Evaluating the Properties of a Coating Material with Polycaprolactone-Degradable Fluorinated Silicon-Containing Waterborne Polyurethane
Abstract
1. Introduction
2. Materials and Methods (Experimental)
2.1. Materials
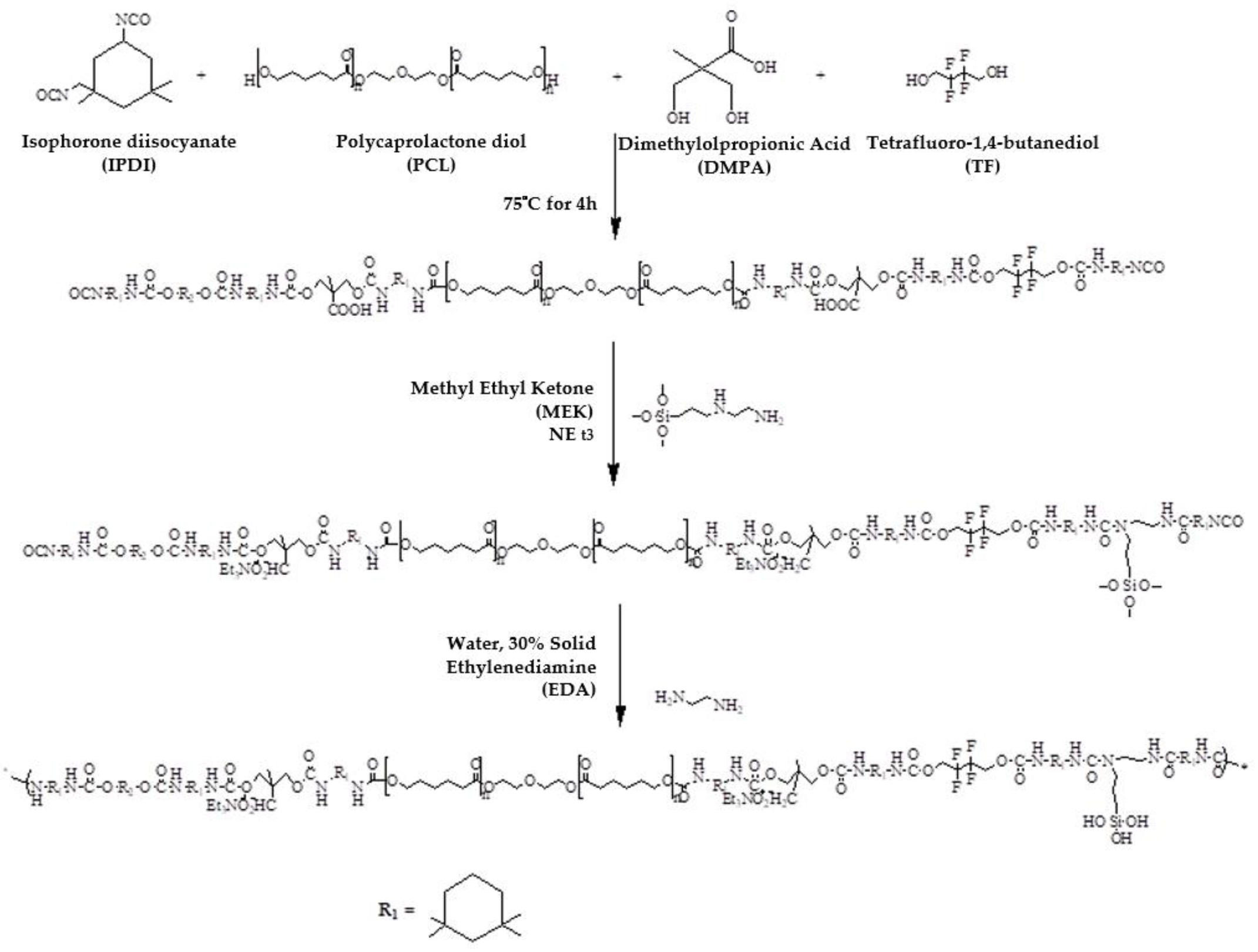
2.2. Synthesis of FSWPUs
