Production of the Hydroxyl Radical and Removal of Formaldehyde by Calcined Green Tuff Powder and Tile
Abstract
1. Introduction
2. Materials and Methods
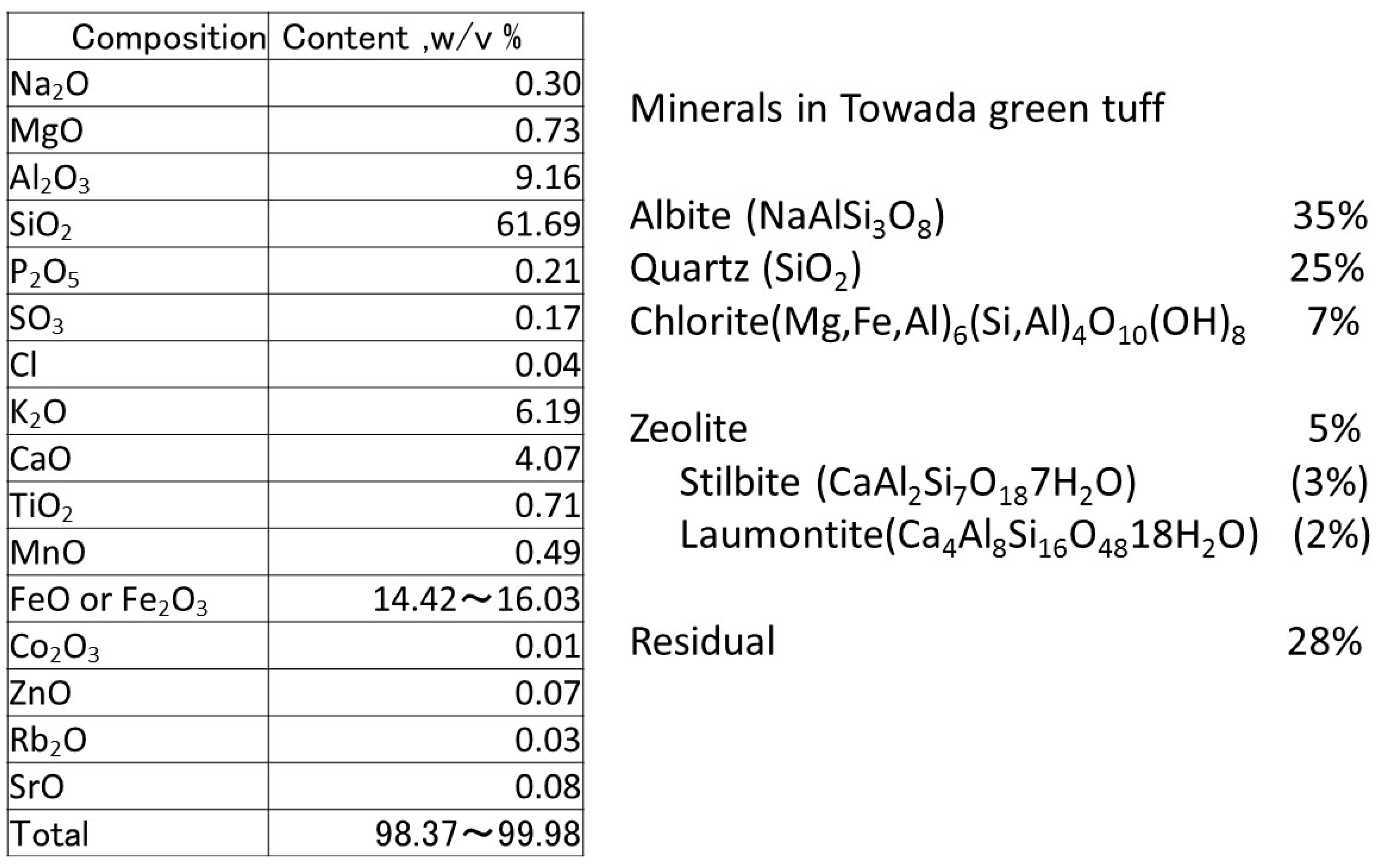
