Deployable Membrane-Based Energy Technologies: the Ethiopian Prospect
Abstract
1. Introduction
1.1. Overview of Currently Employed Energy Sources and Technologies in Ethiopia
1.2. Advances in Renewable Energy Technologies
1.3. Energy Policy and Strategies in Ethiopia
2. Fuel Cell and Hydrogen Technologies
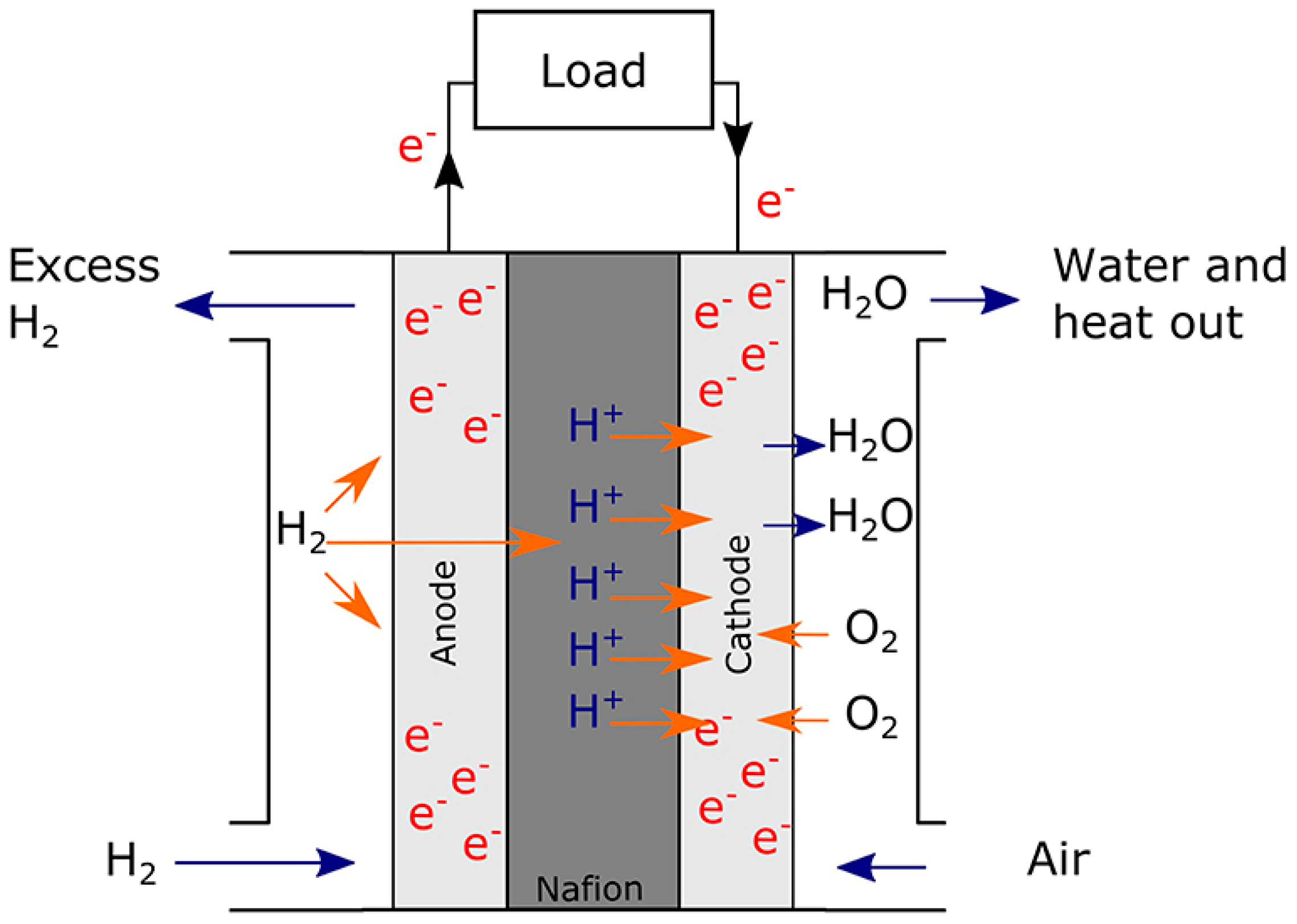
2.1. Fuel Cells
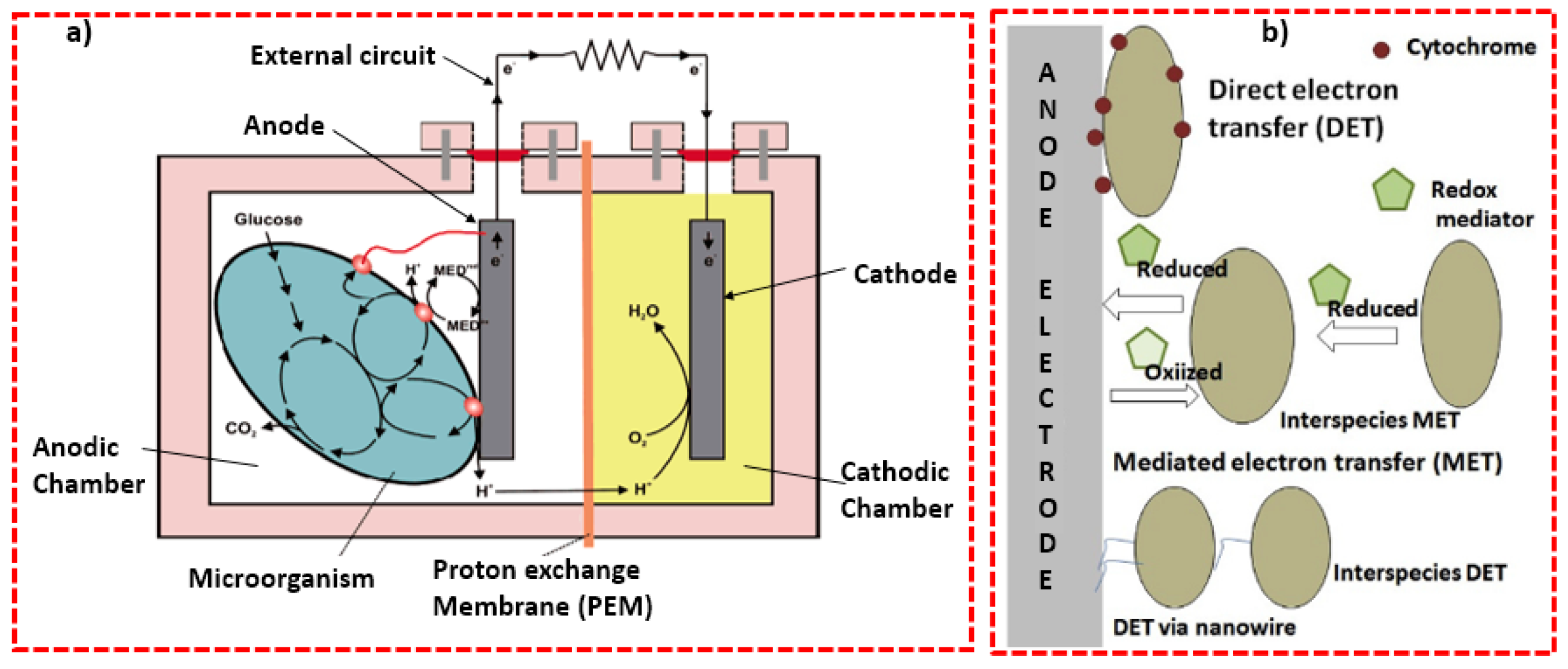
2.2. Microbial Fuel Cell
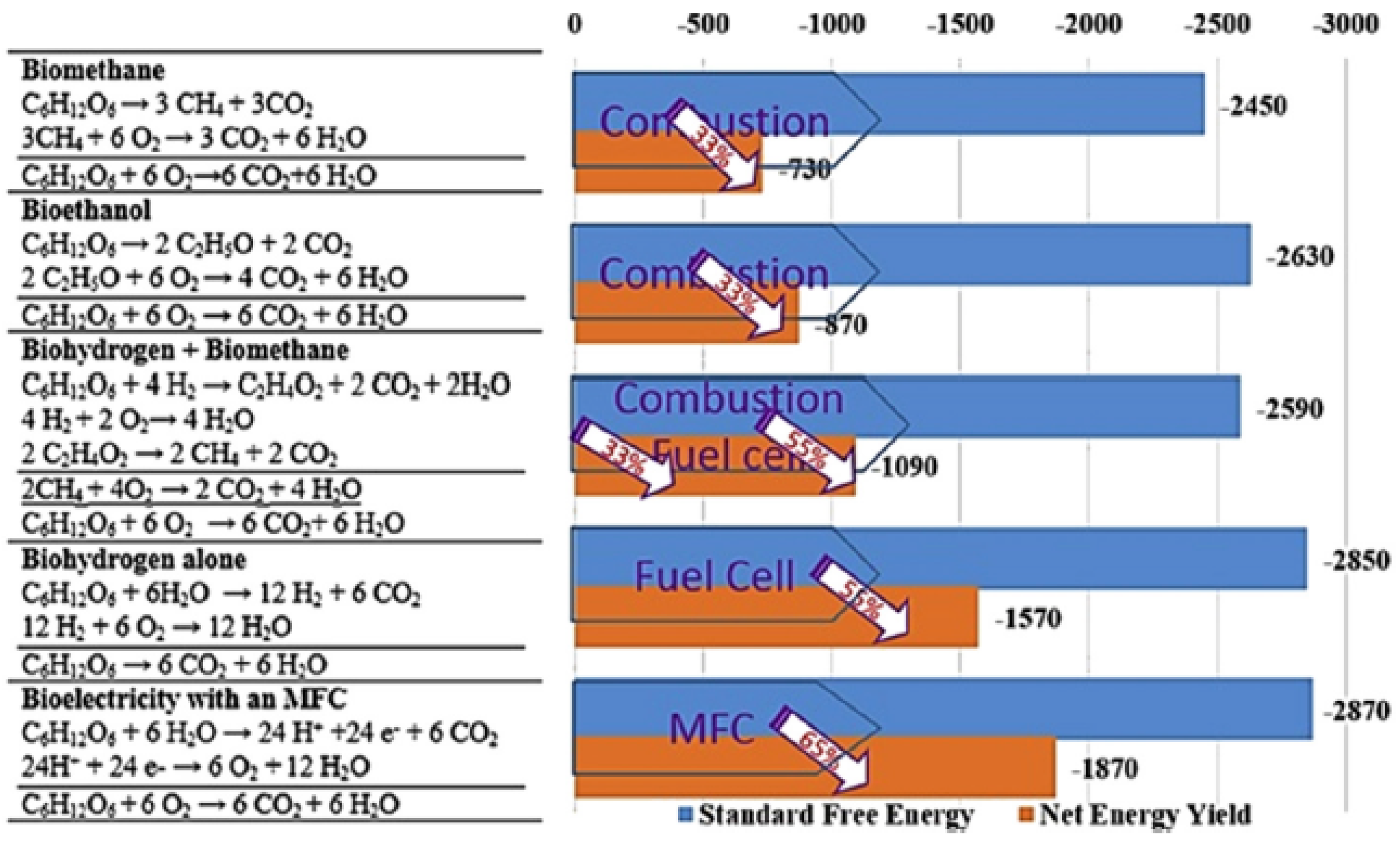
3. Advantages of MFC
3.1. Hydrogen Production Technologies
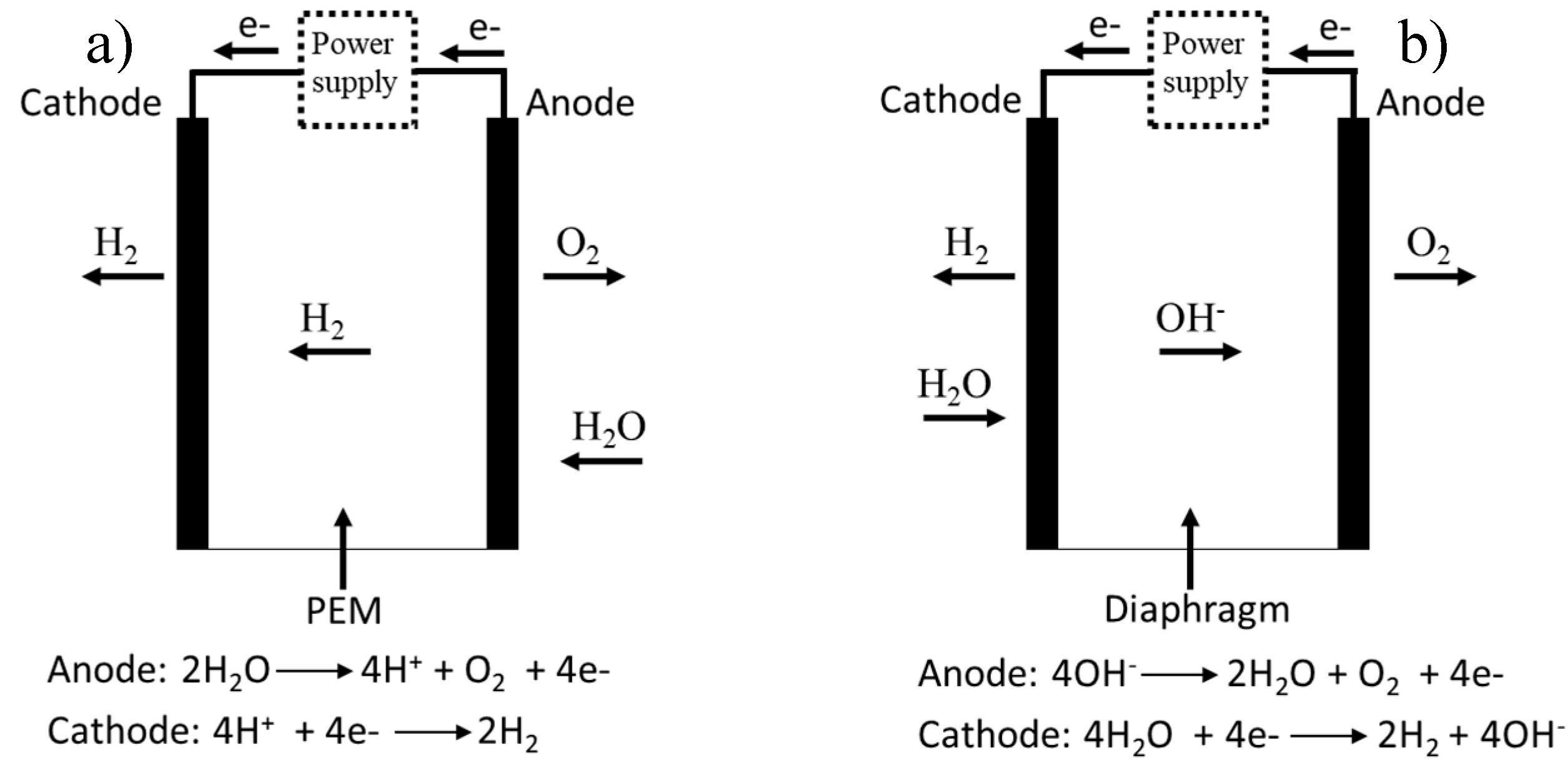
3.1.1. PEM Electrolyzers
3.1.2. Alkaline Electrolyzers
3.1.3. High-Temperature Water Electrolyzers
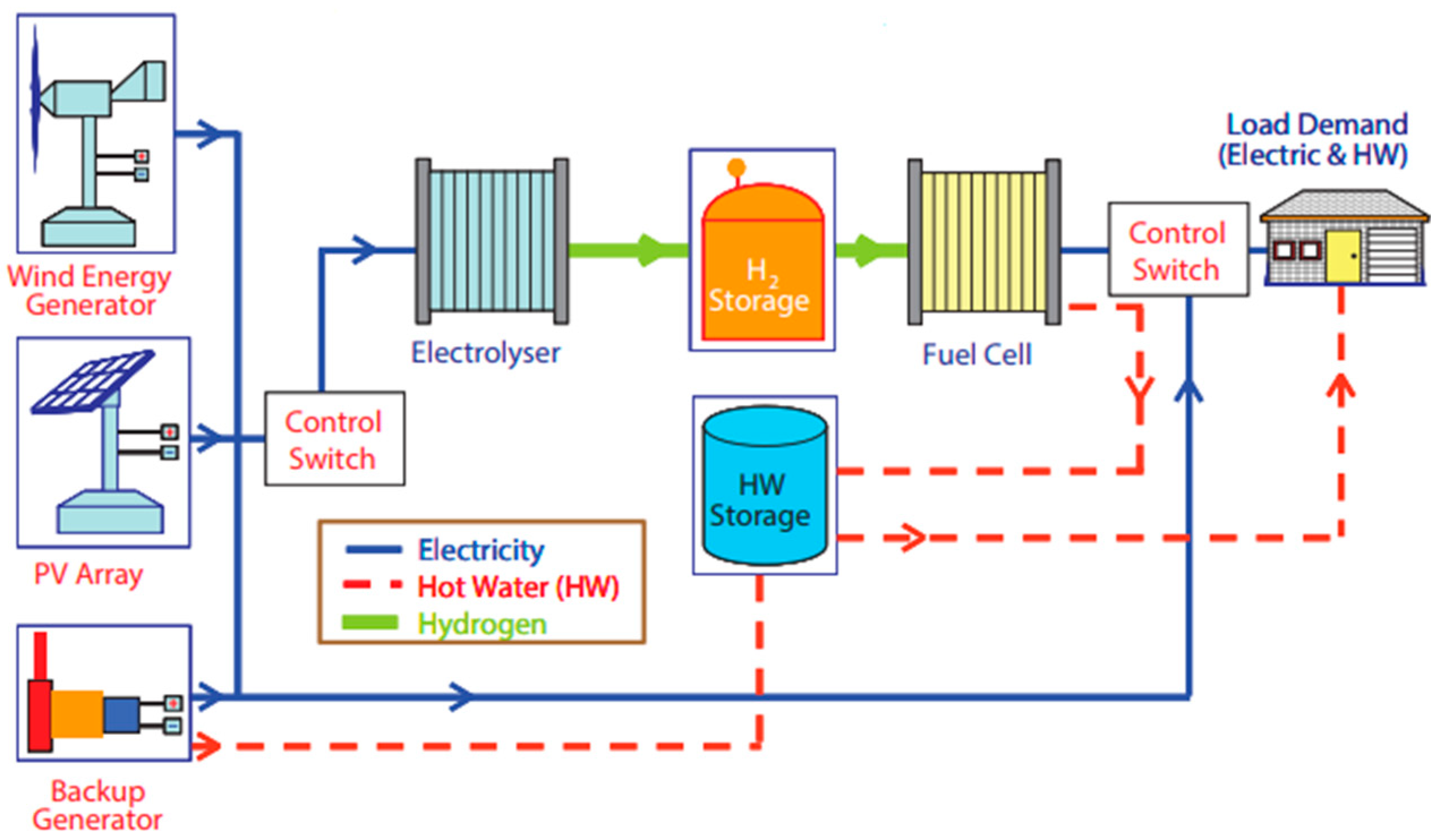
3.1.4. Hydrogen Production from Renewable Power Sources
