Application of Biostimulants Containing Amino Acids to Tomatoes Could Favor Sustainable Cultivation: Implications for Tyrosine, Lysine, and Methionine
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions and Plant Material
2.2. Amino Acid Preparation
2.3. Application of the Treatments
2.4. Parameters Evaluated
2.4.1. Gas Exchange Parameters
2.4.2. Chlorophyll Fluorescence and Concentration Parameters
2.4.3. Growth Parameters
2.4.4. Measuring the Concentration of Mineral Nutrients in Leaf Tissues
2.4.5. Metabolomic Analysis of Leaf Tissues
2.5. Statistical Analysis
3. Results
3.1. Vegetative Growth Parameters
3.2. Physiological Study
3.3. Ionomic Study
3.4. Metabolomic Study
3.4.1. Amino Acids
3.4.2. Organic Acids
3.4.3. Sugars
3.4.4. Other Metabolites
3.5. Principal Component Analysis (PCA) and Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAs | amino acids |
| ACO2 | net assimilation of CO2 |
| CA | cluster analysis |
| Chl HD | chlorophyll in completely developed leaves |
| Chl HB | chlorophyll in leaf buds |
| Fv’/Fm’ | antenna efficiency of photosystem II |
| ΦPSII | quantum efficiency of photosystem II |
| gs | stomatal conductance |
| Lys | lysine |
| Met | methionine |
| PCA | principal component analysis |
| NMR | Nuclear Magnetic Resonance |
| Tyr | tyrosine |
| WUE | water use efficiency |
References
- Tonhati, R.; Mello, S.C.; Momesso, P.; Pedroso, R.M. L-proline alleviates heat stress of tomato plants grown under protected environment. Sci. Hortic. 2020, 268, 109370. [Google Scholar] [CrossRef]
- Chavan, R.F.; Sakhal, B.K. Studies on the effect of exogenous application of salicylic acid on post-harvest quality and shelf life of tomato fruit Cv. Abhinav. Food Res. 2020, 4, 1444–1450. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Datos Sobre Alimentación en Agricultura (FAOSTAT). Available online: http://www.fao.org/faostat/en/#home (accessed on 14 September 2020).
