Experimental Study on the Optimum Preparation of Bentonite–Steel Slag Composite Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Calculation
2.3. Preparation of the BSC
2.4. Single-Factor Experiment
2.5. Orthogonal Experiment
2.6. Adsorption Isotherms
2.7. Microstructure Characterization
3. Results and Discussion
3.1. Single-Factor Experiment
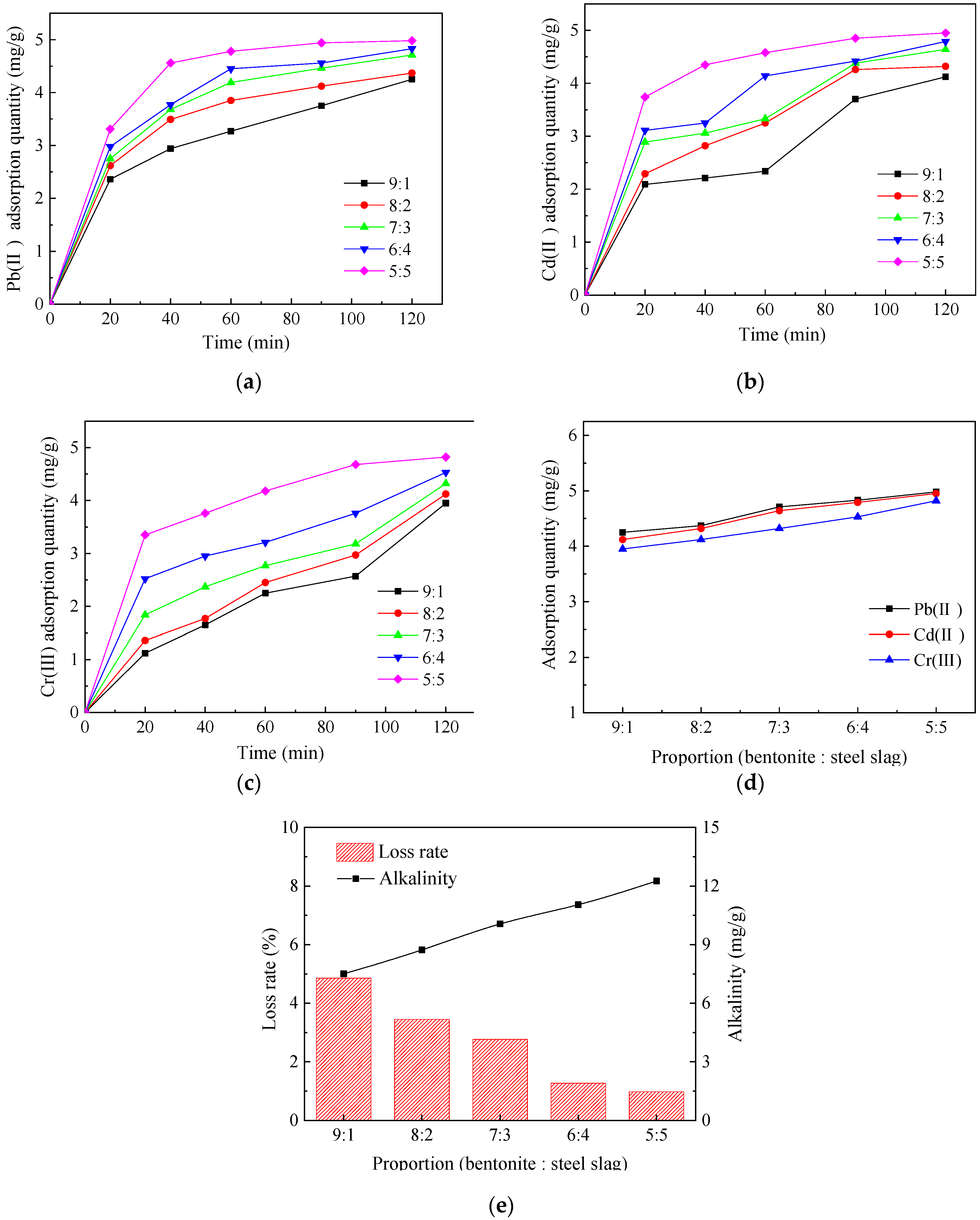
3.1.1. Determination of the Bentonite–Steel Slag Proportion
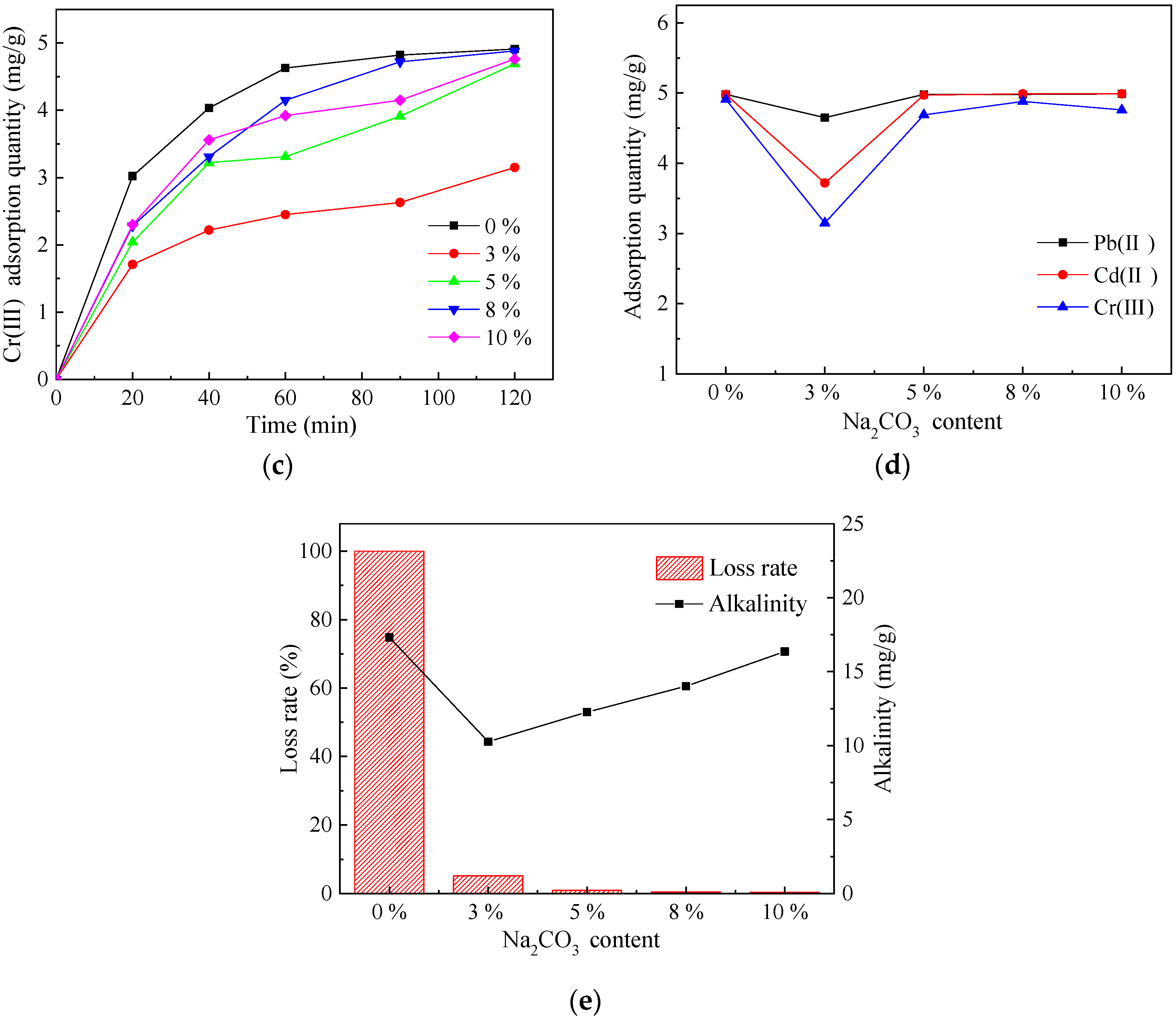
3.1.2. Determination of the Na2CO3 Content
3.1.3. Determination of the Aging Time
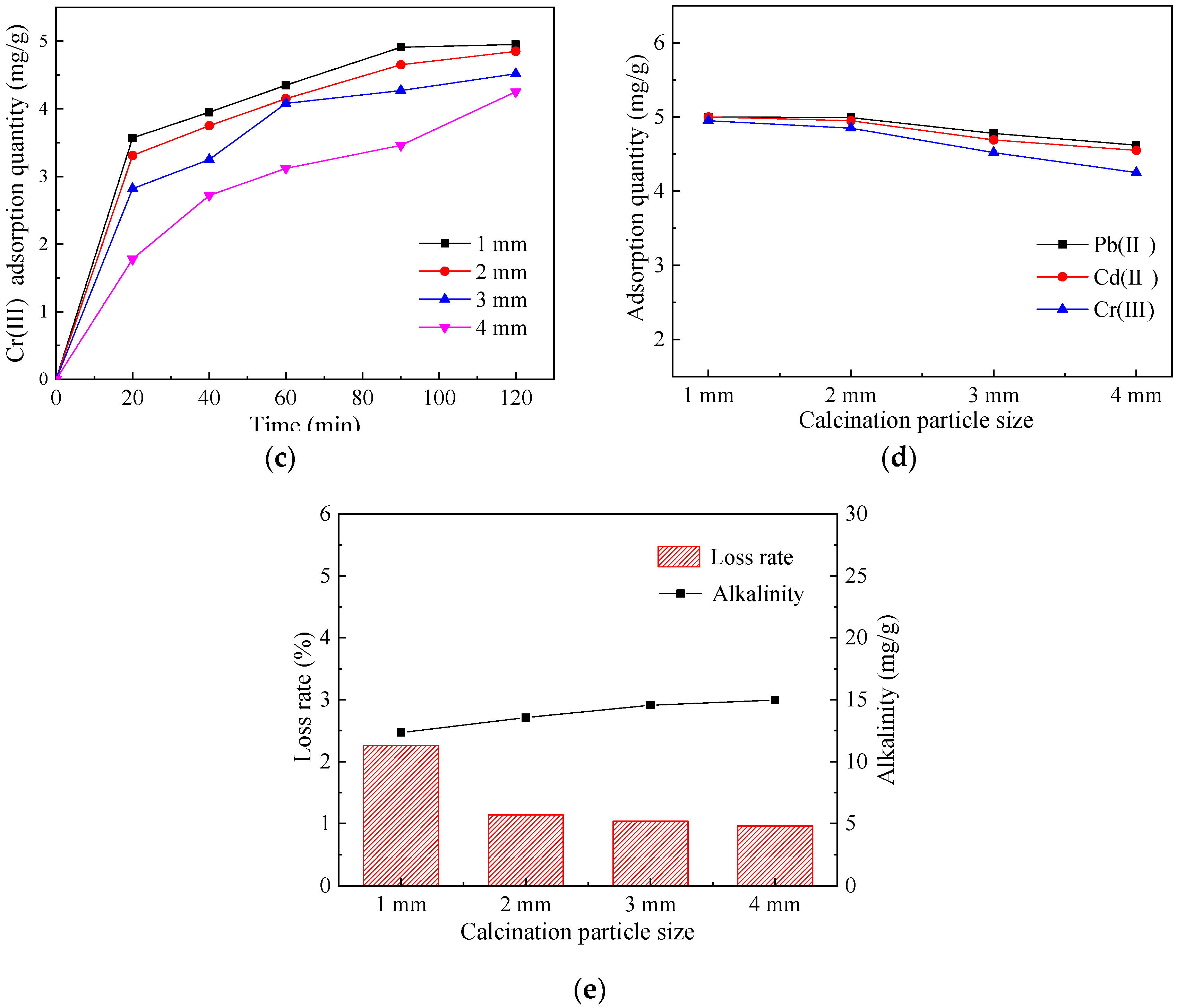
3.1.4. Determination of the Calcination Particle Size
3.1.5. Determination of the Calcination Temperature
3.1.6. Determination of the Calcination Time
3.2. Orthogonal Experimental Study
Range Analysis of Orthogonal Experimental Results
3.3. Adsorption Isotherms
3.4. Results of Microstructure Characterization
3.4.1. SEM and BET Analysis
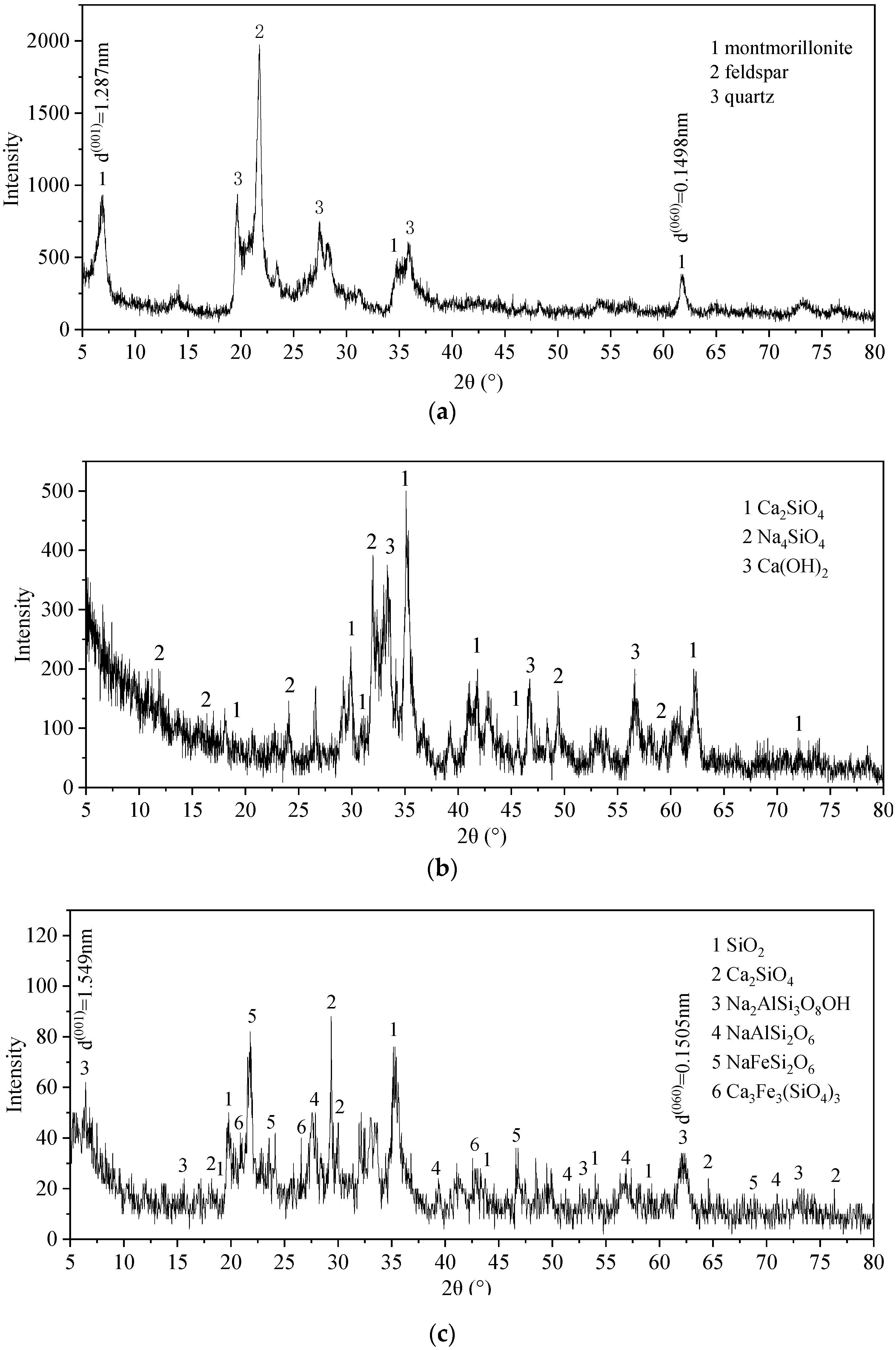
3.4.2. XRD Analysis
3.4.3. FT-IR Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Bessho, M.; Wajima, T.; Ida, T.; Nishiyama, T. Experimental study on prevention of acid mine drainage by silica coating of pyrite waste rocks with amorphous silica solution. Environ. Earth Sci. 2011, 64, 311–318. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Rodriguez, C.; Leiva-Aravena, E.; Serrano, J.; Leiva, E. Occurrence and Removal of Copper and Aluminum in a Stream Confluence Affected by Acid Mine Drainage. Water 2018, 10, 516. [Google Scholar] [CrossRef]
- Gutierrez, M.; Mickus, K.; Camacho, L.M. Abandoned Pb-Zn mining wastes and their mobility as proxy to toxicity: A review. Sci. Total Environ. 2016, 565, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Oldham, C.; Beer, J.; Blodau, C.; Fleckenstein, J.; Jones, L.; Neumann, C.; Peiffer, S. Controls on iron(II) fluxes into waterways impacted by acid mine drainage: A Damkohler analysis of groundwater seepage and iron kinetics. Water Res. 2019, 153, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Balistrieri, L.S.; Seal, R.R.; Piatak, N.M.; Paul, B. Assessing the concentration, speciation, and toxicity of dissolved metals during mixing of acid-mine drainage and ambient river water downstream of the Elizabeth Copper Mine, Vermont, USA. Appl. Geochem. 2007, 22, 930–952. [Google Scholar] [CrossRef]
