Towards Sustainable Agriculture—Agronomic and Economic Effects of Biostimulant Use in Common Bean Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Plant Yielding and Nutritional Value Determination
2.2.1. Plant Yielding
2.2.2. Protein Content
2.3. Nutraceutical Potential
2.3.1. Extract Preparation
2.3.2. Phenolics Content and Antioxidant Capacity Determination
2.4. Economic Analysis
- Wpp—value of yield increase, (EUR·ha−1),
- Ksb—costs of biostimulant use, (EUR·ha−1),
- Pnb—seed yield from the combination with biostimulant application, (t·ha−1),
- Pnk—seed yield from the control combination, (t·ha−1),
- Cn—average price of seeds in a given study year, (EUR·t−1).
- kb—cost of biostimulant purchase, (EUR·ha−1),
- kw—cost of water used for the treatment, (EUR·ha−1),
- kz—cost of performing the treatment, (EUR·ha−1).
2.5. Statistical Analysis
3. Results
3.1. Effect of Biostimulants on Biometric Traits
3.2. Effect of Biostimulant Treatment on Nutraceutical Quality
3.3. Effect of Biostimulant Treatment on the Antioxidant Potential
3.4. Effect of Biostimulant Treatment on Fiber Content
3.5. Economic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Towards the Future We Want. End Hunger and Make the Transition to Sustainable Agricultural and Food Systems. Available online: http://www.fao.org/3/an894e/an894e00.pdf (accessed on 19 July 2019).
- Carvalho, S.; Vasconcelos, M.W. Producing more with less: Strategies and novel technologies for plant-based food biofortification. Food Res. Int. 2013, 54, 961–971. [Google Scholar] [CrossRef]
- IFPRI. Resilience for Food and Nutrition Security; Fan, S., Pandya-Lorch, R., Yosef, S., Eds.; Intl Food Policy Res Inst: Washington, DC, USA, 2014. [Google Scholar]
- Gregory, P.J.; George, T.S. Feeding nine billion: The challenge to sustainable crop production. J. Exp. Bot. 2011, 62, 5233–5239. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A.; Cominelli, E.; Galbiati, M.; Tonelli, C. The future of science: Food and water for life. Plant Cell 2009, 21, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Dymkowska-Malesa, M.; Szparaga, A.; Czerwińska, E. Evaluation of polychlorinated biphenyls content in chosen vegetablesfrom Warmia and Mazury region. Rocz. Ochr. Sr. 2014, 16, 290–299. [Google Scholar]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, M. Worldwide research on plant defense against biotic stresses as improvement for sustainable agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef]
- Rolnictwo zrównoważone sposobem na produkcję bezpiecznej żywności i ochronę środowiska naturalnego. Available online: https://www.cdr.gov.pl/aktualnosci/57-cdr-informuje/2459-rolnictwo-zrownowazone-sposobem-na-produkcje-bezpiecznej-zywnosci-i-ochrone-srodowiska-naturalnego (accessed on 19 July 2019).
- Manzano-Agugliaro, F.; Cañero, R. Economics and environmental analysis of Mediterranean greenhouse crops. Afr. J. Agric. Res. 2010, 5, 3009–3016. [Google Scholar]
- Nuijten, E.; Messmer, M.M.; Lammerts van Bueren, E.T. Concepts and strategies of organic plant breeding in light of novel breeding techniques. Sustainability 2016, 9, 18. [Google Scholar] [CrossRef]
- Gázquez, J.A.; Castellano, N.N.; Manzano-Agugliaro, F. Intelligent low cost telecontrol system for agricultural vehicles in harmful environments. J. Clean. Prod. 2016, 113, 204–215. [Google Scholar] [CrossRef]
- Zapata-Sierra, A.J.; Manzano-Agugliaro, F. Controlled deficit irrigation for orange trees in Mediterranean countries. J. Clean. Prod. 2017, 162, 130–140. [Google Scholar] [CrossRef]
- Monaco, F.; Zasada, I.; Wascher, D.; Glavan, M.; Pintar, M.; Schmutz, U.; Sali, G. Food production and consumption: City regions between localism, agricultural land displacement, and economic competitiveness. Sustainability 2017, 9, 96. [Google Scholar] [CrossRef]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Niccolai, A.; Centritto, M.; Ferrini, F.; Mattii, G.B. Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol. Biochem. 2019, 139, 21–32. [Google Scholar] [CrossRef] [PubMed]
- European Biostimulant Industry Council (EBIC). Available online: http://www.biostimulants.eu/ (accessed on 19 July 2019).