2.3. Dynamic Light Scattering (DLS)
2.4. Gel Permeation Chromatograph (GPC)
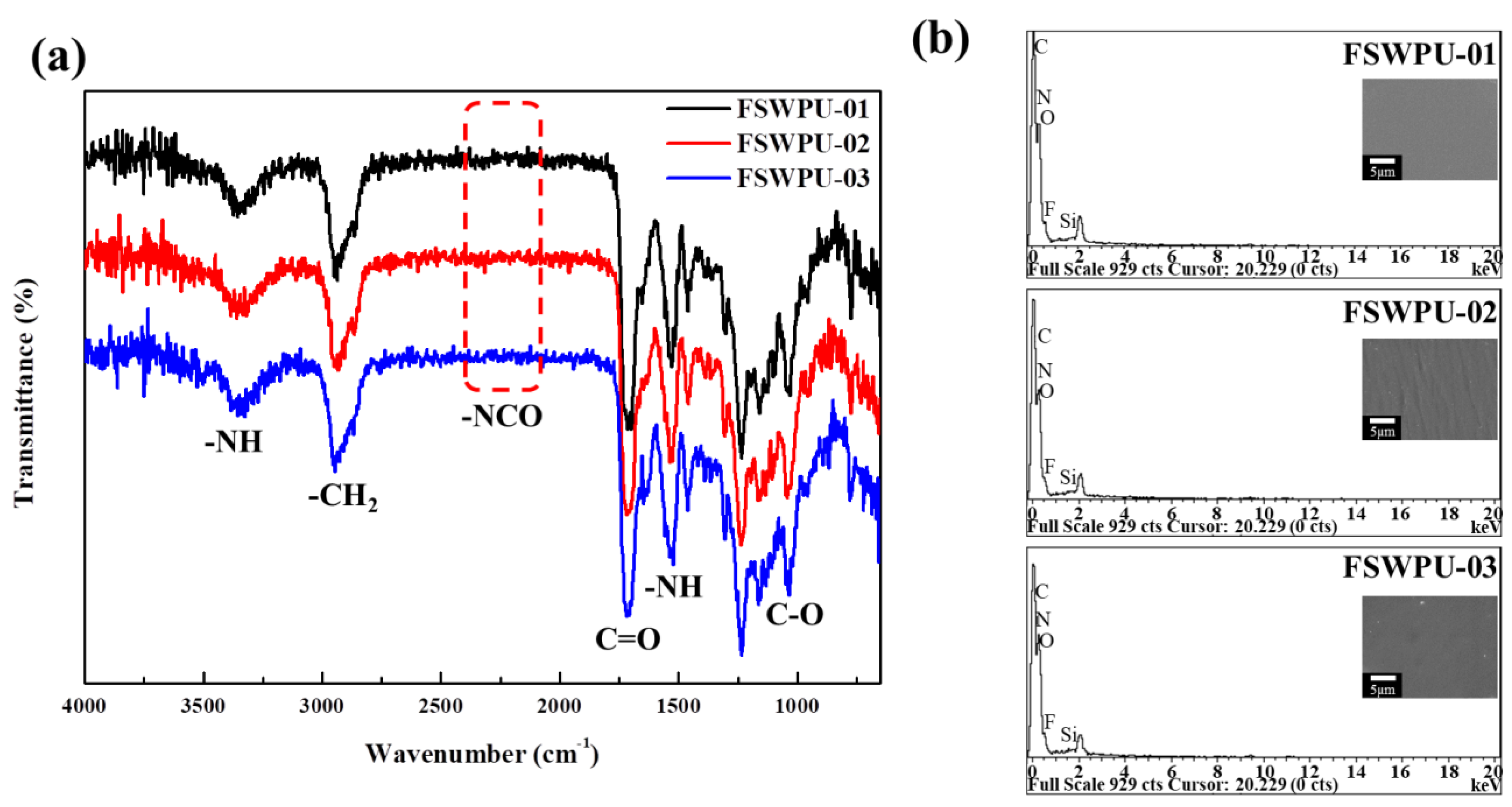
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6. Energy-Dispersive X-ray Spectroscopy (EDS)
2.7. Thermogravimetric Analysis (TGA)
2.8. Dynamic Mechanical Analysis (DMA)
