Conceptual Framework for Biodiversity Assessments in Global Value Chains
Abstract
1. Introduction
Relevance and General Context
2. Requirements for a Biodiversity Methodology in LCA
2.1. Requirements—From an Ecological and Conservation Perspective
2.2. Requirements—From an LCA Perspective
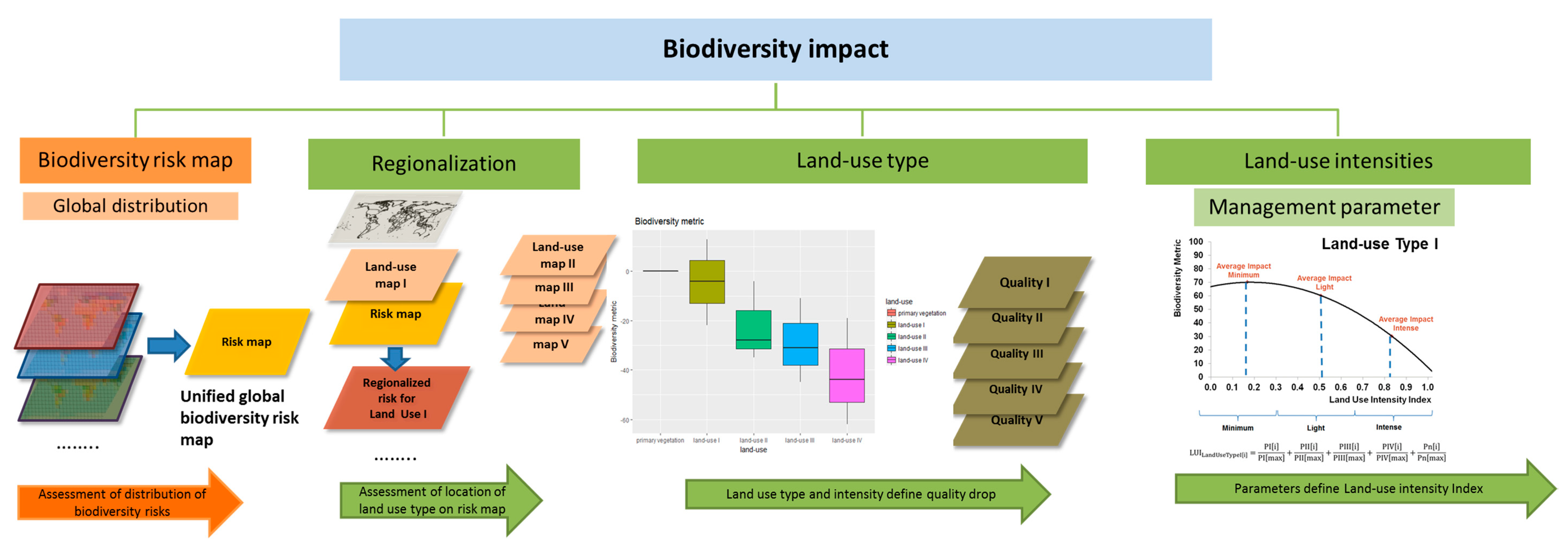
- First, the factor of regionalization. This factor reflects the fact that biodiversity is not evenly distributed across the world and neither are the threats to biodiversity, e.g., there are places with high pressure on biodiversity, places with low anthropogenic disturbances or areas with a high number of species [55,56]. This makes it necessary to have a regionalization factor that can distinguish between different locations of land use, for example: Is it better to use resources from Spain in terms of biodiversity than rather, the same resources, from South Africa? The next step should further pinpoint the location in order to evaluate the impact on a specific region within a country. Here, some regions may still be intact and contain endangered or endemic species; while other regions may already be off-balance, and therefore land use in that region would have fewer negative effects [24,53]. If companies or municipalities want to mitigate their negative impact within their supply chains, it may prove beneficial for biodiversity to source materials from another location or to move the land use to another area.
- Second, the type of land use. A well-developed methodology should be able to assess different types of land use for alternative materials within a production chain [54]. Such land use types include forestry and plantations, agricultural land use, pasture, urban areas, or mining sites. For example, the use of the material ‘wood’ (land use type: forestry) should be comparable to the use of ‘banana leaves’ (land use type: plantation) for one-way plates. In addition, it must be taken into account whether these effects differ depending on the location. This question is especially relevant for the design of new products where different materials and alternative resources can be compared and taken into account in time.
- Third, the degree of land use intensity and suitable management parameters. Depending on the management practices applied in an area, land use has different intensities [22]. It should be possible to distinguish between land use intensities and to quantify their diverse impacts on biodiversity. In this respect, for example, extensive agriculture can be compared with intensive agriculture. Such management practices include the amount of used fertilizers and pesticides, the sampling of exotic or native trees, and the density of livestock. An assessment should quantify which land use practices have higher impacts on biodiversity and identify the influence of specific management parameters. Recommendations should be made as to which land use practices could be changed in order to minimize negative impacts on biodiversity and, if possible, increase positive effects.
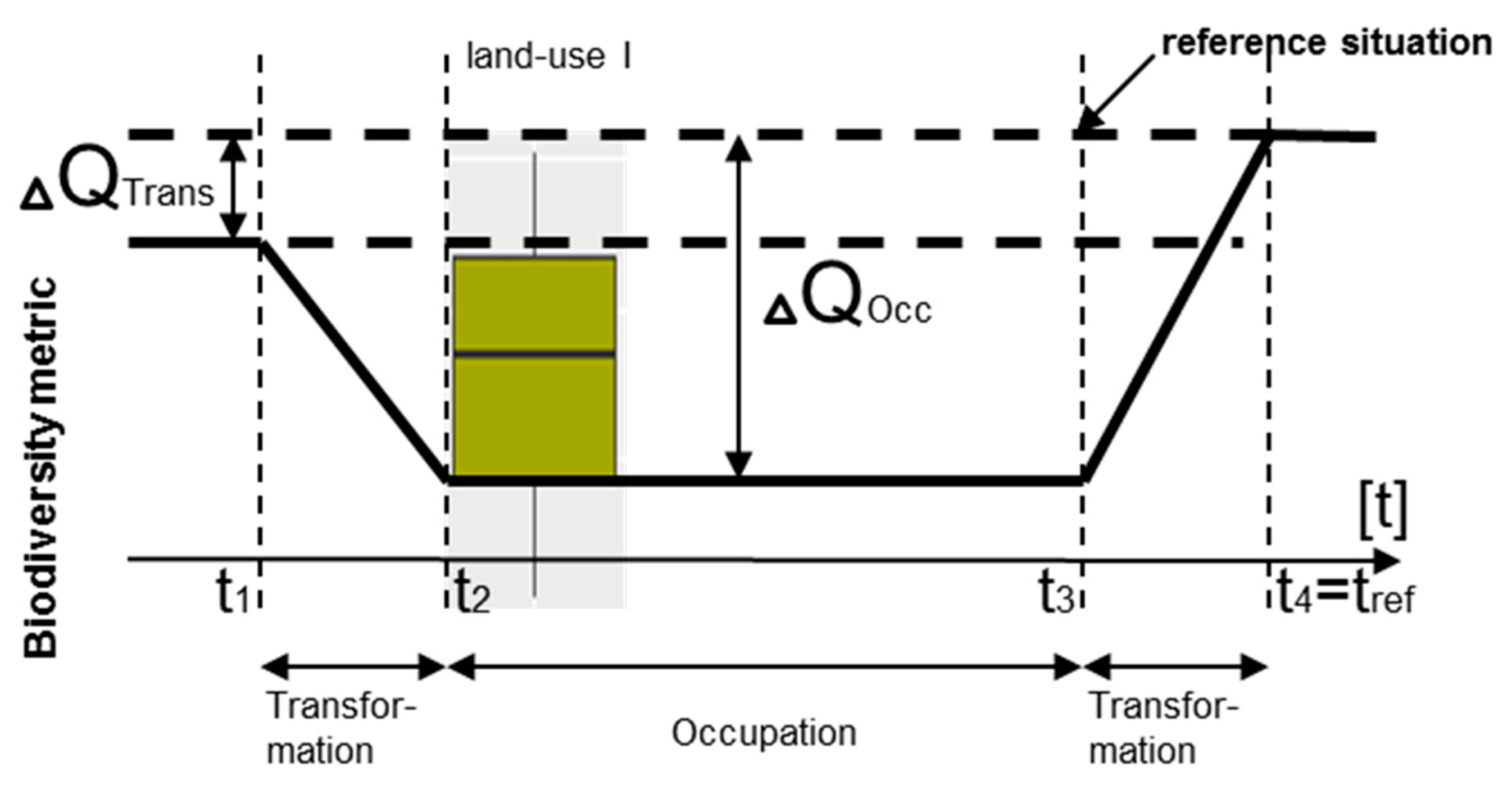
- A biodiversity assessment method should therefore take into account the three above-mentioned levels in order to assess the impact on biodiversity [54,57], compare different sites and types of land uses, and provide recommendations for careful land use practices as well as alternatives. A similar conceptual model has been recommended by the IPCC for assessing land use impacts on climate change [58].