- Beygli, R.A.; Mohaghegh, N.; Rahimi, E. Metal ion adsorption from wastewater by g-C3N4 modified with hydroxyapatite: A case study from Sarcheshmeh Acid Mine Drainage. Res. Chem. Intermediat. 2019, 45, 2255–2268. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Adsorptive Removal of Reactive Black 5 from Wastewater Using Bentonite Clay: Isotherms, Kinetics and Thermodynamics. Sustainability 2015, 7, 15302–15318. [Google Scholar] [CrossRef]
- Sephton, M.G.; Webb, J.A. The role of secondary minerals in remediation of acid mine drainage by Portland cement. J. Hazard. Mater. 2019, 367, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Mamelkina, M.A.; Tuunila, R.; Sillanpaa, M.; Hakkinen, A. Systematic study on sulfate removal from mining waters by electrocoagulation. Sep. Purif. Technol. 2019, 216, 43–50. [Google Scholar] [CrossRef]
- Aguiar, A.O.; Andrade, L.H.; Ricci, B.C.; Pires, W.L.; Miranda, G.A.; Amaral, M.C.S. Gold acid mine drainage treatment by membrane separation processes: An evaluation of the main operational conditions. Sep. Purif. Technol. 2016, 170, 360–369. [Google Scholar] [CrossRef]
- Vital, B.; Bartacek, J.; Ortega-Bravo, J.C.; Jeison, D. Treatment of acid mine drainage by forward osmosis: Heavy metal rejection and reverse flux of draw solution constituents. Chem. Eng. J. 2018, 332, 85–91. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Katsoyiannis, I.A. Recent Advances in Water and Wastewater Treatment with Emphasis in Membrane Treatment Operations. Water 2019, 11, 45. [Google Scholar] [CrossRef]
- Bai, H.; Kang, Y.; Quan, H.E.; Han, Y.; Sun, J.; Feng, Y. Treatment of acid mine drainage by sulfate reducing bacteria with iron in bench scale runs. Bioresour. Technol. 2013, 128, 818–822. [Google Scholar] [CrossRef]
- Yildiz, M.; Yilmaz, T.; Arzum, C.S.; Yurtsever, A.; Kaksonen, A.H.; Ucar, D. Sulfate reduction in acetate- and ethanol-fed bioreactors: Acidic mine drainage treatment and selective metal recovery. Miner. Eng. 2019, 133, 52–59. [Google Scholar] [CrossRef]