2.9. Stress-Strain Testing
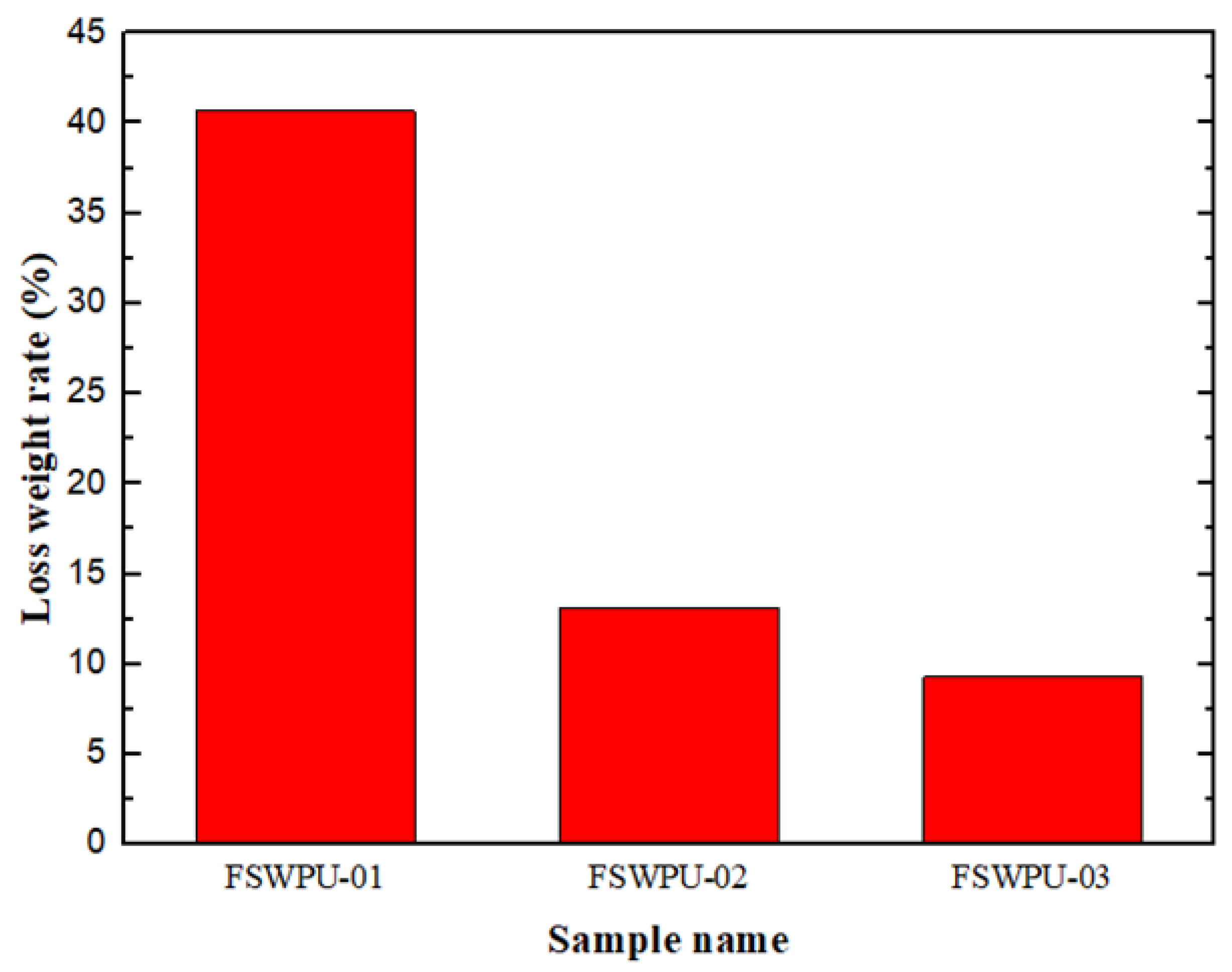
2.10. Hydrolytic Stability
2.11. Contact Angle Measurements
3. Results and Discussion
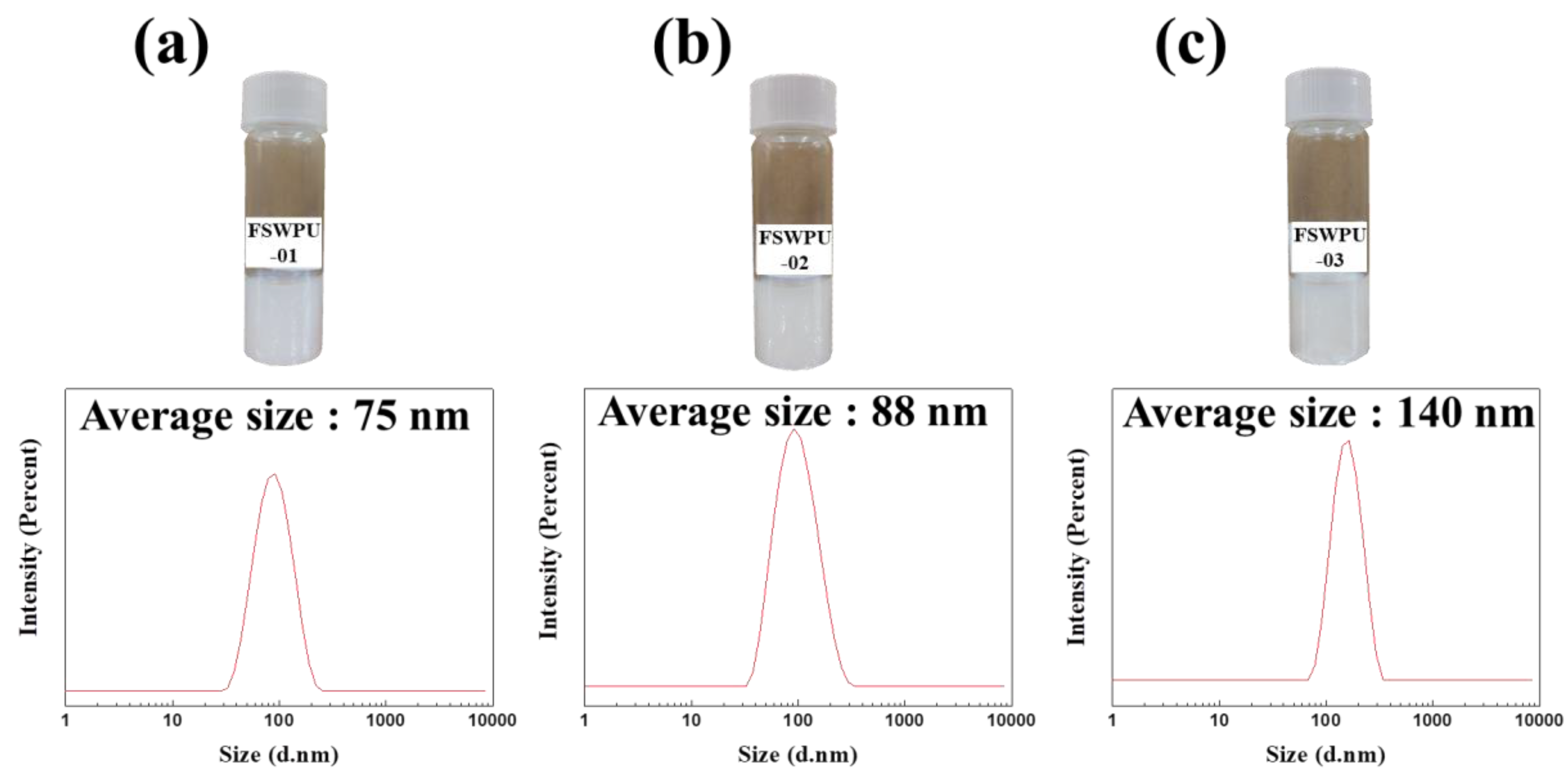
3.1. Emulsion and DLS
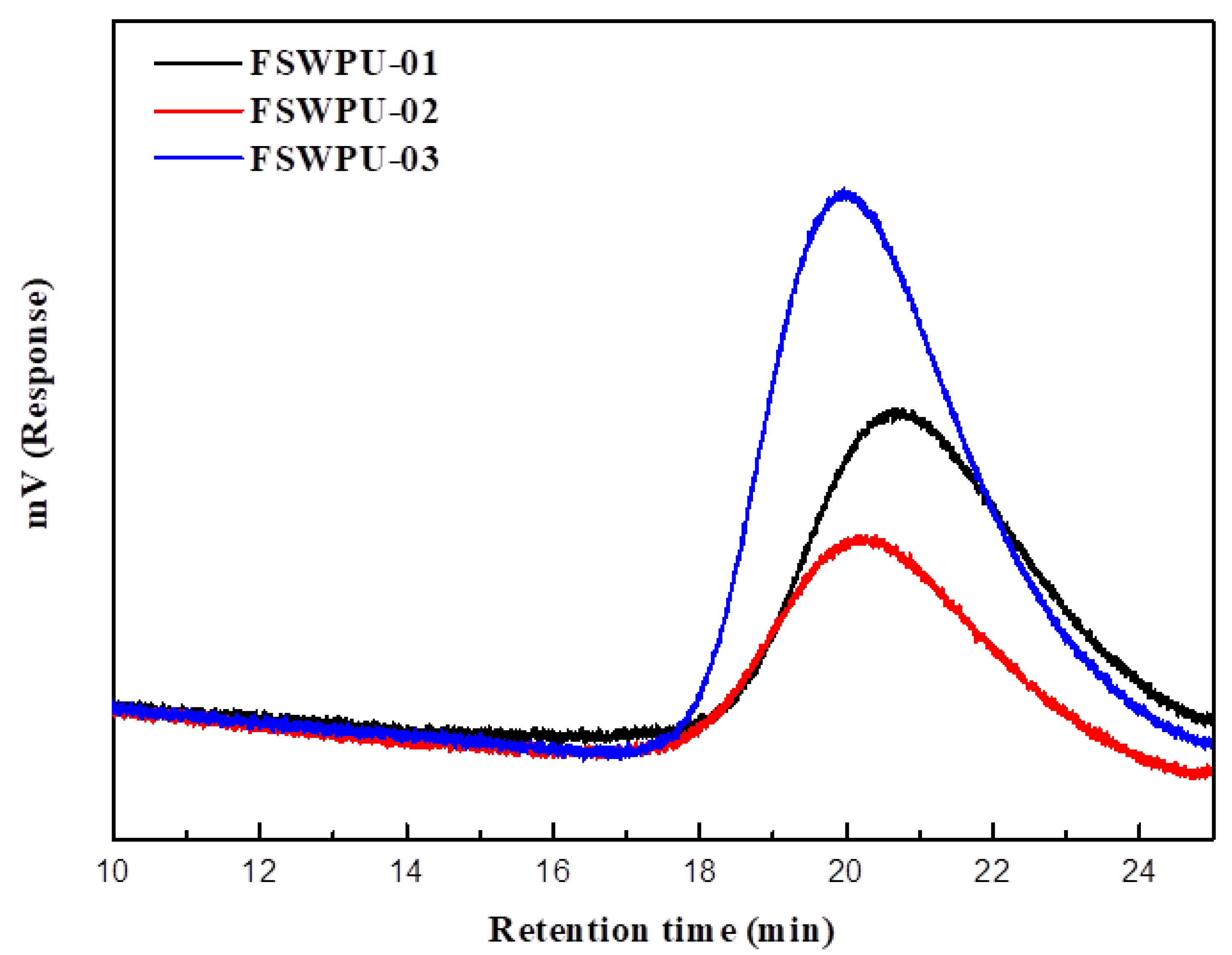