- Parry, M.L. Climate Change and World Agriculture, 1st ed.; Routledge: London, UK, 2019. [Google Scholar]
- Mutale-Joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; El Arroussi, H. Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 1. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Jonhnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling andcrop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 124–133. [Google Scholar] [CrossRef]
- Reglamento (UE) 2019/1009 del Parlamento Europeo y del Consejo de 5 de Junio de 2020. Available online: https://www.boe.es/doue/2019/170/L00001-00114.pdf (accessed on 21 September 2020).
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, 2. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Simón-Grao, S.; Garcia-Sanchez, F.; Alfosea-Simón, M.; Simón, I.; Lidón, V.; Ortega, W.M.R. Study on the foliar aplication of fitomare® on drought tolerance of tomato plants. IJPAES 2016, 6, 15–21. [Google Scholar]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The use of a plant-based biostimulant improves plant performances and fruit quality in tomato plants grown at elevated temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Maach, M.; Boudouasar, K.; Akodad, M.; Skalli, A.; Moumen, A.; Baghour, M. Application of biostimulants improves yield and fruit quality in tomato. Int. J. Veg. Sci. 2020, 1–6. [Google Scholar] [CrossRef]
- Klokić, I.; Koleška, I.; Hasanagić, D.; Murtić, S.; Bosančić, B.; Todorović, V. Biostimulants’ influence on tomato fruit characteristics at conventional and low-input NPK regime. Acta Agric. Scand. B Plant Soil Sci. 2020, 70, 233–240. [Google Scholar] [CrossRef]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef]
- Arruda, P.; Barreto, P. Lysine Catabolism Through the saccharopine pathway: Enzymes and intermediates involved in plant responses to abiotic and biotic stress. Front. Plant Sci. 2020, 11, 587. [Google Scholar] [CrossRef]