- Dean, A.P.; Lynch, S.; Rowland, P.; Toft, B.D.; Pittman, J.K.; White, K.N. Natural Wetlands Are Efficient at Providing Long-Term Metal Remediation of Freshwater Systems Polluted by Acid Mine Drainage. Environ. Sci. Technol. 2013, 47, 12029–12036. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.L.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Jimenez-Castaneda, M.E.; Medina, D.I. Use of Surfactant-Modified Zeolites and Clays for the Removal of Heavy Metals from Water. Water 2017, 9, 235. [Google Scholar] [CrossRef]
- Alakangas, L.; Andersson, E.; Mueller, S. Neutralization/prevention of acid rock drainage using mixtures of alkaline by-products and sulfidic mine wastes. Environ. Sci. Pollut. Res. 2013, 20, 7907–7916. [Google Scholar] [CrossRef] [PubMed]
- Madzivire, G.; Maleka, P.P.; Vadapalli, V.R.K.; Gitari, W.M.; Lindsay, R.; Petrik, L.F. Fate of the naturally occurring radioactive materials during treatment of acid mine drainage with coal fly ash and aluminium hydroxide. J. Environ. Manag. 2014, 133, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Wigniewska, M.; Chibowski, S.; Urban, T.; Terpilowski, K. Investigations of chromium(III) oxide removal from the aqueous suspension using the mixed flocculant composed of anionic and cationic polyacrylamides. J. Hazard. Mater. 2019, 368, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Patra, P.; Singha, K.; Biswas, P.; Sarkar, S.; Pal, S. Graft copolymeric flocculant using functionalized starch towards treatment of blast furnace effluent. Int. J. Biol. Macromol. 2019, 125, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Sephton, M.G.; Webb, J.A.; McKnight, S. Applications of Portland cement blended with fly ash and acid mine drainage treatment sludge to control acid mine drainage generation from waste rocks. Appl. Geochem. 2019, 103, 1–14. [Google Scholar] [CrossRef]
- Name, T.; Sheridan, C. Remediation of acid mine drainage using metallurgical slags. Miner. Eng. 2014, 64, 15–22. [Google Scholar] [CrossRef]
- Badescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation possibilities of exhausted biosorbents loaded with metal ions—A review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef]