3. Biodiversity Impact Assessment in LCA
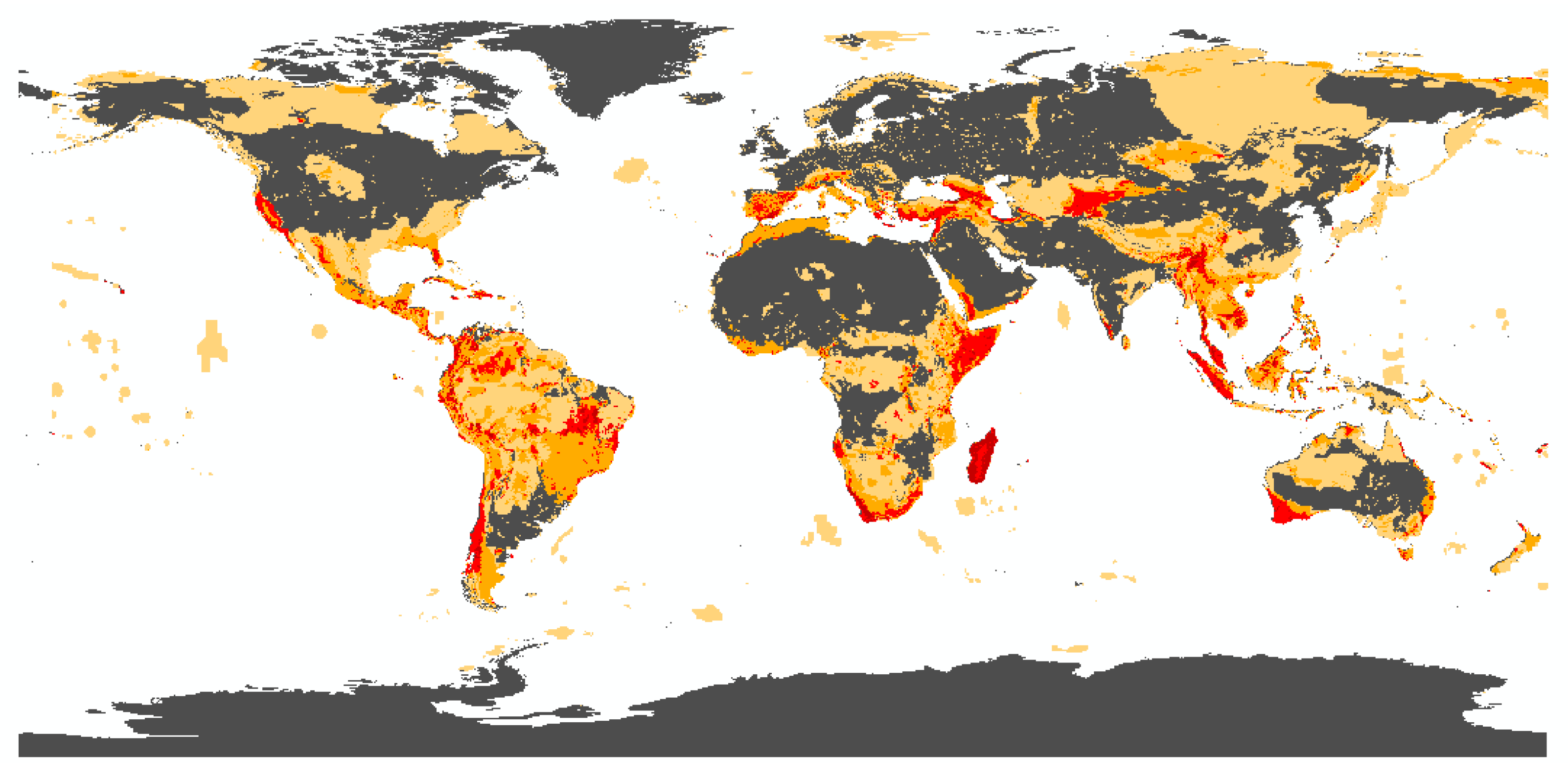
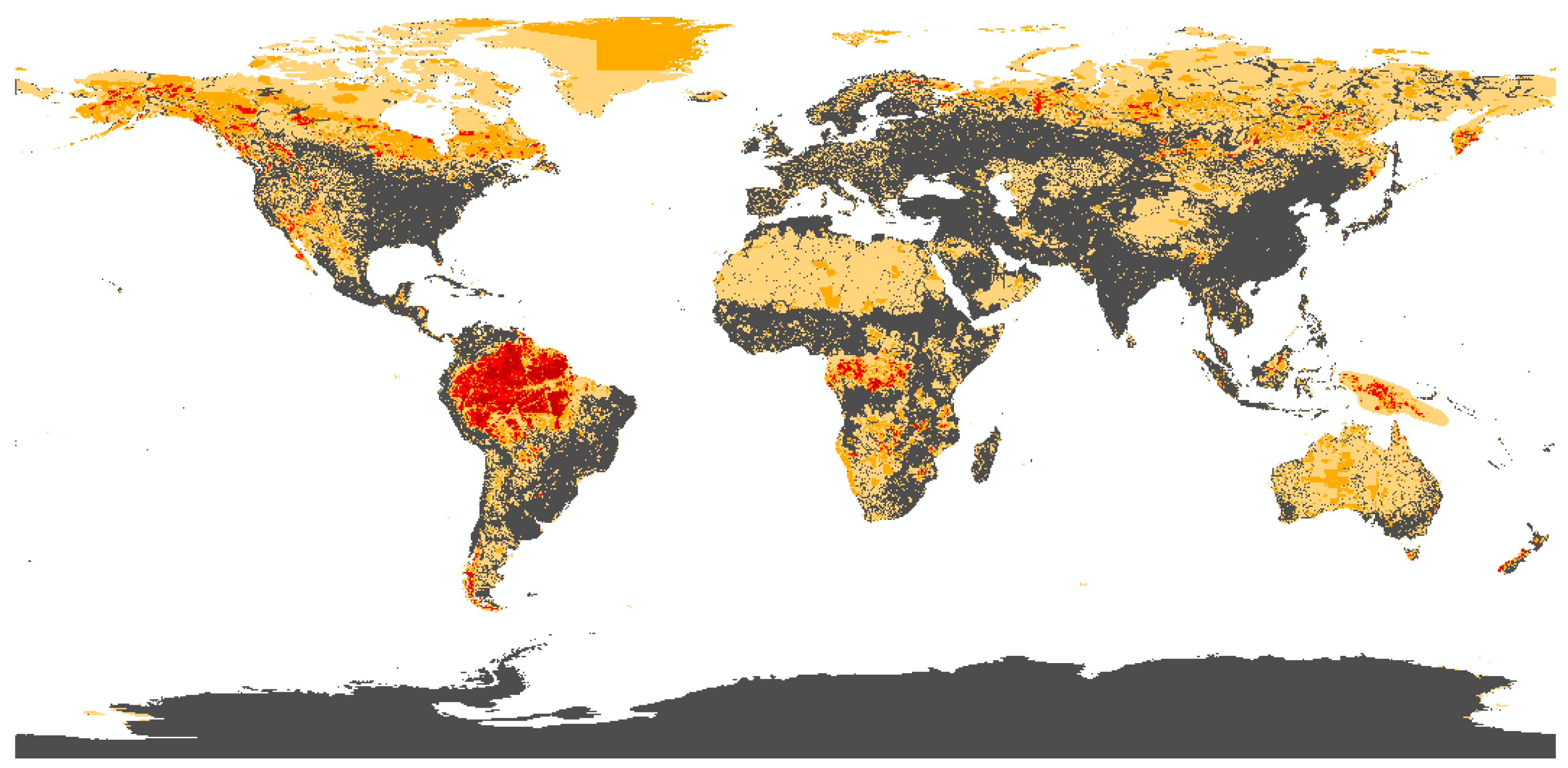
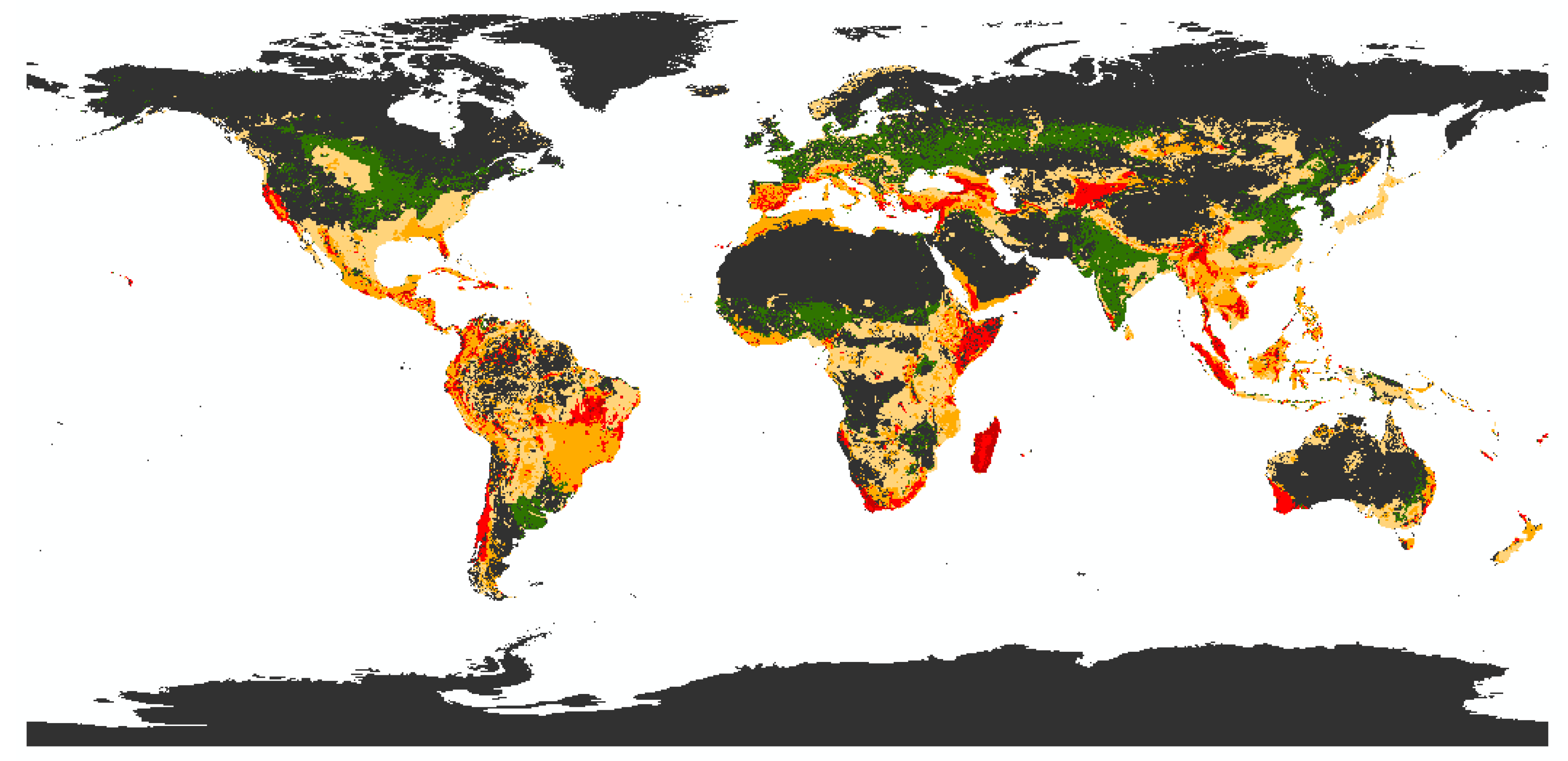
3.1. Biodiversity Risk Map
3.1.1. State of the Art and Research Gaps
3.1.2. Methodological Framework
3.2. Three-Level Hierarchical Biodiversity Life Cycle Impact Analysis
3.2.1. Location—Regionalization of Land Use Types
State of the Art and Research Gaps
Methodological Framework
3.2.2. Land Use Type
State of the Art and Research Gaps
Methodological Framework
3.2.3. Land Use Intensity and Management Parameters
State of the Art and Research Gaps
Methodological Framework
3.3. Summary of the Methodological Framework
4. Discussion
4.1. Major Research Gap and Main Findings
4.2. Advantages and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Author | Location/Distribution of Biodiversity on Global-scale | Land Use Types | Intensities | Management Parameters for Land Use | Operational on Global-scale | Comment | Empirical/Qualitative (e/q) |
|---|---|---|---|---|---|---|---|
| [87] | x | x | No recommendations on activities, only species-level, only Switzerland | e | |||
| [88] | x | x | x | No recommendations on activities, only species-level | e | ||
| [94] | x | x | Qualitative assessment, do not take into account distribution of biodiversity | q | |||
| [89] | x | Only for Netherlands, two models: 1 for species, 1 for ecosystems | e | ||||
| [66,95] | x | x | No recommendations on activities, only Europe | e | |||
| [79] | only mining | Sweden, region in Namibia; Site specific, only for mines | e | ||||
| [80] | grassland and cropland | x | x | Site specific, management options from experts/literature, no global comparison of locations, no comparison in between land use types | e, q | ||
| [67] | x | only forestry | x | x | Difficult to compare different land use types; only land use practices, qualitative scoring | q | |
| [68] | x | x | x | x | Only species richness of vascular plants, no direct quantification of management options | e | |
| [69] | x | Qualitative assessment with interviews per ecoregion, data intensive | q | ||||
| [30] | x | x | No recommendations on parameters, only some ecoregions | e | |||
| [70] | only croplands | x | x | Site specific, only for Germany | e | ||
| [71] | x | x | x | No recommendations on parameters | e | ||
| [72] | x | x | x | No recommendations on parameters | e | ||
| [90] | three land use types | x | Site specific, too data intensive for global-scale, only field and farm level | e, q | |||
| [73] | x | So far only for New Zealand, no intensities, data intensive since CMB has to be developed for all areas | q | ||||
| [91] | Only cropland | intensities for cropland | x | So far only 6 biomes, no management parameters, data intensive, site specific | e | ||
| [74] | x | x | x | no management parameters | e | ||
| [92] | x | x | Data intensive, interviews for each ecoregion necessary, no differentiation of land use types | q | |||
| [75] | x | only cropland | Land use only cropland, no recommendations on activities | e | |||
| [93] | x | x | Only for ‘Temperate Broadleaf and Mixed Forest’ biome, no recommendations on management parameters | e | |||
| [76] | x | x | x | No management parameters | e | ||
| [81] | x | x | Data intensive, parameters for every ecoregion, no differentiation of land use types | e, q | |||
| [38] | x | x | x | x | No recommendations on parameters for intensities | e | |
| [77] | x | x | x | x | Intensities measured at the interval minimum, light, intense, not possible to quantify impact due to specific parameters | e | |
| [78] | only forestry | x | x | So far for forestry, no differentiation between locations and land use types, only for Finland | q |
References
- Ryberg, M.W.; Owsianiak, M.; Richardson, K.; Hauschild, M.Z. Challenges in implementing a Planetary Boundaries based Life-Cycle Impact Assessment methodology. J. Clean. Prod. 2016, 139, 450–459. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science (New York, NY) 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. Planetary Boundaries: Exploring the Safe Operating Space for Humanity. Ecol. Soc. 2009, 14. Available online: http://www.ecologyandsociety.org/vol14/iss2/art32/ (accessed on 20 March 2019). [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A Safe Operating Space for Humanity. Nature 2009, 461, 472–475. Available online: https://www.nature.com/nature/journal/v461/n7263/pdf/461472a.pdf (accessed on 20 July 2017). [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.R.; Harris, N.C.; Colwell, R.K.; Koh, L.P.; Sodhi, N.S. The sixth mass coextinction: Are most endangered species parasites and mutualists? Proc. Biol. Sci. 2009, 276, 3037–3045. [Google Scholar] [CrossRef]