2.1. Materials of Green Tuff Powder
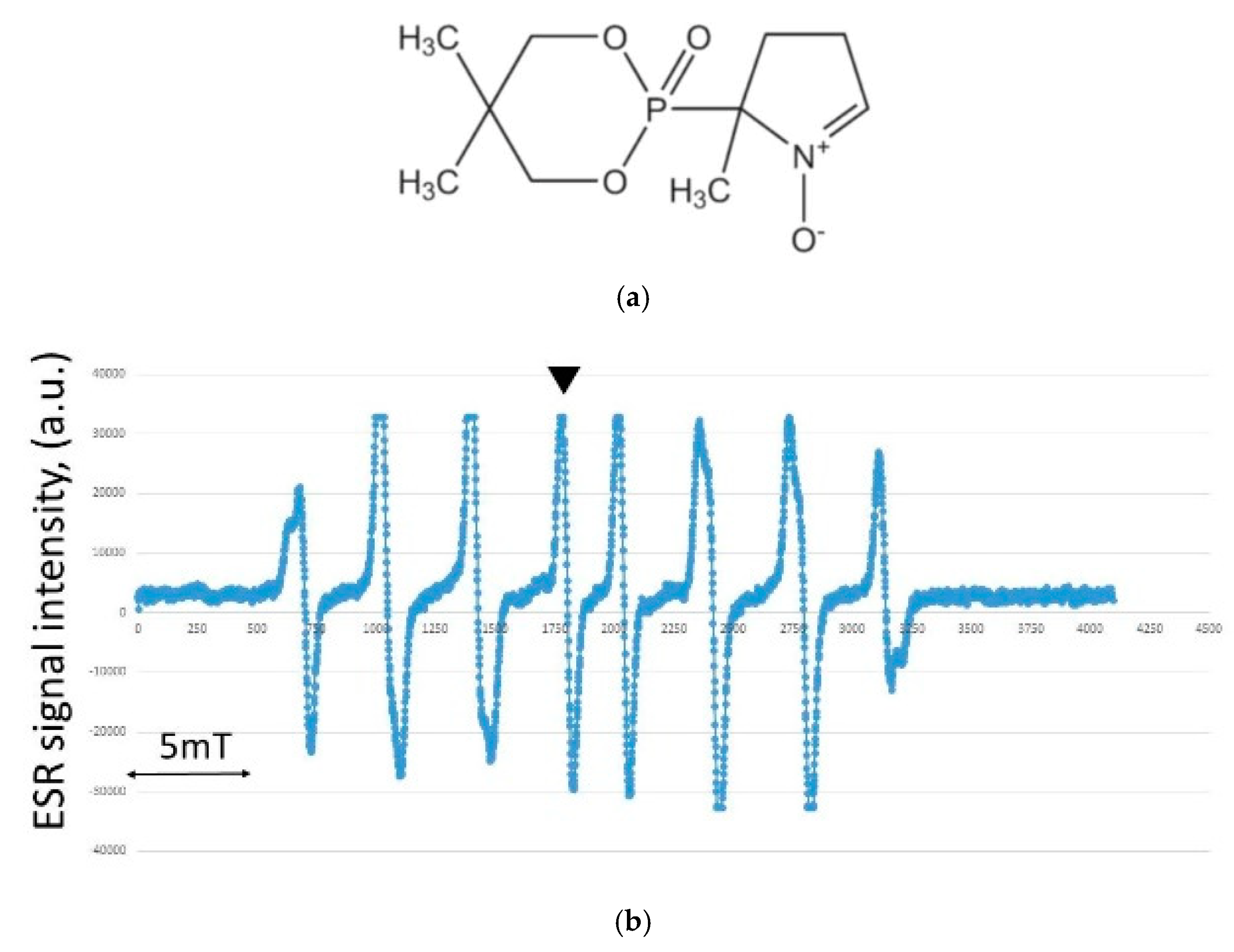
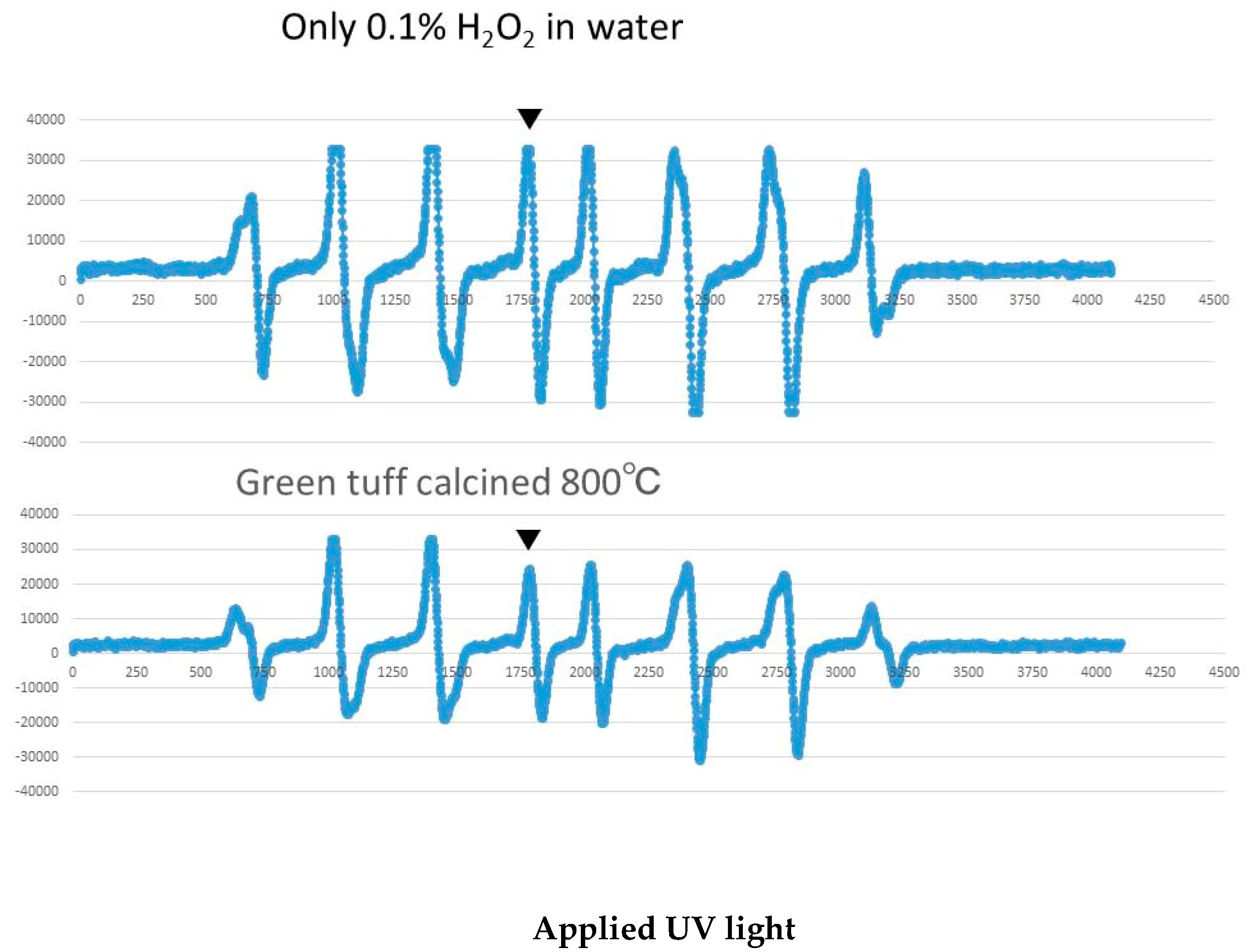
2.2. Hydroxyl Radical Measurement by ESR