- Deng, G.R.; Ma, J.C.; Zhang, X.P.; Zhang, Q.F.; Xiao, Y.Q.; Ma, Q.L.; Wang, S.B. Magnetic natural composite Fe3O4-chitosan@bentonite for removal of heavy metals from acid mine drainage. J. Colloid Interface Sci. 2019, 538, 132–141. [Google Scholar]
- Jun, B.M.; Kim, Y.; Han, J.; Yoon, Y.; Kim, J.; Park, C.M. Preparation of Activated Biochar-Supported Magnetite Composite for Adsorption of Polychlorinated Phenols from Aqueous Solutions. Water 2019, 11, 1899. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, A.X.; Klein, B.; Wang, H.J. Effect of primary flocculant type on a two-step flocculation process on iron ore fine tailings under alkaline environment. Miner. Eng. 2019, 132, 14–21. [Google Scholar] [CrossRef]
- Goldani, E.; Moro, C.C.; Maia, S.M. A Study Employing Differents Clays for Fe and Mn Removal in the Treatment of Acid Mine Drainage. Water Air Soil Pollut. 2013, 224, 1401. [Google Scholar] [CrossRef]
- Farsi, A.; Javid, N.; Malakootian, M. Investigation of adsorption efficiency of Cu2+ and Zn2+ by red soil and activated bentonite from acid copper mine drainage. Desalin. Water Treat. 2019, 144, 172–184. [Google Scholar] [CrossRef]
- Orakwue, E.O.; Asokbunyarat, V.; Rene, E.R.; Lens, P.N.L.; Annachhatre, A. Adsorption of Iron(II) from Acid Mine Drainage Contaminated Groundwater Using Coal Fly Ash, Coal Bottom Ash, and Bentonite Clay. Water Air Soil Pollut. 2016, 227, 74. [Google Scholar] [CrossRef]
- Tsai, T.T.; Kao, C.M.; Wang, J.Y. Remediation of TCE-contaminated groundwater using acid/BOF slag enhanced chemical oxidation. Chemosphere 2011, 83, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Molahid, V.L.M.; Kusin, F.M.; Madzin, Z. Role of multiple substrates (spent mushroom compost, ochre, steel slag, andlimestone) in passive remediation of metal-containing acid mine drainage. Environ. Technol. 2019, 40, 1323–1336. [Google Scholar] [CrossRef]
- Reddy, K.R.; Gopakumar, A.; Chetri, J.K. Critical review of applications of iron and steel slags for carbon sequestration and environmental remediation. Rev. Environ. Sci. Bio Technol. 2019, 18, 127–152. [Google Scholar] [CrossRef]
- Wu, X.; Leung, D.Y.C. Optimization of biodiesel production from camelina oil using orthogonal experiment. Appl. Energy 2011, 88, 3615–3624. [Google Scholar] [CrossRef]