- Pievani, T. The sixth mass extinction: Anthropocene and the human impact on biodiversity. Rend. Fis. Acc. Lincei 2014, 25, 85–93. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are We in the Midst of the Sixth Mass Extinction? A View from the World of Amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. Available online: http://www.pnas.org/content/105/Supplement_1/11466.full.pdf (accessed on 7 June 2017). [CrossRef]
- Wilting, H.C.; Schipper, A.M.; Bakkenes, M.; Meijer, J.R.; Huijbregts, M.A.J. Quantifying Biodiversity Losses Due to Human Consumption: A Global-Scale Footprint Analysis. Environ. Sci. Technol. 2017, 51, 3298–3306. [Google Scholar] [CrossRef]
- Jeffries, M.J. Biodiversity and Conservation, 2nd ed.; Routledge: London, UK, 2006. [Google Scholar]
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Galetti, M.; Alamgir, M.; Crist, E.; Mahmoud, M.I.; Laurance, W.F. World Scientists’ Warning to Humanity: A Second Notice. BioScience 2017, 67, 1026–1028. [Google Scholar] [CrossRef]
- Diamond, J.M. Collapse: How Societies Choose to Fail or Succeed; Penguin Books: New York, NY, USA, 2006. [Google Scholar]
- Weiss, A.E.; Oregon, T.K.; Haney, J.C.; Fascione, N. Social and Ecological Benefits of Restored Wolf Populations. In Proceedings of the Transactions of the 72nd North American Wildlife and Natural Resources Conference, Portland, OR, USA, 20–24 March 2007; pp. 297–319. [Google Scholar]
- Meyer, S.T.; Ptacnik, R.; Hillebrand, H.; Bessler, H.; Buchmann, N.; Ebeling, A.; Eisenhauer, N.; Engels, C.; Fischer, M.; Halle, S.; et al. Biodiversity-multifunctionality relationships depend on identity and number of measured functions. Nat. Ecol. Evol. 2018, 2, 44. [Google Scholar] [CrossRef]
- Soliveres, S.; van der Plas, F.; Manning, P.; Prati, D.; Gossner, M.M.; Renner, S.C.; Alt, F.; Arndt, H.; Baumgartner, V.; Binkenstein, J.; et al. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 2016, 536, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Natural Capital Coalition. Natural Capital Protocol; Natural Capital Coalition: London, UK, 2016. [Google Scholar]
- Liu, J.; Diamond, J. China’s environment in a globalizing world. Nature 2005, 435, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Cesar, H.; Burke, L.; Pet-Soede, L. The Economics of Worldwide Coral Reef Degradation; Cesar Environmental Economics Consulting (CEEC): Arnhem, The Netherlands, 2003. [Google Scholar]
- Manfredi, S.; Allacker, K.; Kirana, C.; Pelletier, N.; de Souza, D.M. Product Environmental Footprint (PEF) Guide; European Commission-Joint Research Centre: Ispra, Italy, 2012. [Google Scholar]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Borger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Dicks, L.V.; Ockendon, N.; Petrovan, S.O.; Smith, R.K. (Eds.) What Works in Conservation 2018; Open Book Publishers: Cambridge, UK, 2018. [Google Scholar]
- Marques, A.; Martins, I.S.; Kastner, T.; Plutzar, C.; Theurl, M.C.; Eisenmenger, N.; Huijbregts, M.A.J.; Wood, R.; Stadler, K.; Bruckner, M.; et al. Increasing impacts of land use on biodiversity and carbon sequestration driven by population and economic growth. Nat. Ecol. Evol. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.P.; Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Chang. 2019, 1. [Google Scholar] [CrossRef]
- Pekin, B.K.; Pijanowski, B.C.; Mac Nally, R. Global land use intensity and the endangerment status of mammal species. Divers. Distrib. 2012, 18, 909–918. [Google Scholar] [CrossRef]
- Nygren, J.; Antikainen, R. Use of Life Cycle Assessment (LCA) in Global Companies; The Finnish Environment Institute: Helsinki, Finland, 2010. [Google Scholar]
- Winter, L.; Lehmann, A.; Finogenova, N.; Finkbeiner, M. including biodiversity in life cycle assessment–State of the art, gaps and research needs. EIA Rev. 2017, 67, 88–100. [Google Scholar] [CrossRef]
- Michelsen, O.; Lindner, J. Why Include Impacts on Biodiversity from Land Use in LCIA and How to Select Useful Indicators? Sustainability 2015, 7, 6278–6302. [Google Scholar] [CrossRef]
- De Souza, D.M.; Flynn, D.F.B.; DeClerck, F.; Rosenbaum, R.K.; de Melo Lisboa, H.; Koellner, T. Land use impacts on biodiversity in LCA: Proposal of characterization factors based on functional diversity. Int. J. Life Cycle Assess. 2013, 18, 1231–1242. [Google Scholar] [CrossRef]
- Schenck, R.C. Land Use and Biodiversity Indicators for Life Cycle Impact Assessment. Int. J. Life Cycle Assess. 2001, 2, 114–117. Available online: https://link.springer.com/content/pdf/10.1007/BF02977848.pdf (accessed on 9 August 2018).