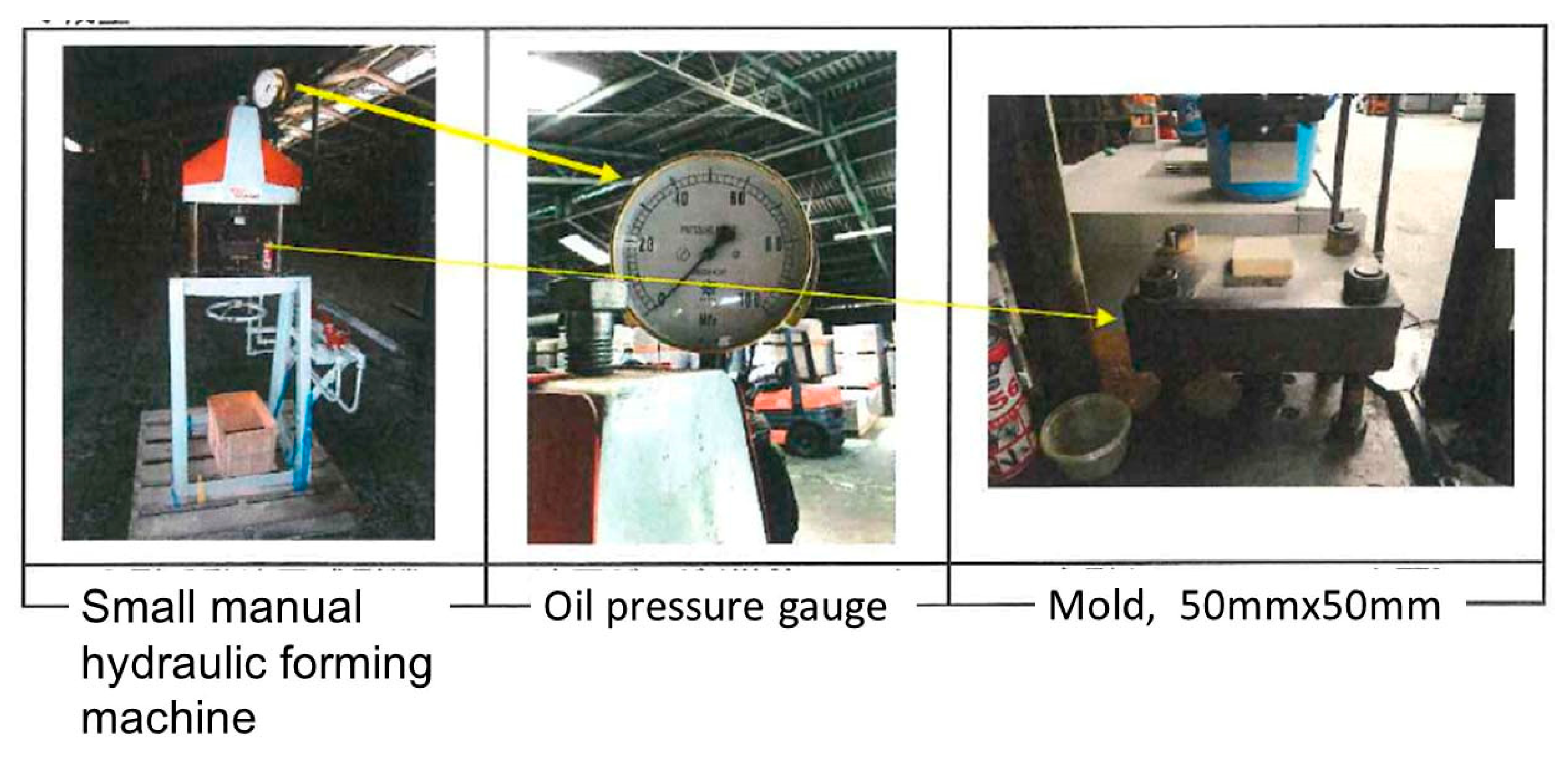
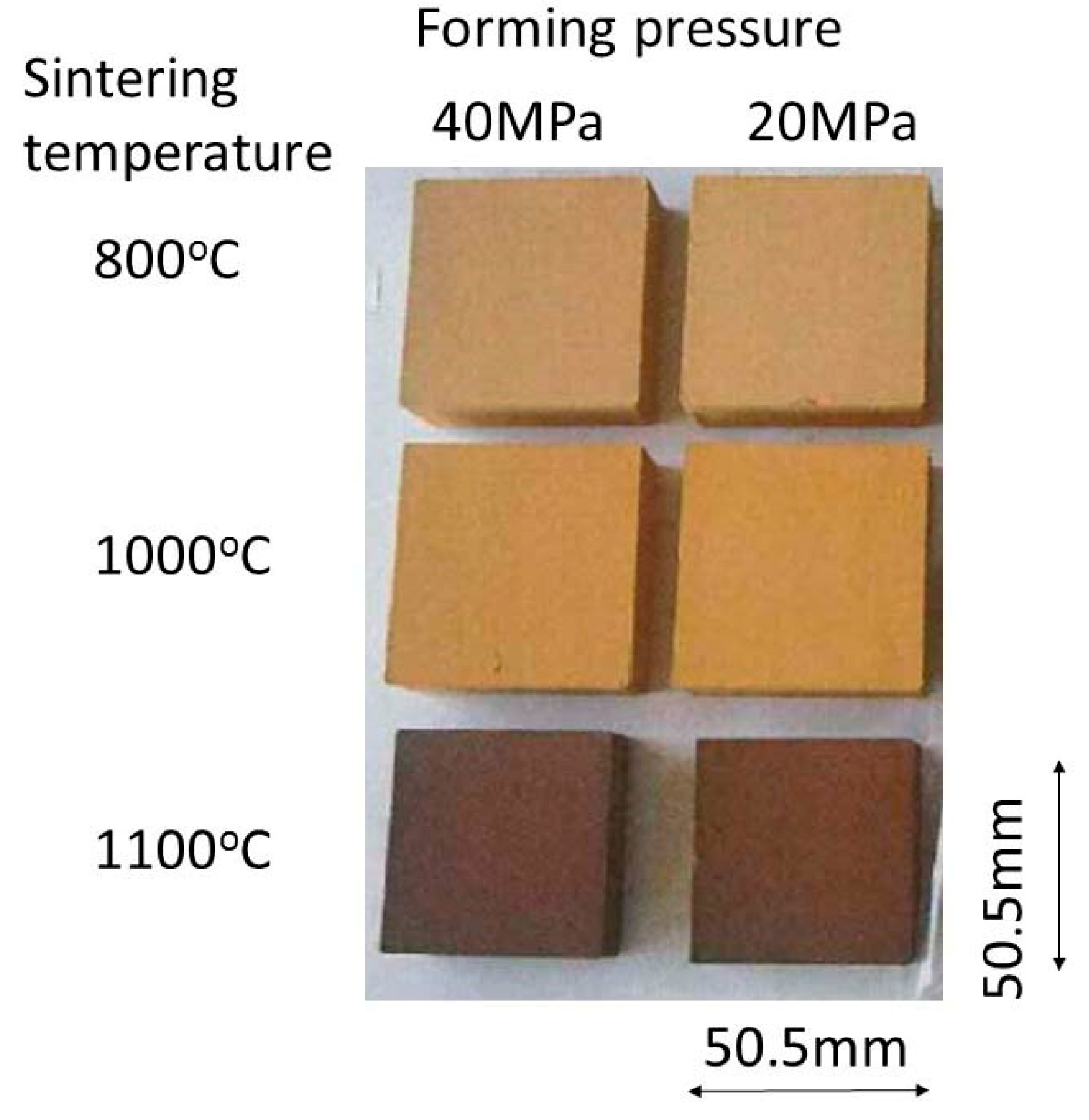
2.3. Production of Tile Using Calcined Green Tuff Powders
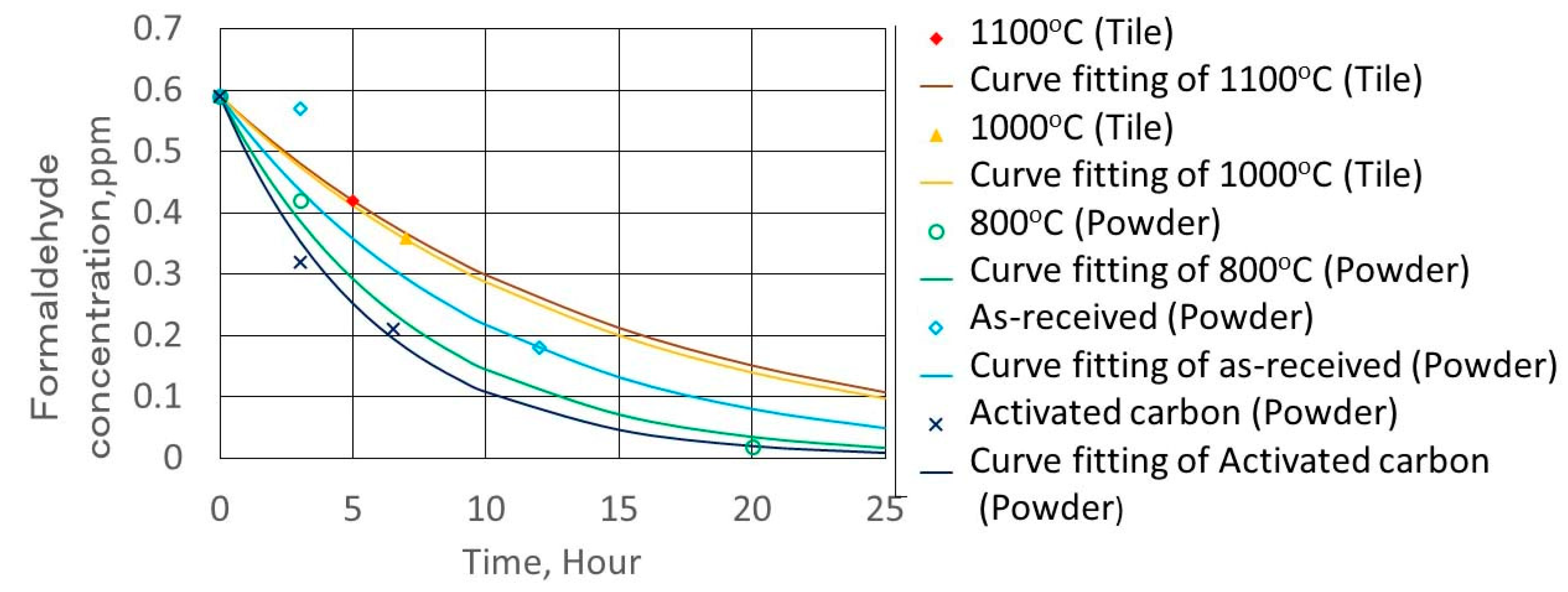
