiC-based refractory paints prepared with alkali aluminosilicate binders. J. Eur. Ceram. Soc. 2011, 31, 2155–2165. [Google Scholar] [CrossRef]
- Bai, C.Y.; Zheng, J.; Rizzi, G.A.; Colombo, P. Low-temperature fabrication of SiC/geopolymer cellular composites. Compos. Part B 2018, 137, 23–30. [Google Scholar] [CrossRef]
- Jia, D.C.; Li, Y.H.; He, P.G.; Fu, S.; Duan, X.M.; Sun, Z.L.; Cai, D.; Li, D.X.; Yang, Z.H.; Zhou, Y. In-situ formation of bulk and porous h-AlN/SiC-based ceramics from geopolymer technique. Ceram. Int. 2019, 45, 24727–24733. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.X. Reexamining calcination of kaolinite for the synthesis of metakaolin geopolymers-roles of dehydroxylation and recrystallization. J. Non Cryst. Solids. 2017, 460, 74–80. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, Y.; Bao, S. Preparation of geopolymers from vanadium tailings by mechanical activation. Constr. Build. Mater. 2017, 145, 236–242. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, S.; Yang, J.K.; Chen, Y.; Ye, N.; Ke, Y.; Tao, S.Y.; Xiao, K.K.; Hu, J.P.; Hou, H.J.; et al. Role of Fe species in geopolymer synthesized from alkali-thermal pretreated Fe-rich Bayer red mud. Constr. Build. Mater. 2019, 200, 398–407. [Google Scholar] [CrossRef]
- Lo, K.W.; Lin, K.L.; Cheng, T.W.; Shiu, H.S. Effect of alkali activation thin film transistor-liquid crystal display waste glass on the mechanical behavior of geopolymers. Constr. Build. Mater. 2018, 162, 724–731. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Arulrajah, A.; Disfani, M.M.; Horpibulsuk, S.; Darmawan, S.; Wang, J. Impact of field conditions on the strength development of a geopolymer stabilized marine clay. Appl. Clay Sci. 2019, 167, 33–42. [Google Scholar] [CrossRef]
- Henon, J.; Alzina, A.; Absi, J.; Smith, D.S.; Rossignol, S. Porosity control of cold consolidated geomaterial foam: Temperature effect. Ceram. Int. 2012, 38, 77–84. [Google Scholar] [CrossRef]
- Tiffo, E.; Mbah, J.B.B.; Belibi, P.D.B.; Djobo, J.N.Y.; Elimbi, A. Physical and mechanical properties of unheated and heated kaolin based-geopolymers with partial replacement of aluminium hydroxide. Mater. Chem. Phys. 2020, 239, 122103. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.X.; León–Patiño, C.A. Geothermal clay-based geopolymer binders: Synthesis and microstructural characterization. Appl. Clay Sci. 2017, 146, 223–229. [Google Scholar] [CrossRef]
- Li, W.; Rao, F.; Song, S.X.; Ma, Q.Y. Deterioration in the microstructure of metakaolin-based geopolymers in marine environment. J. Mater. Res. Technol. 2019, 8, 2747–2752. [Google Scholar] [CrossRef]
- Maekawa, H.; Maekawa, T.; Kawamura, K.; Yokokawa, T. The structural groups of alkali silicate glasses determined from 29Si Mas-NMR. J. Non Cryst. Solid. 1991, 127, 53–64. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.X.; García, R.E.; Estrella, R.M.; Patino, C.L.; Zhang, Y.M. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Lin, K.L.; Lo, K.W.; Cheng, T.W.; Lin, W.T.; Lin, Y.W. Influence of SiC Sludge on the Microstructure of Geopolymers. Materials 2020, 13, 2203. [Google Scholar] [CrossRef]