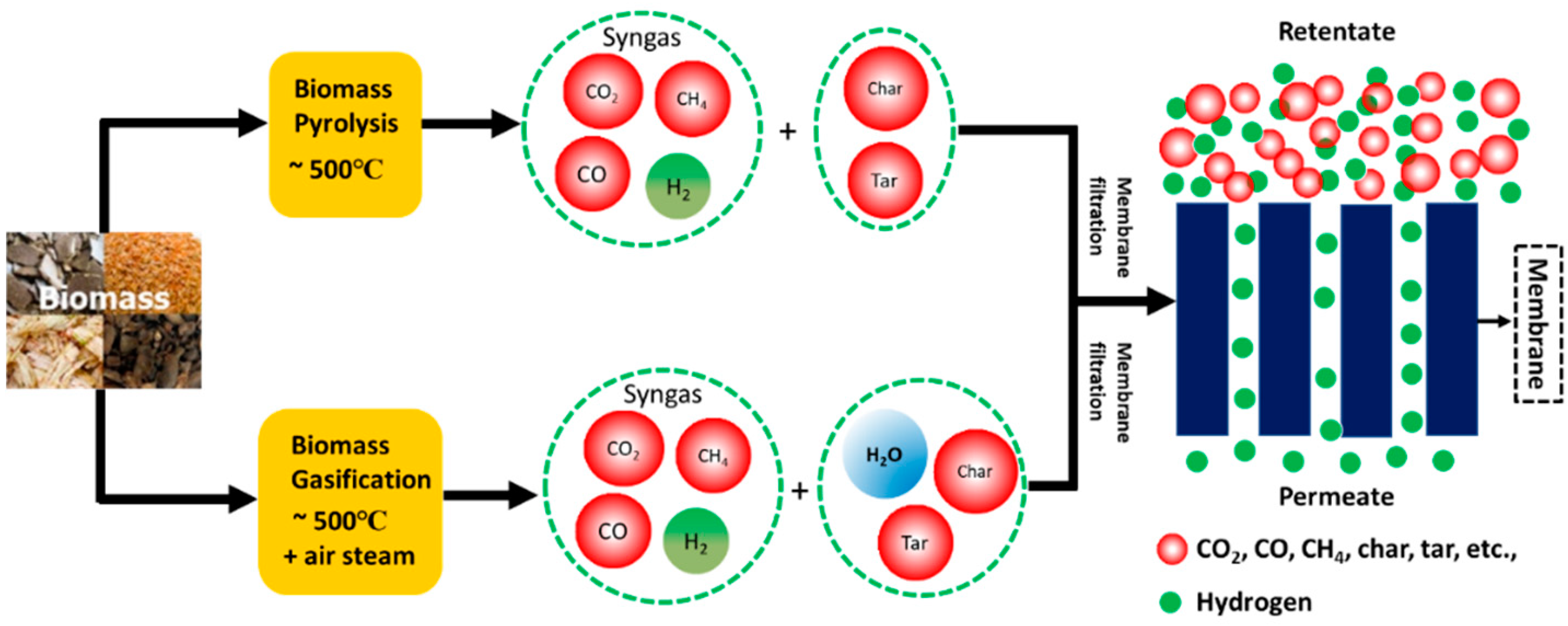
3.1.5. Hydrogen from Biomass
4. Salinity Gradient Energy Technologies
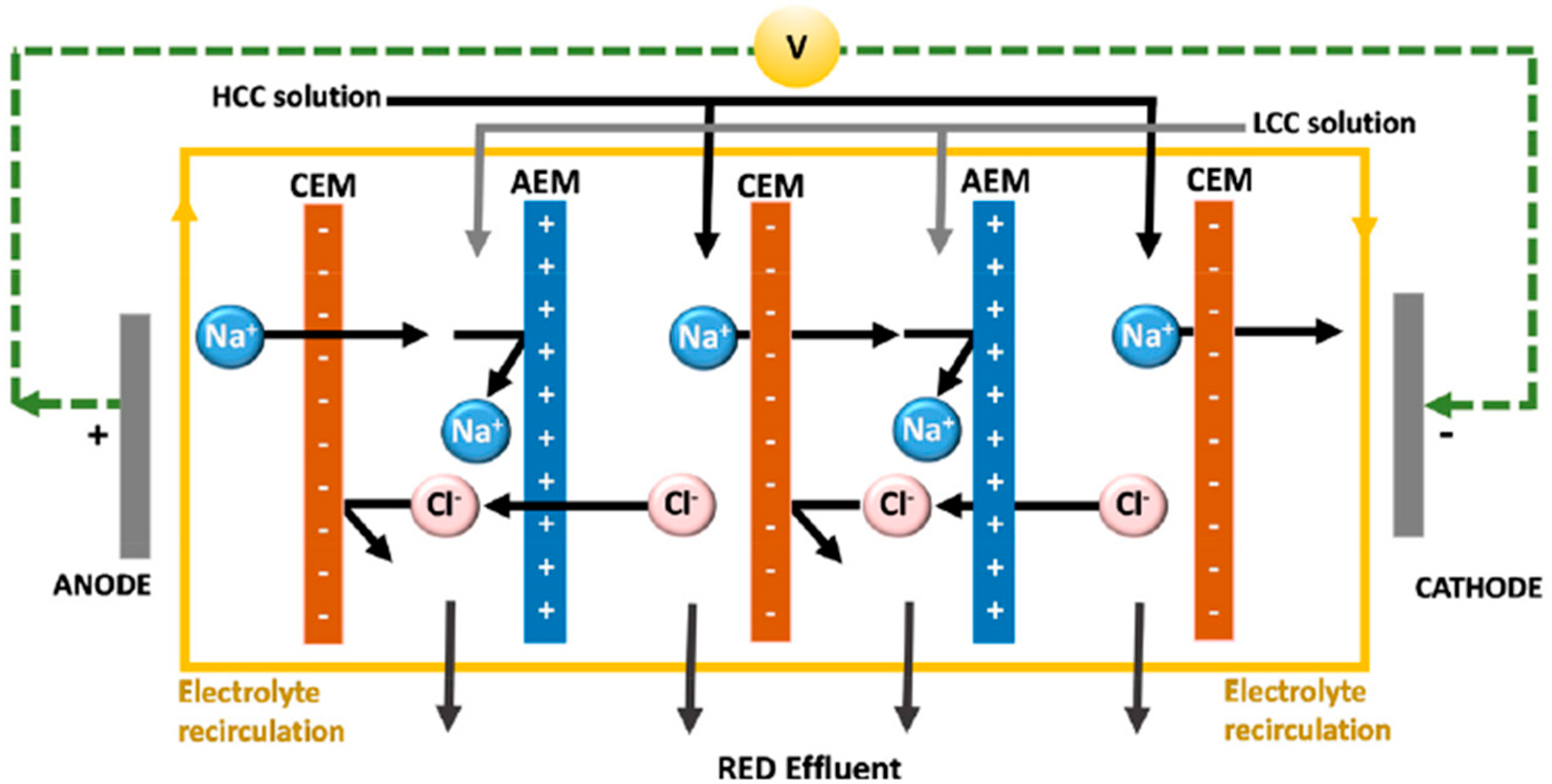
4.1. Reverse Electrodialysis (RED)
4.2. Pressure Retarded Osmosis (PRO)
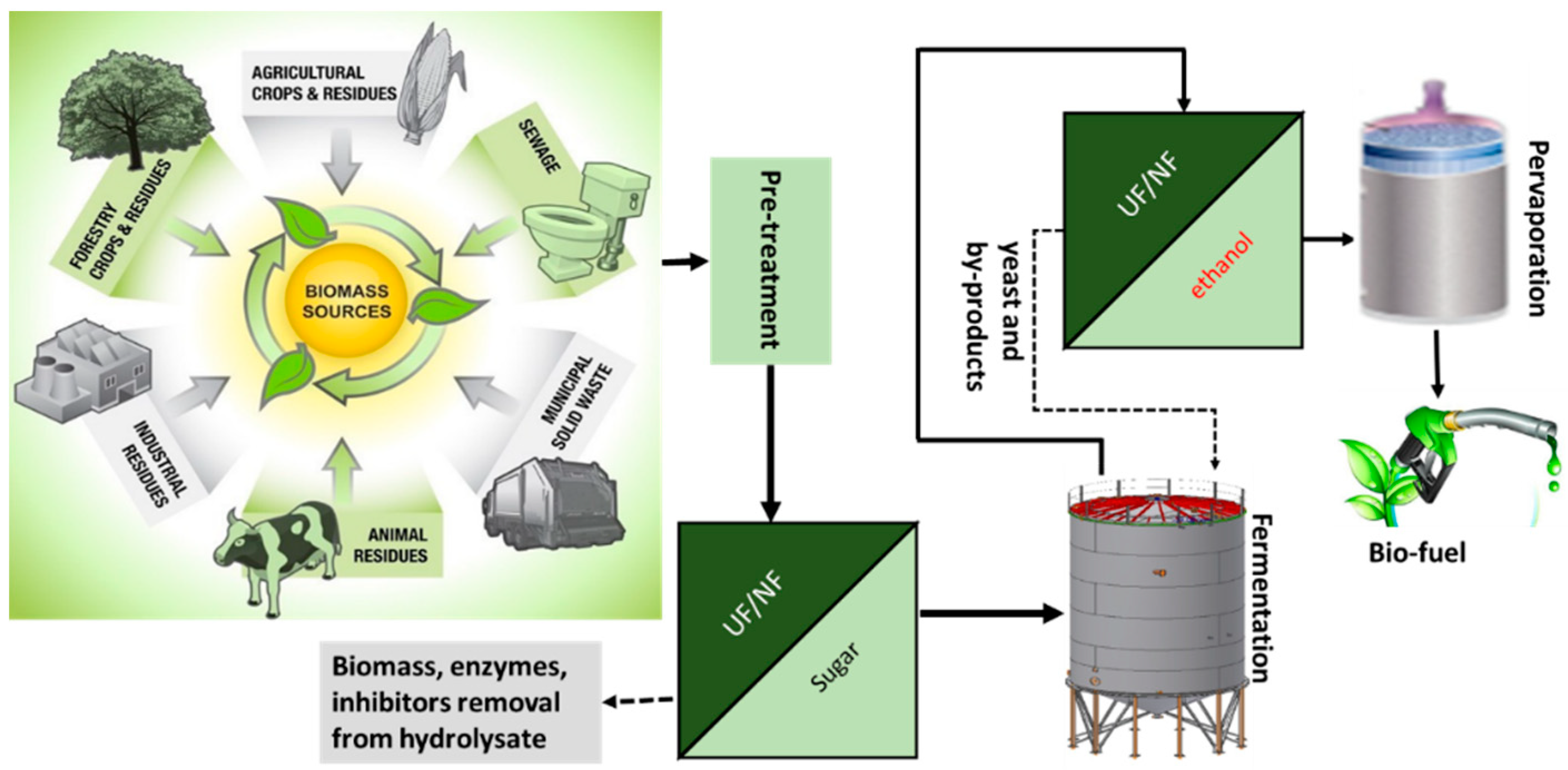
5. Membrane Technology for Advanced Biofuels Production (Bioethanol and Biodiesel)
6. Membranes for Energy Storage Electrochemical Devices
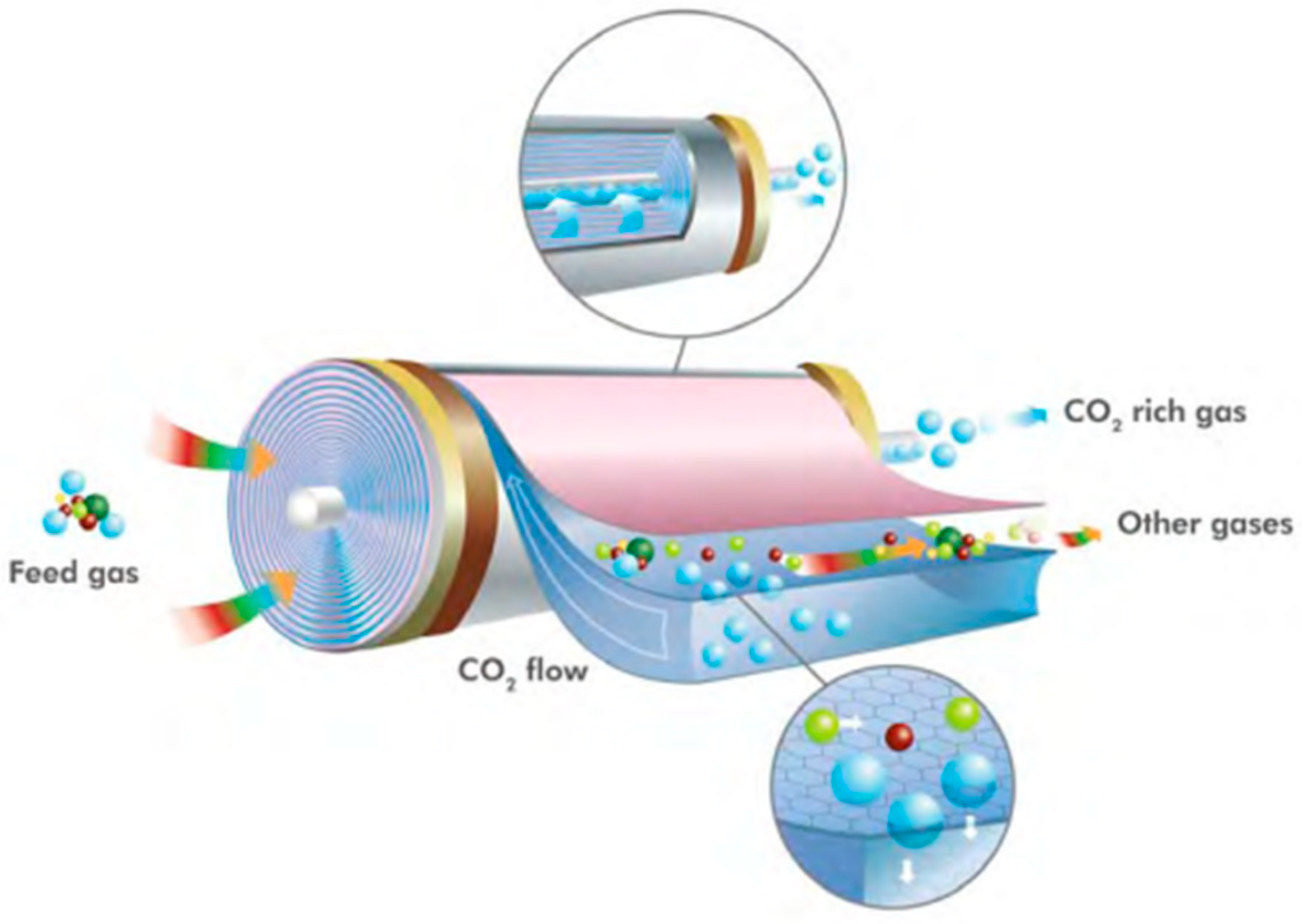
7. CO2 Capture and Use
8. Conclusions and Outlook
- Off-grid power supply: As a clean and versatile energy carrier of electricity, hydrogen has the advantage of being storable and transportable in various ways. When coupled with a fuel cell, hydrogen provides a carbon-free energy pathway, thereby allowing for a flexible and decentralized energy system for