3.2. Gel Permeation Chromatograph (GPC)
3.3. Basic Properties of FSWPUs
3.4. Thermal Properties
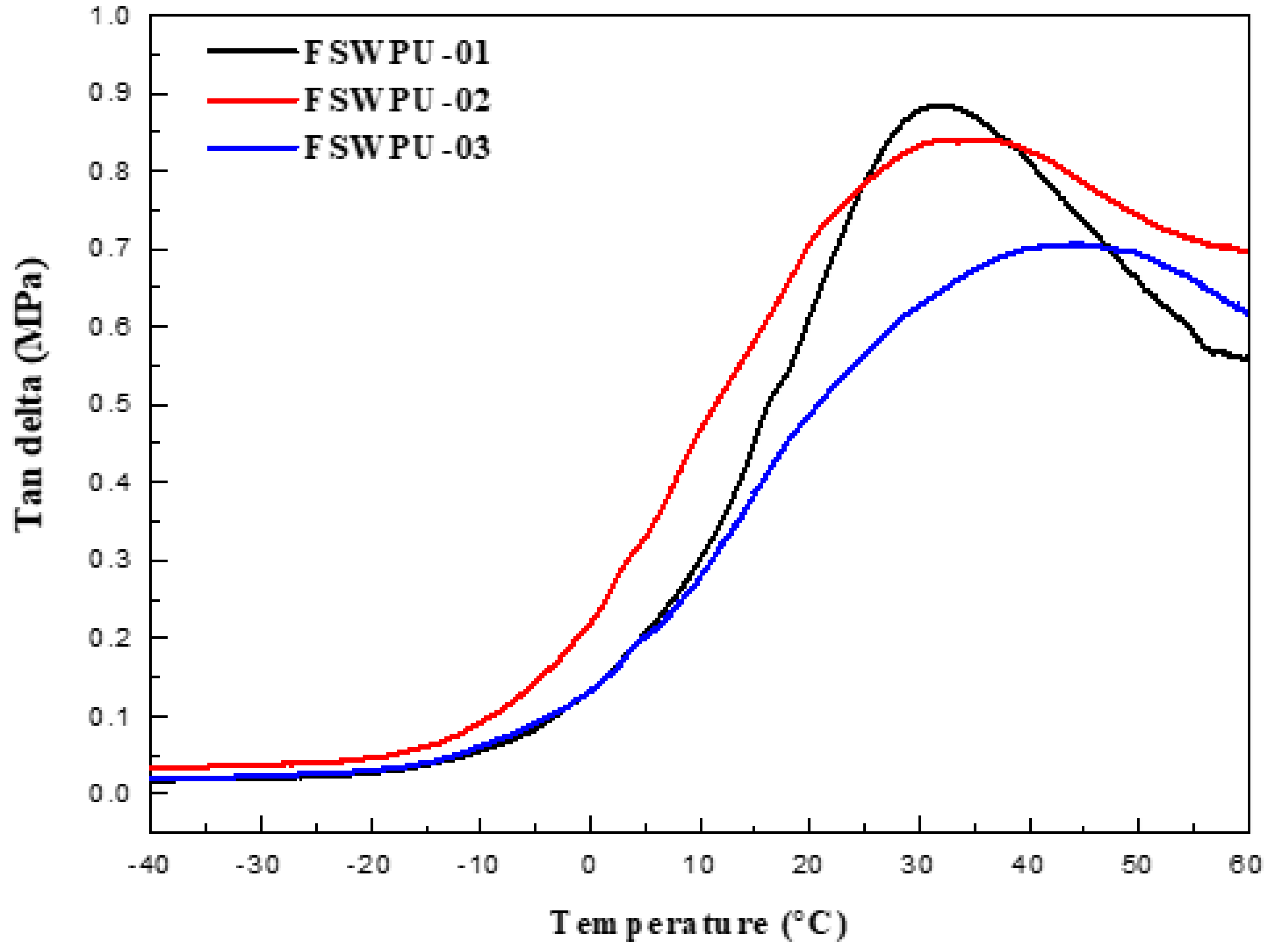
3.5. Dynamic Mechanical Analysis (DMA)
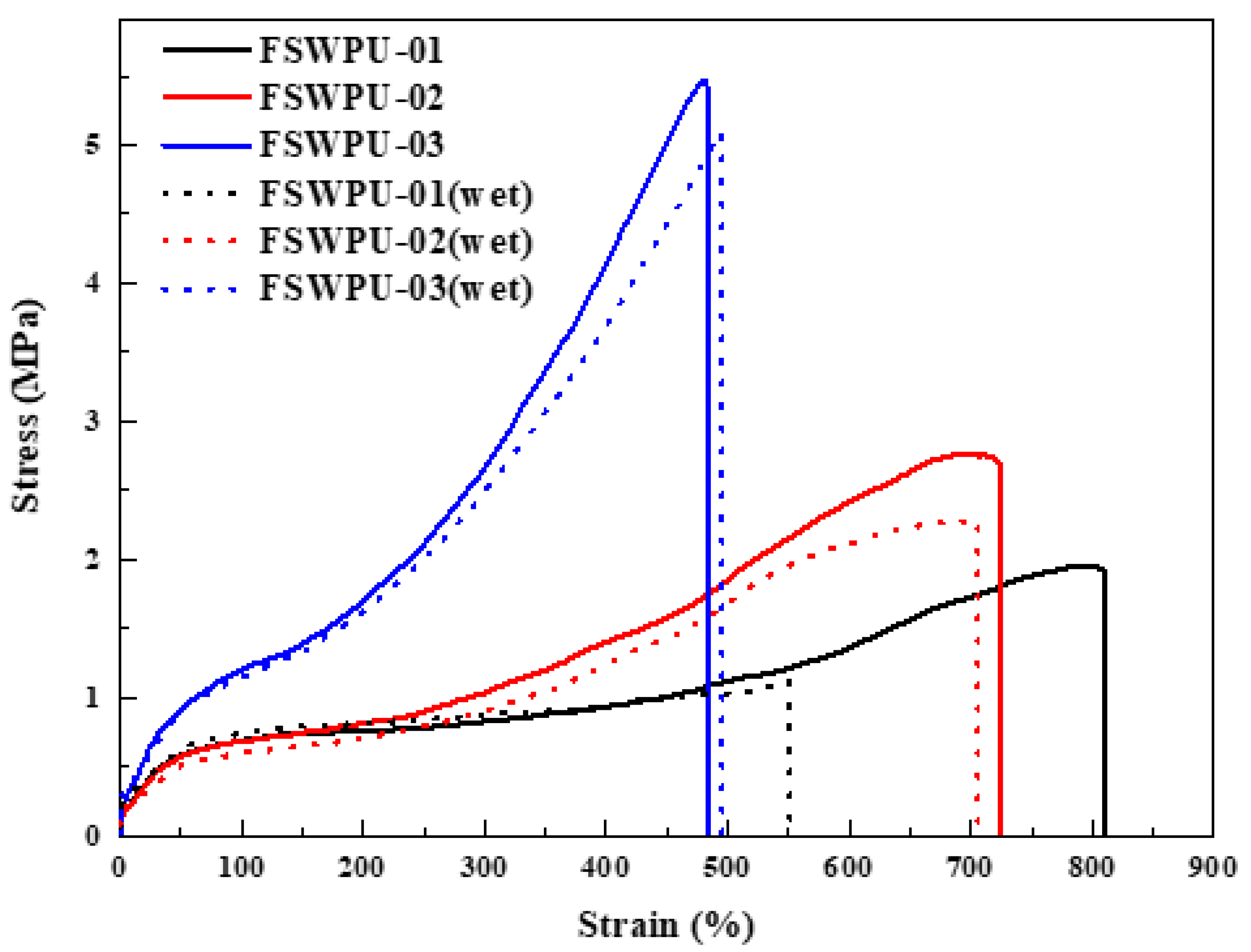
3.6. Tensile Properties, Shear Stress, and Hydrolytic Stability
3.7. Adhesion Test
3.8. Surface Contact Angle
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- PlasticsEurope. Plastics—The Facts. 2017. Available online: https://www.plasticseurope.org/application/files/1115/7236/4388/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 25 March 2020).