- D’Mello, J.P.F. Amino Acids in Higher Plants; D’Mello, J.P.F., Ed.; CABI International: Edinburgh, UK, 2015. [Google Scholar]
- Amir, R.; Hacham, Y.; Galili, G. Cystathionine γ-synthase and threonine synthase operate in concert to regulate carbon flow towards methionine in plants. Trends Plant Sci. 2002, 7, 153–156. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Zavala-Gonzalez, E.A.; Camara-Zapata, J.M.; Martínez-Nicolás, J.J.; Simón, I.; Simón-Grao, S.; García-Sánchez, F. Effect of foliar application of amino acids on the salinity tolerance of tomato plants cultivated under hydroponic system. Sci. Hortic. 2020, 272, 109509. [Google Scholar] [CrossRef]
- Capaldi, F.R.; Gratão, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur metabolism and stress defense responses in plants. Trop. Plant Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef]
- Rai, V.K. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Simón-Grao, S.; Nieves, M.; Cámara-Zapata, J.M.; Martínez-Nicolás, J.J.; Rivero, R.M.; Fernández-Zapata, J.C.; García-Sánchez, F. The Forner Alcaide n° 5 citrus genotype shows a different physiological response to the excess of boron in the irrigation water in relation to its two genotype progenitors. Sci. Hortic. 2019, 245, 19–28. [Google Scholar] [CrossRef]
- Van der Sar, S.; Kim, H.K.; Meissner, A.; Verpoorte, R.; Choi, Y.H. Nuclear Magnetic Resonance spectroscopy for plant metabolite profiling. Handb. Plant Metab. 2013, 57–76. [Google Scholar] [CrossRef]
- Duynhoven, J.V.; Van As, H.; Belton, P.S.; Webb, G.A. Magnetic Resonance in Food Science: Food for Thought, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Becerra-Martínez, E.; Florentino-Ramos, E.; Pérez-Hernández, N.; Zepeda-Vallejo, L.G.; Villa-Ruano, N.; Velázquez-Ponce, M.; García-Mendoza, F.; Bañuelos-Hernández, A.E. 1H NMR-based metabolomic fingerprinting to determine metabolite levels in serrano peppers (Capsicum annum L.) grown in two different regions. Food Res. Int. 2017, 102, 163–170. [Google Scholar] [CrossRef]