2.4. Formaldehyde Adsorption Experimental Method
3. Results and Discussion
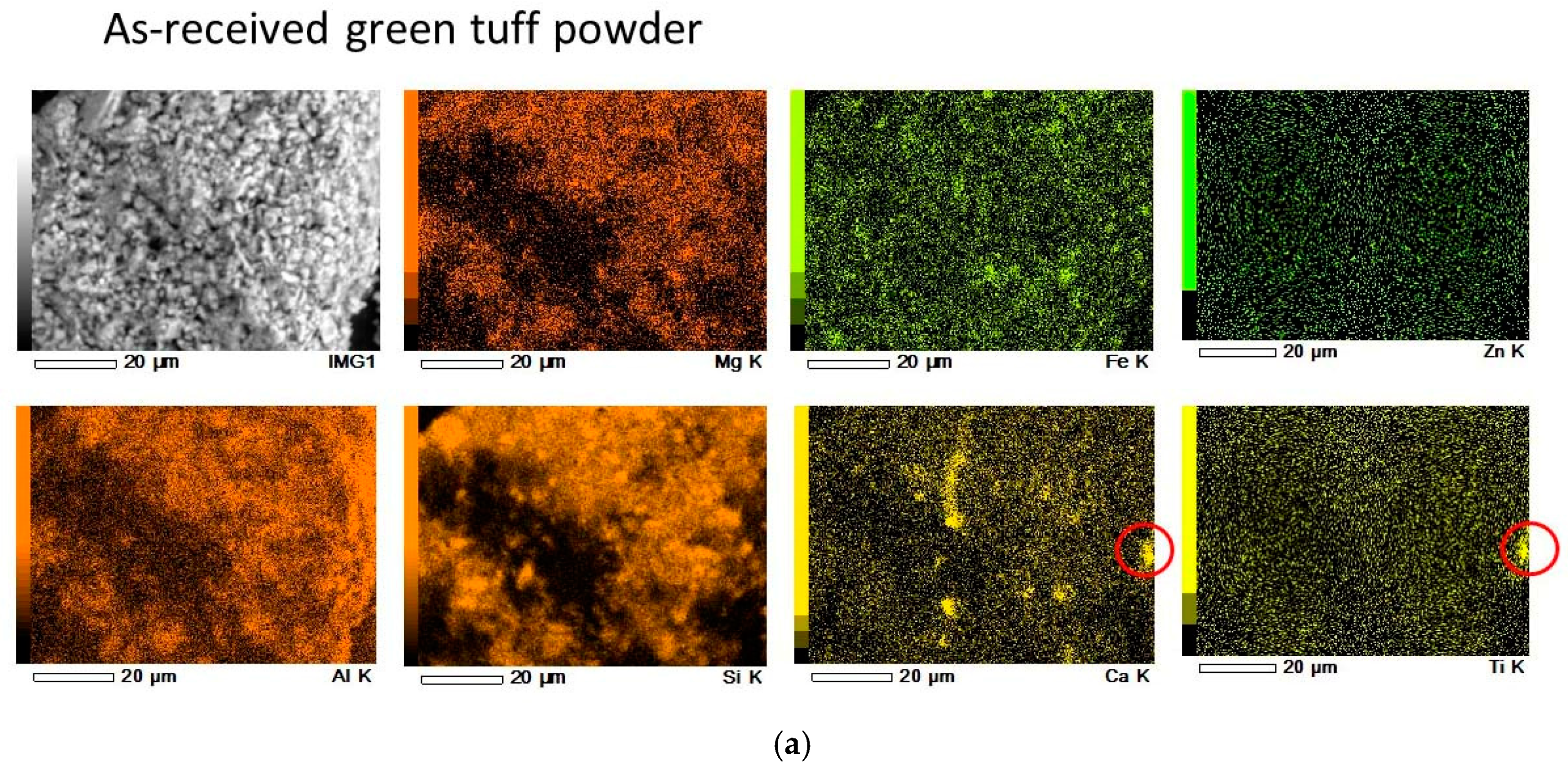
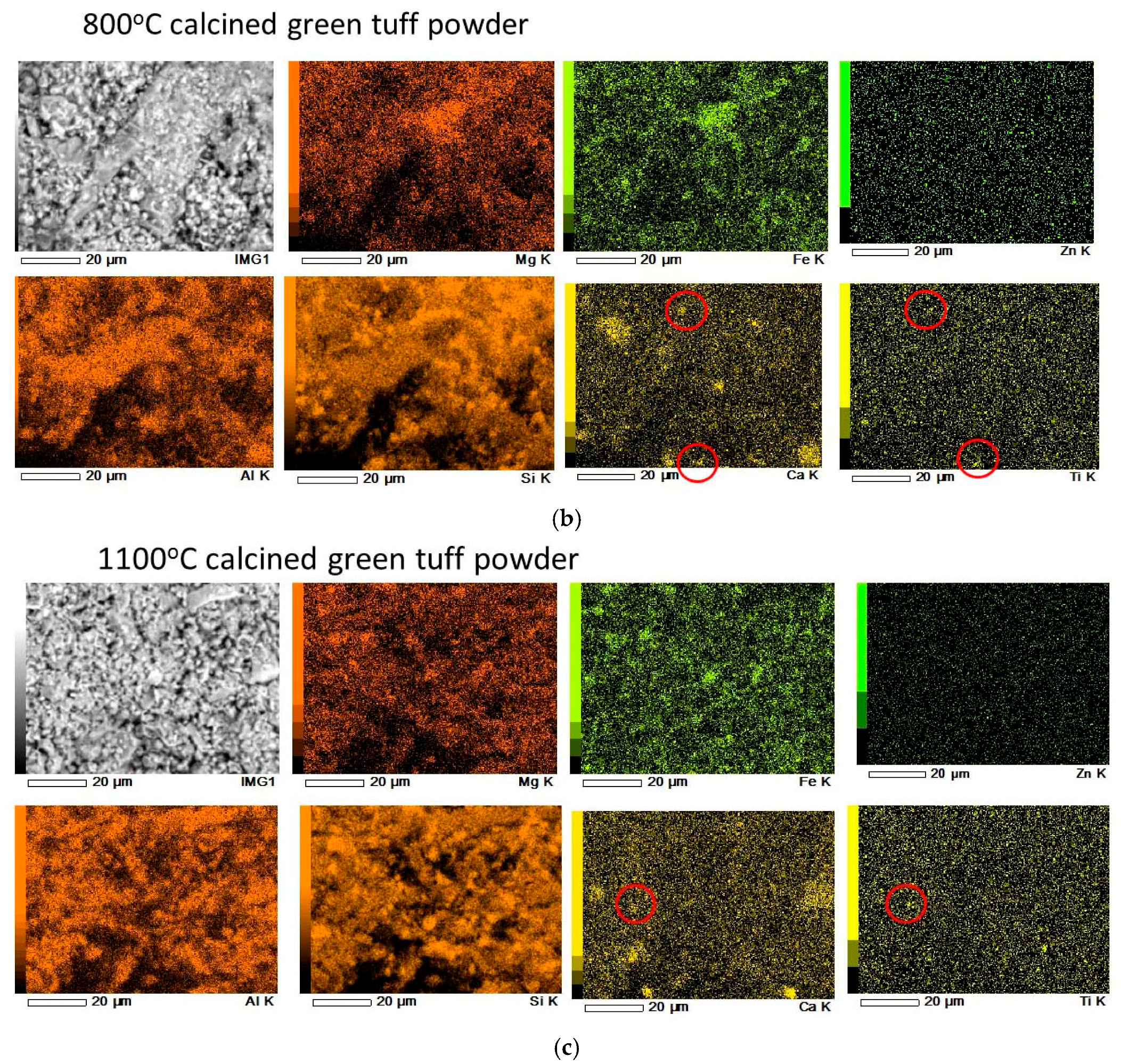