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hamauzu, Y. Phenolic compounds, ascorbic acid, carotenoides and antioxidant properties of green, red and yellow bell peppers. J. Food Agr. Environ. 2003, 1, 22–27. [Google Scholar]
- Ertani, A.; Pizzeghello, D.; Baglieri, A.; Cadil, V.; Tambone, F.; Gennari, M.; Nardi, S. Humic-like substances from agro-industrial residues affect growth and nitrogen assimilation in maize (Zea mays L.) plantlets. J. Geochem. Explor. 2013, 129, 103–111. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wójtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of growth, yield, and the nutraceutical and antioxidative potential of soybean through the use of synthetic biostimulants. Front. Plant Sci. 2018, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Caradonia, F.; Battaglia, V.; ·Righi, L.; Pascali, G.; La Torre, A. Plant biostimulant regulatory framework: Prospects in europe and current situation at international level. J. Plant Growth Regul. 2018, 38, 438–448. [Google Scholar] [CrossRef]
- Traon, D.; Amat, L.; Zotz, F.; du Jardin, P. A Legal Framework for Plant Biostimulants and Agronomic Fertiliser Additives in the EU; European Comission: Brussels, Belgium, 2014. [Google Scholar]
- Chojnacka, K.; Michalak, I.; Dmytryk, A.; Wilk, R.; Górecki, H. Innovative natural plant growth biostimulants. In Advances in Fertilizer Technology; Shishir, S., Pant, K.K., Govil, J.N., Eds.; Studium Press LLC: Houston, TX, USA, 2014; Volume 21, pp. 451–489. [Google Scholar]
- Paradikovic, N.; Vinkovic, T.; Vrcek, I.V.; Zuntar, I.; Bojic, M.; Medic-Saric, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [PubMed]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Depo, K.; Erlichowska, B.; Deszcz, E. Effect of applying a biostimulant containing seaweed and amino acids on the content of fiber fractions in three soybean cultivars. Legume Res. 2019, 42, 341–347. [Google Scholar]
- Michałek, W.; Kocira, A.; Findura, P.; Szparaga, A.; Kocira, S. The Influence of Biostimulant Asahi SL on the Photosynthetic Activity of Selected Cultivars of Phaseolus vulgaris L. Rocz. Ochr. Sr. 2018, 20, 1286–1301. [Google Scholar]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and Biochemical Responses of Glycine max (L.) Merr. to the Use of Seaweed Extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S. Generalized logistic functions in modelling emergence of Brassica napus L. PLoS ONE 2018, 13, e0201980. [Google Scholar] [CrossRef] [PubMed]
- Biesaga-Kościelniak, J.; Kościelniak, J.; Filek, M.; Marcińska, I.; Krekule, J.; Machackova, I.; Kuboń, M. The effect of plant growth regulators and their interaction with electric current on winter wheat development. Acta Physiol. Plant. 2010, 32, 987–995. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Report. Conclusion on the Peer Reviewofsodium Nitroguaiacolate, Sodium o-Nitrophenolate and Sodium p-Nitrophenolate 191, 1–130. 2008. Available online: http://www.efsa.europa.eu/en/efsajournal/doc/191r.pdf (accessed on 19 August 2019).
- Environmental Protection Agency (EPA). Government Registration. 2014. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/--.pdf (accessed on 19 August 2019).
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. The role of nitrophenol on delaying abscission of tomato flowersand fruit. Food Agric. Environ. 2004, 2, 183–186. [Google Scholar]
- Kazda, J.; Herda, G.; Spitzer, T.; Řičařová, V.; Przybysz, A.; Gawrońska, H. Effect of nitrophenolates on pod damage caused by the brassica pod midge on the photosynthetic appa-ratus and yield of winter oilseed rape. J. Pest Sci. 2015, 88, 235–247. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a beneficial element for crop production. Front. Plant Sci. 2017, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Hołubowicz-Kliza, G. Uprawa Fasoli. Instrukcja Upowszechnieniowa Nr 208; IUNG-PIB: Puławy, Poland, 2015. [Google Scholar]
- Caproni, L.; Raggi, L.; Tissi, C.; Howlett, S.; Torricelli, R.; Negri, V. Multi-environment evaluation and genetic characterisation of common bean breeding lines for organic farming systems. Sustainability 2018, 10, 777. [Google Scholar] [CrossRef]
- Pet, E.; Pet, I.; Dragomir, N.; Dragomir, C. Researches concerning the economic efficiency achieved successive to the application of biologically-active products in smooth brome crop. Lucr. Stiin Ńifice Zooteh. Biotehnol. 2008, 41, 347–351. [Google Scholar]
- Kocira, S.; Kocira, A.; Kornas, R.; Koszel, M.; Szmigielski, M.; Krajewska, M.; Szparaga, A.; Krzysiak, Z. Effects of seaweed extract on yield and protein content of two common bean (Phaseolus vulgaris L.) cultivars. Legume Res. 2018, 41, 589–593. [Google Scholar] [CrossRef]
- Redmile-Gordon, M.A.; Armenise, E.; White, R.P.; Hirsch, P.R.; Goulding, K.W.T. A comparison of two colorimetric assays, based upon Lowry and Bradford techniques, to estimate total protein in soil extracts. Soil Biol. Biochem. 2013, 67, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Gawlik-Dziki, U.; Kowalczyk, D.; Złotek, U. Impact of germination time and type of illumination on the antioxidant compounds and antioxidant capacity of lens culinaris sprouts. Sci. Hortic. 2012, 140, 87–95. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lamaison, J.L.C.; Carnet, A. Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D. C) en fonction de la vegetation. Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of diet ary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Alvarez, D.; Giuffrida, F.; Vanrobaeys, F.; Golay, P.A.; Cotting, C.; Lardeau, A.; Keely, B.J. High-throughput methods to assess lipophilic and hydrophilic antioxidant capacity of food extracts in vitro. J Agric. Food Chem. 2008, 56, 3470–3477. [Google Scholar] [CrossRef]
- Sancho, R.A.S.; Pavan, V.; Pastore, G.M. Effect of in vitro digestion on bioactive compounds and antioxidant activity of common bean seed coats. Food Res. Int. 2015, 76, 74–78. [Google Scholar] [CrossRef]
- Protocol: Extraction and Determination of Proline. Available online: https://www.researchgate.net/publication/_PROTOCOL_Extraction_and_determination_of_proline (accessed on 19 July 2019).
