A Brief Note on the Heap Leaching Technologies for the Recovery of Valuable Metals
Abstract
1. Introduction
2. Heap Leaching of Mines
2.1. Heap Leaching Advantages and Economic factors
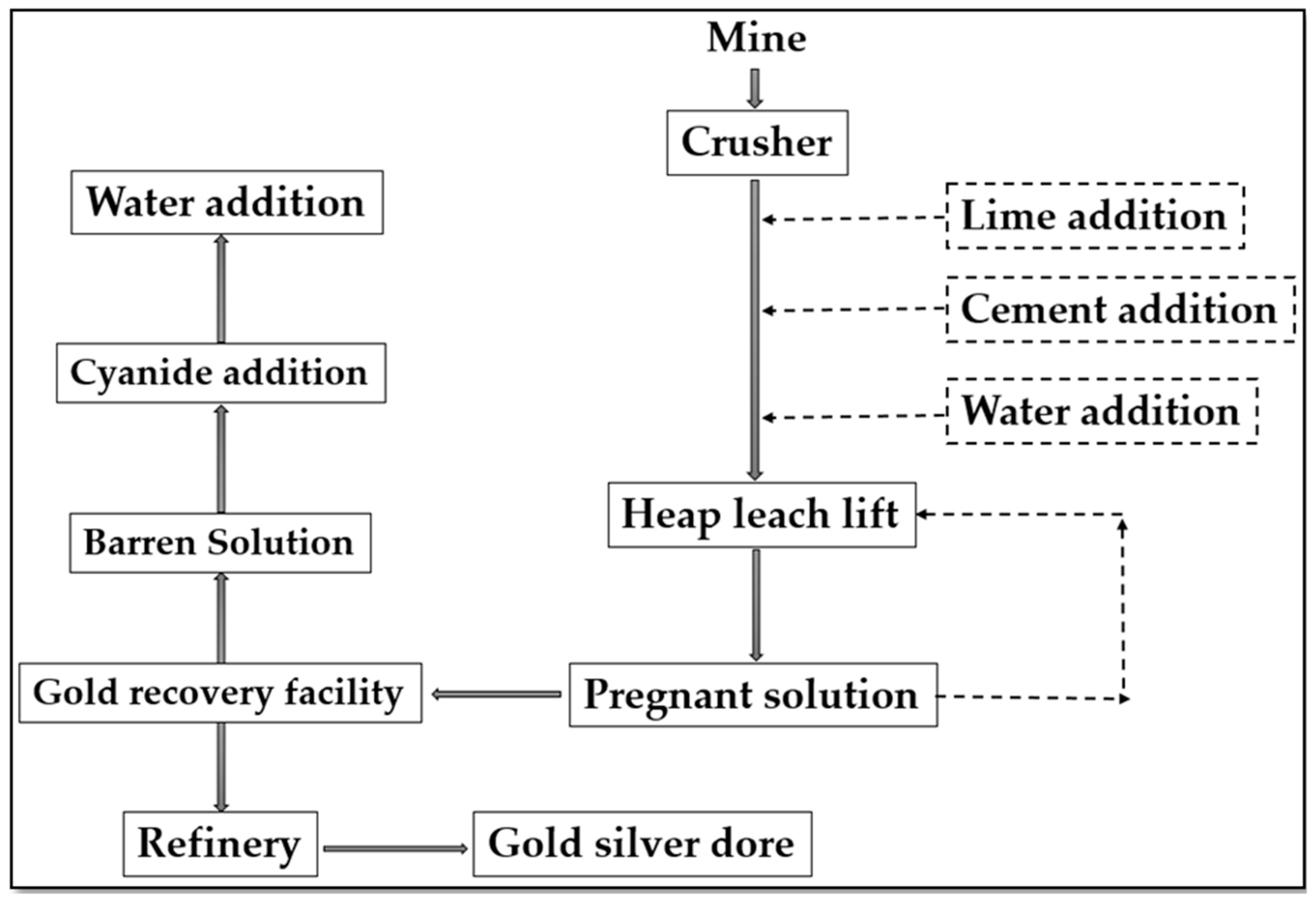
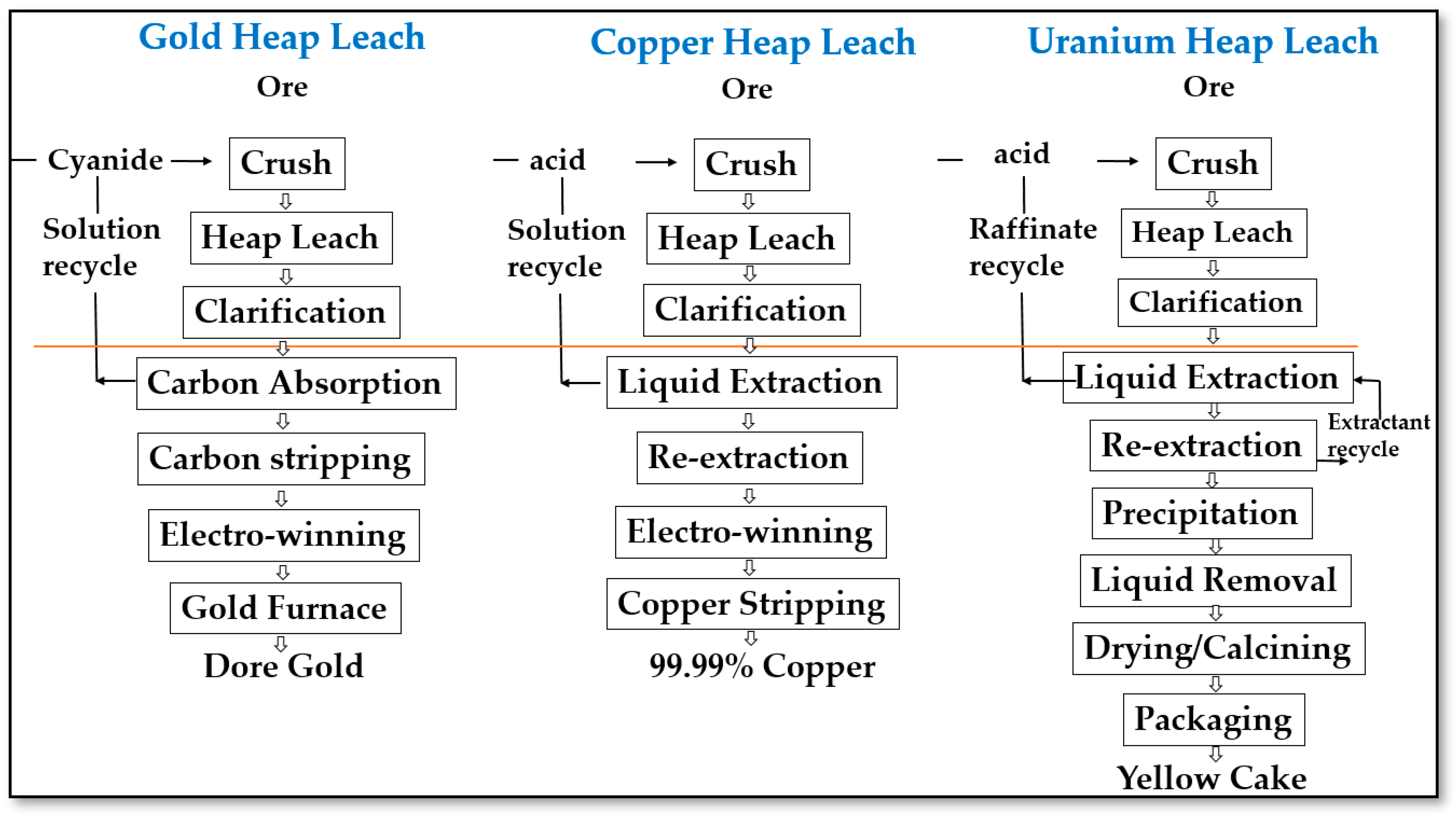
2.2. Heap Leaching of gold and silver
2.3. Heap Leaching of Copper
2.4. Heap Leaching of Uranium
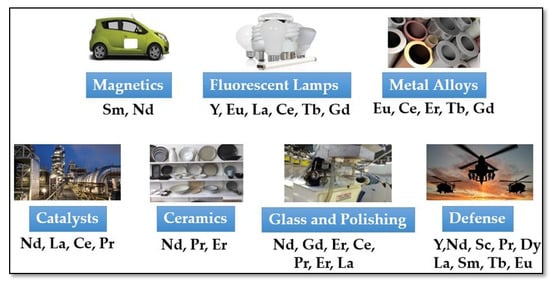
3. Rare Earth Elements Recovery by Heap Leaching
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kappes, D.W. Precious metal heap leach design and practice. In Mineral Processing Plant Design, Practice, and Control; Mular, A.L., Haibe, D.N., Barrett, D.J., Eds.; Society for Mining, Metallurgy, and Exploration: Denver, CO, USA, 2002; Volume 2, pp. 1606–1630. ISBN 0-87335-223-8. [Google Scholar]
- Terry, M.; David, P. Experience-based approach to successful heap leach pad design. Min. World 2015, 12, 28–35. [Google Scholar]
- Caner, Z. Heap leaching technique in mining, within the context of best available techniques (BAT), Report. Euromines 2013, 1–33. Available online: http://www.euromines.org/files/mining-europe/mining- techniques/batforheapleaching-feb2013-c.zanbak-euromines.pdf (accessed on 6 June 2019).
- Basov, V. Heap Leach: Mining Breakthrough Technology. Min. Com News 2015, 1–4. Available online: http://www.mining.com/heap-leach-minings-breakthrough-technology/ (accessed on 6 June 2019).
- John, C. Small but mighty. Int. Min. 2012, 26–65. Available online: https://im-mining.com/ (accessed on 10 June 2019).
- Breitenbach, A.J. Overview study of several geomembrane liner failures under high fill load conditions. In Geosynthetics’97 Conference Proceedings; Industrial Fabrics Association International: Long Beach, CA, USA, 1997; pp. 1045–1062. [Google Scholar]