- El-Sherbeny, M.; da Teixeira Silva, J.A. Foliar treatment with proline and tyrosine affect the growth and yield of beetroot and some pigments in beetroot leaves. J. Hortic. Res. 2013, 21, 95–99. [Google Scholar] [CrossRef]
- Merwad, A.R.M.A.; Desoky, E.S.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Akram, N.A.; Umm, E.H.; Ashraf, M.; Ashraf, M.; Sadiq, M. Exogenous application of L-methionine mitigates the drought-induced oddities in biochemical and anatomical responses of bitter gourd (Momordica charantia L.). Sci. Hortic. 2020, 267, 109333. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation Effects of Foliar Applied glycine and glutamine amino acids on lettuce growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Yordanova, R.; Popava, L. Effect of exogenous treatment with salicylic acid or photosynthetic activity and antioxidant capacity of chilled wheat plants. Gen. Appl. Plant Physiol. 2007, 33, 155–170. [Google Scholar]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol. Plant 2011, 142, 35–46. [Google Scholar] [CrossRef]
- Kong, D.; Hu, H.C.; Okuma, E.; Lee, Y.; Lee, H.S.; Munemasa, S.; Cho, D.; Ju, C.; Pedoeim, L.; Rodriguez, B.; et al. L-Met activates arabidopsis GLR Ca2+ channels upstream of ROS production and regulates stomatal movement. Cell Rep. 2016, 17, 2553–2561. [Google Scholar] [CrossRef]
- Bakry, B.A.; Sadak, M.S.; Abd El-Monem, A.A. Physiological aspects of tyrosine and salicylic acid on morphological, yield and biochemical constituents of peanut plants. PJBS 2020, 23, 375–384. [Google Scholar]
- Sadak, M.S.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar]