- Guo, J.K.; Zhang, Y.T.; Che, S.Q. Performance analysis and experimental study on rainfall water purification with an extensive green roof matrix layer in Shanghai, China. Water Sci. Technol. 2018, 77, 670–681. [Google Scholar] [CrossRef]
- Cao, L.; Shen, W.G.; Huang, J.Q.; Yang, Y.; Zhang, D.; Huang, X.Q.; Lv, Z.J.; Ji, X.L. Process to utilize crushed steel slag in cement industry directly: Multi-phased clinker sintering technology. J. Clean. Prod. 2019, 217, 520–529. [Google Scholar] [CrossRef]
- Barisic, I.; Markovic, B.; Zagvozda, M. Freeze-thaw resistance assessment of cement-bound steel slag aggregate for pavement structures. Int. J. Pavement Eng. 2019, 20, 448–457. [Google Scholar] [CrossRef]
- Dimitrova, S.V.; Mehandgiev, D.R. Lead removal from aqueous solutions by granulated blast-furnace slag. Water Res. 1998, 32, 3289–3292. [Google Scholar] [CrossRef]
- Ishwarya, G.; Singh, B.; Deshwal, S.; Bhattacharyya, S.K. Effect of sodium carbonate/sodium silicate activator on the rheology, geopolymerization and strength of fly ash/slag geopolymer pastes. Cem. Concr. Comp. 2019, 97, 226–238. [Google Scholar]
- Martinez, L.R.; Escalante, G.J.I. Alkali activated composite binders of waste silica soda lime glass and blast furnace slag: Strength as a function of the composition. Constr. Build. Mater. 2016, 119, 119–129. [Google Scholar] [CrossRef]
- Gil, A.; Amiri, M.J.; Abedi-Koupai, J.; Eslamian, S. Adsorption/reduction of Hg(II) and Pb(II) from aqueous solutions by using bone ash/nZVI composite: Effects of aging time, Fe loading quantity and co-existing ions. Environ. Sci. Pollut. Res. 2018, 25, 2814–2829. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cui, X.L.; Li, Y.G.; Zhang, Q.H.; Wang, H.Z. The Effect of Aging Time on the Properties of Mg-Al-CO3 Layered Double Hydroxides and Its Application as a Catalyst Support for TiO2. J. Nanosci. Nanotechnol. 2016, 16, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zhu, L.H.; Luo, Z.H.; Lei, M.; Zhang, S.C.; Tang, H.Q. Sono-assisted preparation of magnetic magnesium-aluminum layered double hydroxides and their application for removing fluoride. Ultrason. Sonochem. 2011, 18, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, G.H.; Lo, I.M.C. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef]