- Senninger Mining Solutions. Heap Leaching Guide. 2012, pp. 1–6. Available online: http://www. synsamm.co.za/wp-content/uploads/2018/09/Heap-Leaching-Brochure-English.pdf (accessed on 6 June 2019).
- Bartlett, R.W. Metal extraction from ores by heap leaching. Met. Mater. Trans. B 1997, 28, 529–545. [Google Scholar] [CrossRef]
- John, L.U. Pressure leaching coming of age or has it been here for along time? Rpm Glob. Perspect. 2015, 130, 1–3. [Google Scholar]
- Michael Kerr, E.; Aurora, I. Polymeric Combinations Used as Copper and Precious Metal Heap Leaching Agglomeration Aids. U.S.Patent 5,833,937, 10 November 1998. [Google Scholar]
- Anthony, S.; Purkiss, R. Heap Leaching Base Metals from Oxide Ores. Patent WO2004031422A1, 10 January 2004. [Google Scholar]
- Qin, W.Q.; Li, W.Z.; Lan, Z.Y.; Qiu, G.Z. Simulated small-scale pilot plant heap leaching of low-grade oxide zinc ore with integrated selective extraction of zinc. Miner. Eng. 2007, 20, 694–700. [Google Scholar] [CrossRef]
- Sherlock, W.K. Issues affecting heap biooxidation of low-grade refractory gold ore:formation of secondary sulfates, ore lithology, alteration and sulfide mineralogy at gold quarry, Carlin, Nevada. Master’s Thesis, University of Nevada, Reno, Reno, NA, USA, June 2010. [Google Scholar]
- Mellado, M.E.; Gálvez, E.D.; Cisternas, L.A. On the optimization off low rates on copper heap leaching operations. Int. J. Min. Proc. 2011, 101, 75–80. [Google Scholar] [CrossRef]
- Zanbak, C. Heap leaching technique in mining with in the context of best available techniques (BAT)—Introductory Statement by Euromines. Technol. Rep. 2012, 1–33. [Google Scholar] [CrossRef]