- Teillard, F.; Maia de Souza, D.; Thoma, G.; Gerber, P.J.; Finn, J.A.; Bode, M. What does Life-Cycle Assessment of agricultural products need for more meaningful inclusion of biodiversity? J. Appl. Ecol. 2016, 53, 1422–1429. [Google Scholar] [CrossRef]
- Curran, M.; de Souza, D.M.; Anton, A.; Teixeira, R.F.M.; Michelsen, O.; Vidal-Legaz, B.; Sala, S.; Mila i Canals, L. How Well Does LCA Model Land Use Impacts on Biodiversity?—A Comparison with Approaches from Ecology and Conservation. Environ. Sci. Technol. 2016, 50, 2782–2795. [Google Scholar] [CrossRef]
- Marques, A.; Verones, F.; Kok, M.T.J.; Huijbregts, M.A.J.; Pereira, H.M. How to quantify biodiversity footprints of consumption? A review of multi-regional input–Output analysis and life cycle assessment. Curr. Opin. Environ. Sustain. 2017, 29, 75–81. [Google Scholar] [CrossRef]
- Teixeira, R.; Morais, T.; Domingos, T.A. Practical Comparison of Regionalized Land Use and Biodiversity Life Cycle Impact Assessment Models Using Livestock Production as a Case Study. Sustainability 2018, 10, 4089. [Google Scholar] [CrossRef]
- Vrasdonk, E.; Palme, U.; Lennartsson, T. Reference situations for biodiversity in life cycle assessments: Conceptual bridging between LCA and conservation biology. Int. J. Life Cycle Assess. 2019, 3, 38. [Google Scholar] [CrossRef]
- UNEP CBD. Convention on Biological Diversity; United Nations: New York, NY, USA, 1992. [Google Scholar]
- Chaudhary, A.; Pourfaraj, V.; Mooers, A.O.; Cowie, R. Projecting global land use-driven evolutionary history loss. Divers. Distrib. 2018, 24, 158–167. [Google Scholar] [CrossRef]
- Srivastava, D.S.; Cadotte, M.W.; MacDonald, A.A.M.; Marushia, R.G.; Mirotchnick, N. Phylogenetic diversity and the functioning of ecosystems. Ecol. Lett. 2012, 15, 637–648. [Google Scholar] [CrossRef]
- Collen, B.; Turvey, S.T.; Waterman, C.; Meredith, H.M.R.; Kuhn, T.S.; Baillie, J.E.M.; Isaac, N.J.B. Investing in evolutionary history: Implementing a phylogenetic approach for mammal conservation. Philos. Trans. R. Soc. Lond. Ser. B 2011, 366, 2611–2622. [Google Scholar] [CrossRef]
- Wittig, R.; Niekisch, M. Biodiversität: Grundlagen, Gefährdung, Schutz; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Schmitt, C.B. A Tough Choice: Approaches towards the Setting of Global Conservation Priorities. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 23–42. [Google Scholar]
- Mittermeier, R.A.; Mittermeier, C.G.; Brooks, T.M.; Pilgrim, J.D.; Konstant, W.R.; da Fonseca, G.A.B.; Kormos, C. Wilderness and Biodiversity Conservation. Proc. Natl. Acad. Sci. USA 2003, 100, 10309–10313. Available online: https://pdfs.semanticscholar.org/3dae/0f08967bf0bb819a06f803db2168b2f2f9f2.pdf (accessed on 17 August 2017). [CrossRef]
- Mittermeier, R.A.; Gil, P.R.; Hoffmann, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; Fonseca, D.A.; Gustavo, A.B. Hotspots Revisited; CEMEX: San Pedro Garza García, Mexico, 2004. [Google Scholar]
- Olson, D.M.; Dinerstein, E. The Global 200: Priority Ecoregions for Global Conservation-ANNEX. Ann. Mo. Bot. Gard. 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol. Lett. 2005, 8, 23–29. [Google Scholar] [CrossRef]
- Bryant, D.; Nielsen, D.; Tangley, L.; Sizer, N. The Last Frontier Forests: Ecosystems & Economies on the Edge; What is the Status of the World’s Remaining Large, Natural Forest Ecosystems? World Resources Inst. Forest Frontiers Initiative: Washington, DC, USA, 1997. [Google Scholar]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The Human Footprint and the Last of the Wild. BioScience 2002, 52, 891. [Google Scholar] [CrossRef]
- Isaac, N.J.B.; Turvey, S.T.; Collen, B.; Waterman, C.; Baillie, J.E.M. Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLoS ONE 2007, 2, e296. [Google Scholar] [CrossRef] [PubMed]
- Safi, K.; Armour-Marshall, K.; Baillie, J.E.M.; Isaac, N.J.B. Global patterns of evolutionary distinct and globally endangered amphibians and mammals. PLoS ONE 2013, 8, e63582. [Google Scholar] [CrossRef] [PubMed]
- WWF; IUCN. Centres of Plant Diversity: A Guide and Strategy for Their Conservation, 1st ed.; World Wide Fund for Nature: Gland, Switzerland; Cambridge, UK, 1994–1997. [Google Scholar]
- Stattersfield, A.J.; Crosby, M.J.; Long, A.J.; Wege, D.C. Endemic Bird Areas of the World: Priorities for Biodiversity Conservation; BirdLife International: Cambridge, UK, 1998. [Google Scholar]
- Brooks, T.M.; Mittermeier, R.A.; da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global biodiversity conservation priorities. Science (New York, NY) 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- UNEP/SETAC Life Cycle Initiative. Global Guidance for life cycle impact assessment indicators, 1st ed.; United Nations Environment Programme: Nairobi, Kenya, 2016. [Google Scholar]
- Lanzerath, D.; Mutke, J.; Barthlott, W.; Baumgärtner, S.; Becker, C.; Spranger, T.M. Biodiversität; Alber: Freiburg, Germany, 2008. [Google Scholar]
- Grenyer, R.; Orme, C.D.L.; Jackson, S.F.; Thomas, G.H.; Davies, R.G.; Davies, T.J.; Jones, K.E.; Olson, V.A.; Ridgely, R.S.; Rasmussen, P.C.; et al. Global distribution and conservation of rare and threatened vertebrates. Nature 2006, 444, 93–96. [Google Scholar] [CrossRef]
- Koellner, T.; de Baan, L.; Beck, T.; Brandão, M.; Civit, B.; Goedkoop, M.; Margni, M.; i Canals, L.M.; Müller-Wenk, R.; Weidema, B.; et al. Principles for life cycle inventories of land use on a global scale. Int. J. Life Cycle Assess. 2013, 18, 1203–1215. [Google Scholar] [CrossRef]
- Paustian, K.; Ravindranath, N.H.; van Amstel, A.; Gytarsky, M.; Kurz, W.A.; Ogle, S.; Richards, G.; Somogyi, Z. 2006 IPCC Guidelines for National Greenhouse Gas Inventories. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Institute for Global Environmental Strategies: Hayama, Japan, 2006; pp. 1–21. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. Available online: https://www.nature.com/nature/journal/v403/n6772/pdf/403853a0.pdf (accessed on 19 October 2017). [CrossRef]
- Gordon, E.A.; Franco, O.E.; Tyrrell, M.L. Protecting Biodiversity: A Guide to Criteria Used by Global Conservation Organizations (2005); Global Institute of Sustainable Forestry, Yale School of Forestry & Environmental Studies: New Haven, CT, USA, 2005. [Google Scholar]
- Orme, C.D.L.; Davies, R.G.; Burgess, M.; Eigenbrod, F.; Pickup, N.; Olson, V.A.; Webster, A.J.; Ding, T.-S.; Rasmussen, P.C.; Ridgely, R.S.; et al. Global hotspots of species richness are not congruent with endemism or threat. Nature 2005, 436, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Orgiazzi, A.; Panagos, P.; Yigini, Y.; Dunbar, M.B.; Gardi, C.; Montanarella, L.; Ballabio, C. A knowledge-based approach to estimating the magnitude and spatial patterns of potential threats to soil biodiversity. Sci. Total Environ. 2016, 545–546, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Orgiazzi, A.; Bardgett, R.D.; Barrios, E.; Behan-Pelletier, V.; Briones, M.J.I. (Eds.) Global Soil Biodiversity Atlas; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Ballesteros-Mejia, L.; Kitching, I.J.; Jetz, W.; Beck, J. Putting insects on the map: Near-global variation in sphingid moth richness along spatial and environmental gradients. Ecography 2017, 40, 698–708. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Döring, M.; Eisenhauer, N.; Gardi, C.; Ladau, J.; Leff, J.W.; Lentendu, G.; Lindo, Z.; Rillig, M.C.; Russell, D.; et al. Toward a global platform for linking soil biodiversity data. Front. Ecol. Evol. 2015, 3, 2189. [Google Scholar] [CrossRef]
- Koellner, T.; Scholz, R.W. Assessment of land use impacts on the natural environment. Int. J. Life Cycle Assess. 2008, 13, 32–48. [Google Scholar] [CrossRef]
- Michelsen, O. Assessment of land use impact on biodiversity. Int. J. Life Cycle Assess. 2008, 13, 22–31. [Google Scholar] [CrossRef]
- Schmidt, J.H. Development of LCIA characterisation factors for land use impacts on biodiversity. J. Clean. Prod. 2008, 16, 1929–1942. [Google Scholar] [CrossRef]
- Penman, T.D.; Law, B.S.; Ximenes, F. A proposal for accounting for biodiversity in life cycle assessment. Biodivers. Conserv. 2010, 19, 3245–3254. [Google Scholar] [CrossRef]
- Urban, B.; Haaren, C.v.; Kanning, H.; Krahl, J.; Munack, A. Spatially Differentiated Examination of Biodiversity in LCA (Life Cycle Assessment) on National Scale Exemplified by Biofuels. VTI Agric. For. Res. 2012, 3, 65–67. Available online: http://literatur.thuenen.de/digbib_extern/bitv/dn050629.pdf (accessed on 1 August 2017).