- Liu, Z.M.; Li, X.; Xu, L.C.; Liu, R.R. A novel combined process of MFPAC coagulation and mineralized refuse adsorption for landfill leachate pretreatment. Desalin. Water Treat. 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Moon, D.H.; Wazne, M.; Cheong, K.H.; Chang, Y.Y.; Baek, K.; Ok, Y.S.; Park, J.H. Stabilization of As-, Pb-, and Cu-contaminated soil using calcined oyster shells and steel slag. Environ. Sci. Pollut. Res. 2015, 22, 11162–11169. [Google Scholar] [CrossRef]
- Magagane, N.; Masindi, V.; Ramakokovhu, M.M.; Shongwe, M.B.; Muedi, K.L. Facile thermal activation of non-reactive cryptocrystalline magnesite and its application on the treatment of acid mine drainage. J. Environ. Manage. 2019, 236, 499–509. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.W.; Chen, Y.M.; Zhan, L.T.; Li, Z.Z.; Tang, Q. Adsorption behavior and mechanism of Cd(II) on loess soil from China. J. Hazard. Mater. 2009, 172, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Guo, Q.X.; Men, X.P.; Ren, S.Y.; Jin, W.L.; Shen, B.J. Preparation of modified bentonite by polyhedral oligomeric silsesquioxane and sodium dodecyl sulfate in aqueous phase and its adsorption property. Mater. Lett. 2019, 253, 71–73. [Google Scholar] [CrossRef]
- Ma, J.Z.; Khan, M.A.; Xia, M.Z.; Fu, C.L.; Zhu, S.D.; Chu, Y.T.; Lei, W.; Wang, F.Y. Effective adsorption of heavy metal ions by sodium lignosulfonate reformed montmorillonite. Int. J. Biol. Macromol. 2019, 138, 188–197. [Google Scholar] [CrossRef] [PubMed]










| Constituent | Mass Percentage (%) | |
|---|---|---|
| Bentonite | Steel Slag | |
| SiO2 | 71.39 | 12.33 |
| Al2O3 | 14.4 | 0.18 |
| Na2O | 1.98 | 0 |
| Fe2O3 | 1.71 | 38.83 |
| MgO | 1.52 | 10 |
| CaO | 1.20 | 32.73 |
| K2O | 0.44 | 0 |
| TiO2 | <0.1 | 0 |
| MnO | 0 | 3.29 |
| P2O5 | 0 | 1.12 |
| Levels | Factors | |||
|---|---|---|---|---|
| Ratio (Bentonite: Steel Slag) | Na2CO3 Content (%) | Calcination Temperature (°C) | Calcination Time (min) | |
| 1 | 7:3 | 3 | 450 | 40 |
| 2 | 6:4 | 5 | 500 | 60 |
| 3 | 5:5 | 8 | 550 | 90 |