| Composition | SiC Sludge | MK |
|---|---|---|
| SiO2 (wt %) | 75.40 | 51.80 |
| Al2O3 (wt %) | 0.80 | 43.00 |
| Fe2O3 (wt %) | 0.58 | 1.30 |
| CaO (wt %) | 0.09 | 0.25 |
| MgO (wt %) | N.D. 1 | N.D. 1 |
| SO3 (wt %) | 0.06 | N.D. 1 |
| Na2O (wt %) | N.D. 1 | 0.04 |
| K2O (wt %) | 0.01 | 0.32 |
| SiC (wt %) | 23.00 | N.D. 1 |
| Mix Designation | NS/SS | MK (g) | SiC Sludge (g) | Sodium Silicate Solution (g) | 10 M NaOH (g) | Si/Al Mole Ratios |
|---|---|---|---|---|---|---|
| 0.8NS0SCS | 0.8 | 500 | 0 | 240 | 260 | 1.01 |
| 0.8NS10SCS | 450 | 50 | 240 | 260 | 1.36 | |
| 0.8NS20SCS | 400 | 100 | 240 | 260 | 1.24 | |
| 0.8NS30SCS | 350 | 150 | 240 | 260 | 1.74 | |
| 0.8NS40SCS | 300 | 200 | 240 | 260 | 2.60 | |
| 1.2NS0SCS | 1.2 | 500 | 0 | 315 | 185 | 0.98 |
| 1.2NS10SCS | 450 | 50 | 315 | 185 | 1.34 | |
| 1.2NS20SCS | 400 | 100 | 315 | 185 | 1.22 | |
| 1.2NS30SCS | 350 | 150 | 315 | 185 | 1.87 | |
| 1.2NS40SCS | 300 | 200 | 315 | 185 | 2.72 | |
| 1.6NS0SCS | 1.6 | 500 | 0 | 374 | 126 | 1.16 |
| 1.6NS10SCS | 450 | 50 | 374 | 126 | 1.51 | |
| 1.6NS20SCS | 400 | 100 | 374 | 126 | 1.43 | |
| 1.6NS30SCS | 350 | 150 | 374 | 126 | 1.92 | |
| 1.6NS40SCS | 300 | 200 | 374 | 126 | 2.89 | |
| 2.0NS0SCS | 2.0 | 500 | 0 | 421 | 79 | 1.07 |
| 2.0NS10SCS | 450 | 50 | 421 | 79 | 1.48 | |
| 2.0NS20SCS | 400 | 100 | 421 | 79 | 1.23 | |
| 2.0NS30SCS | 350 | 150 | 421 | 79 | 1.78 | |
| 2.0NS40SCS | 300 | 200 | 421 | 79 | 2.67 |
| Samples | NS/SS | Curing Time (Days) | Temperature (°C) | ||
|---|---|---|---|---|---|
| 50–230 | 230–400 | 400–750 | |||
| 0.8NS0SCS | 0.8 | 1 | 2.58 | 10.21 | 2.61 |
| 28 | 3.70 | 11.81 | 4.24 | ||
| 56 | 4.37 | 12.36 | 4.68 | ||
| 0.8NS10SCS | 1 | 2.77 | 10.20 | 2.35 | |
| 28 | 3.83 | 11.69 | 4.24 | ||
| 56 | 4.42 | 12.36 | 4.62 | ||
| 0.8NS20SCS | 1 | 3.72 | 10.09 | 2.33 | |
| 28 | 3.83 | 11.66 | 4.03 | ||
| 56 | 4.48 | 12.32 | 4.24 | ||
| 0.8NS30SCS | 1 | 3.49 | 10.08 | 2.22 | |
| 28 | 3.94 | 11.64 | 3.68 | ||
| 56 | 4.48 | 12.24 | 4.03 | ||
| 0.8NS40SCS | 1 | 3.49 | 10.06 | 1.95 | |
| 28 | 3.94 | 11.59 | 3.52 | ||
| 56 | 4.50 | 12.21 | 3.68 | ||
| 1.2NS0SCS | 1.2 | 1 | 3.49 | 10.97 | 2.73 |
| 28 | 3.94 | 12.51 | 4.09 | ||
| 56 | 4.51 | 12.96 | 4.77 | ||
| 1.2NS10SCS | 1 | 3.49 | 10.96 | 2.72 | |
| 28 | 4.14 | 12.49 | 4.07 | ||
| 56 | 4.55 | 12.93 | 4.34 | ||
| 1.2NS20SCS | 1 | 3.52 | 10.93 | 2.64 | |
| 28 | 4.21 | 12.46 | 3.99 | ||
| 56 | 4.55 | 12.86 | 4.33 | ||
| 1.2NS30SCS | 1 | 3.52 | 10.92 | 2.64 | |
| 28 | 4.25 | 12.42 | 3.96 | ||
| 56 | 4.61 | 12.75 | 4.21 | ||
| 1.2NS40SCS | 1 | 3.58 | 10.88 | 2.57 | |
| 28 | 4.25 | 12.39 | 3.90 | ||
| 56 | 4.61 | 12.65 | 4.14 | ||
| Samples | Curing Time | Silicate Derivatives (%) | Deconvoluted Fractions of Silicon Centers (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4(0Al) | Q4(1Al) | Q4(2Al) | Q4(3Al) | Q4(4Al) | ||
| 0.8NS0SCS | 1 day | 10.07 | 1.41 | 2.11 | 0.66 | 5.62 | 16.17 | 35.85 | 28.11 |
| 0.8NS10SCS | 8.72 | 2.53 | 2.30 | 1.30 | 7.59 | 18.64 | 33.82 | 25.10 | |