- De Baan, L.; Alkemade, R.; Koellner, T. Land use impacts on biodiversity in LCA: A global approach. Int. J. Life Cycle Assess. 2013, 18, 1216–1230. [Google Scholar] [CrossRef]
- De Baan, L.; Mutel, C.L.; Curran, M.; Hellweg, S.; Koellner, T. Land use in life cycle assessment: Global characterization factors based on regional and global potential species extinction. Environ. Sci. Technol. 2013, 47, 9281–9290. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Michelsen, O. Land use impacts on biodiversity from kiwifruit production in New Zealand assessed with global and national datasets. Int. J. Life Cycle Assess. 2014, 19, 285–296. [Google Scholar] [CrossRef]
- Chaudhary, A.; Verones, F.; de Baan, L.; Hellweg, S. Quantifying Land Use Impacts on Biodiversity: Combining Species-Area Models and Vulnerability Indicators. Environ. Sci. Technol. 2015, 49, 9987–9995. [Google Scholar] [CrossRef] [PubMed]
- De Baan, L.; Curran, M.; Rondinini, C.; Visconti, P.; Hellweg, S.; Koellner, T. High-resolution assessment of land use impacts on biodiversity in life cycle assessment using species habitat suitability models. Environ. Sci. Technol. 2015, 49, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Mooers, A.O. Terrestrial Vertebrate Biodiversity Loss under Future Global Land Use Change Scenarios. Sustainability 2018, 10, 2764. [Google Scholar] [CrossRef]
- Chaudhary, A.; Brooks, T.M. Land Use Intensity-Specific Global Characterization Factors to Assess Product Biodiversity Footprints. Environ. Sci. Technol. 2018, 52, 5094–5104. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Lehesvirta, T.; Schenker, U.; Lundquist, L.; Koski, O.; Gueye, S.; Taylor, R.; Humbert, S. Capturing the potential biodiversity effects of forestry practices in life cycle assessment. Int. J. Life Cycle Assess. 2018, 23, 1192–1200. [Google Scholar] [CrossRef]
- Burke, A.; Kyläkorpi, L.; Rydgren, B.; Schneeweiss, R. Testing a Scandinavian biodiversity assessment tool in an African desert environment. J. Environ. Manag. 2008, 42, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Jeanneret, P.; Baumgartner, D.U.; Knuchel, R.F.; Gaillard, G. (Eds.) Integration of biodiversity as impact category for LCA in agriculture (SALCA-Biodiversity). In Proceedings of the 6th International Conference on LCA in the Agri-Food Sector, Zürich, Switzerland, 12–14 November 2008. [Google Scholar]
- Winter, L.; Pflugmacher, S.; Berger, M.; Finkbeiner, M. Biodiversity Impact Assessment (BIA+)-methodological framework for screening biodiversity. Integr. Environ. Assess. Manag. 2017, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.M.; Venter, O.; Lee, J.; Jones, K.R.; Robinson, J.G.; Possingham, H.P.; Allan, J.R. Protect the last of the wild. Nature 2018, 563, 27–30. [Google Scholar] [CrossRef]
- Betts, M.G.; Wolf, C.; Ripple, W.J.; Phalan, B.; Millers, K.A.; Duarte, A.; Butchart, S.H.M.; Levi, T. Global forest loss disproportionately erodes biodiversity in intact landscapes. Nature 2017, 547, 441–444. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Mittermeier, C.G.; Robles, G.P.; Pilgrim, J.D. Wilderness: Earth’s Last Wild Places; CEMEX: San Pedro Garza García, Mexico, 2002. [Google Scholar]
- Potapov, P.; Hansen, M.C.; Laestadius, L.; Turubanova, S.; Yaroshenko, A.; Thies, C.; Smith, W.; Zhuravleva, I.; Komarova, A.; Minnemeyer, S.; et al. The Last Frontiers of Wilderness: Tracking Loss of Intact Forest Landscapes from 2000 to 2013. Sci. Adv. 2017, 3, 1–13. Available online: http://advances.sciencemag.org/content/advances/3/1/e1600821.full.pdf (accessed on 17 May 2018). [CrossRef]
- Potapov, P.; Yaroshenko, A.; Turubanova, S.; Dubinin, M.; Laestadius, L.; Thies, C.; Aksenov, D.; Egorov, A.; Yesipova, Y.; Glushkov, I.; et al. Mapping the World’s Intact Forest Landscapes by Remote Sensing. Ecol. Soc. 2008, 13. [Google Scholar] [CrossRef]
- Koellner, T. Species-pool effect potentials (SPEP) as a yardstick to evaluate and-use impacts on biodiversity. J. Clean. Prod. 2000, 8, 293–311. [Google Scholar] [CrossRef]
- Lindeijer, E. Biodiversity and life support impacts of land use in LCA. J. Clean. Prod. 2000, 8, 313–319. [Google Scholar] [CrossRef]
- Vogtländer, J.G.; Lindeijer, E.; Witte, J.-P.M.; Hendriks, C. Characterizing the change of land-use based on flora: Application for EIA and LCA. J. Clean. Prod. 2004, 12, 47–57. [Google Scholar] [CrossRef]
- Jeanneret, P.; Baumgartner, D.U.; Freiermuth Knuchel, R.; Koch, B.; Gaillard, G. An expert system for integrating biodiversity into agricultural life-cycle assessment. Ecol. Indic. 2014, 46, 224–231. [Google Scholar] [CrossRef]
- Elshout, P.M.F.; van Zelm, R.; Karuppiah, R.; Laurenzi, I.J.; Huijbregts, M.A.J. A spatially explicit data-driven approach to assess the effect of agricultural land occupation on species groups. Int. J. Life Cycle Assess. 2014, 19, 758–769. [Google Scholar] [CrossRef]
- Lindner, J.P. Quantitative Darstellung der Wirkungen Landnutzender Prozesse auf die Biodiversität in Ökobilanzen; Fraunhofer Verlag: Stuttgart, Germany, 2016. [Google Scholar]
- Knudsen, M.T.; Hermansen, J.E.; Cederberg, C.; Herzog, F.; Vale, J.; Jeanneret, P.; Sarthou, J.-P.; Friedel, J.K.; Balazs, K.; Fjellstad, W.; et al. Characterization factors for land use impacts on biodiversity in life cycle assessment based on direct measures of plant species richness in European farmland in the ‘Temperate Broadleaf and Mixed Forest’ biome. Sci. Total Environ. 2017, 580, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Brentrup, F.; Küsters, J.; Lammel, J.; Kuhlmann, H. Life Cycle Impact assessment of land use based on the hemeroby concept. Int. J. Life Cycle Assess. 2002, 7, 339. [Google Scholar] [CrossRef]
- Koellner, T.; Scholz, R.W. Assessment of Land Use Impacts on the Natural Environment. Part 1: An Analytical Framework for Pure Land Occupation and Land Use Change (8 pp). Int. J. Life Cycle Assess. 2007, 12, 16–23. [Google Scholar] [CrossRef]
- De Schryver, A.M.; Goedkoop, M.J.; Leuven, R.S.E.W.; Huijbregts, M.A.J. Uncertainties in the application of the species area relationship for characterisation factors of land occupation in life cycle assessment. Int J. Life Cycle Assess. 2010, 15, 682–691. [Google Scholar] [CrossRef]
- Milà i Canals, L.; Bauer, C.; Depestele, J.; Dubreuil, A.; Freiermuth Knuchel, R.; Gaillard, G.; Michelsen, O.; Müller-Wenk, R.; Rydgren, B. Key Elements in a Framework for Land Use Impact Assessment Within LCA. Int. J. Life Cycle Assess. 2007, 12, 5–15. [Google Scholar] [CrossRef]
- Turner, W.R.; Brandon, K.; Brooks, T.M.; Costanza, R.; da Fonseca, G.A.B.; Portela, R. Global Conservation of Biodiversity and Ecosystem Services. BioScience 2007, 57, 868–873. [Google Scholar] [CrossRef]
- Dobrovolski, R.; Loyola, R.D.; Guilhaumon, F.; Gouveia, S.F.; Diniz-Filho, J.A.F. Global agricultural expansion and carnivore conservation biogeography. Biol. Conserv. 2013, 165, 162–170. [Google Scholar] [CrossRef]
- Dobrovolski, R.; Diniz-Filho, J.A.F.; Loyola, R.D.; de Marco Júnior, P. Agricultural expansion and the fate of global conservation priorities. Biodivers. Conserv. 2011, 20, 2445–2459. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Mittermeier, C.G. Megadiversity: Earth’s Biologically Wealthiest Nations, 1st ed.; CEMEX: San Pedro Garza García, Mexico, 1997. [Google Scholar]
- Alliance for Zero Extinction. 2018 Global AZE Map. Available online: http://zeroextinction.org/site-identification/2018-global-aze-map/ (accessed on 20 March 2019).
- Myers, N. Threatened Biotas: “Hot Spots” in Tropical Forests. Environmentalist 1988, 8, 187–208. Available online: https://pdfs.semanticscholar.org/9e44/07cd1b3982fe929eaeaca7d2eb640bcb0413.pdf (accessed on 17 August 2017). [CrossRef]
- BirdLife International. World Database of Key Biodiversity Areas. Available online: http://www.keybiodiversityareas.org/home (accessed on 20 March 2019).
- Gumbs, R.; Gray, C.L.; Wearn, O.R.; Owen, N.R. Tetrapods on the EDGE: Overcoming data limitations to identify phylogenetic conservation priorities. PLoS ONE 2018, 13, e0194680. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Abell, R.; Allnutt, T.; Carpenter, C.; McClenachan, L.; D’Amico, J.; Hurley, P.; Kassem, K.; Strand, H.; et al. The Global 200: A Representation Approach to Conserving the Earth’s Distinctive Ecoregions; Conservation Science Program; World Wildlife Fund-US: Washington, DC, USA, 2000. [Google Scholar]
- Langhammer, P.F.; Butchart, S.H.M.; Brooks, T.M. Key Biodiversity Areas. In Encyclopedia of the Anthropocene; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–345. [Google Scholar]
- UNEP-WCMC; IUCN. Protected Planet: The World Database on Protected Areas (WDPA) [Jan 2019]. 2019. Available online: https://www.protectedplanet.net/c/terms-and-conditions (accessed on 30 January 2019).
- Jenkins, C.N.; Pimm, S.L.; Joppa, L.N. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, E2602–E2610. [Google Scholar] [CrossRef]
- Hurtt, G.C.; Chini, L.P.; Frolking, S.; Betts, R.A.; Feddema, J.; Fischer, G.; Fisk, J.P.; Hibbard, K.; Houghton, R.A.; Janetos, A.; et al. Harmonization of land-use scenarios for the period 1500–2100: 600 years of global gridded annual land-use transitions, wood harvest, and resulting secondary lands. Clim. Chang. 2011, 109, 117–161. [Google Scholar] [CrossRef]
- Kehoe, L.; Romero-Muñoz, A.; Polaina, E.; Estes, L.; Kuemmerle, T. Biodiversity at risk under future cropland expansion and intensification. Nat. Ecol. Evol. 2017, 8, 1129–1135. [Google Scholar] [CrossRef]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.; Güneralp, B.; Hutyra, L.R. Global Grid of Probabilities of Urban Expansion to 2030; NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2015. [Google Scholar]
- IBAT. Integrated Biodiversity Assessment Tool (IBAT)–Fact Sheet; IUCN: Washington, WA, USA, 2015. [Google Scholar]
- Koellner, T.; de Baan, L.; Beck, T.; Brandão, M.; Civit, B.; Margni, M.; i Canals, L.M.; Saad, R.; de Souza, D.M.; Müller-Wenk, R. UNEP-SETAC guideline on global land use impact assessment on biodiversity and ecosystem services in LCA. Int. J. Life Cycle Assess. 2013, 18, 1188–1202. [Google Scholar] [CrossRef]
- Kowarik, I.; Naturnähe, N. Hemerobie als Bewertungskriterien. In Handbuch Naturschutz und Lanschaftspflege: Grundlagen und Anwendungen der Ökosystemforschung; Konold, W., Böcker, R., Harapicke, U., Eds.; Ecomed: Landsberg, Germany, 1999; pp. 1–18. [Google Scholar]
- Hudson, L.N.; Newbold, T.; Contu, S.; Hill, S.L.L.; Lysenko, I.; De Palma, A.; Phillips, H.R.P.; Alhusseini, T.I.; Bedford, F.E.; Bennett, D.J.; et al. The database of the PREDICTS (Projecting Responses of Ecological Diversity in Changing Terrestrial Systems) project. Ecol. Evol. 2017, 7, 145–188. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.N.; Newbold, T.; Contu, S.; Hill, S.L.L.; Lysenko, I.; De Palma, A.; Phillips, H.R.P.; Alhusseini, T.I.; Bedford, F.E.; Bennett, D.J.; et al. The 2016 Release of the PREDICTS Database. Available online: https://doi.org/10.5519/0066354 (accessed on 20 March 2019).