- Mwase, J.M.; Petersen, J.; Eksteen, J.J. A conceptual flow sheet for heap leaching of platinum group metals (PGMs) from a low-grade ore concentrate. Hydrometallurgy 2012, 111–112, 129–135. [Google Scholar] [CrossRef]
- Mwase, J.M.; Petersen, J.; Eksteen, J.J. A novel sequential heap leach process for treating crushed Plat reef ore. Hydrometallurgy 2014, 141, 97–104. [Google Scholar] [CrossRef]
- Trujilloc, J.Y.; Cisternas, L.A.; Gálveza, E.D.; Mellado, M.E. Optimal design and planning of heap leaching process. Application to copper oxide leaching. Chem. Eng. Res. Des. 2014, 92, 308–317. [Google Scholar] [CrossRef]
- Watling, H.R.; Shiers, D.W.; Collinson, D.M. Extremophiles in mineral sulphide heaps: Some bacterial responses to variable temperature, acidity and solution composition. Microorganisms 2015, 3, 364–390. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current scenario of chalcopyrite bioleaching: A review on the recent advances to its heap-leach technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Nathsarma, K.C.; Srinivasa Rao, K.; Behari Sukla, L. Heap bioleaching of chalcopyrite: A review. Min. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Rautenbach, G.F. Heap Leaching of Copper. Patent WO2015059551A1, 30 April 2015. [Google Scholar]
- Pingitore, N.E. Extraction and Recovery of Yttrium and Rare Earth Elements. U.S. Patent US20160138133A1, 19 May 2016. [Google Scholar]
- Ngantung, B.; Agustin, R. Increasing flux rate to shorten leaching period and ramp-up production. Am. Inst. Phys. (Aip) Conf. Proc. 2017, 1805, 030009. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Franzidis, J.P.; Petersen, J. Heap leaching technology-current state, innovations, and future directions: A review. Min. Proc. Ext. Met. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef]