3.1. Calcined Green Tuff Powder
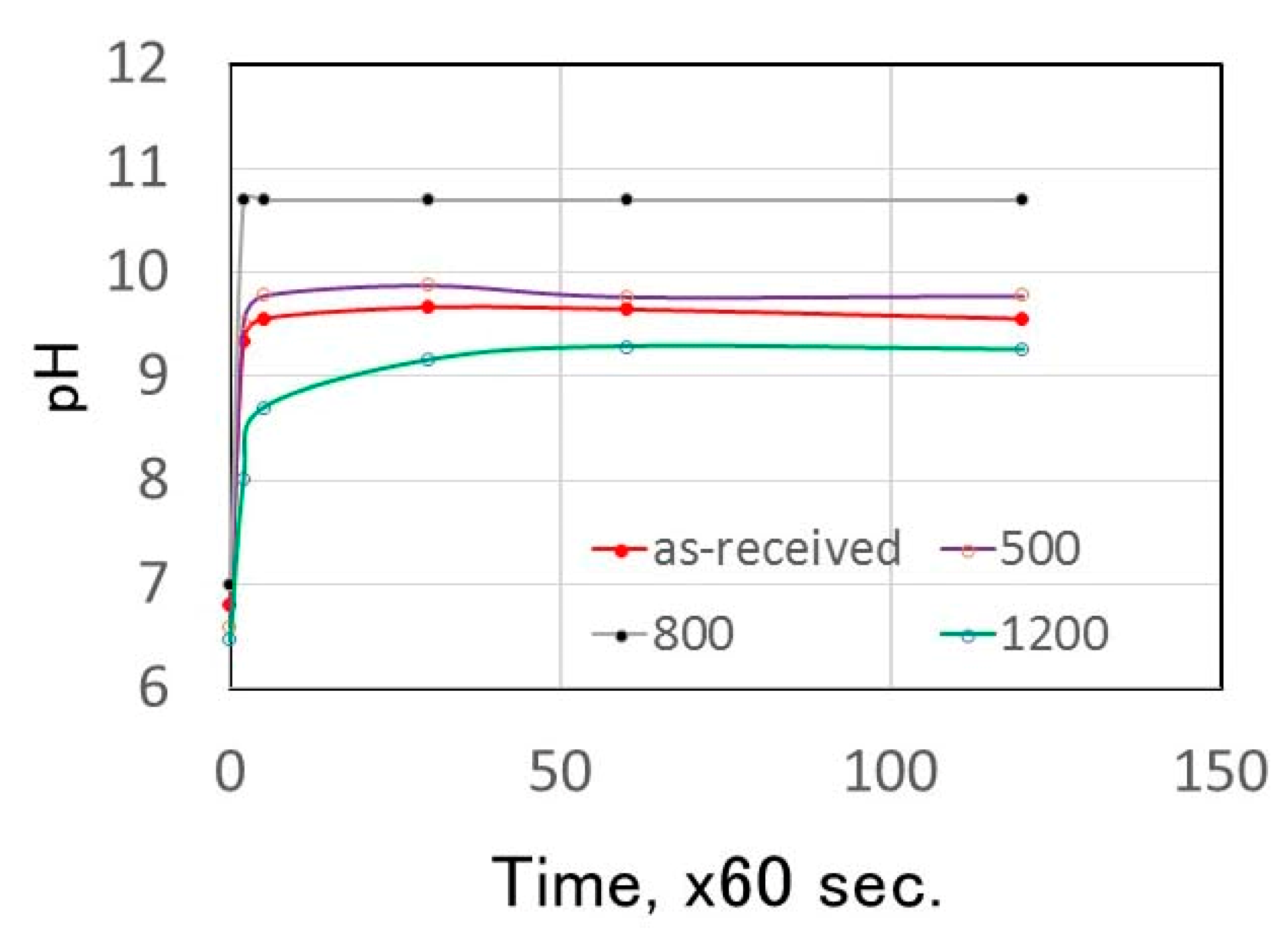
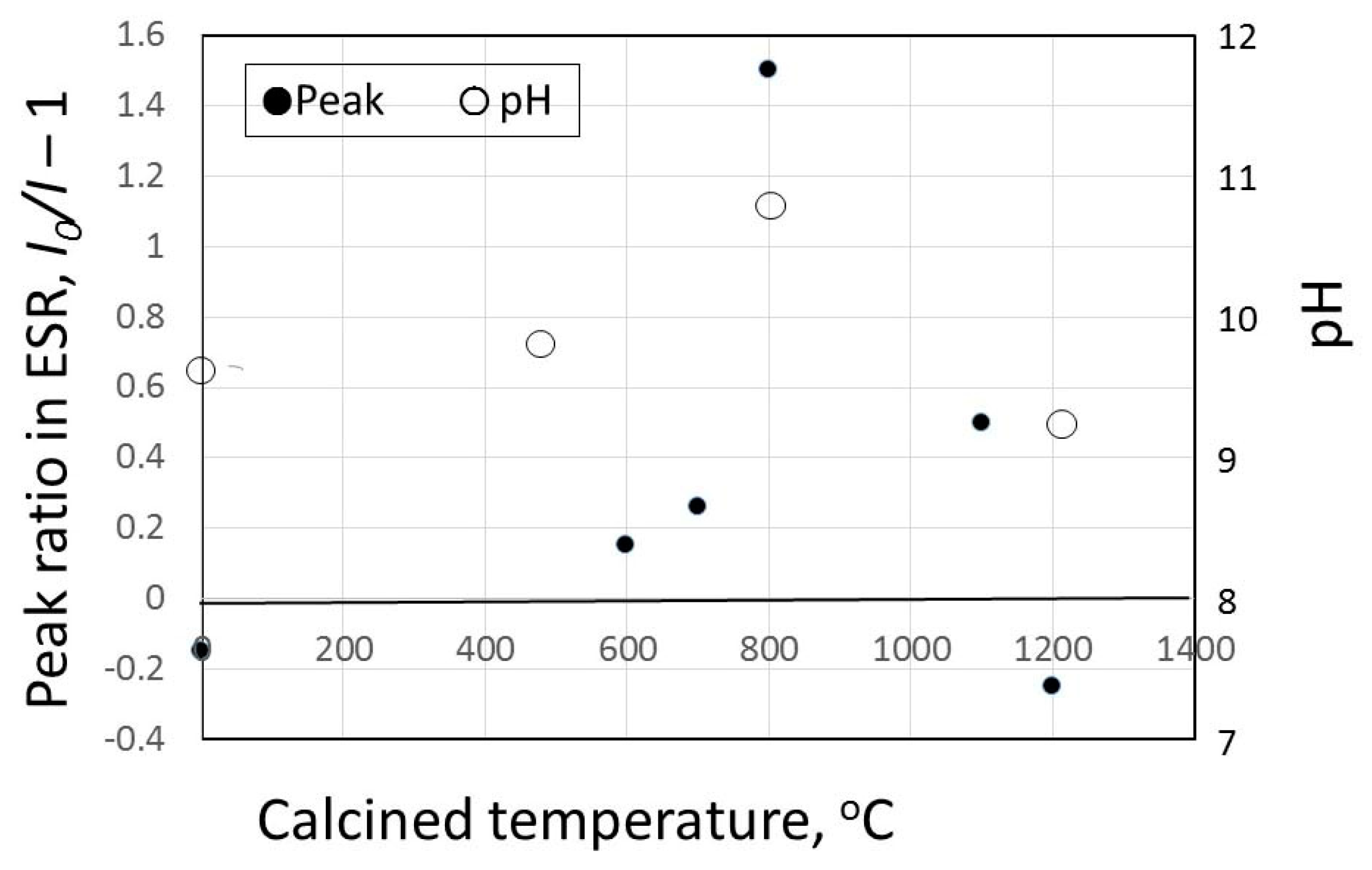
3.2. pH Change of Calcined Green Tuff Powder