- Van-Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Szparaga, A. Wybrane Właściwości Fizyczne, Mechaniczne, Chemiczne i Plon Nasion Fasoli Zwykłej (Phaseolus Vulgaris L.) w Zależności od Metody Aplikacji Biostymulatorów; Polskie Towarzystwo Inżynierii Rolniczej: Kraków, Polska, 2019. [Google Scholar]
- Stutte, C.A.; Clark, T.H. Radiolabeled Studies of Atonik in Cotton Using HPLC. In Arysta LifeScience Report; Altheimer Laboratory, University of Arkansas: Fayetteville, SC, USA, 1990. [Google Scholar]
- Djanaguiraman, M.; Devi, D.D.; Sheeba, J.A.; Bangarusamy, U.; Babu, C. Effect of oxidative stress on abscission of tomato fruits and its regulation by nitrophenols. Trop. Agric. Res. 2004, 16, 25–36. [Google Scholar]
- Djanaguiraman, M.; Sheeba, J.A.; Devi, D.D.; Bangarusamy, U. Effect of Atonik seed treatment on seedling physiology of cotton and tomato. J. Biol. Sci. 2005, 5, 163–169. [Google Scholar]
- Davies, P.J. Plant Hormones and Their Role in Plant Growth and Development; Martinus Nijhoff Publishers: Boston, MA, USA, 1987; p. 681. [Google Scholar] [CrossRef]
- Kurzumi, S.; Maruyama, A.; Fulio, T. Purification on characterisation of ascorbic acid phosphorylating enzyme from Pseudomonas azotocolligans. Agric. Biol. Chem. 1990, 54, 3235–3239. [Google Scholar] [CrossRef]
- Przybysz, A.; Gawronska, H.; Gajc-Wolska, J. Biological mode of action of a nitrophenolates-based biostimulant: Case study. Front. Plant Sci. 2014, 5, 713. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B.; Gruisseau, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants, 2nd ed.; Wiley Blackwell: Oxford, UK, 2015; p. 1051. [Google Scholar]
- Khan, S.; Basra, S.M.A.; Afzal, I.; Wahid, A. Screening of Moringa landraces for leaf extract as biostimulant. Int. J. Agric. Biol. 2017, 19, 999–1006. [Google Scholar] [CrossRef]
- Edreva, A. The importance of non-photosynthetic pigments and cinnamic acid derivatives in photoprotection. Agric. Ecosyst. Environ. 2005, 106, 135–146. [Google Scholar] [CrossRef]
- Tsuda, T.; Kato, Y.; Osawa, T. Mechanism for the peroxynitrite scavenging activity by anthocyanins. FEBS Lett. 2000, 484, 207–210. [Google Scholar] [CrossRef]
- Maurya, V.K.; Kumar, D.; Pathak, C.; Tiwari, B.S. Involvement of Reactive Species of Oxygen and Nitrogen in Triggering Programmed Cell Death in Plants. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; pp. 257–278. [Google Scholar] [CrossRef]
- Olvera-Carrillo, Y.; Luis Reyes, J.; Covarrubias, A.A. Late embryogenesis abundant proteins: Versatile players in the plant adaptation to water limiting environments. Plant Signal. Behav. 2011, 6, 586–589. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlo rophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasisa more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Wang, S.M.; Jing, R.L.; Mao, X.G. Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J. Plant Physiol. 2009, 166, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Lakkineni, K.; Zhang, Z.; Verma, D.P.S. Removal of feedback inhibition of d 1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protectionof plants from osmotic stress. Plant Physiol. 