| 0.8NS20SCS | 9.22 | 2.16 | 2.55 | 2.85 | 5.89 | 19.23 | 34.15 | 23.95 | |
| 0.8NS30SCS | 4.36 | 3.11 | 2.08 | 4.12 | 10.79 | 21.79 | 34.24 | 19.51 | |
| 0.8NS40SCS | 1.56 | 3.31 | 4.28 | 3.43 | 17.66 | 25.46 | 26.38 | 17.92 | |
| 0.8NS0SCS | 28 days | 10.22 | 4.62 | 4.39 | 0.46 | 7.06 | 16.06 | 34.32 | 22.87 |
| 0.8NS10SCS | 10.58 | 5.06 | 2.99 | 0.43 | 3.45 | 14.26 | 36.79 | 26.44 | |
| 0.8NS20SCS | 11.26 | 4.77 | 2.29 | 1.74 | 4.96 | 16.22 | 34.53 | 24.23 | |
| 0.8NS30SCS | 10.42 | 4.59 | 2.08 | 0.58 | 5.84 | 18.13 | 36.27 | 22.09 | |
| 0.8NS40SCS | 4.13 | 3.90 | 4.82 | 4.24 | 20.05 | 24.91 | 29.59 | 8.36 | |
| 1.2NS0SCS | 1 day | 7.74 | 3.61 | 3.10 | 2.45 | 9.81 | 20.39 | 33.55 | 19.35 |
| 1.2NS10SCS | 8.05 | 4.14 | 2.76 | 1.73 | 8.05 | 20.25 | 34.98 | 20.04 | |
| 1.2NS20SCS | 7.17 | 4.01 | 3.59 | 3.57 | 9.50 | 20.68 | 34.18 | 17.30 | |
| 1.2NS30SCS | 3.94 | 5.03 | 4.81 | 3.94 | 10.36 | 24.02 | 32.26 | 15.64 | |
| 1.2NS40SCS | 3.08 | 3.74 | 4.17 | 6.18 | 21.53 | 25.71 | 26.37 | 9.22 | |
| 1.2NS0SCS | 28 days | 6.44 | 5.77 | 4.89 | 2.94 | 10.88 | 19.99 | 34.43 | 14.66 |
| 1.2NS10SCS | 6.17 | 3.97 | 3.08 | 3.50 | 9.03 | 22.47 | 33.71 | 18.07 | |
| 1.2NS20SCS | 2.41 | 6.03 | 5.07 | 2.72 | 11.10 | 21.00 | 35.72 | 15.95 | |
| 1.2NS30SCS | 5.59 | 3.30 | 4.94 | 2.05 | 14.21 | 22.30 | 34.11 | 13.50 | |
| 1.2NS40SCS | 0.84 | 4.60 | 5.85 | 5.73 | 21.95 | 25.71 | 27.38 | 7.94 | |
| 1.6NS0SCS | 1 day | 5.00 | 3.81 | 3.81 | 3.40 | 13.58 | 25.66 | 30.69 | 14.05 |
| 1.6NS10SCS | 5.87 | 4.51 | 4.96 | 4.81 | 14.46 | 23.80 | 31.50 | 10.09 | |
| 1.6NS20SCS | 4.91 | 5.40 | 3.93 | 6.62 | 16.00 | 27.18 | 29.37 | 6.59 | |
| 1.6NS30SCS | 2.23 | 3.57 | 4.01 | 6.64 | 18.05 | 27.85 | 29.63 | 8.02 | |
| 1.6NS40SCS | 0.89 | 2.23 | 6.01 | 9.19 | 27.82 | 28.93 | 17.81 | 7.12 | |
| 1.6NS0SCS | 28 days | 5.03 | 1.76 | 3.27 | 4.94 | 12.63 | 24.54 | 30.97 | 16.86 |
| 1.6NS10SCS | 5.13 | 3.50 | 3.27 | 2.28 | 9.70 | 23.92 | 33.63 | 18.57 | |
| 1.6NS20SCS | 2.41 | 3.07 | 3.07 | 2.32 | 11.78 | 24.30 | 33.87 | 19.18 | |
| 1.6NS30SCS | 0.92 | 4.16 | 4.16 | 5.46 | 18.26 | 27.51 | 29.13 | 10.40 | |
| 1.6NS40SCS | 1.69 | 3.74 | 4.12 | 9.75 | 26.59 | 26.78 | 21.53 | 5.80 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-L.; Lo, K.-W.; Cheng, T.-W.; Lin, W.-T.; Lin, Y.-W. Utilization of Silicon Carbide Sludge as Metakaolin-Based Geopolymer Materials. Sustainability 2020, 12, 7333. https://doi.org/10.3390/su12187333
Lin K-L, Lo K-W, Cheng T-W, Lin W-T, Lin Y-W. Utilization of Silicon Carbide Sludge as Metakaolin-Based Geopolymer Materials. Sustainability. 2020; 12(18):7333. https://doi.org/10.3390/su12187333
Chicago/Turabian StyleLin, Kae-Long, Kang-Wei Lo, Ta-Wui Cheng, Wei-Ting Lin, and Ya-Wen Lin. 2020. "Utilization of Silicon Carbide Sludge as Metakaolin-Based Geopolymer Materials" Sustainability 12, no. 18: 7333. https://doi.org/10.3390/su12187333
APA StyleLin, K.-L., Lo, K.-W., Cheng, T.-W., Lin, W.-T., & Lin, Y.-W. (2020). Utilization of Silicon Carbide Sludge as Metakaolin-Based Geopolymer Materials. Sustainability, 12(18), 7333. https://doi.org/10.3390/su12187333