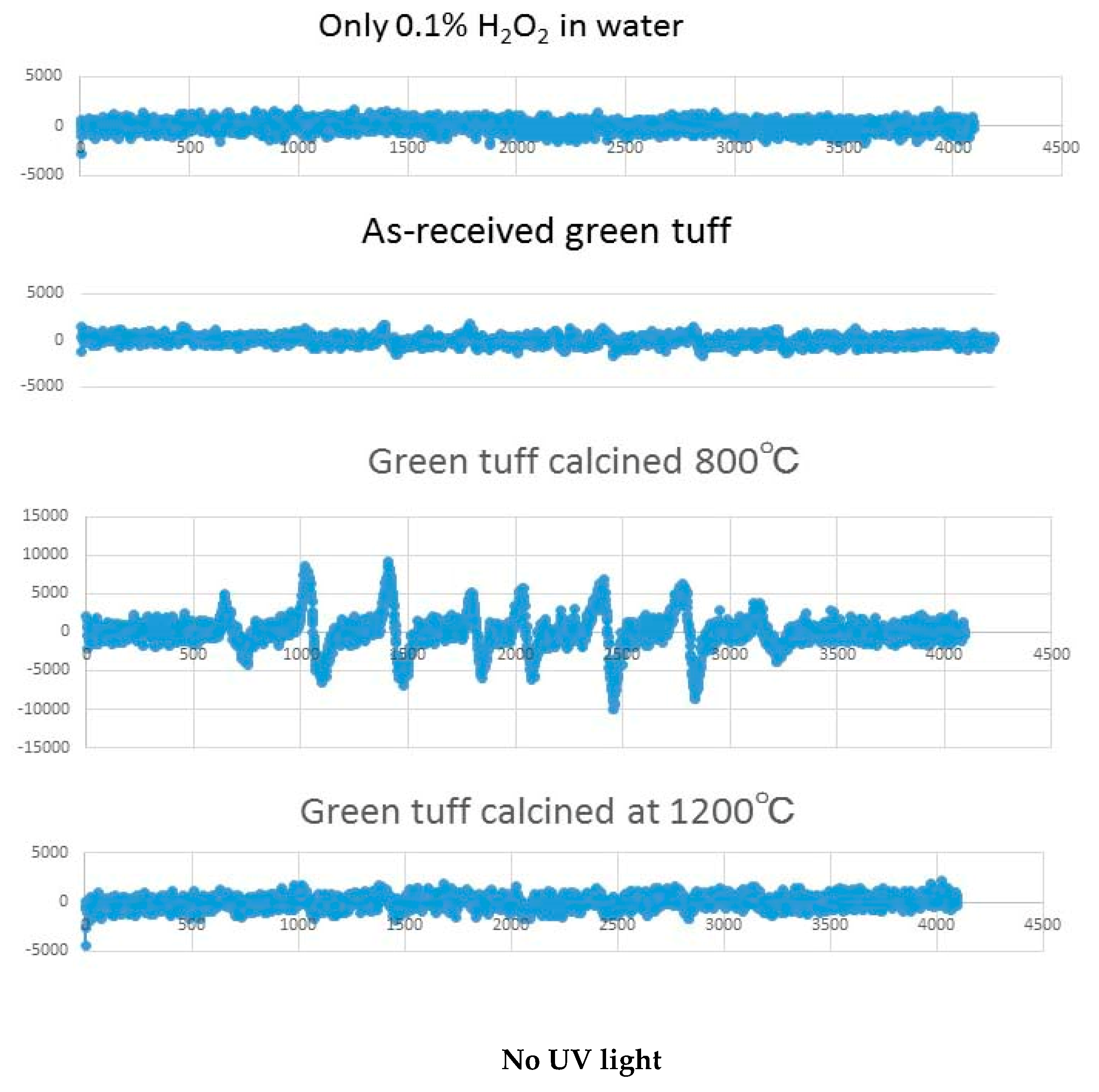
3.3. Results of Hydroxyl Radical Measurement
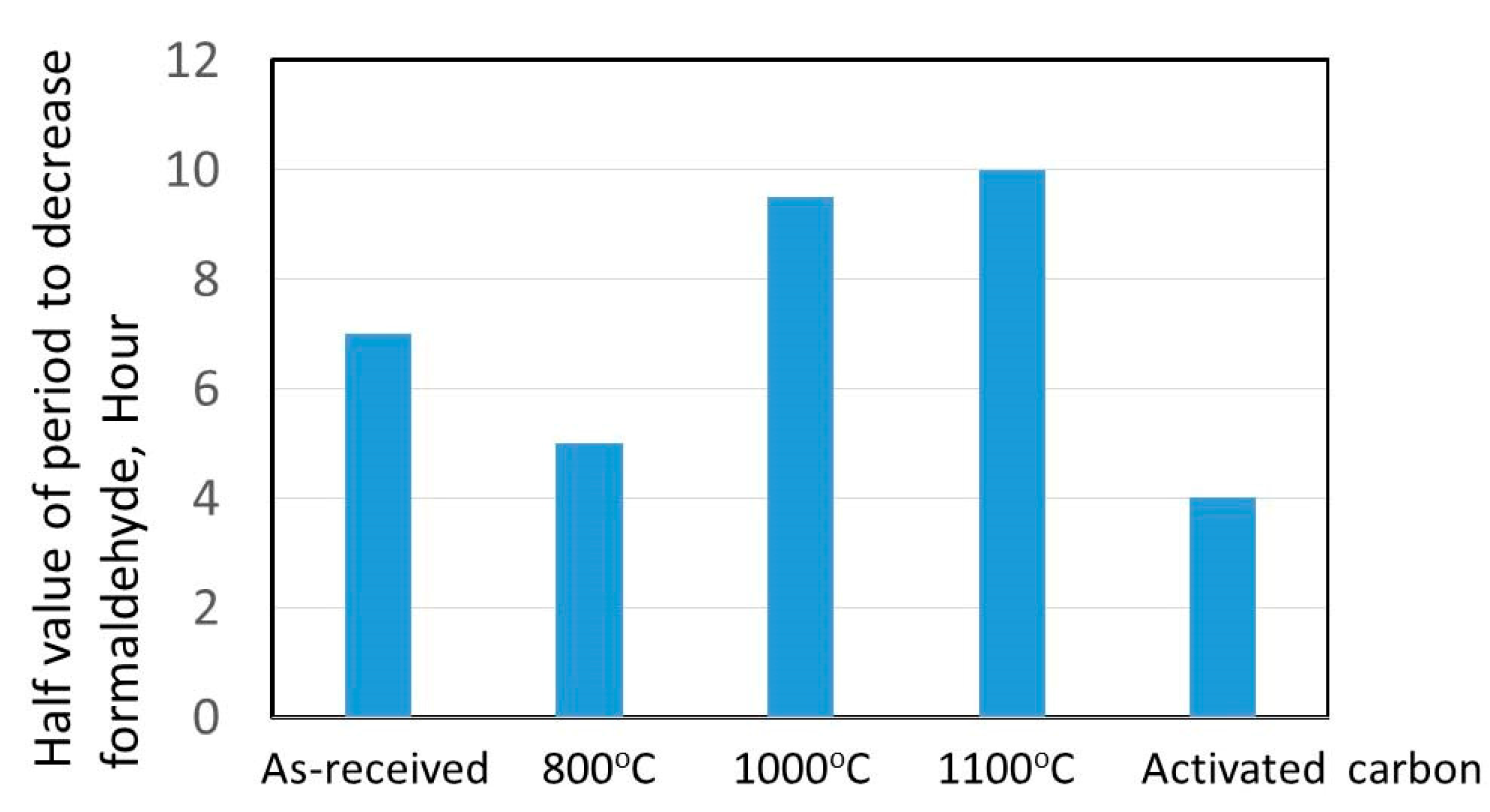
3.4. Formaldehyde Adsorption Result
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EPA. 42 U.S. Code §7412. Hazardous Air Pollutants. 1992. Available online: https://www.law.cornell.edu/uscode/text/42/7412 (accessed on 19 June 2019).