2000, 122, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.E.A.; de Camargo e Castro, P.R.; Gaziola, S.A.; Azevedo, R.A. Is seaweed extract an elicitor compound? Changing proline content in drought-stressed bean plants. Comun. Sci. 2018, 9, 292–297. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Sheeba, J.A.; Devi, D.D.; Bangarusamy, U. Cotton leaf senescence can be delayed by nitrophenolate spray through enhanced antioxidant defence system. J. Agron. Crop Sci. 2009, 195, 213–224. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Patanè, C.; Scordia, D.; Testa, G.; Cosentino, S.L. Physiological screening for drought tolerance in Mediterranean long-storage tomato. Plant Sci. 2016, 249, 25–34. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol Biochem 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Brzóska, F.; Sliwinski, B. Jakość pasz objętościowych w żywieniu przeżuwaczy i metody jej oceny Cz. II. Metody analizy i oceny wartości pokarmowej pasz objętościowych. Wiadomości Zootech. 2011, 4, 57–68. [Google Scholar]
- Silva, R.A.; Santos, J.L.; Oliveira, L.S.; Soares, M.R.S.; dos Santos, S.M.S. Biostimulants on mineral nutrition and fiber quality of cotton crop. Rev. Brasil. Engenharia Agrícol. Ambiental. 2016, 20, 1062–1066. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhao, P.; Han, L.; Jiao, G.; Zheng, Y. Overexpression of a Profilin (GhPFN2) promotes the progression of developmental phases in cotton fibers. Plant Cell Physiol. 2010, 51, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Souffrant, W.B. Effect of dietary fibre on ileal digestibility and endogenous nitrogen losses in the pig. Anim. Feed Sci. Technol. 2001, 90, 93–102. [Google Scholar] [CrossRef]
- Southgate, D.A.T.; Spiller, G.A.; White, M.; McPherson, R. Polysaccharides food additives that contribute to dietary fiber. In Dietary Fiber in Human Nutrition; Spiller, G.A., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 2001; pp. 29–33. [Google Scholar]
- Hikawczuk, T.; Szuba-Trznadel, A.; Wiliczkiewicz, A. Polisacharydy nieskrobiowe w żywieniu kurcząt brojlerów i prosiąt. Przegląd Hod. 2015, 3, 21–22. [Google Scholar]
- Choct, M. Feed non-starch polysaccharides: Chemical structures and nutritional significance. Feed Mill. Int. 1997, 191, 13–27. [Google Scholar]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physiochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Annison, G. The chemistry of dietary fiber. In Dietary Fiber and Beyond-Australian Perspectives; Samman, S., Anisson, G., Eds.; Nutrition Society of Australia Inc.: Perth, Australia, 1993; pp. 1–18. [Google Scholar]
- Knudsen, K.E.B. Carbohydrates and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Chojnacka, K. Innovative bio-products for agriculture. Open Chem. 2015, 13, 932–937. [Google Scholar] [CrossRef]
- Polo, J.; Mata, P. Evaluation of a Biostimulant (Pepton) Based in Enzymatic Hydrolyzed Animal Protein in Comparison to Seaweed Extracts on Root Development, Vegetative Growth, Flowering, and Yield of Gold Cherry Tomatoes Grown under Low Stress Ambient Field Conditions. Front. Plant Sci. 2018, 8, 2261. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Szafranska, K. Biostimulators: A new trend towards solving an old problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar] [CrossRef] [PubMed]