various applications. For instance, there is a possibility of converting the largely available biomass directly into hydrogen which can be converted (transported if required) to electricity to fuel cells. Thus, the implementation of such technologies enhances the potential of powering the large majority of the Ethiopian population residing in off-grid remote areas. It is, therefore, essential to consider the fuel cell and hydrogen technologies in the strategic plan of the Ethiopian energy policy.
- Transportation: Given its high energy density of approximately 120 MJ/kg that is about three times that of diesel or gasoline, hydrogen can also play an important role in the transport sector, for instance in fueling railway and Automotive including heavy load vehicles, trucks, buses, etc and even ships and aircraft. This would largely reduce the import expense and use of fossil fuels.
- Chemical production: Other secondary application of hydrogen includes the chemical industry, for example in making fertilizer, with a huge advantage for a country such as Ethiopia heavily relying on agriculture.
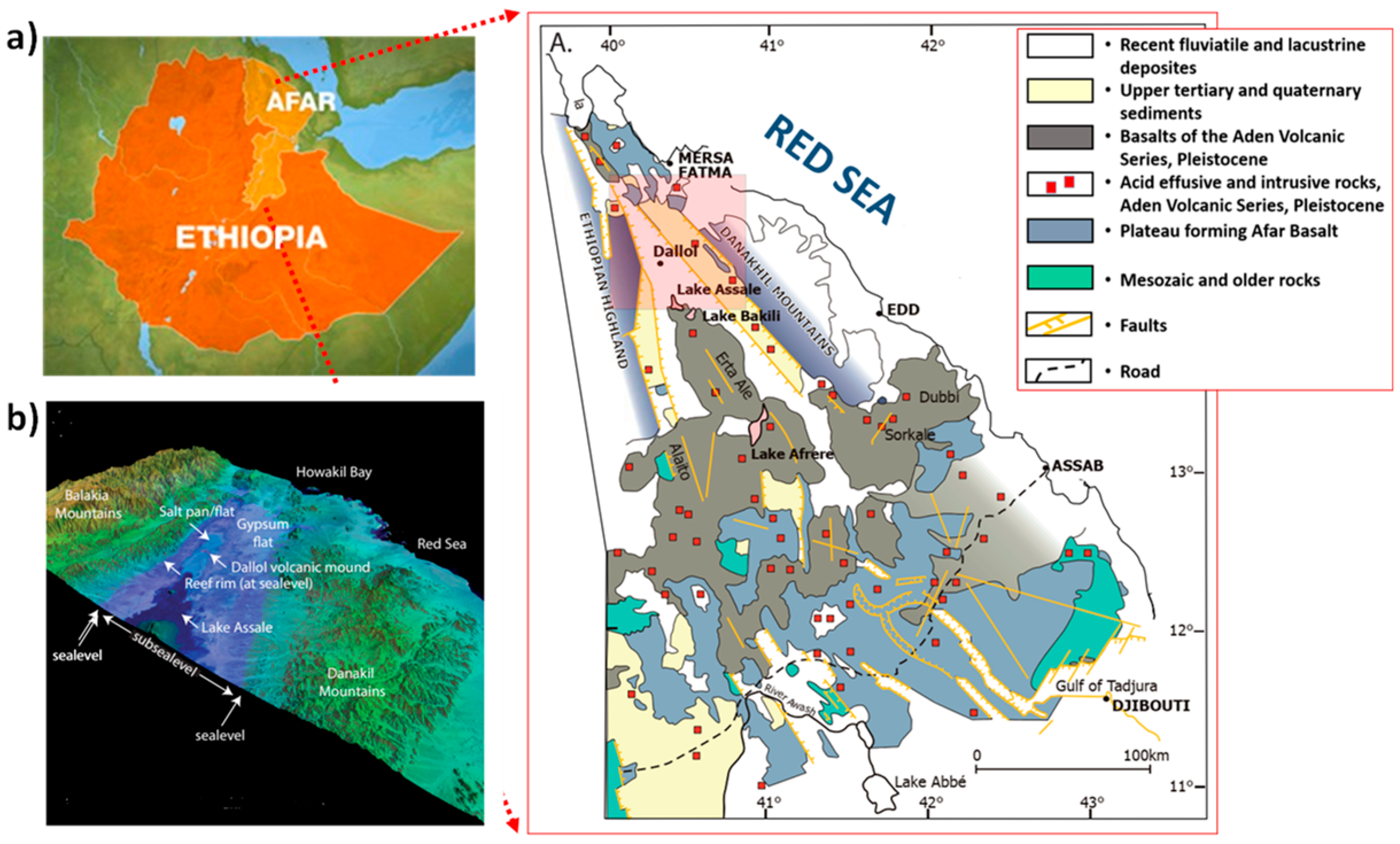
- Natural salt lakes: Lake Karum, which is located in the northern part of the country (Afar region) in the geological depression termed “Denakil Depression”. Also, a mining settlement called “Dallol” is located in the north of Lake Karum. These kinds of salty sites can be a potential source of brine and hence SGP considering the huge availability of freshwater such as rivers and lakes in different parts of Ethiopia.