- Hudson, L.N.; Newbold, T.; Contu, S.; Hill, S.L.L.; Lysenko, I.; De Palma, A.; Phillips, H.R.P.; Senior, R.A.; Bennett, D.J.; Booth, H.; et al. The PREDICTS database: A global database of how local terrestrial biodiversity responds to human impacts. Ecol. Evol. 2014, 4, 4701–4735. [Google Scholar] [CrossRef] [PubMed]
- Purvis, A.; Newbold, T.; de Palma, A.; Contu, S.; Hill, S.L.L.; Sanchez-Ortiz, K.; Phillips, H.R.P.; Hudson, L.N.; Lysenko, I.; Börger, L.; et al. Modelling and Projecting the Response of Local Terrestrial Biodiversity Worldwide to Land Use and Related Pressures: The PREDICTS Project. In Next Generation Biomonitoring; Bohan, D.A., Dumbrell, A.J., Woodward, G., Jackson, M., Eds.; AP Academic Press an imprint of Elsevier: Oxford, UK; London, UK; Cambridge, MA, USA; San Diego, CA, USA, 2018; Volume 58, pp. 201–241. [Google Scholar]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Gray, C.L.; Scharlemann, J.P.W.; Börger, L.; Phillips, H.R.P.; Sheil, D.; Lysenko, I.; et al. Global patterns of terrestrial assemblage turnover within and among land uses. Ecography 2016, 39, 1151–1163. [Google Scholar] [CrossRef]
- Scholes, R.J.; Biggs, R. A biodiversity intactness index. Nature 2005, 434, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.S.; Pereira, H.M. Improving extinction projections across scales and habitats using the countryside species-area relationship. Sci. Rep. 2017, 7, 25. [Google Scholar] [CrossRef]
- Scherr, S.J.; McNeely, J.A. Biodiversity conservation and agricultural sustainability: Towards a new paradigm of ‘ecoagriculture’ landscapes. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 477–494. [Google Scholar] [CrossRef]
- Herzog, F.; Steiner, B.; Bailey, D.; Baudry, J.; Billeter, R.; Bukácek, R.; De Blust, G.; De Cock, R.; Dirksen, J.; Dormann, C.F.; et al. Assessing the intensity of temperate European agriculture at the landscape scale. Eur. J. Agron. 2006, 24, 165–181. [Google Scholar] [CrossRef]
- Blüthgen, N.; Dormann, C.F.; Prati, D.; Klaus, V.H.; Kleinebecker, T.; Hölzel, N.; Alt, F.; Boch, S.; Gockel, S.; Hemp, A.; et al. A quantitative index of land-use intensity in grasslands: Integrating mowing, grazing and fertilization. Basic Appl. Ecol. 2012, 13, 207–220. [Google Scholar] [CrossRef]
- Kuemmerle, T.; Erb, K.; Meyfroidt, P.; Müller, D.; Verburg, P.H.; Estel, S.; Haberl, H.; Hostert, P.; Jepsen, M.R.; Kastner, T.; et al. Challenges and opportunities in mapping land use intensity globally. Curr. Opin. Environ. Sustain. 2013, 5, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Erb, K.-H.; Haberl, H.; Jepsen, M.R.; Kuemmerle, T.; Lindner, M.; Müller, D.; Verburg, P.H.; Reenberg, A. A conceptual framework for analysing and measuring land-use intensity. Curr. Opin. Environ. Sustain. 2013, 5, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Ramankutty, N.; Hertel, T.; Lee, H.-L.; Rose, S.K. Global spatial data of 18 Agro-ecological Zones (AEZs); Global Agricultural Land Use Data for Integrated Assessment Modeling. In Human-Induced Climate Change: An Interdisciplinary Assessment; Department of Agricultural Economics, Purdue University: West Lafayette, IN, USA, 2007. [Google Scholar]
- Ruiz-Martinez, I.; Marraccini, E.; Debolini, M.; Bonari, E. Indicators of agricultural intensity and intensification: A review of the literature. Ital. J. Agron. 2015, 10, 74. [Google Scholar] [CrossRef]
- Joppa, L.N.; O’Connor, B.; Visconti, P.; Smith, C.; Geldmann, J.; Hoffmann, M.; Watson, J.E.M.; Butchart, S.H.M.; Virah-Sawmy, M.; Halpern, B.S.; et al. Big Data and Biodiversity. Filling in biodiversity threat gaps. Science (New York, NY) 2016, 352, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Puletti, N.; Giannetti, F.; Chirici, G.; Canullo, R. Deadwood distribution in European forests. J. MAPS 2017, 13, 733–736. [Google Scholar] [CrossRef]
- Potter, P.; Ramankutty, N.; Bennett, E.M.; Donner, S.D. Global Fertilizer and Manure, Version 1: Nitrogen Fertilizer Application; NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2011. [Google Scholar]
- Potter, P.; Ramankutty, N.; Bennett, E.M.; Donner, S.D. Characterizing the Spatial Patterns of Global Fertilizer Application and Manure Production. Earth Interact. 2010, 14, 1–22. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data (accessed on 16 January 2019).
- Brown de Colstoun, E.C.; Huang, C.; Wang, P.; Tilton, J.C.; Tan, B.; Phillips, J.; Niemczura, S.; Ling, P.-Y.; Wolfe, R.E. Global Man-Made Impervious Surface (GMIS) Dataset from Landsat; NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2017. [Google Scholar]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef] [PubMed]
- Rugani, B.; Rocchini, D. Positioning of remotely sensed spectral heterogeneity in the framework of life cycle impact assessment on biodiversity. Ecol. Indic. 2016, 61, 923–927. [Google Scholar] [CrossRef]
- Bruel, A.; Troussier, N.; Guillaume, B.; Sirina, N. Considering Ecosystem Services in Life Cycle Assessment to Evaluate Environmental Externalities. Procedia CIRP 2016, 48, 382–387. [Google Scholar] [CrossRef]
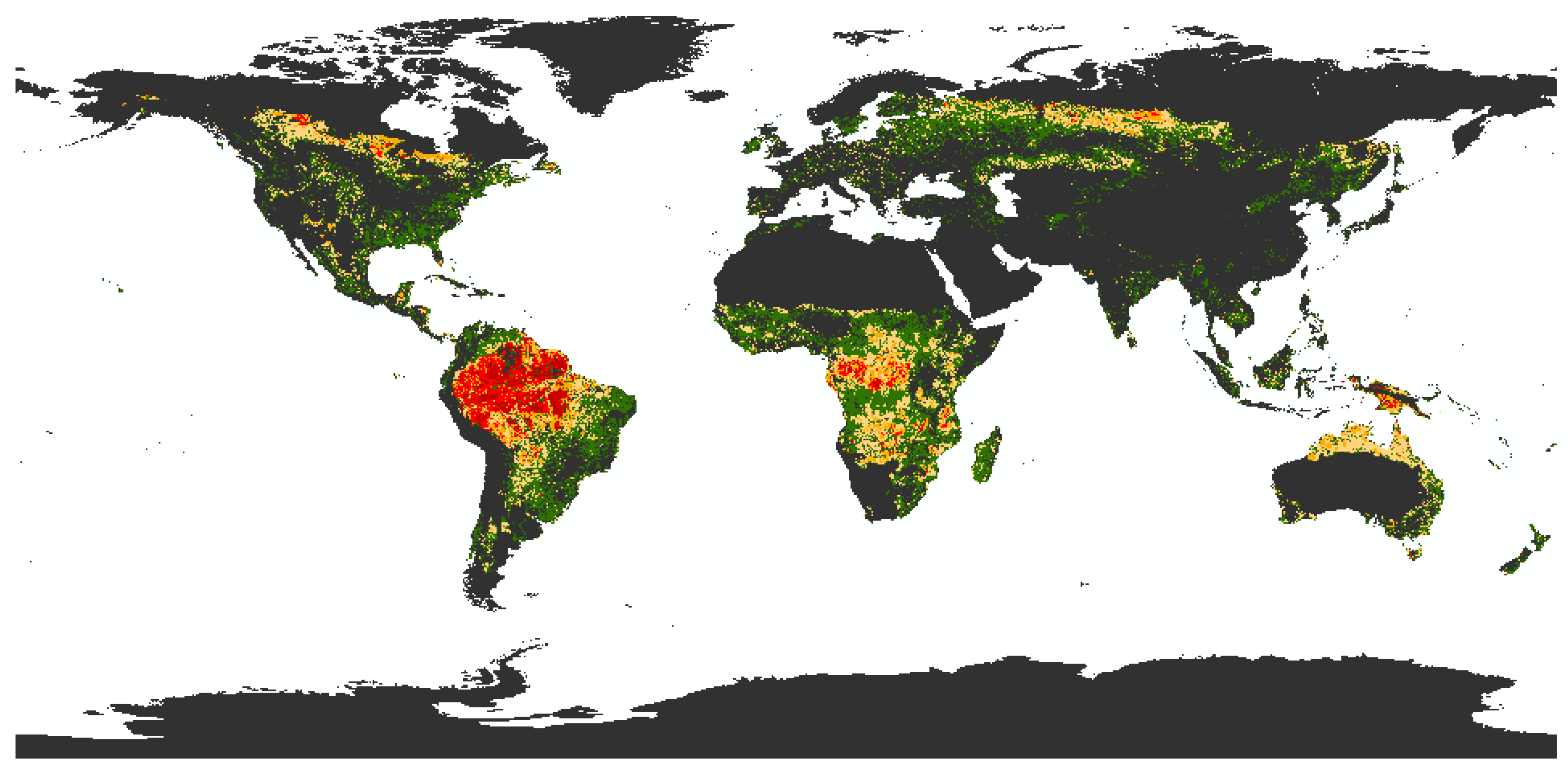