- Zarzecka, K.; Gugała, M.; Głuszczak, B.; Mystkowska, I. Ekonomiczne uzasadnienie stosowania herbicydów i biostymulatorów w uprawie ziemniaków jadalnych. Rocz. Nauk. Stowarzyszenia Rol. Agrobiz. 2018, 20, 169–173. [Google Scholar]
- Santoso, D.; Gunawan, A.; Budiani, A.; Sari, D.A.; Priyono, D. Plant biostimulant to improve crops productivity and planters profit. Earth Environ. Sci. 2018, 183, 12–17. [Google Scholar] [CrossRef]
- Mystkowska, I. Wpływ zróżnicowanej techniki odchwaszczania i stosowania biostymulatorów na efektywność ekonomiczną uprawy ziemniaków jadalnych. Rocz. Nauk. Stowarzyszenia Rol. Agrobiz. 2017, 19, 190–193. [Google Scholar] [CrossRef]
- Gugała, M.; Sikorska, A.; Zarzecka, K.; Krasnodębska, E.; Kapela, K.; Mystkowska, I. Opłacalność stosowania biostymulatorów wzrostu w uprawie rzepaku ozimego. Rocz. Nauk. Stowarzyszenia Rol. Agrobiz. 2017, 19, 92–96. [Google Scholar] [CrossRef][Green Version]
- Anderson, A.B.J.; de Lima, S.F.; Vendruscolo, E.P.; Félix Alvarez, R.D.C.; Merquides Contardi, L. Análise econômica da produção do milho doce cultivado com aplicação de bioestimulante via semente. Rev. Fac. Agron. La Plata 2016, 115, 119–127. [Google Scholar]
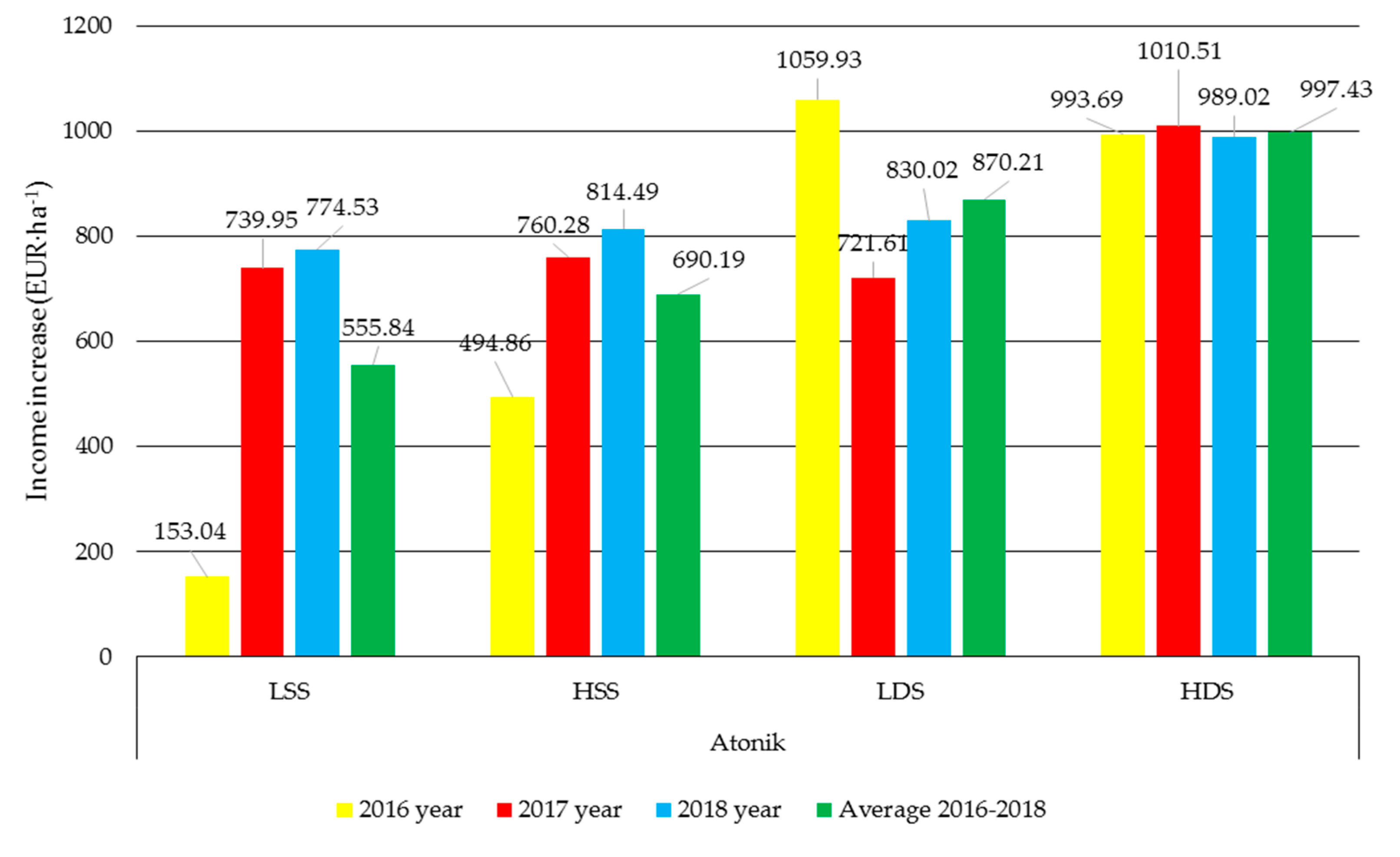
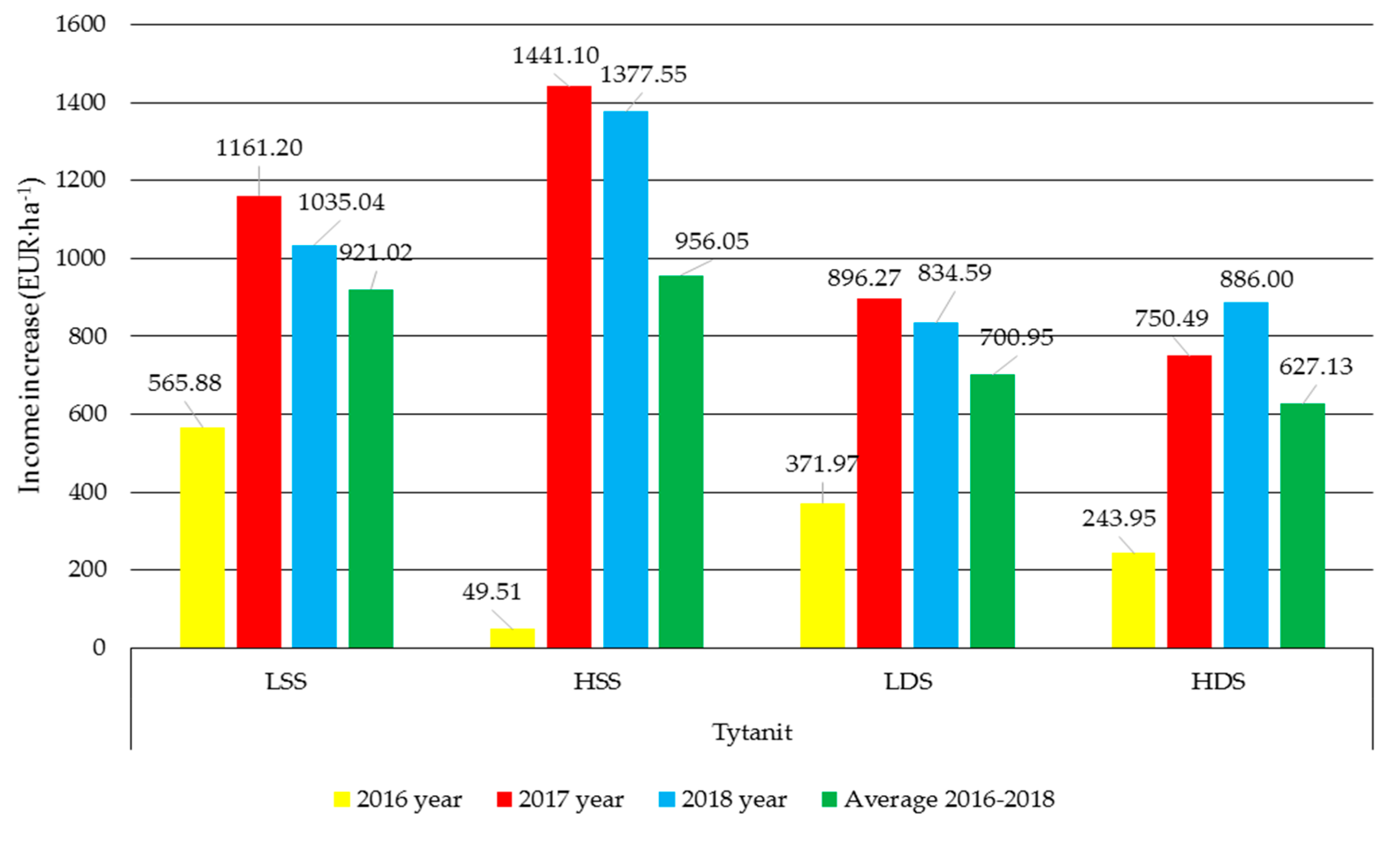
| Biostimulant | Formulation |
|---|---|
| Atonik | sodium p-nitrophenolate NaC6H4NO3 (3.75g/L), sodium o-nitrophenolate NaC6H4NO3 (2.5 g/L), sodium 5-nitroguaiacolate NaC7H6NO4 (1.25g/L); dissolved in water |
| Tytanit | Ti as titanium ascorbate (8.5 g/L); Mg as magnesium sulphate MgSO4 (40.8 g/L); S as magnesium sulphate MgSO4 (54.4 g/L) |
| Biostimulant | Number of Sprays and Plant Developmental Stages (BBCH) in Which the Biostimulants Were Applied | Concentration | Volume of Working Solution/Working Pressure |
|---|---|---|---|
| Atonik | Single spraying: BBCH 13–15 (LSS) | 0.1% | 300 L·ha−1/0.30 MPa |
| Single spraying: BBCH 13–15 (HSS) | 0.2% | ||
| Double spraying: BBCH 13–15, BBCH 61 (LDS) | 0.1% | ||
| Double spraying: BBCH 13–15, BBCH 61 (HDS) | 0.2% | ||
| Tytanit | Single spraying: BBCH 13–15 (LSS) | 0.07% | 300 L·ha−1/0.30 MPa |
| Single spraying: BBCH 13–15 (HSS) | 0.13% | ||
| Double spraying: BBCH 13–15, BBCH 61 (LDS) | 0.07% | ||
| Double spraying: BBCH 13–15, BBCH 61 (HDS) | 0.13% |
| Month | Year | Average from 2002 to 2015 | ||||||
|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | ||||||
| T (°C) Average (min/max) | Rainfall (mm) | T (°C) Average (min/max) | Rainfall (mm) | T (°C) Average (min/max) | Rainfall (mm) | T (°C) | Rainfall (mm) | |
| IV | 9.2 (−1.2/22.6) | 68.4 | 7.7 (−1.6/23.3) | 37.2 | 11.5 (−1.0/23.1) | 29.6 | 8.6 | 41.9 |
| V | 13.8 (2.6/26.7) | 61.3 | 13.7 (−1.4/26.9) | 100.0 | 14.2 (1.9/25.8) | 54.7 | 12.6 | 64.1 |
| VI | 18.1 (4.2/31.5) | 97.1 | 18.3 (5.7/30.2) | 38.6 | 18.0(5.2/30.6) | 77.1 | 17.8 | 68.3 |
| VII | 19.5 (8.8/31.2) | 107.6 | 18.5 (5.3/32.9) | 61.1 | 19.1(7.6/32.4) | 93.7 | 18.8 | 79.4 |
| VIII | 18.2 (7.1/30.7) | 95.3 | 19.5 (4.3/34.4) | 25.5 | 19.8 (6.3/31.9) | 64.5 | 19.5 | 71.5 |
| IX | 15.2 (1.6/28.7) | 41.2 | 13.2 (−0.3/27.3) | 100.4 | 15.1 (1.9/26.9) | 44.3 | 14.0 | 69.6 |
| Average/Total | 17.1 | 470.9 | 15.2 | 362.8 | 16.3 | 363.9 | 15.2 | 394.8 |
| Specification | Unit | Value |
|---|---|---|
| Price of bean seeds | EUR·t−1 | 934.28 |