- Measuring Global Water Vapor and Formaldehyde. Science News. 14 April 2010. Available online: https://www.sciencedaily.com/releases/2010/04/100409134731.htm (accessed on 19 June 2019).
- Green Tuff. 2013. Available online: http://www.oki-geopark.jp/en/episode/geohistory/stage2/green-tuff/ (accessed on 19 June 2019).
- Towada Green-Tuff AgroScience Co., Ltd. 2018. Available online: https://towadagreentuff.com/company.html (accessed on 19 June 2019).
- Mitsumori, H.; Aiba, N.; Saito, M.; Nagasawa, Y. A Study on the Use of Towada Stones in Food Processing. Bull. Seirei Women’s J. Coll. 2011, 39, 14–25. [Google Scholar] [CrossRef]
- Sugai, Y.; Sasaki, K.; Matsubaya, O.; Naka, H.; Tanaka, F. The abilities of Hinai-Green Tuff to adjust pH and activate of microorganisms. Shigen-To-Sozai 2005, 121, 513–520. [Google Scholar] [CrossRef]
- Nakamura, T.; Okawa, H.; Kawamura, Y.; Takahata, S.; Naka, H.; Sugawara, K. Research on the New Method to Precipitate Hinai Green Tuff Suspension using Ultrasound Irradiation. Resour. Process. 2009, 56, 13–20. [Google Scholar] [CrossRef]
- Sugai, Y.; Sasaki, K.; Takahata, S.; Naka, H. Characteristics of functional wall material containing a green tuff. Soc. Heat. Air-Cond. Sanit. Eng. Jpn. 2007, 32, 1–10. [Google Scholar] [CrossRef]
- LIXIL (INAX), ECOCARAT. 2019. Available online: https://ecocarat.jp/features/, https://ecocarat.jp/features/3.html (accessed on 19 June 2019).
- Grabowska, E.; Marchelek, E.M.; Paszkiewicz-Gawron, M.; Zaleska-Medynska, A. 3-Metal Oxide Photocatalysts, Metal Oxide-Based Photocatalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128116340. [Google Scholar]
- Davari, N.; Farhadian, M.; Nazar, A.R.S.; Homayoonfal, M. Degradation of diphenhydramine by the photocatalysis of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: Structural and operational comparison. J. Environ. Chem. Eng. 2017, 5, 5707–5720. [Google Scholar] [CrossRef]
- Ma, R.Y.; Wang, L.; Wang, H.; Liu, Z.Y.; Xing, M.Y.; Zhu, L.F.; Meng, X.J.; Xiao, F.S. Solid acids accelerate the photocatalytic hydrogen peroxide synthesis over a hybrid catalyst of Titania nanotube with carbon dot. Appl. Catal. B Environ. 2019, 244, 594–603. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Minguzzi, A.; Rondinini, S.; Vertova, A. Achieving efficient H2O2 production by a visible-light absorbing, highly stable photosensitized TiO2. Appl. Catal. B Environ. 2019, 244, 303–312. [Google Scholar] [CrossRef]
- Duke, F.R.; Haas, T.W. The Homogeneous Base-Catalyzed Decomposition of Hydrogen Peroxide. J. Phys. Chem. 1961, 65, 304–306. [Google Scholar] [CrossRef]
- Hoshiba, K.; Ponou, J.; Dodbiba, G.; Ito, H.; Sase, T.; Matsui, H.; Fujita, T. Effect of Calcination Temperature on the Hydroxyl Radical Generation of Calcined Dolomite Suspension. J. MMIJ 2018, 134, 151–157. [Google Scholar] [CrossRef]
- Liu, L.M.; Zhao, J. Formaldehyde adsorption and decomposition on rutile (110): A first-principles study. Surf. Sci. 2016, 652, 156–162. [Google Scholar] [CrossRef]
- Oka, T.; Midoricawa, M.; Saiki, S.; Muroya, Y.; Kamibayasshi, M.; Yamashita, M.; Anzai, K. Spin-Trapping Reactions of a Novel Gauchetype Radical Trapper G-CYPMPO. Anal. Chem. 2011, 83, 9600–9604. [Google Scholar] [CrossRef] [PubMed]
- Kohri, S.; Fujii, H.; Oowada, S.; Endoh, N.; Sueishi, Y.; Kusakabe, M.; Shimmei, M.; Kotake, Y. An oxygen radical absorbance capacity-like assay that directly quantifies the antioxidant’s scavenging capacity against AAPH-derived free radicals. Anal. Biochem. 2009, 386, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, T.; Venugopal, G.; Durairaj, A.; Vasanthkumar, S.; Huang, X. Utilization of the internal electric field in semiconductor photocatalysis: A short review. J. Ind. Eng. Chem. 2019, 72, 18–30. [Google Scholar] [CrossRef]
- Wang, F.; Smith, D.W.; El-Din, M.G. Application of advanced oxidation methods for landfill leachate treatment—A review. J. Environ. Eng. Sci. 2003, 2, 413–427. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, T.; Zhang, L.; Dodbiba, G.; Anh, J.-W.; Wei, Y.; Kurokawa, H.; Matsui, H.; Yamamoto, S.; Kawaguchi, H. Production of the Hydroxyl Radical and Removal of Formaldehyde by Calcined Green Tuff Powder and Tile. Sustainability 2019, 11, 3390. https://doi.org/10.3390/su11123390
Fujita T, Zhang L, Dodbiba G, Anh J-W, Wei Y, Kurokawa H, Matsui H, Yamamoto S, Kawaguchi H. Production of the Hydroxyl Radical and Removal of Formaldehyde by Calcined Green Tuff Powder and Tile. Sustainability. 2019; 11(12):3390. https://doi.org/10.3390/su11123390
Chicago/Turabian StyleFujita, Toyohisa, Lanyin Zhang, Gjergj Dodbiba, Ji-Whan Anh, Yuezhou Wei, Hiromi Kurokawa, Hirofumi Matsui, Shigeki Yamamoto, and Hiroshi Kawaguchi. 2019. "Production of the Hydroxyl Radical and Removal of Formaldehyde by Calcined Green Tuff Powder and Tile" Sustainability 11, no. 12: 3390. https://doi.org/10.3390/su11123390
APA StyleFujita, T., Zhang, L., Dodbiba, G., Anh, J.-W., Wei, Y., Kurokawa, H., Matsui, H., Yamamoto, S., & Kawaguchi, H. (2019). Production of the Hydroxyl Radical and Removal of Formaldehyde by Calcined Green Tuff Powder and Tile. Sustainability, 11(12), 3390. https://doi.org/10.3390/su11123390