- Molaie, H.; Panahi, B.; Tajabadipour, A. The effect of foliar application of some amino acid compounds on photosynthesis and yield of two commercial cultivars in pistachio orchards of Kerman province in Iran. IJACS 2013, 5, 2827–2830. [Google Scholar]
- Watanabe, T.; Maejima, E.; Yoshiruma, T.; Urayama, M.; Yamauchi, A.; Owadano, M.; Okada, R.; Osaki, M.; Kanayama, Y.; Shinano, T. The ionomic study of vegetable crops. PLoS ONE 2016, 11, e0160273. [Google Scholar] [CrossRef]
- Padgett, P.E.; Leonard, R.T. Free amino acid levels and the regulation of nitrate uptake in maize cell suspension cultures. J. Exp. Bot. 1996, 47, 871–883. [Google Scholar] [CrossRef][Green Version]
- Xing, Y.; Zhu, Z.L.; Wang, F.; Zhang, X.; Li, B.Y.; Liu, Z.X.; Wu, X.X.; Ge, S.F.; Jiang, Y.M. Role of calcium as a possible regulator of growth and nitrate nitrogen metabolism in apple dwarf rootstock seedlings. Sci. Hortic. 2021, 276, 109740. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 155–166. [Google Scholar]
- Pervaiz, A.; Iqbal, A.; Khalid, A.; Manzoor, A.; Noreen, S.; Ayaz, A.; Zafar, Z.; Athar, H.; Ashraf, M. Proline induced modulation in physiological responses in wheat plants. J. Agric. Environ. Sci. 2019, 8, 112–119. [Google Scholar] [CrossRef]
- Joshi, V.; Joung, J.G.; Fei, Z.; Jander, G. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef]
- Li, M.; Guo, R.; Jiao, Y.; Jin, X.; Zhang, H.; Shi, L. Comparison of salt tolerance in Soja based on metabolomics of seedling roots. Front. Plant Sci. 2017, 8, 1101. [Google Scholar] [CrossRef]
- Zhong, M.; Yuan, Y.; Shu, S.; Sun, J.; Guo, S.; Yuan, R.; Tang, Y. Effects of exogenous putrescine on glycolysis and Krebs cycle metabolism in cucumber leaves subjected to salt stress. Plant Growth Regul. 2016, 79, 319–330. [Google Scholar] [CrossRef]
- Carillo, P.; Woo, S.L.; Comite, E.; El-nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of trichoderma harzianum, 6-pentyl-α-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]