| Price biostimulant Atonik | EUR·l−1 | 29.87 |
| Price biostimulant Tytanit | EUR·l−1 | 12.85 |
| Price water | EUR·m−3 | 1.87 |
| Prince of the application | EUR·ha−1 | 14.02 |
| Parameters | Biostimulant Treatment | Biostimulant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Atonik | Tytanit | ||||||||
| Season | Average | Season | Average | ||||||
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | ||||
| 1000 seed weight (g 1000−1) | C | 177.1a | 152.8bc | 151.7b | 160.5a | 177.1c | 152.8b | 151.7b | 160.5a |
| LSS | 174.7a | 132.4a | 132.8a | 146.6a | 171.4b | 160.3cd | 161.1bc | 164.3a | |
| HSS | 174.2a | 156.8c | 155.1b | 162.0a | 155.1a | 164.7d | 164.0c | 161.3a | |
| LDS | 193.9b | 155.6c | 156.4b | 168.6a | 183.3d | 138.8a | 137.4a | 153.2a | |
| HDS | 176.4a | 148.6b | 148.7b | 157.9a | 169.5b | 157.8bc | 155.7bc | 161.0a | |
| Seed yield (g m−2) | C | 241.8a | 278.4a | 286.8a | 269.0a | 241.8a | 278.4a | 286.8a | 269.0a |
| LSS | 260.6b | 360.0b | 372.1b | 330.9ab | 304.2e | 404.5bc | 399.4b | 369.4a | |
| HSS | 299.6c | 364.6b | 378.8b | 347.7ab | 250.8b | 436.3c | 437.9c | 375.0a | |
| LDS | 358.5d | 358.9b | 378.9b | 365.4ab | 283.7d | 376.4b | 378.2b | 346.1a | |
| HDS | 354.7d | 393.1b | 399.2b | 382.3b | 272.1c | 362.9b | 385.8b | 340.3a | |
| Number of pods (per m−2) | C | 350a | 421a | 428a | 400a | 350a | 421a | 428a | 400a |
| LSS | 598b | 605c | 588d | 597b | 471b | 606c | 598d | 558bc | |
| HSS | 613bc | 530b | 538c | 560b | 527c | 497b | 512b | 512ab | |
| LDS | 627cd | 473a | 486b | 529b | 535c | 584c | 575c | 565bc | |
| HDS | 644d | 627c | 623e | 631b | 670d | 617c | 618d | 635c | |
| Number of seeds (per m−2) | C | 1366a | 1822a | 1890a | 1693a | 1366a | 1822a | 1890a | 1693a |
| LSS | 1492b | 2719c | 2850d | 2354a | 1775d | 2522c | 2480b | 2259a | |
| HSS | 1720c | 2325b | 2400b | 2148a | 1617c | 2648c | 2670c | 2312a | |
| LDS | 1849d | 2305b | 2421b | 2192a | 1548b | 2711c | 2753d | 2337a | |
| HDS | 2012e | 2644c | 2685c | 2447a | 1605c | 2299b | 2478b | 2127a | |
| Parameters | Biostimulant Treatment | Biostimulant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Atonik | Tytanit | ||||||||
| Season | Average | Season | Average | ||||||
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | ||||
| Protein (mg g−1 FM) | C | 4970.7a | 5181.7ab | 5050.5a | 5067.6a | 4970.7a | 5181.7a | 4968.2a | 5040.2a |
| LSS | 5723.0c | 4782.1a | 5759.5c | 5421.5a | 5860.0b | 4701.1a | 5824.2c | 5461.8a | |
| HSS | 5216.3ab | 5588.7b | 5299.1ab | 5368.0a | 5872.8b | 4839.2a | 5795.5c | 5502.5a | |
| LDS | 5540.2bc | 4600.6a | 5573.2bc | 5238.0a | 5506.1b | 4797.4a | 5489.2b | 5264.2a | |
| HDS | 5711.3bc | 5186.5ab | 5664.7c | 5520.8a | 5964.5b | 4869.7a | 5919.7c | 5584.6a | |
| FRAP (µM trolox/mL) | C | 945.7a | 5181.7a | 959.2a | 2362.2a | 945.7ab | 1148.7b | 918.6ab | 1004.3ab |
| LSS | 1020.7a | 4782.1a | 1042.9ab | 2281.9a | 1084.0bc | 983.4a | 1071.4cd | 1046.3ab | |
| HSS | 1076.6a | 5588.7a | 1079.9ab | 2581.7a | 1019.5bc | 1110.0ab | 989.9bc | 1039.8ab | |
| LDS | 1003.8a | 4600.6a | 1038.7ab | 2214.4a | 792.6a | 985.9a | 793.5a | 857.3a | |
| HDS | 1131.0a | 5186.5a | 1135.4b | 2484.3a | 1204.6c | 1074.7ab | 1164.7d | 1148.0b | |
| ABTS (µM trolox/mL) | C | 946.1a | 1003.1ab | 984.3ab | 977.8a | 946.1a | 1003.1a | 955.4a | 968.2a |