- Brine solutions: Brine can be found in the waste stream of geothermal power production sites. There exist established geothermal sites in Ethiopia, for example, the Aluto-Langano geothermal power station which is the oldest one with a net power generation capacity of 7.3 MW, and prospective areas, for example, in the Afar depression. Coupling the SGP from hypersaline water from geothermal wells enables a unique synergy that increases the overall economic viability of geothermal power plants.
- Saline groundwater and industrial wastewater: Such types of feed resources can also be exploited for SGP; however, they require exhaustive assessment to determine the SGP potential of such sources in the country.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EIA. Today in Energy. Available online: https://www.eia.gov/todayinenergy/detail.php?id=41433 (accessed on 14 October 2020).
- IEA. Key World Energy Statistics. 2020. Available online: https://www.iea.org/reports/key-world-energy-statistics-2020 (accessed on 14 October 2020).
- Tufa, R.A.; Curcio, E.; Fontananova, E.; Di Profio, G. 3.8 Membrane-Based Processes for Sustainable Power Generation Using Water: Pressure-Retarded Osmosis (PRO), Reverse Electrodialysis (RED), and Capacitive Mixing (CAPMIX). Compr. Membr. Sci. Eng. 2017, 2, 206–248. [Google Scholar]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Crespo, J.G.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Liang, P.; Duan, R.; Jiang, Y.; Zhang, X.; Qiu, Y.; Huang, X. One-year operation of 1000-L modularized microbial fuel cell for municipal wastewater treatment. Water Res. 2018, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Pan, Z.; An, L.; Zhao, T.; Tang, Z. Advances and challenges in alkaline anion exchange membrane fuel cells. Prog. Energy Combust. Sci. 2018, 66, 141–175. [Google Scholar] [CrossRef]
- Tufa, R.A.; Chandac, D.; Tundisa, L.; Hnátb, J.; Bouzekb, K.; Veermand, J.; Fontananovae, E.; Di Profioe, G.; Curcioa, E. Salinity gradient power driven water electrolysis for hydrogen production. Chem. Eng. 2017, 60. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiUiM-lhtTsAhVSBKYKHS8HCPAQFjAAegQIAhAC&url=https%3A%2F%2Fwww.aidic.it%2Fcet%2F17%2F60%2F048.pdf&usg=AOvVaw17Y5SPXwcteB66mGQc44wS (accessed on 3 October 2020).
- Tufa, R.A.; Hnát, J.; Němeček, M.; Kodým, R.; Curcio, E.; Bouzek, K. Hydrogen production from industrial wastewaters: An integrated reverse electrodialysis-Water electrolysis energy system. J. Clean. Prod. 2018, 203, 418–426. [Google Scholar] [CrossRef]
- Yin, H.; Yip, A.C. A review on the production and purification of biomass-derived hydrogen using emerging membrane technologies. Catalysts 2017, 7, 297. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Bank, W. Access to Electricity, Urban (% of Urban Population)—Ethiopia. 2018. Available online: https://data.worldbank.org/indicator/EG.ELC.RNWX.KH?locations=ET (accessed on 10 September 2020).
- Gabaldon Moreno, A. Renewables-Driven Membrane Distillation for Drinking Water Purification: Main Ethiopian Rift Valley Case Study. Master’s Thesis, School of Industrial Engineering and Management, Stockholm, Sweden, 2018. [Google Scholar]
- Issa, M. Energy Report—Ethiopia; Embassy of Sweden: Addis Abeba, Ethiopia, 2016. [Google Scholar]
- Beyene, G.; Kumie, A.; Edwards, R.; Troncoso, K. Opportunities for Transition To Clean Household Energy in Ethiopia: Application of the Household Energy Assessment Rapid Tool (HEART); World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Mondal, M.A.H.; Bryan, E.; Ringler, C.; Mekonnen, D.; Rosegrant, M.J.E. Ethiopian energy status and demand scenarios: Prospects to improve energy efficiency and mitigate GHG emissions. Energy 2018, 149, 161–172. [Google Scholar] [CrossRef]
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate change and the impact of greenhouse gasses: CO2 and NO, friends and foes of plant oxidative stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- AEP. Ethiopia: Lotus Energy to Install Power Plant (500 MW) for Effort Group. Available online: https://africa-energy-portal.org/news/ethiopia-lotus-energy-install-power-plant-500-mw-effort-group (accessed on 25 September 2020).
- Multiconsult. Metahara 100 MW Solar PV Power Plant in Ethiopia. Available online: https://www.multiconsultgroup.com/projects/metahara-solar-pv-plant-ethiopia/ (accessed on 21 August 2020).
- Bank, W. Federal Democratic Republic of Ethiopia Ethiopia Electrification Program; 119032-ET; Government of Ethiopia: Addis Abeba, Ethiopia, 2018. Available online: www.documents.worldbank.org (accessed on 10 September 2020).
- Federal Democratic Republic of Ethiopia Ministry of Water and Energy. Scaling-Up Renewable Energy Program Ethiopia Investment Plan (Draft Final); Federal Democratic Republic of Ethiopia Ministry of Water and Energy: Addis Ababa, Ethiopia, 2012.
- Kirubakaran, A.; Jain, S.; Nema, R.K. A review on fuel cell technologies and power electronic interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Stephen, A.J.; Rees, N.V.; Mikheenko, I.; Macaskie, L.E. Platinum and Palladium Bio-Synthesized Nanoparticles as Sustainable Fuel Cell Catalysts. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Samimi, F.; Rahimpour, M.R. Chapter 14—Direct Methanol Fuel Cell. In Methanol; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 381–397. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. London. Ser. Bcontaining Pap. A Biol. Character 1911, 84, 260–276. [Google Scholar]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Hassett, D.J.; Gu, T. Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J. Chem. Technol. Biotechnol. 2013, 88, 508–518. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial Phenazine Production Enhances Electron Transfer in Biofuel Cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Microbial Fuel Cells—Challenges and Applications. Environ. Sci. Technol. 2006, 40, 5172–5180. [Google Scholar] [CrossRef]
- Mekuto, L.; Olowolafe, A.V.; Pandit, S.; Dyantyi, N.; Nomngongo, P.; Huberts, R. Microalgae as a biocathode and feedstock in anode chamber for a self-sustainable microbial fuel cell technology: A review. South Afr. J. Chem. Eng. 2020, 31, 7–16. [Google Scholar] [CrossRef]
- Schröder, U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 2007, 9, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Peng, L.; You, S.-J.; Wang, J.-Y. Carbon nanotubes as electrode modifier promoting direct electron transfer from Shewanella oneidensis. Biosens. Bioelectron. 2010, 25, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Freguia, S.; Masuda, M.; Tsujimura, S.; Kano, K. Lactococcus lactis catalyses electricity generation at microbial fuel cell anodes via excretion of a soluble quinone. Bioelectrochemistry 2009, 76, 14–18. [Google Scholar] [CrossRef]
- Keck, A.; Conradt, D.; Mahler, A.; Stolz, A.; Mattes, R.; Klein, J. Identification and functional analysis of the genes for naphthalenesulfonate catabolism by Sphingomonas xenophaga BN6. Microbiology 2006, 152, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, F.; Zhou, S.; Huang, D.; Ni, J. A study of electron-shuttle mechanism in Klebsiella pneumoniae based-microbial fuel cells. Chin. Sci. Bull. 2010, 55, 99–104. [Google Scholar] [CrossRef]
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ahn, Y. Continuous electricity generation in stacked air cathode microbial fuel cell treating domestic wastewater. J. Environ. Manag. 2013, 130, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Serra, M.; Coma, M.; Balaguer, M.D.; Colprim, J. Simultaneous domestic wastewater treatment and renewable energy production using microbial fuel cells (MFCs). Water Sci. Technol. 2011, 64, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Ohmori, H.; Ishida, M.; Waki, M.; Tanaka, Y. Treatment of cow-waste slurry by a microbial fuel cell and the properties of the treated slurry as a liquid manure. Anim. Sci. J. 2006, 77, 634–638. [Google Scholar] [CrossRef]
- Sathian, S.; Rajasimman, M.; Radha, G.; Shanmugapriya, V.; Karthikeyan, C. Performance of SBR for the treatment of textile dye wastewater: Optimization and kinetic studies. Alex. Eng. J. 2014, 53, 417–426. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, B.; Kong, L.; Xue, A.; Ni, J. Electricity generation from molasses wastewater by an anaerobic baffled stacking microbial fuel cell. J. Chem. Technol. Biotechnol. 2011, 86, 406–413. [Google Scholar] [CrossRef]
- Habermann, W.; Pommer, E.H. Biological fuel cells with sulphide storage capacity. Appl. Microbiol. Biotechnol. 1991, 35, 128–133. [Google Scholar] [CrossRef]
- Water, A.A.; Authority, S. Environmental and Social Impact Assessment of the Wastewater Treatment Plant and Sewer Lines Expansion and Rehabilitation in The Kaliti Catchment; Addis Ababa Water and Sewerage Authority (AAWSA)Water, Sanitation Rehabilitation and Development Project Office: Addis Ababa, Ethiopia, 2014.