- Li, J.W.; Cheng, Y.H.; Lee, H.T.; Tsen, W.C.; Chiu, C.W.; Suen, M.C. Properties and degradation of castor oil-based fluoridated biopolyurethanes with different lengths of fluorinated segments. RSC Adv. 2019, 9, 31133–31149. [Google Scholar] [CrossRef]
- Deng, L.; Yu, Y.; Zhang, H.; Wang, Q.; Yu, R. The effects of biodegradable mulch film on the growth, yield, and water use efficiency of cotton and maize in an arid region. Sustainability 2019, 11, 7039. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly (lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar]
- Liu, Y.; Liang, H.; Li, S.; Liu, D.; Long, Y.; Liang, G.; Zhu, F. Preparation of waterborne polyurethane with high solid content and elasticity. J. Polym. Res. 2019, 26, 146. [Google Scholar] [CrossRef]
- Li, J.W.; Lee, H.T.; Tsai, H.A.; Suen, M.C.; Chiu, C.W. Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders. Polymers 2018, 10, 1292. [Google Scholar] [CrossRef]
- Palmisani, J.; Di Gilio, A.; Cisternino, E.; Tutino, M.; de Gennaro, G. Volatile Organic Compound (VOC) Emissions from a Personal Care Polymer-Based Item: Simulation of the Inhalation Exposure Scenario Indoors under Actual Conditions of Use. Sustainability 2020, 12, 2577. [Google Scholar] [CrossRef]
- Kozicki, M.; Piasecki, M.; Goljan, A.; Deptuła, H.; Niesłochowski, A. Emission of volatile organic compounds (VOCs) from dispersion and cementitious waterproofing products. Sustainability 2018, 10, 2178. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Protection of Environment Chapter I-Environmental Protection Agency (Continued) Subchapter C-Air Programs (Continued) Part 59-National Volatile Organic Compound Emission Standards for Consumer and Commercial Products; Office of the Federal Register: Washington, DC, USA, 1998. [Google Scholar]
- Bhargava, S.; Kubota, M.; Lewis, R.D.; Advani, S.G.; Prasad, A.K.; Deitzel, J.M. Ultraviolet, water, and thermal aging studies of a waterborne polyurethane elastomer-based high reflectivity coating. Prog. Org. Coat. 2015, 79, 75–82. [Google Scholar] [CrossRef]
- Paris Agreement. Adoption of the Paris Agreement; FCCC/CP/2015/L.9/Rev.1; United Nations Framework Convention on Climate Change: Le Bourget, France, 2015. [Google Scholar]
- Fuensanta, M.; Jofre-Reche, J.A.; Rodríguez-Llansola, F.; Costa, V.; Iglesias, J.I.; Martín-Martínez, J.M. Structural characterization of polyurethane ureas and waterborne polyurethane urea dispersions made with mixtures of polyester polyol and polycarbonate diol. Prog. Org. Coat. 2017, 112, 141–152. [Google Scholar] [CrossRef]
- Anderle, G.A.; Lenhard, S.L.; Lubnin, A.V.; Snow, G.E.; Tamareselvy, K. Plasticized Waterborne Polyurethane Dispersions and Manufacturing Process. US Patent 6,576,702, 10 June 2003. [Google Scholar]
- Zhou, X.; Li, Y.; Fang, C.; Li, S.; Cheng, Y.; Lei, W.; Meng, X. Recent advances in synthesis of waterborne polyurethane and their application in water-based ink: A review. J. Mater. Sci. Technol. 2015, 31, 708–722. [Google Scholar] [CrossRef]
- Lindstrom, U.M. Organic Reactions in Water: Principles, Strategies and Applications; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Zhang, S.F.; Wang, R.M.; He, Y.F.; Song, P.F.; Wu, Z.M. Waterborne polyurethane-acrylic copolymers crosslinked core–shell nanoparticles for humidity-sensitive coatings. Prog. Org. Coat. 2013, 76, 729–735. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, Y.; Xu, C.; Xie, A.; Yang, M.; Yang, S.; Chen, H. Study on synthesis and thickening property of hyperbranched waterborne polyurethane. Prog. Org. Coat. 2013, 76, 1302–1307. [Google Scholar] [CrossRef]
- Li, K.; Shen, Y.; Fei, G.; Wang, H.; Li, J. Preparation and properties of castor oil/pentaerythritol triacrylate-based UV curable waterborne polyurethane acrylate. Prog. Org. Coat. 2015, 78, 146–154. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, F.; Xu, B.; Xu, J.; Jiang, Y.; Yang, D.; Li, P. Preparation, mechanical properties and surface morphologies of waterborne fluorinated polyurethane-acrylate. Prog. Org. Coat. 2013, 76, 876–883. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. One-pot sonochemical synthesis of superhydrophobic organic–inorganic hybrid coatings on cotton cellulose. Cellulose 2013, 20, 3039–3051. [Google Scholar] [CrossRef]