| LSS | 873.3a | 1543.4ab | 923.9a | 1113.5a | 1184.9ab | 1391.1ab | 1191.6bc | 1255.9ab | |
| HSS | 1129.8ab | 1206.8ab | 1121.2bc | 1152.6a | 1230.5ab | 1567.5b | 1170.4b | 1322.8b | |
| LDS | 1147.9ab | 1659.0b | 1138.7bc | 1315.2a | 1130.4ab | 1262.9ab | 1118.9ab | 1170.7ab | |
| HDS | 1283.5b | 959.5a | 1251.9c | 1165.0a | 1366.8b | 1310.2ab | 1345.7c | 1340.9b | |
| Prolina (µM mL−1) | C | 5.912a | 4.982ab | 5.651a | 5.515a | 5.912a | 4.982a | 5.465a | 5.453a |
| LSS | 4.672a | 5.418b | 4.580a | 4.890a | 4.758a | 4.140a | 4.737a | 4.545a | |
| HSS | 5.026a | 4.604ab | 4.594a | 4.741a | 4.969a | 5.286a | 4.765a | 5.007a | |
| LDS | 5.616a | 4.255a | 5.179a | 5.017a | 4.978a | 5.543a | 4.758a | 5.093a | |
| HDS | 5.318a | 4.708ab | 5.190a | 5.072a | 5.187a | 5.552a | 4.618a | 5.119a | |
| Parameters | Biostimulant Treatment | Biostimulant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Atonik | Tytanit | ||||||||
| Season | Average | Season | Average | ||||||
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | ||||
| Anthocyanins (mg g−1 DM) | C | 0.010b | 0.007b | 0.009a | 0.009a | 0.010b | 0.007b | 0.011b | 0.009ab |
| LSS | 0.009ab | 0.009c | 0.009a | 0.009a | 0.010b | 0.009c | 0.010b | 0.010b | |
| HSS | 0.010b | 0.007b | 0.009a | 0.009a | 0.013c | 0.006b | 0.014c | 0.011b | |
| LDS | 0.008a | 0.004a | 0.007a | 0.006a | 0.014c | 0.015d | 0.014c | 0.014b | |
| HDS | 0.009ab | 0.009c | 0.009a | 0.009a | 0.004a | 0.003a | 0.003a | 0.003a | |
| Total flavonoids (mg g−1 DM) | C | 1166.3b | 1094.7b | 1134.9b | 1132.0a | 1166.3c | 1094.7a | 1210.0c | 1157.0a |
| LSS | 1007.1a | 1161.4c | 970.8a | 1046.4a | 787.6b | 1569.5e | 822.7b | 1059.9a | |
| HSS | 1305.1d | 898.0a | 1258.2c | 1153.8a | 1476.1e | 1449.6d | 1503.5e | 1476.4a | |
| LDS | 1190.9c | 1191.1d | 1138.8b | 1173.6a | 682.3a | 1316.8c | 651.2a | 883.4a | |
| HDS | 1778.9e | 1110.5b | 1724.8d | 1538.1a | 1302.5d | 1253.7b | 1329.1d | 1295.1a | |
| Reducing power (mg TE g−1 DM) | C | 2.459b | 2.967b | 2.302b | 2.576a | 2.459b | 2.967c | 2.638b | 2.688a |
| LSS | 0.043a | 2.794a | 0.166a | 1.001a | 3.182c | 2.694c | 3.407c | 3.094a | |
| HSS | 2.643c | 4.357d | 2.466b | 3.155a | 3.223c | 0.139a | 3.450c | 2.271a | |
| LDS | 2.684c | 2.776a | 2.421b | 2.627a | 2.178a | 2.723d | 1.930a | 2.277a | |
| HDS | 3.172d | 3.852c | 2.933c | 3.319a | 2.162a | 2.474b | 1.999a | 2.212a | |
| Total phenols (mg g−1 DM) | C | 23.555b | 26.252b | 22.082b | 23.963a | 23.555a | 26.252a | 25.083a | 24.963a |
| LSS | 22.460a | 29.000d | 20.983a | 24.148a | 28.846b | 31.199b | 30.096c | 30.047a | |
| HSS | 28.011d | 28.631c | 26.129d | 27.590ab | 28.898b | 43.981e | 30.185c | 34.355a | |
| LDS | 24.391c | 23.103a | 22.930c | 23.475a | 28.871b | 42.812d | 27.578b | 33.087a | |
| HDS | 38.258e | 29.154d | 36.847e | 34.753b | 28.809b | 36.912c | 27.310b | 31.010a | |
| Parameters | Biostimulant Treatment | Biostimulant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Atonik | Tytanit | ||||||||
| Season | Average | Season | Average | ||||||
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | ||||
| NDF (% DM) | C | 10.919a | 26.601e | 9.110a | 15.543a | 10.919d | 24.583c | 12.539c | 16.014a |