- Fikreyesus, D.; Turpeinen, M.; Gebre, G.; Nebsu, B.; Ermias, M. Ethiopia Solid Waste & Landfill, Country Profile and Action Plan Report; Global Methane Initiative: Washington, DC, USA, 2011. [Google Scholar]
- Wu, S.; Li, H.; Zhou, X.; Liang, P.; Zhang, X.; Jiang, Y.; Huang, X. A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment. Water Res. 2016, 98, 396–403. [Google Scholar] [CrossRef]
- Kuntke, P.; Śmiech, K.M.; Bruning, H.; Zeeman, G.; Saakes, M.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res. 2012, 46, 2627–2636. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Liang, P.; Liu, P.; Huang, X. Microbial fuel cell sensors for water quality early warning systems: Fundamentals, signal resolution, optimization and future challenges. Renew. Sustain. Energy Rev. 2018, 81, 292–305. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, F.; Grimaud, J.; Hurst, J.; He, Z. Long-term investigation of microbial fuel cells treating primary sludge or digested sludge. Bioresour. Technol. 2013, 136, 509–514. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, Z.; Grimaud, J.; Hurst, J.; He, Z. In situ investigation of tubular microbial fuel cells deployed in an aeration tank at a municipal wastewater treatment plant. Bioresour. Technol. 2013, 136, 316–321. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, Z.; Grimaud, J.; Hurst, J.; He, Z. Long-Term Performance of Liter-Scale Microbial Fuel Cells Treating Primary Effluent Installed in a Municipal Wastewater Treatment Facility. Environ. Sci. Technol. 2013, 47, 4941–4948. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Badwal, S.P.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol 2015, 4, 2249–8958. [Google Scholar]
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production–A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Gabisa, E.W.; Gheewala, S.H. Potential of bio-energy production in Ethiopia based on available biomass residues. Biomass Bioenergy 2018, 111, 77–87. [Google Scholar] [CrossRef]
- Kaygusuz, K. Energy for sustainable development: A case of developing countries. Renew. Sustain. Energy Rev. 2012, 16, 1116–1126. [Google Scholar] [CrossRef]
- Guta, D.D. Assessment of biomass fuel resource potential and utilization in Ethiopia: Sourcing strategies for renewable energies. Int. J. Renew. Energy Res. 2012, 2, 131–139. [Google Scholar]
- Brackish Water Desalination System; Afar Region, Ethiopia, Unpublished; 2008.
- Sołowski, G.; Shalaby, M.S.; Abdallah, H.; Shaban, A.M.; Cenian, A. Production of hydrogen from biomass and its separation using membrane technology. Renew. Sustain. Energy Rev. 2018, 82, 3152–3167. [Google Scholar] [CrossRef]
- UNEP. In Sustainability of Biogas and Solid Biomass Value Chains in Ethiopia; Technical Report; UNEP: Nairobi, Kenya, 2018.
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Doubled Power Density from Salinity Gradients at Reduced Intermembrane Distance. Environ. Sci. Technol. 2011, 45, 7089–7095. [Google Scholar] [CrossRef]
- Besha, A.T.; Tsehaye, M.T.; Aili, D.; Zhang, W.; Tufa, R.A. Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis. Membranes 2020, 10, 7. [Google Scholar] [CrossRef]
- Avci, A.H.; Sarkar, P.; Tufa, R.A.; Messana, D.; Argurio, P.; Fontananova, E.; Di Profio, G.; Curcio, E. Effect of Mg2+ ions on energy generation by Reverse Electrodialysis. J. Membr. Sci. 2016, 520, 499–506. [Google Scholar] [CrossRef]
- Tufa, R.A.; Chanda, D.; Tundis, L.; Hnát, J.; Bouzek, K.; Veerman, J.; Fontananova, E.; Di Profio, G.; Curcio, E. Salinity gradient power driven water electrolysis for hydrogen production. Chem. Eng. Trans. 2017, 60, 283–288. [Google Scholar] [CrossRef]
- Hatzell, M.C.; Ivanov, I.; Cusick, R.D.; Zhu, X.; Logan, B.E. Comparison of hydrogen production and electrical power generation for energy capture in closed-loop ammonium bicarbonate reverse electrodialysis systems. Phys. Chem. Chem. Phys. 2014, 16, 1632–1638. [Google Scholar] [CrossRef]
- Tedesco, M.; Scalici, C.; Vaccari, D.; Cipollina, A.; Tamburini, A.; Micale, G. Performance of the first reverse electrodialysis pilot plant for power production from saline waters and concentrated brines. J. Membr. Sci. 2016, 500, 33–45. [Google Scholar] [CrossRef]
- Tufa, R.A.; Curcio, E.; Brauns, E.; van Baak, W.; Fontananova, E.; Di Profio, G. Membrane distillation and reverse electrodialysis for near-zero liquid discharge and low energy seawater desalination. J. Membr. Sci. 2015, 496, 325–333. [Google Scholar] [CrossRef]
- Kim, Y.; Logan, B.E. Hydrogen production from inexhaustible supplies of fresh and salt water using microbial reverse-electrodialysis electrolysis cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16176–16181. [Google Scholar] [CrossRef]
- Tufa, R.A.; Rugiero, E.; Chanda, D.; Hnàt, J.; van Baak, W.; Veerman, J.; Fontananova, E.; Di Profio, G.; Drioli, E.; Bouzek, K.; et al. Salinity gradient power-reverse electrodialysis and alkaline polymer electrolyte water electrolysis for hydrogen production. J. Membr. Sci. 2016, 514, 155–164. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Chu, K.; Coronell, O. Energy storage by reversible electrodialysis: The concentration battery. J. Membr. Sci. 2015, 495, 502–516. [Google Scholar] [CrossRef]
- Loeb, S.; Van Hessen, F.; Shahaf, D. Production of energy from concentrated brines by pressure-retarded osmosis: II. Experimental results and projected energy costs. J. Membr. Sci. 1976, 1, 249–269. [Google Scholar] [CrossRef]
- Loeb, S. Method and Apparatus for Generating Power Utilizing Pressure-Retarded-Osmosis. U.S. Patent 3,9062,50A, 16 September 1975. [Google Scholar]
- Mehta, G.D. Further results on the performance of present-day osmotic membranes in various osmotic regions. J. Membr. Sci. 1982, 10, 3–19. [Google Scholar] [CrossRef]
- Lee, K.L.; Baker, R.W.; Lonsdale, H.K. Membranes for power generation by pressure-retarded osmosis. J. Membr. Sci. 1981, 8, 141–171. [Google Scholar] [CrossRef]
- Aaberg, R.J. Osmotic power: A new and powerful renewable energy source? Refocus 2003, 4, 48–50. [Google Scholar] [CrossRef]
- Thorsen, T.; Holt, T. Finding hidden energy in membrane processes. Filtr. Sep. 2005, 42, 28–30. [Google Scholar] [CrossRef]
- Achilli, A.; Childress, A.E. Pressure retarded osmosis: From the vision of Sidney Loeb to the first prototype installation—Review. Desalination 2010, 261, 205–211. [Google Scholar] [CrossRef]
- ForwardOsmosisTech. Inquiry about Commercially Available PRO Membranes. Available online: https://www.forwardosmosistech.com/inquiry-about-commercially-available-pro-membranes/ (accessed on 21 October 2020).