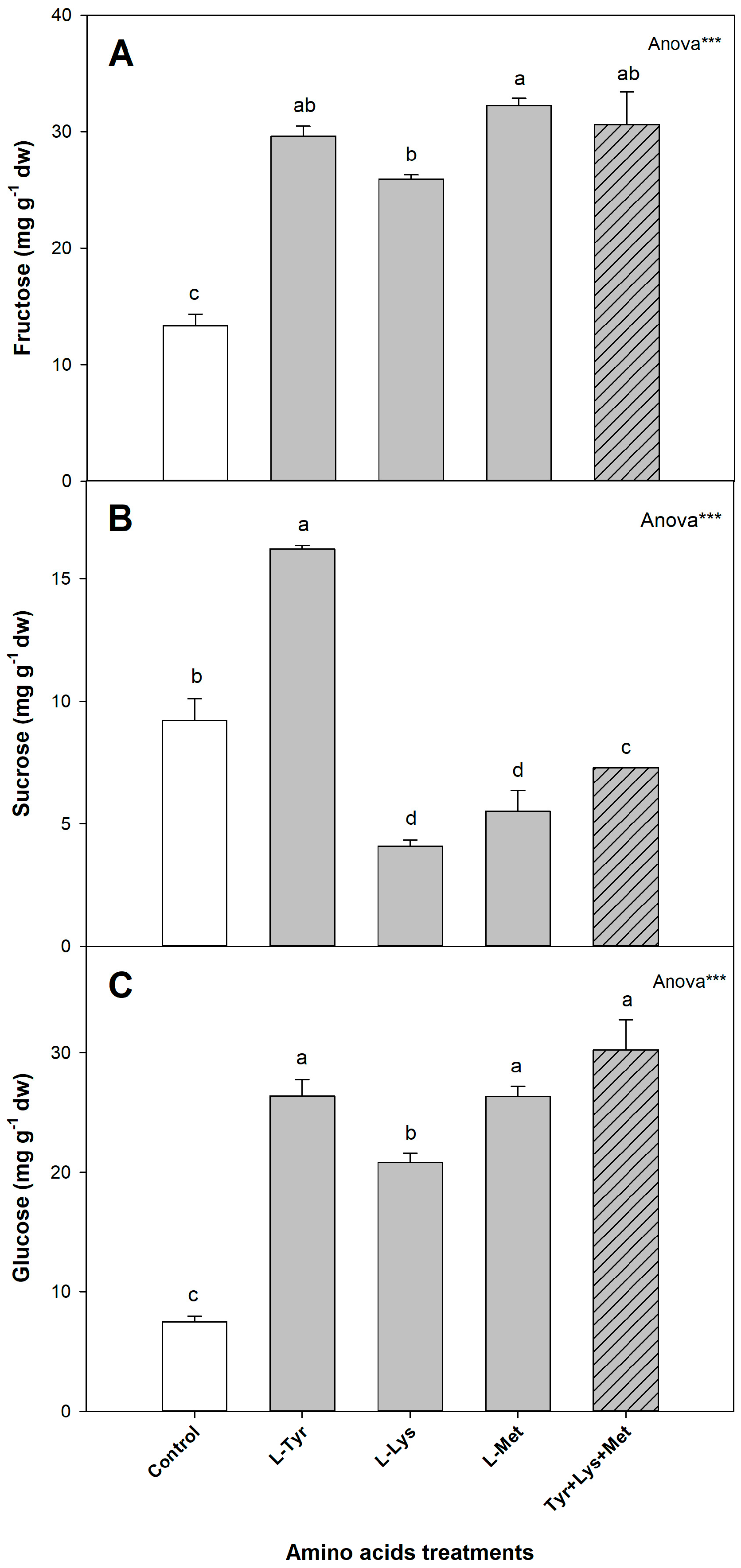
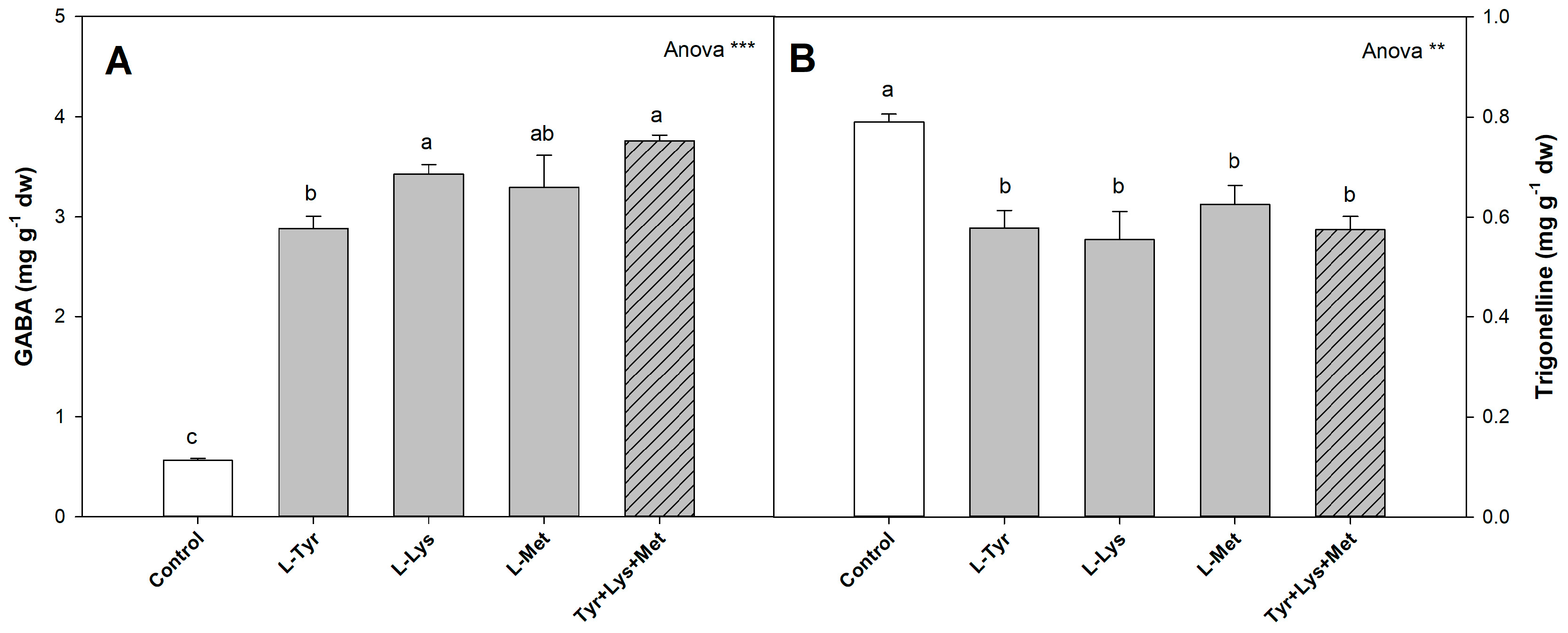
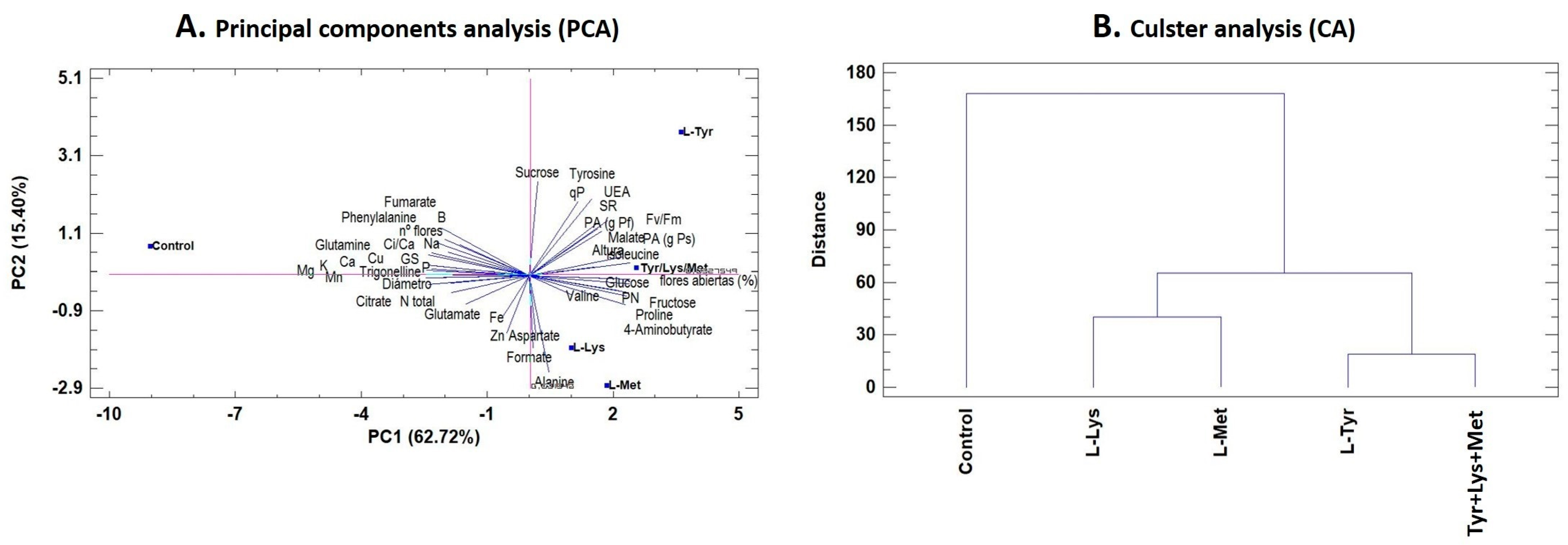
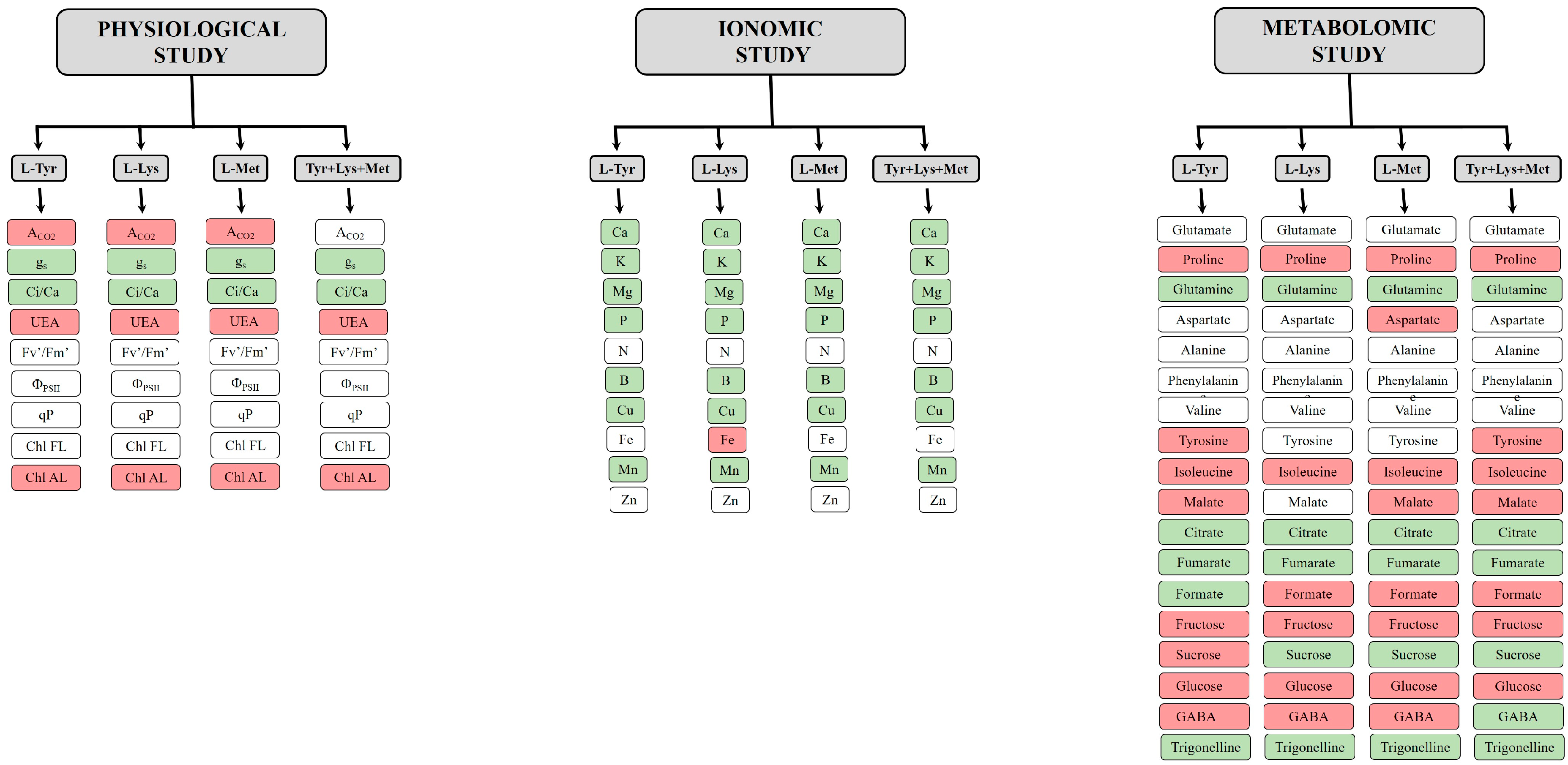
| Treatments | Height (cm) | Stem Diameter (mm) | Sh (g dw) |
|---|---|---|---|
| Control (w/o AAs) | 39.3 ± 0.5 | 10.8 ± 0.1 a | 13.3 ± 0.4 b |
| L-Tyr | 41.0 ± 1.2 | 10.0 ± 0.2 b | 21.2 ± 1.8 a |
| L-Lys | 39.0 ± 1.4 | 10.1 ± 0.2 ab | 19.0 ± 0.5 a |
| L-Met | 41.5 ± 1.3 | 10.2 ± 0.2 ab | 19.0 ± 0.4 a |
| Tyr + Lys + Met | 41.6 ± 2.0 | 9.3 ± 0.3 c | 18.8 ± 0.7 a |
| ANOVA | ns | ** | *** |
| Gas Exchange Parameters | |||||
| Treatments | ACO2 (μmol m−2 s−1) | gs (mmol m−2 s−1) | WUE (μmol CO2 mmol−1 H2O) | ||
| Control (w/o AAs) | 16.8 ± 1.5 b | 1196.3 ± 105.7 a | 2.38 ± 0.35 c | ||
| L-Tyr | 22.0 ± 1.3 a | 162.8 ± 18.4 c | 10.08 ± 0.70 a | ||
| L-Lys | 22.0 ± 1.4 a | 498.7 ± 51.9 b | 5.18 ± 0.15 b | ||
| L-Met | 21.6 ± 0.8 a | 250.4 ± 21.7 c | 5.79 ± 0.21 b | ||
| Tyr + Lys + Met | 19.6 ± 0.3 ab | 507.6 ± 46.5 b | 5.45 ± 0.24 b | ||
| ANOVA | * | *** | *** | ||
| Chl Fluorescence Parameters | |||||
| Treatments | Fv’/Fm’ | ΦPSII | qP | Chl HD (SPAD) | Chl HB (SPAD) |
| Control (w/o AAs) | 0.76 ± 0.011 | 0.70 ± 0.014 | 0.93 ± 0.005 | 24.8 ± 2.5 ab | 20.1 ± 1.8 c |
| L-Tyr | 0.80 ± 0.001 | 0.75 ± 0.003 | 0.94 ± 0.005 | 26.4 ± 2.4 ab | 45.1 ± 2.8 ab |
| L-Lys | 0.79 ± 0.013 | 0.74 ± 0.014 | 0.93 ± 0.003 | 30.7 ± 1.7 a | 46.8 ± 3.6 a |
| L-Met | 0.76 ± 0.030 | 0.71 ± 0.029 | 0.92 ± 0.006 | 30.8 ± 2.8 a | 38.2 ± 3.7 ab |
| Tyr + Lys + Met | 0.78 ± 0.013 | 0.73 ± 0.009 | 0.93 ± 0.008 | 20.2 ± 2.0 b | 36.5 ± 2.2 b |
| ANOVA | ns | ns | ns | * | *** |
| Macronutrients (g 100 g−1 dw) | ||||||||||
| Treatments | Ca | K | Mg | Na | P | N | ||||
| Control (sin AAs) | 4.22 ± 0.22 a | 4.25 ± 0.14 a | 1.03 ± 0.07 a | 0.34 ± 0.02 a | 0.46 ± 0.03 a | 5.04 ± 0.21 | ||||