| LSS | 12.606c | 14.521b | 11.337c | 12.821a | 8.405a | 11.494a | 6.811a | 8.903a | |
| HSS | 22.628e | 11.341a | 20.852e | 18.274a | 9.339b | 17.147b | 10.910b | 12.465a | |
| LDS | 14.328d | 16.447c | 12.632d | 14.469a | 12.694e | 16.631b | 10.896b | 13.407a | |
| HDS | 11.558b | 17.699d | 10.123b | 13.127a | 10.176c | 9.454a | 11.706bc | 10.445a | |
| ADF (% DM) | C | 8.651b | 8.702c | 7.842b | 8.398a | 8.651b | 8.702b | 7.769b | 8.374a |
| LSS | 9.474c | 12.600e | 8.659c | 10.244a | 10.429d | 6.062a | 9.566c | 8.686a | |
| HSS | 9.867c | 6.027a | 8.890c | 8.261a | 6.754a | 10.048c | 7.584b | 8.129a | |
| LDS | 9.921c | 8.920d | 9.063c | 9.301a | 6.840a | 12.730e | 5.976a | 8.515a | |
| HDS | 7.249a | 6.892b | 6.403a | 6.848a | 9.018c | 11.423d | 9.877c | 10.106a | |
| ADL (% DM) | C | 1.676b | 1.322b | 1.514b | 1.504a | 1.676d | 1.322b | 1.939c | 1.646a |
| LSS | 1.678b | 3.849d | 1.481b | 2.336a | 3.322e | 0.591a | 3.208d | 2.374a | |
| HSS | 2.041c | 0.632a | 1.885c | 1.519a | 0.722b | 1.537c | 0.895b | 1.051a | |
| LDS | 4.915d | 1.468c | 4.690d | 3.691a | 0.398a | 4.279d | 0.162a | 1.613a | |
| HDS | 0.643a | 1.443c | 0.487a | 0.858a | 1.002c | 4.614e | 0.789b | 2.135a | |
| HCEL (% DM) | C | 2.267a | 17.899e | 1.268a | 7.145a | 2.267c | 15.881c | 4.770c | 7.639a |
| LSS | 3.132b | 1.920a | 2.678b | 2.577a | 0.000a | 5.432b | 0.000a | 1.811a | |
| HSS | 12.761d | 5.314b | 11.962c | 10.012a | 2.585d | 7.099b | 3.326bc | 4.337a | |
| LDS | 4.407c | 7.527c | 3.569b | 5.168a | 5.855e | 3.901ab | 4.920c | 4.892a | |
| HDS | 4.310c | 10.807d | 3.720b | 6.279a | 1.157b | 0.000a | 1.829b | 0.995a | |
| CEL (% DM) | C | 6.976b | 7.380b | 6.328bc | 6.895a | 6.976c | 7.380c | 5.829a | 6.728a |
| LSS | 7.796c | 8.751c | 7.178c | 7.908a | 7.106c | 5.471a | 6.358b | 6.312a | |
| HSS | 7.825c | 5.395a | 7.005c | 6.742a | 6.033a | 8.511d | 6.689b | 7.078a | |
| LDS | 5.006a | 7.452b | 4.373a | 5.610a | 6.441b | 8.451d | 5.813a | 6.902a | |
| HDS | 6.605b | 5.448a | 5.916b | 5.990a | 8.017d | 6.809b | 9.088c | 7.971a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szparaga, A.; Kuboń, M.; Kocira, S.; Czerwińska, E.; Pawłowska, A.; Hara, P.; Kobus, Z.; Kwaśniewski, D. Towards Sustainable Agriculture—Agronomic and Economic Effects of Biostimulant Use in Common Bean Cultivation. Sustainability 2019, 11, 4575. https://doi.org/10.3390/su11174575
Szparaga A, Kuboń M, Kocira S, Czerwińska E, Pawłowska A, Hara P, Kobus Z, Kwaśniewski D. Towards Sustainable Agriculture—Agronomic and Economic Effects of Biostimulant Use in Common Bean Cultivation. Sustainability. 2019; 11(17):4575. https://doi.org/10.3390/su11174575
Chicago/Turabian StyleSzparaga, Agnieszka, Maciej Kuboń, Sławomir Kocira, Ewa Czerwińska, Anna Pawłowska, Patryk Hara, Zbigniew Kobus, and Dariusz Kwaśniewski. 2019. "Towards Sustainable Agriculture—Agronomic and Economic Effects of Biostimulant Use in Common Bean Cultivation" Sustainability 11, no. 17: 4575. https://doi.org/10.3390/su11174575
APA StyleSzparaga, A., Kuboń, M., Kocira, S., Czerwińska, E., Pawłowska, A., Hara, P., Kobus, Z., & Kwaśniewski, D. (2019). Towards Sustainable Agriculture—Agronomic and Economic Effects of Biostimulant Use in Common Bean Cultivation. Sustainability, 11(17), 4575. https://doi.org/10.3390/su11174575