- Yu, F.; Cao, L.; Meng, Z.; Lin, N.; Liu, X.Y. Crosslinked waterborne polyurethane with high waterproof performance. Polym. Chem. 2016, 7, 3913–3922. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Tian, X.; Xue, M.; Ding, X.; Ye, X.; Cui, Z. Surface and mechanical properties of hydrophobic silica contained hybrid films of waterborne polyurethane and fluorinated polymethacrylate. Polymer 2014, 55, 187–194. [Google Scholar] [CrossRef]
- Fu, H.; Yan, C.; Zhou, W.; Huang, H. Nano-SiO2/fluorinated waterborne polyurethane nanocomposite adhesive for laminated films. J. Ind. Eng. Chem. 2014, 20, 1623–1632. [Google Scholar] [CrossRef]
- Jin, K.; Li, L.; Torkelson, J.M. Recyclable Crosslinked Polymer Networks via One-Step Controlled Radical Polymerization. Adv. Mater. 2016, 28, 6746–6750. [Google Scholar] [CrossRef]
- Li, J.W.; Tsen, W.C.; Tsou, C.H.; Suen, M.C.; Chiu, C.W. Synthetic Environmentally Friendly Castor Oil Based-Polyurethane with Carbon Black as a Microphase Separation Promoter. Polymers 2019, 11, 1333. [Google Scholar] [CrossRef]
- Su, T.; Wang, G.Y.; Xu, X.D.; Hu, C.P. Preparation and properties of waterborne polyurethaneurea consisting of fluorinated siloxane units. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3365–3373. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Feng, Y. The reinforcing effect of crosslinkable waterborne polyurethane/polysiloxane composite emulsion by aqueous sol–gel method. J. Coat. Technol. Res. 2020, 17, 243–253. [Google Scholar] [CrossRef]
- Li, S.; Chen, F.; Zhang, B.; Luo, Z.; Li, H.; Zhao, T. Structure and improved thermal stability of phenolic resin containing silicon and boron elements. Polym. Degrad. Stab. 2016, 133, 321–329. [Google Scholar] [CrossRef]
- Hui, B.; Ye, L. Highly heat-resistant silicon-containing polyurethane-imide copolymers: Synthesis and thermal mechanical stability. Eur. Polym. J. 2017, 91, 337–353. [Google Scholar] [CrossRef]
- Zhang, P.; Fan, H.; Tian, S.; Chen, Y.; Yan, J. Synergistic effect of phosphorus–nitrogen and silicon-containing chain extenders on the mechanical properties, flame retardancy and thermal degradation behavior of waterborne polyurethane. RSC Adv. 2016, 6, 72409–72422. [Google Scholar] [CrossRef]
- Król, P.; Król, B. Surface free energy of polyurethane coatings with improved hydrophobicity. Colloid. Polym. Sci. 2012, 290, 879–893. [Google Scholar] [CrossRef]
- Wang, S.; Yaszemski, M.J.; Knight, A.M.; Gruetzmacher, J.A.; Windebank, A.J.; Lu, L. Photo-crosslinked poly (ε-caprolactone fumarate) networks for guided peripheral nerve regeneration: Material properties and preliminary biological evaluations. Acta Biomater. 2009, 5, 1531–1542. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, L.; Han, D.; Li, W.; Chen, Y.; Wang, X.; Ning, L. Effects of fluorine and silicon components on the hydrophobicity failure behavior of acrylic polyurethane coatings. J. Coat. Technol. Res. 2017, 14, 691–699. [Google Scholar] [CrossRef]
- Li, J.W.; Cheng, Y.H.; Lee, H.T.; Wang, C.C.; Chiu, C.W.; Suen, M.C. Synthesis and properties of castor oil-based polyurethane containing short fluorinated segment. J. Appl. Polym. Sci. 2020, e49062. [Google Scholar] [CrossRef]
- Komada, M.; Nakanishi, Y.; Matsumoto, T.; Kotera, M.; Hongo, C.; Nishino, T. Enhancement of adhesion by applying amine primer to isotactic polypropylene and open time dependence of primer effect. Int. J. Adhes. Adhes. 2018, 84, 173–177. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Arbelaiz, A.; Saralegi, A.; Fernández-d’Arlas, B.; Eceiza, A.; Corcuera, M.A. Relationship between reagents molar ratio and dispersion stability and film properties of waterborne polyurethanes. Surf. A Physicochem. Eng. Asp. 2015, 482, 554–561. [Google Scholar] [CrossRef]