| Encoding | Proportion (Bentonite: Steel Slag) | Na2CO3 Content (%) | Calcination Temperature (°C) | Calcination Time (min) | Adsorption Quantity (mg/g) | Alkalinity (mg/g) | Loss Rate (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Pb(II) | Cd(II) | Cr(III) | |||||||
| A1B1C1D1 | 7:3 | 3 | 450 | 40 | 4.19 | 4.09 | 3.99 | 13.16 | 100 |
| A1B2C2D2 | 7:3 | 5 | 500 | 60 | 3.12 | 2.67 | 2.56 | 9.02 | 1.79 |
| A1B3C3D3 | 7:3 | 8 | 550 | 90 | 1.82 | 2.35 | 2.22 | 8.15 | 1.21 |
| A2B1C2D3 | 6:4 | 3 | 500 | 90 | 2.29 | 2.51 | 2.45 | 9.01 | 1.75 |
| A2B2C3D1 | 6:4 | 5 | 550 | 40 | 2.27 | 3.14 | 3.34 | 9.58 | 1.19 |
| A2B3C1D2 | 6:4 | 8 | 450 | 60 | 4.06 | 3.69 | 3.55 | 13.94 | 7.01 |
| A3B1C3D2 | 5:5 | 3 | 550 | 60 | 2.67 | 2.94 | 2.78 | 10.14 | 0.87 |
| A3B2C1D3 | 5:5 | 5 | 450 | 90 | 3.82 | 3.92 | 3.82 | 14.04 | 3.82 |
| A3B3C2D1 | 5:5 | 8 | 500 | 40 | 3.31 | 3.51 | 3.55 | 12.26 | 2.62 |
| k Value | Pb(II) Adsorption Quantity | Cd(II) Adsorption Quantity | Cr(III) Adsorption Quantity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proportion | Na2CO3 Content | Calcination Temperature | Calcination Time | Proportion | Na2CO3 Content | Calcination Temperature | Calcination Time | Proportion | Na2CO3 Content | Calcination Temperature | Calcination Time | |
| k1 | 3.04 | 3.05 | 4.02 | 3.26 | 3.04 | 3.18 | 3.90 | 3.58 | 2.92 | 3.07 | 3.79 | 3.63 |
| k2 | 2.87 | 3.07 | 2.91 | 3.28 | 3.11 | 3.24 | 2.90 | 3.10 | 3.11 | 3.24 | 2.85 | 2.96 |
| k3 | 3.27 | 3.06 | 2.25 | 2.64 | 3.46 | 3.18 | 2.81 | 2.93 | 3.38 | 3.11 | 2.78 | 2.83 |
| R | 0.40 | 0.02 | 1.77 | 0.64 | 0.42 | 0.06 | 1.09 | 0.65 | 0.46 | 0.17 | 1.01 | 0.80 |
| optimum level | 5:5 | 5% | 450 °C | 60 min | 5:5 | 5% | 450 °C | 40 min | 5:5 | 5% | 450 °C | 40 min |
| Dominant factors rank | ① Calcination temperature ② Calcination time ③ Proportion ④ Na2CO3 content | ① Calcination temperature ② Calcination time ③ Proportion ④ Na2CO3 content | ① Calcination temperature ② Calcination time ③ Proportion ④ Na2CO3 content | |||||||||
| k value | Alkalinity | Loss Rate | ||||||
|---|---|---|---|---|---|---|---|---|
| Proportion | Na2CO3 Content | Calcination Temperature | Calcination Time | Proportion | Na2CO3 Content | Calcination Temperature | Calcination Time | |