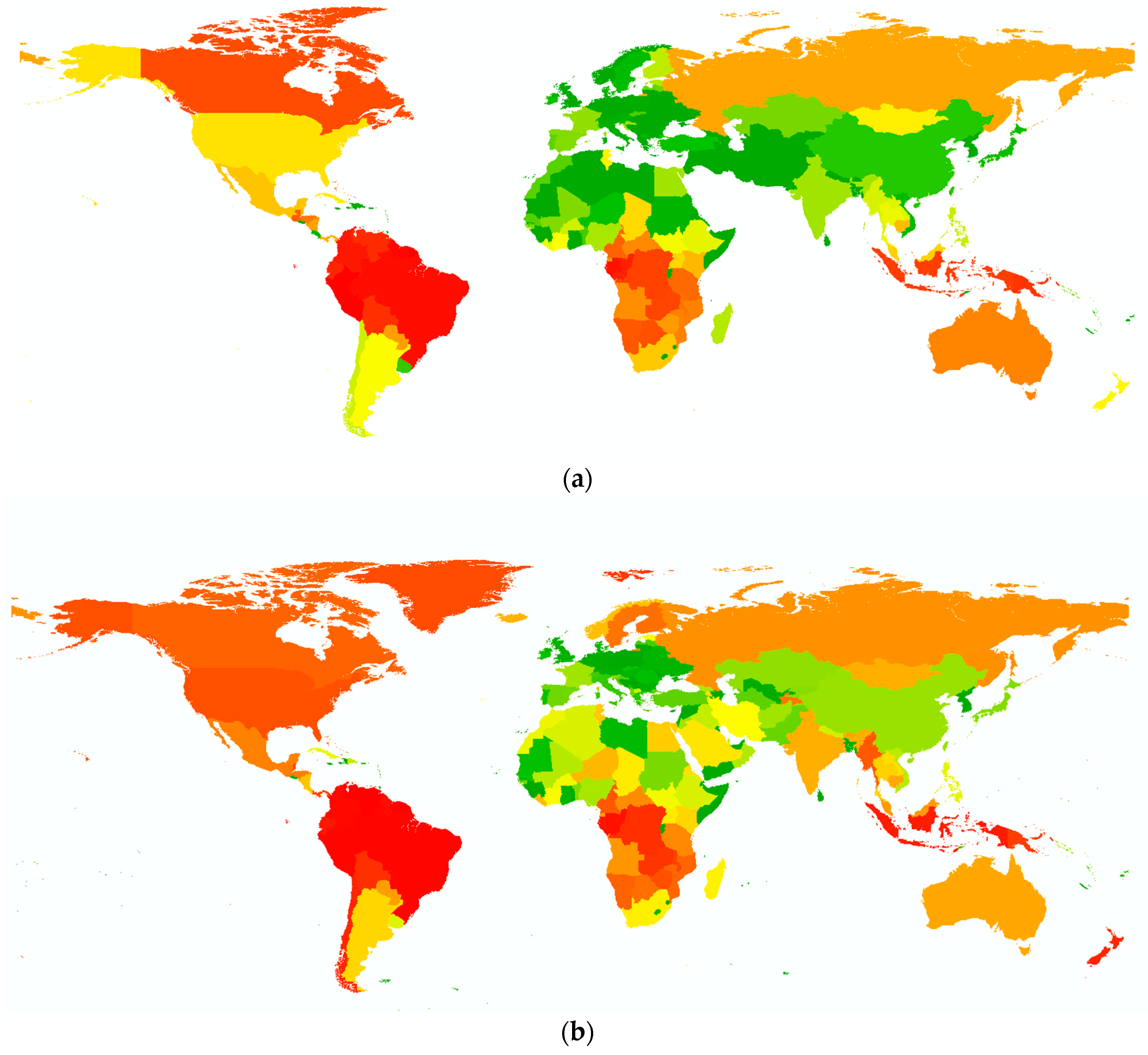
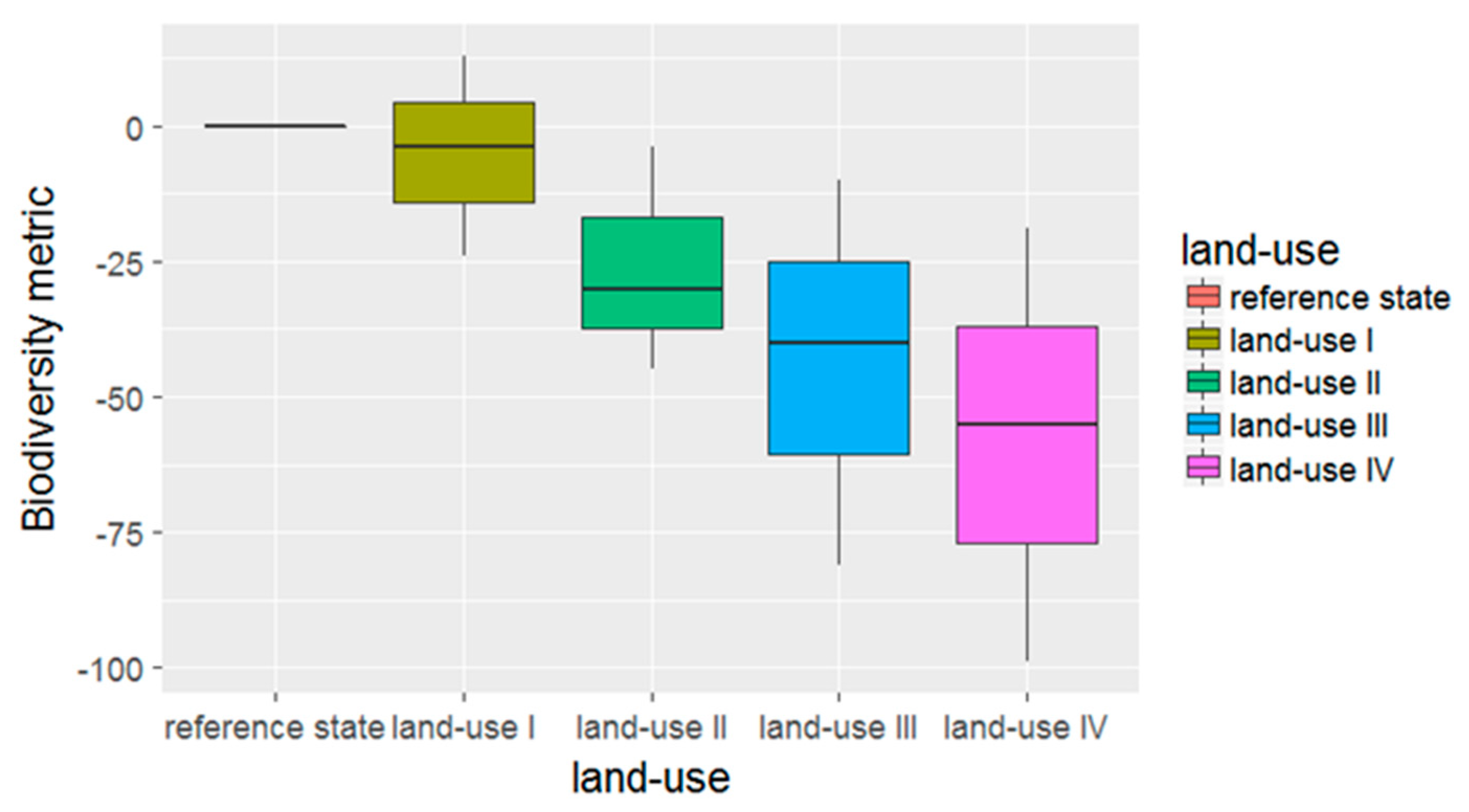
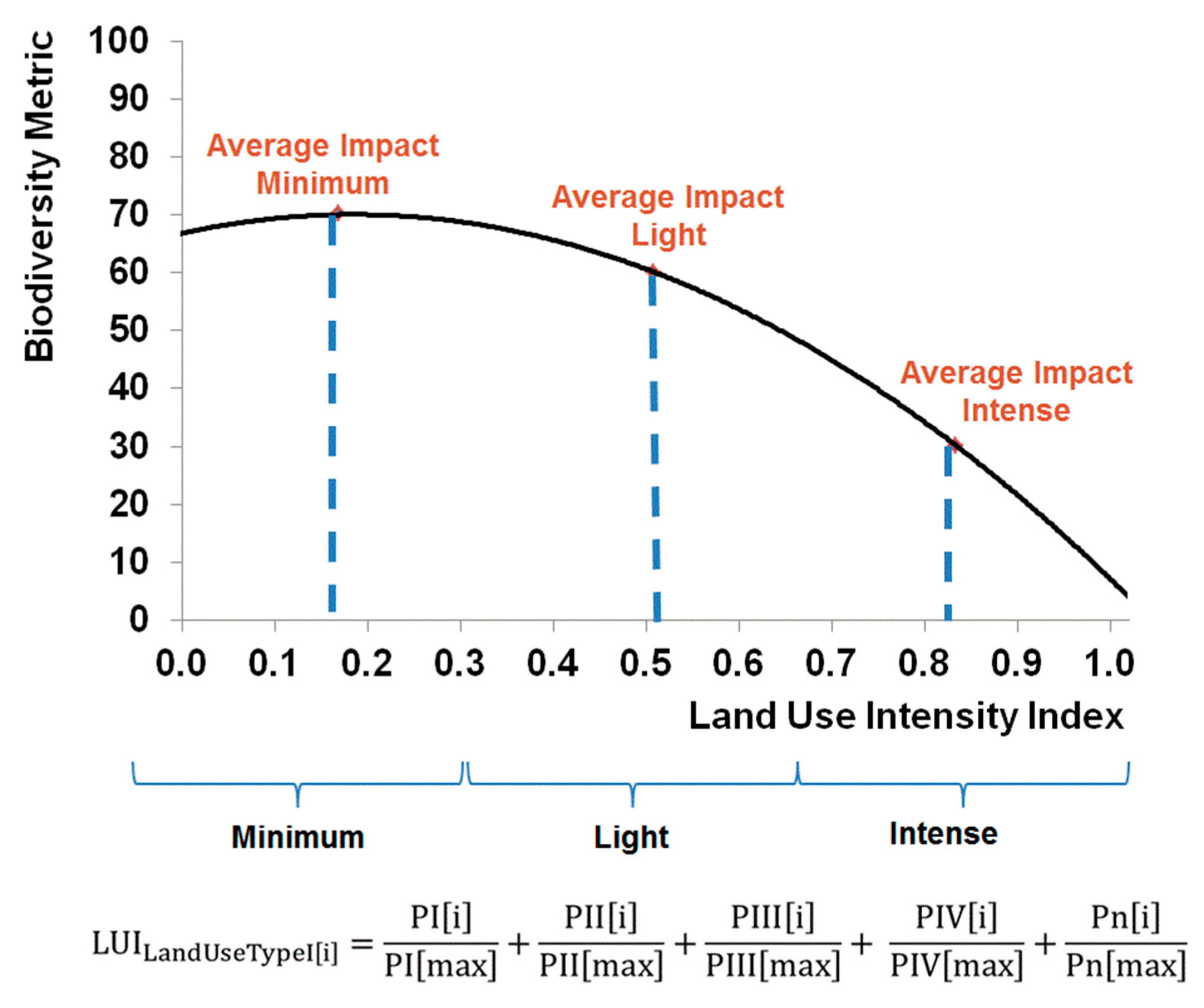
| Reactive | Proactive | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Regionalization/Location | Land Use Type | Irreplaceability (i) Vulnerability (v) | Valuation | Biodiversity-Level | Number of Endemic Species | Taxonomic Uniqueness | Unusual Phenomena | Rare Habitats, Ecosystems | Number of Species (Richness) | Threat Level | Habitat Loss, (Degradation High) | Intact Habitat/Ecosystem |
| Irreplaceability | Vulnerability | ||||||||||||
| [87] | x | i | Richness | Species | x | ||||||||
| [88] | x | x | i | Biomass production | Species | x | |||||||
| [94] | x | v | Hemeroby | Ecosystem | x | ||||||||
| [89] | x | i | Richness, rare ecosystems | Species, Ecosystems | x | x | |||||||
| [66,95] | x | v, i | Threatened species, habitat loss (SARs) | Species | x | x | x | ||||||
| [79] | mining | v, i | Richness, threat, endemism, rare biotopes | Species, Ecosystems | x | x | x | x | |||||
| [80] | x grassland, cropland | v, i | Richness, threat | Species | x | x | |||||||
| [67] | x | forestry | v, i | Ecosystem scarcity, vulnerability | Ecosystem | x | x | ||||||
| [68] | x | x | v, i | Habitat loss (SAR), ecosystem vulnerability | Species, Ecosystems | x | x | ||||||
| [69] | x | v | Ecosystem quality, threatened species | Species, Ecosystems | x | x | |||||||
| [30] | x | v, i | Species traits, richness | Species (functional diversity) | x | x | |||||||
| [70] | croplands | v, i | Rare and vulnerable areas | Ecosystem | x | x | |||||||
| [71] | x | x | v | Richness | Species | x | |||||||
| [96] | x | v, i | Habitat loss (SAR) | Species | x | x | |||||||
| [72] | x | three land use types | v, i | Habitat loss, extinction (SAR) | Species | x | |||||||
| [90] | x | v | Richness, indicator species | Species | x | ||||||||
| [73] | x | cropland | v, i | Scarcities, vulnerability, hemeroby | Ecosystems | x | x | x | |||||
| [91] | x | v | Richness | Species | x | ||||||||
| [74] | x | x | v, i | Habitat loss (SAR), endemism, threat | Species | x | x | x | |||||
| [92] | x | cropland | v, i | Rarity rated richness | Species, Ecosystems | x | x | ||||||
| [75] | x | v, i | Habitat, threat level, rarity | Species | x | x | x | ||||||
| [93] | x | x | v | Richness | Species | x | |||||||
| [38] | x | v | Habitat loss (cSARs), endemism | Species | x | x | x | ||||||
| [76] | x | x | v, i | PD, Habitat loss (cSARs) | Species, Phylogeny | x | x | x | |||||
| [81] | forestry | v, i | Species richness, ecoregion scarcity, endemism, threat | Species, Ecosystems | x | x | x | x | |||||
| [78] | x | v | Hemeroby | Ecosystems | x | ||||||||
| Reference/Author | Name | Creation Date | Biodiversity Level | Taxa | Vulnerability | Irreplaceability |
|---|---|---|---|---|---|---|
| [44,59,103] | Biodiversity Hotspot (BH) | 2016 (1999) | Ecosystem, species | Vascular plants | High | High |
| [104,107] | Key Biodiversity Areas (KBA) | 2014 | Species, ecosystems | Birds, mammals, reptiles, amphibians, vascular plants, conifer, algae, fungi, lichens, liverworts, mosses, etc. | High | High |
| [46] | Crisis Ecoregions (CE) | 2005 | Ecosystem | No focus | High | High |
| [30,42,83,86] | High Biodiversity Wilderness Areas (HBWA) | 2002 | Ecosystem | Vascular plants, vertebrates | Low | High |
| [48] | Last of the Wild (LtW) | 2002 | Ecosystem | No focus | Low | - |
| [47,85,86] | Intact Forest Landscapes (IFL) | 2013 (1997) | Ecosystem | No focus | Low | - |
| [104] | Important Bird Areas (IBA) | 2014 (1980) | Ecosystem, species | Birds | High | High |
| [104] | Important Plant Areas (IPA) | 1995 | Species, Ecosystem | Vascular plants, algae, fungi, lichens, liverworts, mosses | High | High |
| [45] | Global 200 Ecoregions | 1998 | Ecosystem, species | No focus | - | High |
| [52,104] | Endemic Bird Areas (EBA) | 1998 | Ecosystem, species | Birds | - | High |
| [51] | Centers of Plant Diversity (CPD) | 2013 (data 1994–1997) | Species, Genes | Vascular plants | - | High |
| [102] | Alliance for Zero Extinction (AZE) | 2005 | Species | Birds, mammals, reptiles, amphibians, conifers | High | High |
| [50] | Evolutionarily Distinct and Globally Endangered (EDGE) | 2013 | Species, Genes | Mammals, amphibians | High | High |
| [108] | Protected areas | 2018 | Ecosystem | No focus | Low | - |
| [109] | Threatened Species | 2013 | Species | Mammals, amphibians, birds | High | - |
| [109] | Species richness, endemic species | 2013 | Species | Mammals, amphibians, birds | - | High |
| Land Use Flows | Management Parameter | ||||||
|---|---|---|---|---|---|---|---|
| Land Use Type [22,117,118,119] | Sub Types [110] | Land Use Intensity (LUI) [22,117,118,119] | Land Use Intensity (LUI) Index | Management Parameter for LUI | Data type | Indicator [Unit] | Data Source |
| Primary vegetation | Forested/Non forested | Minimal (0.0–0.33) Light (0.34–0.66) Intense (0.67–>1.0) | 0.0–>1.0 | Tree age | global maps, primary data | years | primary data |
| Wood harvesting rates | global maps, primary data | units kg C | [110], primary data | ||||