- Guo, Y.H.; Guo, J.J.; Miao, H.; Teng, L.J.; Huang, Z. Properties and paper sizing application of waterborne polyurethane emulsions synthesized with isophorone diisocyanate. Prog. Org. Coat. 2014, 77, 988–996. [Google Scholar] [CrossRef]
- Hajializadeh, S.; Barikani, M.; Bellah, S.M. Synthesis and characterization of multiwall carbon nanotube/waterborne polyurethane nanocomposites. Polym. Int. 2017, 66, 1074–1083. [Google Scholar] [CrossRef]


| Designation | PCL 1 (moles) | IPDI 2 (moles) | TF 3 (mole) | DMPA 4 (moles) | TEA 5 (moles) | AEAPTMS 6 (moles) | EDA 7 (moles) | Si 8 (%) |
|---|---|---|---|---|---|---|---|---|
| FSWPU-01 | 2.2 | 4 | 0.3 | 0.8 | 0.8 | 0 | 0.7 | 0 |
| FSWPU-02 | 2.1 | 4 | 0.3 | 0.8 | 0.8 | 0.1 | 0.7 | 1 |
| FSWPU-03 | 2 | 4 | 0.3 | 0.8 | 0.8 | 0.2 | 0.7 | 2 |
| Sample | Retention Time of the Peak (min) | (x 104) | (× 104) | / |
|---|---|---|---|---|
| FSWPU-01 | 20.7 | 1.88 | 2.63 | 1.40 |
| FSWPU-02 | 20.2 | 2.64 | 3.46 | 1.31 |
| FSWPU-03 | 20.0 | 2.57 | 3.58 | 1.39 |
| Sample Name | C (%) | N (%) | O (%) | F (%) | Si (%) |
|---|---|---|---|---|---|
| FSWPU-01 | 48.35 | 38.14 | 11.33 | 2.15 | 0.03 |
| FSWPU-02 | 47.21 | 37.35 | 13.67 | 1.73 | 0.04 |
| FSWPU-03 | 48.52 | 38.11 | 12.28 | 1.03 | 0.06 |
| Designation | TGA | DMA | |||
|---|---|---|---|---|---|
| Tonset (oC) | Tmax | 700 °C Residue | Tgd from Tan δ (°C) | Tan δmax | |
| FSWPU-01 | 295.1 | 330.6 | 1.7% | 31.71 | 0.8845 |
| FSWPU-02 | 300.7 | 333.8 | 2.4% | 35.59 | 0.8397 |
| FSWPU-03 | 302.8 | 339.0 | 2.9% | 44.26 | 0.7058 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Shear Stress | ||
|---|---|---|---|---|---|---|
| Original a | Wet b | Original a | Wet b | (MPa) | ||
| FSWPU-01 | 2.0 | 1.1 | 811 | 551 | 0.03 | 0.78 |
| FSWPU-02 | 2.8 | 2.3 | 724 | 706 | 0.04 | 1.16 |
| FSWPU-03 | 5.5 | 5.1 | 484 | 495 | 0.11 | 1.41 |
| Sample Name | Contact Angle (o) | Surface Free Energy (mJ/m2) | |||
|---|---|---|---|---|---|
| Water | CH2I2 | ||||
| Control | 41.5 ± 1.8 | 48.5 ± 0.3 | 23.11 | 33.31 | 56.42 |
| FSWPU-01 | 80.4 ± 2.6 | 45.4 ± 0.6 | 32.82 | 4.85 | 37.67 |
| FSWPU-02 | 84.3 ± 1.3 | 44.3 ± 0.4 | 34.50 | 3.11 | 37.61 |
| FSWPU-03 | 92.1 ± 3.2 | 44.2 ± 0.6 | 36.66 | 0.91 | 37.57 |
| Sample Name | Soft Segment + other Auxiliary | Tensile Strength (MPa) | Elongation at Break (%) | Water Contact Angle (o) | Ref. |
|---|---|---|---|---|---|
| FSWPU-01 | PCL530 | 2.0 | 810 | 41.5 ± 1.8 | This study |
| FSWPU-03 | PCL530 | 5.5 | 484 | 92.1 ± 3.2 | This study |
| PU2-1 | PCL2000 | 5.5 ± 0.2 | 1197 | -- | [36] |
| PU3-1 | PCL2000 | 7.9 ± 0.4 | 735 | -- | [36] |
| B2 | PCL2000 | 8.64 | 715 | -- | [37] |
| C1 | PCL2000 | 6.82 | 638 | -- | [37] |
| WBPU-CNT0.5 | PCL2000+ MWCNT a | 12 | 160 | -- | [38] |
| WBPU-CNT1.0 | PCL2000+ MWCNT | 4 | 260 | -- | [38] |
| FPU-3 | PTMG2000 b+ SiO2 NPs c | 38 | 920 | 150.6 | [22] |
| SiO2/FWPU-0 | PBA2000 d+ SiO2 NPs | -- | -- | 61.29 | [23] |
| SiO2/FWPU-20 | PBA2000+ SiO2 NPs | -- | -- | 101.26 | [23] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-T.; Wang, W.-H.; Hung, W.-H. Evaluating the Properties of a Coating Material with Polycaprolactone-Degradable Fluorinated Silicon-Containing Waterborne Polyurethane. Sustainability 2020, 12, 3745. https://doi.org/10.3390/su12093745
Hsu Y-T, Wang W-H, Hung W-H. Evaluating the Properties of a Coating Material with Polycaprolactone-Degradable Fluorinated Silicon-Containing Waterborne Polyurethane. Sustainability. 2020; 12(9):3745. https://doi.org/10.3390/su12093745
Chicago/Turabian StyleHsu, Yao-Tang, Wen-Hsin Wang, and Wei-Hsi Hung. 2020. "Evaluating the Properties of a Coating Material with Polycaprolactone-Degradable Fluorinated Silicon-Containing Waterborne Polyurethane" Sustainability 12, no. 9: 3745. https://doi.org/10.3390/su12093745
APA StyleHsu, Y.-T., Wang, W.-H., & Hung, W.-H. (2020). Evaluating the Properties of a Coating Material with Polycaprolactone-Degradable Fluorinated Silicon-Containing Waterborne Polyurethane. Sustainability, 12(9), 3745. https://doi.org/10.3390/su12093745