| L-Tyr | 2.24 ± 0.07 b | 3.00 ± 0.13 b | 0.61 ± 0.04 b | 0.14 ± 0.01 bc | 0.28 ± 0.02 b | 4.63 ± 0.14 | ||||
| L-Lys | 2.45 ± 0.12 b | 3.25 ± 0.04 b | 0.72 ± 0.03 b | 0.13 ± 0.01 c | 0.33 ± 0.02 b | 4.82 ± 0.32 | ||||
| L-Met | 2.48 ± 0.06 b | 3.22 ± 0.17 b | 0.65 ± 0.04 b | 0.14 ± 0.00 c | 0.30 ± 0.03 b | 4.75 ± 0.20 | ||||
| Tyr + Lys + Met | 2.29 ± 0.16 b | 3.12 ± 0.16 b | 0.69 ± 0.05 b | 0.18 ± 0.01 b | 0.32 ± 0.02 b | 4.33 ± 0.07 | ||||
| ANOVA | *** | *** | *** | *** | *** | ns | ||||
| Micronutrients (ppm) | ||||||||||
| Treatments | B | Cu | Fe | Mn | Zn | |||||
| Control (sin AAs) | 49.1 ± 3.7 a | 9.1 ± 0.4 a | 172.6 ± 11.7 b | 78.3 ± 5.3 a | 28.0 ± 1.0 | |||||
| L-Tyr | 37.8 ± 1.3 bc | 5.0 ± 0.2 b | 144.5 ± 10.9 b | 56.8 ± 1.9 b | 26.1 ± 1.1 | |||||
| L-Lys | 39.6 ± 2.5 b | 5.0 ± 0.5 b | 210.8 ± 8.5 a | 65.4 ± 1.7 b | 31.4 ± 2.5 | |||||
| L-Met | 31.6 ± 1.4 c | 5.3 ± 0.3 b | 149.7 ± 9.6 b | 53.5 ± 4.8 b | 28.4 ± 2.0 | |||||
| Tyr + Lys + Met | 40.2 ± 1.4 b | 4.5 ± 0.3 b | 157.7 ± 8.0 b | 59.1 ± 3.5 b | 24.5 ± 1.3 | |||||
| ANOVA | *** | *** | ** | ** | Ns | |||||
| Amino Acids (mg g−1 dw) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | Glutamate | Proline | Glutamine | Aspartate | Alanine | Phenylalanine | Valine | Tyrosine | Isoleucine |
| Control (w/o AAs) | 7.71 ± 0.80 | 5.08 ± 0.41 b | 4.01 ± 0.33 a | 2.53 ± 0.19 b | 0.52 ± 0.04 | 0.27 ± 0.01 | 0.22 ± 0.01 | 0.15 ± 0.01 c | 0.17 ± 0.01 b |
| L-Tyr | 7.36 ± 0.68 | 9.08 ± 0.81 a | 1.82 ± 0.15 c | 2.51 ± 0.23 b | 0.47 ± 0.02 | 0.24 ± 0.02 | 0.24 ± 0.00 | 0.92 ± 0.08 a | 0.28 ± 0.00 a |
| L-Lys | 7.79 ± 0.82 | 9.76 ± 1.05 a | 1.45 ± 0.06 c | 2.54 ± 0.14 b | 0.60 ±0.03 | 0.20 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.01 c | 0.26 ± 0.02 a |
| L-Met | 7.38 ± 0.34 | 8.90 ± 0.79 a | 1.84 ± 0.14 c | 3.23 ± 0.18 a | 0.64 ± 0.04 | 0.24 ± 0.02 | 0.25 ± 0.03 | 0.20 ± 0.02 c | 0.28 ± 0.03 a |