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Curcio, E.; Poerio, T.; Mazzei, R.; Di Profio, G.; Drioli, E.; Giorno, L. Treatment of Olive Mill Wastewater by Forward Osmosis. Sep. Purif. Technol. 2015, 147, 292–302. [Google Scholar] [CrossRef]
- Sarp, S.; Li, Z.; Saththasivam, J. Pressure Retarded Osmosis (PRO): Past experiences, current developments, and future prospects. Desalination 2016, 389, 2–14. [Google Scholar] [CrossRef]
- Cavalazzi, B.; Barbieri, R.; Gómez, F.; Capaccioni, B.; Olsson-Francis, K.; Pondrelli, M.; Rossi, A.; Hickman-Lewis, K.; Agangi, A.; Gasparotto, G.J.A. The Dallol geothermal area, Northern Afar (Ethiopia)—An exceptional planetary field analog on Earth. Astrobiology 2019, 19, 553–578. [Google Scholar] [CrossRef]
- Feyissa, D. The political economy of salt in the Afar Regional State in northeast Ethiopia. Rev. Afr. Political Econ. 2011, 38, 7–21. [Google Scholar] [CrossRef]
- Warren, J.K. Danakhil Potash, Ethiopia: Is the Present Geology the Key? SaltWork Consultants: Seacliff Park, Australia, 2015. [Google Scholar]
- Wei, P.; Cheng, L.-H.; Zhang, L.; Xu, X.-H.; Chen, H.-L.; Gao, C.-J. A review of membrane technology for bioethanol production. Renew. Sustain. Energy Rev. 2014, 30, 388–400. [Google Scholar] [CrossRef]
- Basile, A.; Gugliuzza, A.; Iulianelli, A.; Morrone, P. Membrane technology for carbon dioxide (CO2) capture in power plants. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 113–159. [Google Scholar]
- Curcio, S. Membranes for advanced biofuels production. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 361–410. [Google Scholar]
- Mazzei, R.; Piacentini, E.; Yihdego Gebreyohannes, A.; Giorno, L. Membrane bioreactors in food, pharmaceutical and biofuel applications: State of the art, progresses and perspectives. Curr. Org. Chem. 2017, 21, 1671–1701. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Mazzei, R.; Curcio, E.; Poerio, T.; Drioli, E.; Giorno, L. Study on the in situ enzymatic self-cleansing of microfiltration membrane for valorization of olive mill wastewater. Ind. Eng. Chem. Res. 2013, 52, 10396–10405. [Google Scholar] [CrossRef]
- Giorno, L.; Drioli, E. Biocatalytic membrane reactors: Applications and perspectives. Trends Biotechnol. 2000, 18, 339–349. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Bilad, M.R.; Verbiest, T.; Courtin, C.M.; Dornez, E.; Giorno, L.; Curcio, E.; Vankelecom, I.F.J. Nanoscale tuning of enzyme localization for enhanced reactor performance in a novel magnetic-responsive biocatalytic membrane reactor. J. Membr. Sci. 2015, 487, 209–220. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Dharmjeet, M.; Swusten, T.; Mertens, M.; Verspreet, J.; Verbiest, T.; Courtin, C.M.; Vankelecom, I.F.J. Simultaneous glucose production from cellulose and fouling reduction using a magnetic responsive membrane reactor with superparamagnetic nanoparticles carrying cellulolytic enzymes. Bioresour. Technol. 2018, 263, 532–540. [Google Scholar] [CrossRef]
- Mahboubi, A.; Ylitervo, P.; Doyen, W.; De Wever, H.; Taherzadeh, M.J. Reverse membrane bioreactor: Introduction to a new technology for biofuel production. Biotechnol. Adv. 2016, 34, 954–975. [Google Scholar] [CrossRef]
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef]
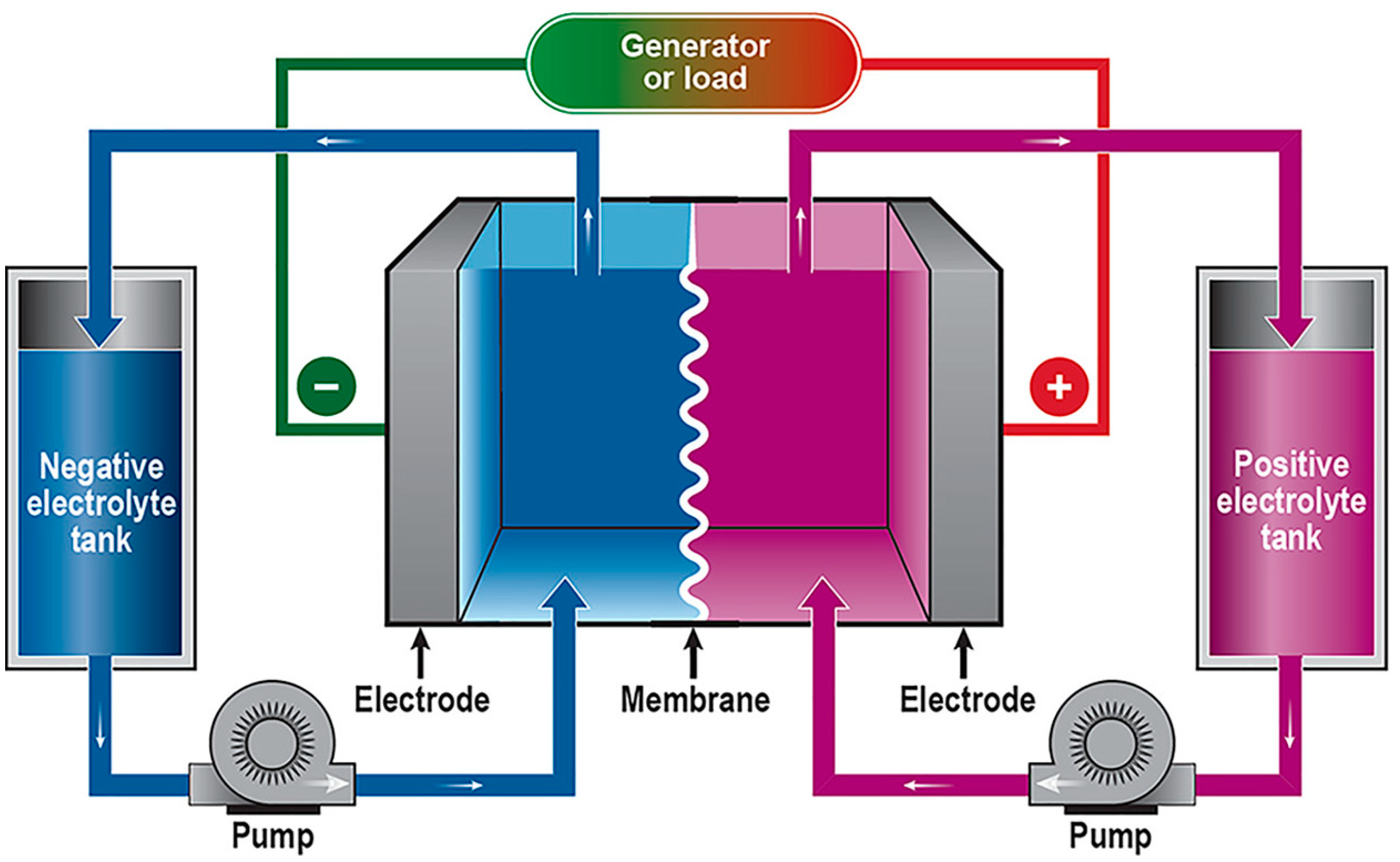
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Flow batteries: Current status and trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G. Renewable energy and energy storage systems. Energy 2017, 136, 1–6. [Google Scholar] [CrossRef]
- Guney, M.S.; Tepe, Y. Classification and assessment of energy storage systems. Renew. Sustain. Energy Rev. 2017, 75, 1187–1197. [Google Scholar] [CrossRef]
- Amrouche, S.O.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrogen Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- Associates, M. Overview of Energy Storage Methods; Mora Associates Ltd.: Aéroport Strasbourg, France, 2007. [Google Scholar]
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Battery Separators—Types and Importance in the Performance of Battery. Available online: https://components101.com/articles/battery-seperators-types-and-importance (accessed on 25 June 2020).
- Roy, P.; Srivastava, S.K. Nanomaterials for Electrochemical Energy Storage Devices; Wiley Online Library: Hoboken, NJ, USA, 2019. [Google Scholar]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C. Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries. Energies 2019, 12, 4702. [Google Scholar] [CrossRef]
- Sum, E.; Rychcik, M.; Skyllas-kazacos, M. Investigation of the V(V)/V(IV) system for use in the positive half-cell of a redox battery. J. Power Sources 1985, 16, 85–95. [Google Scholar] [CrossRef]
- Cha, S.-H. Recent development of nanocomposite membranes for vanadium redox flow batteries. J. Nanomater. 2015, 2015, 207525. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Gierke, T.D.; Munn, G.; Wilson, F. The morphology in nafion perfluorinated membrane products, as determined by wide-and small-angle X-ray studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar] [CrossRef]
- Schulte, D.; Drillkens, J.; Schulte, B.; Sauer, D. Nafion hybrid membranes for use in redox flow batteries. J. Electrochem. Soc. 2010, 157, A989. [Google Scholar] [CrossRef]
- Dai, W.; Yu, L.; Li, Z.; Yan, J.; Liu, L.; Xi, J.; Qiu, X. Sulfonated poly (ether ether ketone)/graphene composite membrane for vanadium redox flow battery. Electrochim. Acta 2014, 132, 200–207. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef]
- Hu, B.; Seefeldt, C.; DeBruler, C.; Liu, T.L. Boosting the energy efficiency and power performance of neutral aqueous organic redox flow batteries. J. Mater. Chem. A 2017, 5, 22137–22145. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Xu, Z.; Yang, Z. Poly (phenylene oxide)-Based Ion-Exchange Membranes for Aqueous Organic Redox Flow Battery. Ind. Eng. Chem. Res. 2019, 58, 10707–10712. [Google Scholar] [CrossRef]
- Janoschka, T.; Martin, N.; Martin, U.; Friebe, C.; Morgenstern, S.; Hiller, H.; Hager, M.D.; Schubert, U.S. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 2015, 527, 78–81. [Google Scholar] [CrossRef]
- Wei, X.; Pan, W.; Duan, W.; Hollas, A.; Yang, Z.; Li, B.; Nie, Z.; Liu, J.; Reed, D.; Wang, W. Materials and systems for organic redox flow batteries: Status and challenges. Acs Energy Lett. 2017, 2, 2187–2204. [Google Scholar] [CrossRef]