| Dead Wood volume | regional maps (Europe), primary data | Average deadwood volume [m3 ha−1] | [133] primary data | ||||
| Fire frequency | global/regional maps, primary data | fire density per km2 | MODIS, primary data | ||||
| Biomass density | global maps, primary data | kg C/m2 | [110], primary data | ||||
| Set aside areas/buffer zones | primary data, satellite images | Ratio Field size/buffer zone size [%] | Satellite images, primary data | ||||
| Secondary vegetation | Forested/Non forested | Minimal (0.0–0.33) Light (0.34–0.66) Intense (0.67–>1.0) | 0.0–>1.0 | Mean age/tree age | global maps, primary data | years | [110], primary data |
| Wood harvesting rates | global maps, primary data | units kg C | [110], primary data | ||||
| Dead Wood volume | maps (Europe), primary data | Average deadwood volume (m3 ha−1) | [133] | ||||
| Fire frequency | global/regional maps, primary data | fire density per km2 | MODIS, primary data | ||||
| Set aside areas/buffer zones | primary data, satellite images | Ratio Field size/buffer zone size [%] | Satellite images, primary data | ||||
| Biomass density | global maps, primary data | kg C/m2 | [110] | ||||
| Native vegetation | global maps, primary data | [%] native vegetation per land use type | [110] | ||||
| Cropland | C3 annual/C3 perennial/C4 annual/C4 perennial/C3 nitrogen fixing | Minimal (0.0–0.33) Light (0.34–0.66) Intense (0.67–>1.0) | 0.0–>1.0 | Fertilizer | global maps, primary data | kg nitrogen ha−1·year−1 | [134,135] |
| Irrigated/flooded | Global maps, primary data | Percentage/grid cell | [136] | ||||
| Pesticide | Global maps, FAO statistics, primary data | tons of active ingredients | [136] | ||||
| Mechanization (tillage) | FAO statistics, primary data | hectare per tractor | [136] | ||||
| Set aside areas | primary data, satellite images | Ratio Field size/buffer zone size [%] | Satellite images, primary data | ||||
| Mixed cropping | Global maps, primary data | C3/C4 and nitrogen fixing plants per grid cell [%] | [110] | ||||
| Native vegetation | global maps, primary data | [%] native vegetation per land use type | [110] | ||||
| Pasture | Managed pasture/Rangeland pasture | Minimal (0.0–0.33) Light (0.34–0.66) Intense (0.67–>1.0) | 0.0–>1.0 | Livestock density | global maps primary data | livestock units ha−1·year−1 | [136] |
| livestock manure | FAO statistics, global maps primary data | kg/ha | [134,135,136] | ||||
| Pesticides | global maps | ||||||
| Mowing frequency | primary data, statistics | times per year | |||||
| Set aside areas/buffer zones | primary data, satellite images | Ratio Field size/buffer zone size [%] | Satellite images, primary data | ||||
| Native vegetation | global maps primary data | [%] native vegetation per land use type | [110] | ||||
| Plantation | Minimal (0.0–0.33) Light (0.34–0.66) Intense (0.67–>1.0) | 0.0–>1.0 | Mixed cropping/Tree diversity | Global maps primary data | [%] | [110] | |
| Fertilizer | global maps primary data, Fertilizer perennial plants | kg nitrogen ha−1·year−1 | [110,134,135] | ||||
| Pesticide | Global maps, FAO statistics primary data | tons of active ingredients | [136] | ||||
| Irrigation | Global maps primary data | [%] Percentage/grid cell | [110,136] | ||||
| Mechanization | FAO statistics, primary data | hectare per tractor | [136] | ||||
| Harvesting rates | FAO statistics, primary data | ||||||
| Native vegetation | global maps primary data | [%] native vegetation per land use type | [110] | ||||
| Set aside areas | primary data, satellite images | Ratio Field size/buffer zone size [%] | Satellite images, primary data | ||||
| Urban | Minimal (0.0–0.33) Light (0.34–0.66) Intense (0.67–>1.0) | 0.0–>1.0 | Green spaces | satellite images, primary data | [%] green space per urban area | Sentinel data, NDVI maps https://modis.gsfc.nasa.gov/data/dataprod/mod13.php | |
| Degree of sealing | global maps primary data | [%] imperviousness | [137] | ||||
| Native vegetation | global maps primary data | [%] native vegetation per land use type | [110] | ||||
| Light pollution | global maps, statistics primary data | artificial sky brightness [mcd/m2] | [138] | ||||
| Set aside areas | primary data, satellite images | Ratio Field size/buffer zone size [%] | Satellite images, primary data | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, S.D.; Lindner, J.P.; Francisco, J. Conceptual Framework for Biodiversity Assessments in Global Value Chains. Sustainability 2019, 11, 1841. https://doi.org/10.3390/su11071841
Maier SD, Lindner JP, Francisco J. Conceptual Framework for Biodiversity Assessments in Global Value Chains. Sustainability. 2019; 11(7):1841. https://doi.org/10.3390/su11071841
Chicago/Turabian StyleMaier, Stephanie D., Jan Paul Lindner, and Javier Francisco. 2019. "Conceptual Framework for Biodiversity Assessments in Global Value Chains" Sustainability 11, no. 7: 1841. https://doi.org/10.3390/su11071841
APA StyleMaier, S. D., Lindner, J. P., & Francisco, J. (2019). Conceptual Framework for Biodiversity Assessments in Global Value Chains. Sustainability, 11(7), 1841. https://doi.org/10.3390/su11071841