| Tyr + Lys + Met | 7.44 ± 0.77 | 8.98 ± 0.50 a | 2.99 ± 0.03 b | 2.24 ± 0.07 b | 0.58 ± 0.04 | 0.20 ± 0.01 | 0.22 ± 0.02 | 0.68 ± 0.03 b | 0.25 ± 0.02 a |
| ANOVA | ns | ** | *** | * | ns | ns | ns | *** | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfosea-Simón, M.; Simón-Grao, S.; Zavala-Gonzalez, E.A.; Cámara-Zapata, J.M.; Simón, I.; Martínez-Nicolás, J.J.; Lidón, V.; Rodríguez-Ortega, W.M.; García-Sánchez, F. Application of Biostimulants Containing Amino Acids to Tomatoes Could Favor Sustainable Cultivation: Implications for Tyrosine, Lysine, and Methionine. Sustainability 2020, 12, 9729. https://doi.org/10.3390/su12229729
Alfosea-Simón M, Simón-Grao S, Zavala-Gonzalez EA, Cámara-Zapata JM, Simón I, Martínez-Nicolás JJ, Lidón V, Rodríguez-Ortega WM, García-Sánchez F. Application of Biostimulants Containing Amino Acids to Tomatoes Could Favor Sustainable Cultivation: Implications for Tyrosine, Lysine, and Methionine. Sustainability. 2020; 12(22):9729. https://doi.org/10.3390/su12229729
Chicago/Turabian StyleAlfosea-Simón, Marina, Silvia Simón-Grao, Ernesto A. Zavala-Gonzalez, Jose María Cámara-Zapata, Inmaculada Simón, Juan José Martínez-Nicolás, Vicente Lidón, Wilbert M. Rodríguez-Ortega, and Francisco García-Sánchez. 2020. "Application of Biostimulants Containing Amino Acids to Tomatoes Could Favor Sustainable Cultivation: Implications for Tyrosine, Lysine, and Methionine" Sustainability 12, no. 22: 9729. https://doi.org/10.3390/su12229729
APA StyleAlfosea-Simón, M., Simón-Grao, S., Zavala-Gonzalez, E. A., Cámara-Zapata, J. M., Simón, I., Martínez-Nicolás, J. J., Lidón, V., Rodríguez-Ortega, W. M., & García-Sánchez, F. (2020). Application of Biostimulants Containing Amino Acids to Tomatoes Could Favor Sustainable Cultivation: Implications for Tyrosine, Lysine, and Methionine. Sustainability, 12(22), 9729. https://doi.org/10.3390/su12229729