- Chen, H.; Cong, G.; Lu, Y.-C. Recent progress in organic redox flow batteries: Active materials, electrolytes and membranes. J. Energy Chem. 2018, 27, 1304–1325. [Google Scholar] [CrossRef]
- Wong, S. Building Capacity for CO2 Capture and Storage in the APEC Region; Asia-Pacific Economic Cooperation: Singapore, 2009. [Google Scholar]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
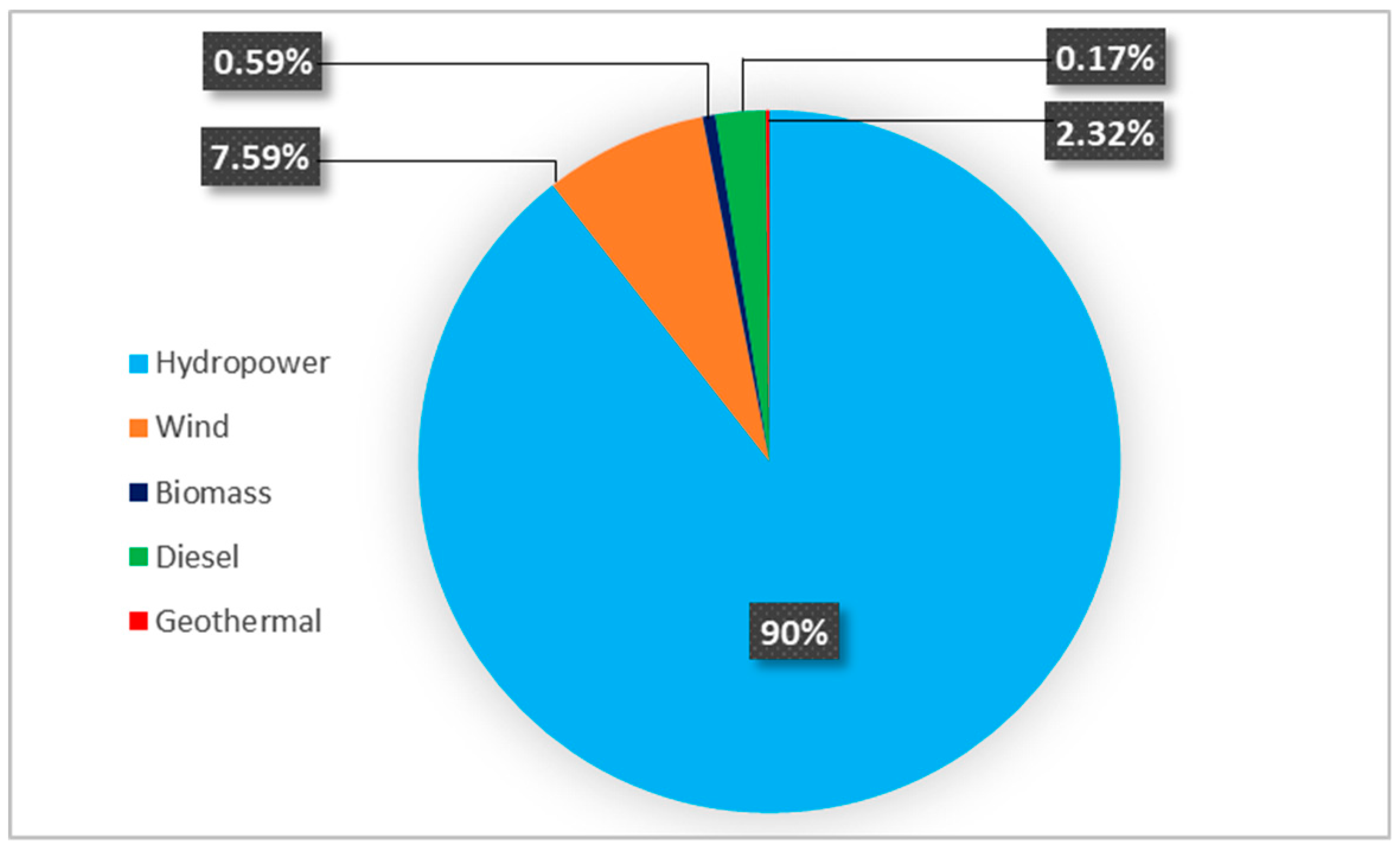


| Sources | Unit | Potential | Exploited | Exploited (%) |
|---|---|---|---|---|
| Hydropower | MW | 45,000 | 3180 | ~7 |
| Solar (day) | kWh/m2 | 5.2 | – | <1 |
| Wind (power speed) | GW m/s | 1350 > 7 a | 0.324 | <1 |
| Geothermal | MW | 7000 | 7.3 | <1 |
| Wood | Million T | 1120 | 560 | 50 |
| Agricultural waste | Million T | 15–20 | ~6 | 30 |
| Natural gas | Trillion m3 (2013) | <0.1 | 0 | 0 |
| Coal | Million T | >300 | – | 0 |
| Oil Shale | Million T | 253 | – | 0 |
| Bio-gas | Households | 1–3 million | 17 896 | <1 |
| Power Plants | Capacity (MW) | Operational Since | Type |
|---|---|---|---|
| Aba Samuel | 6.6 | 1932 | Hydropower |
| Koka | 43.2 | 1960 | Hydropower |
| Tis Abay I | 11.4 | 1964 | Hydropower |
| Awash II | 32 | 1966 | Hydropower |
| Awash III | 32 | 1971 | Hydropower |
| Fincha | 134 | 1973/2003 | Hydropower |
| Melka Wakena | 153 | 1998 | Hydropower |
| Alutto Langano | 7.3 | 1999 | Geothermal |
| Tis Abay II | 73 | 2001 | Hydropower |
| Gilgel Gibe I | 184 | 2004 | Hydropower |
| Kaliti | 14 | 2004 | Diesel |
| Dire Dawa | 38 | 2004 | Diesel |
| Awash 7 kilo | 35 | 2004 | Diesel |
| Tekeze | 300 | 2009 | Hydropower |
| Gilgel Gibe II | 420 | 2010 | Hydropower |
| Beles | 460 | 2010 | Hydropower |
| Adama I | 51 | 2010 | Wind |
| Fincha Amerti Neshi | 97 | 2011 | Hydropower |
| Ashegoda | 120 | 2012 | Wind |
| Adama II | 153 | 2015 | Wind |
| Gilgel Gibe III | 1870 | 2015 | Hydropower |
| Ayisha | 300 | – | Wind |
| Debre Birhan | 100 | – | Wind |
| Asela | 100 | – | Wind |
| Mesebo Harena | 42 | – | Wind |
| Galema I | 250 | – | Wind |
| Metahara | 100 | – | Solar |
| Gad and Dicheto | 250 | – | Solar |
| Tigray | 500 | – | Solar |
| Type of Fuel Cell (FC) | Electrolyte | Operating Temperature (°C) | Power Output | Electrical Efficiency (%) | Applications | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Polymer electrolyte membrane (PEMFC) * | Solid organic polymer-Perfluoro sulfonic acid | 50–100 | <1 kW–250 kW | 53–58 (transportation) 25–35 (stationary) |
|
|
|
| Alkaline fuel cell (AFC) | Aqueous solution of potassium hydroxide soaked in matrix | 90–100 | 10 kW–100 kW | 60 |
|
|
|
| Phosphoric acid fuel cell (PAFC) | Liquid phosphoric acid soaked in a matrix | 150–200 | 50 kW–1 MW (250 kW typical module) | > 40 |
|
|
|
| Molten carbonate fuel cell (MCFC) | Liquid solution of Li2 CO3, Na2 CO3 and/or K2 CO3 | 150–200 | <1 kW–1 MW (250 kW typical module) | 45–47 |
|
|
|
| Solid oxide fuel cell (SOFC) | Yttria stabilized zirconia | 600–700 | <1 kW–3 MW | 35–40 |
|
|
|
| Parameters * | Polymeric | Microporous | Dense * |
|---|---|---|---|
| Typical composition | Polyimide; Cellulose acetate | Silica; Zeolites; Metal-organic frameworks | Palladium;Palladium alloys |
| Separation mechanism | Solution-diffusion | Molecular-sieving | Solution-diffusion |
| Driving force * | Partial pressure difference | Partial pressure difference | Partial pressure difference |
| Operation temperature | ≤110 °C | ≤1000 °C | 150–700 °C |
| Relative permeability | Low-moderate | Moderate- high | Low |
| Typical selectivity | Moderate | Low-moderate | Very- high |
| Relative cost | Low | Low-moderate | Moderate- high |
| Draw Solution Concentration (M NaCl) | Draw Solution Pressure (bar) | Membrane | Power Density (W/m2) |
|---|---|---|---|
| 0.60 | 9.7 | CTA flat sheet | 2.7 |
| 0.59 | 13.0 | TFC flat sheet | 9.0 |
| 1.03 | 9.7 | CTA flat sheet | 4.0 |
| 1.00 | 20.7 | TFC flat sheet | 14.1 |
| 1.00 | 15.0 | TFC flat sheet | 12.0 |
| 1.06 | – | TFC flat sheet | 11.4 |
| 2.07 | 12.6 | CTA falt sheet | 3.2 |
| 3.00 | 48.0 | TFC flat sheet | 60.0 |
| 1.00 | 15.0 | Dual layer hollow fiber | 5.1 |
| 1.00 | 20.0 | TFC hollow fiber | 7.6 |
| 1.00 | 15.0 | TFC hollow fiber | 16.5 |
| 1.00 | 15.0 | TFC hollow fiber | 20.9 |
| 1.00 | 20.0 | TFC hollow fiber | 24.0 |
| Energy Storage | Working Principle | Typical Examples |
|---|---|---|
| Chemical | Energy is stored in the chemical bonds of atoms and molecules. | Hydrogen, synthetic natural gas, biofuels and thermochemical energy storage. |
| Electrochemical | Convert chemical energy into electrical energy. Energy available as electric current at a defined voltage and time | Electrochemical batteries (primary and secondary cell or battery) and electrochemical capacitors. |
| Mechanical | Convert mechanical energy into electrical energy. | Flywheel system, pumped hydro storage system and compressed air energy storage system. |
| Electrical | Realized by applying supercapacitor and magnetic storage. | Capacitor, supercapacitor and superconducting magnet. |
| Thermal | Store thermal energy by heating/cooling different media in enclosures. | Sensible heat system, latent heat system, absorption and adsorption system |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besha, A.T.; Tsehaye, M.T.; Tiruye, G.A.; Gebreyohannes, A.Y.; Awoke, A.; Tufa, R.A. Deployable Membrane-Based Energy Technologies: the Ethiopian Prospect. Sustainability 2020, 12, 8792. https://doi.org/10.3390/su12218792
Besha AT, Tsehaye MT, Tiruye GA, Gebreyohannes AY, Awoke A, Tufa RA. Deployable Membrane-Based Energy Technologies: the Ethiopian Prospect. Sustainability. 2020; 12(21):8792. https://doi.org/10.3390/su12218792
Chicago/Turabian StyleBesha, Abreham Tesfaye, Misgina Tilahun Tsehaye, Girum Ayalneh Tiruye, Abaynesh Yihdego Gebreyohannes, Aymere Awoke, and Ramato Ashu Tufa. 2020. "Deployable Membrane-Based Energy Technologies: the Ethiopian Prospect" Sustainability 12, no. 21: 8792. https://doi.org/10.3390/su12218792
APA StyleBesha, A. T., Tsehaye, M. T., Tiruye, G. A., Gebreyohannes, A. Y., Awoke, A., & Tufa, R. A. (2020). Deployable Membrane-Based Energy Technologies: the Ethiopian Prospect. Sustainability, 12(21), 8792. https://doi.org/10.3390/su12218792