| k1 | 10.11 | 10.77 | 13.71 | 11.67 | 34.33 | 34.21 | 36.94 | 34.6 |
| k2 | 10.84 | 10.88 | 10.10 | 11.03 | 3.32 | 2.27 | 2.05 | 3.22 |
| k3 | 12.15 | 11.45 | 9.29 | 10.40 | 2.44 | 3.61 | 1.09 | 2.26 |
| R | 2.04 | 0.68 | 4.42 | 1.27 | 31.89 | 31.94 | 35.85 | 32.34 |
| optimum level | 5:5 | 8% | 450 °C | 40 min | 5:5 | 5% | 550 °C | 90 min |
| Dominant factors rank | ① Calcination temperature ② Proportion ③ Calcination time ④ Na2CO3 content | ① Calcination temperature ② Calcination time ③ Na2CO3 content ④ Proportion | ||||||
| Isothermal Models | Pb(II) | Cd(II) | Cr(III) |
|---|---|---|---|
| Langmuir isotherm | |||
| (mg/g) | 53.53 | 28.97 | 17.81 |
| (L/mg) | 0.0105 | 0.0259 | 0.0154 |
| 0.997 | 0.998 | 0.999 | |
| Freundlich isotherm | |||
| (L/g) | 1.68 | 1.83 | 0.96 |
| 1.69 | 1.91 | 1.98 | |
| 0.986 | 0.966 | 0.957 | |
| D–R isotherm | |||
| (mg/g) | 214.69 | 101.89 | 44.32 |
| (mol2/kJ2) | 0.0059 | 0.0053 | 0.0061 |
| (kJ/mol) | −9.19 | −9.74 | −9.01 |
| 0.995 | 0.985 | 0.979 | |
| BET isotherm | |||
| (mg/g) | 53.46 | 28.96 | 17.76 |
| 0.997 | 0.998 | 0.999 |
| Sample | SBET (m2/g) | Average Pore Radius (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| Bentonite | 76.5 | 6.2 | 0.18 |
| Steel slag | 0.4 | 5.3 | 0.06 |
| BSC before calcination | 50.7 | 4.5 | 0.11 |
| Optimum BSC | 130.6 | 8.4 | 5.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, X.; Xiao, L.; Liang, B. Experimental Study on the Optimum Preparation of Bentonite–Steel Slag Composite Particles. Sustainability 2020, 12, 18. https://doi.org/10.3390/su12010018
Zhan X, Xiao L, Liang B. Experimental Study on the Optimum Preparation of Bentonite–Steel Slag Composite Particles. Sustainability. 2020; 12(1):18. https://doi.org/10.3390/su12010018
Chicago/Turabian StyleZhan, Xinhui, Liping Xiao, and Bing Liang. 2020. "Experimental Study on the Optimum Preparation of Bentonite–Steel Slag Composite Particles" Sustainability 12, no. 1: 18. https://doi.org/10.3390/su12010018
APA StyleZhan, X., Xiao, L., & Liang, B. (2020). Experimental Study on the Optimum Preparation of Bentonite–Steel Slag Composite Particles. Sustainability, 12(1), 18. https://doi.org/10.3390/su12010018