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores-A brief overview. Hydrometallurgy 2016, 165, 206–212. [Google Scholar] [CrossRef]
- Hernández, I.F.; Ordóñez, J.I.; Robles, P.A.; Gálvez, E.D.; Cisternas, L.A. A methodology for design and operation of heap leaching systems. Miner. Process. Extr. Metall. Rev. 2017, 38, 180–192. [Google Scholar] [CrossRef]
- Svetlov, A.; Seleznev, S.; Makarov, D.; Selivanova, E.; Masloboev, V.; Nesterov, D. Heap leaching and perspectives of bioleaching technology for the processing of low-grade copper-nickel sulfide ores in Murmansk Region, Russia. J. Pol. Min. Eng. Soc. (Inz. Miner.) 2017, 39, 51–59. [Google Scholar] [CrossRef]
- McBride, D.; Gebhardt, J.; Croft, N.; Cross, M. Heap Leaching: Modelling and forecasting using CFD technology. Minerals 2018, 8, 9. [Google Scholar] [CrossRef]
- Tetteh, G.M.; Lartey, E.O. Texture and Gold grade as guides to blending of conglomerate ore. Int. J. Sci. Technol. Res. 2018, 7, 61–66. [Google Scholar]
- Brown, P. Alous Copper-Silver mine project scoping study; D114506/R17712; Odyssey Resources Ltd.: Longueuil, QC, Canada, 2007; pp. 1–68. [Google Scholar]
- Sulliden. Clear Path to Production for Shahuindo: A Low Cost Heap Leach Gold Project in Peru; Resource investing in Latin America and Canada; Ministry of Mines and Energy, Peru and National Society of Mining, Oil, & Energy: Lima, Peru, 2013; pp. 1–51.
- Star Gold Inc. Scoping Study, Longstreet Gold Project, Nye County, NV, USA; A-Z Mining Professionals Limited: Thunder Bay, ON, Canada, 2014; pp. 1–110. [Google Scholar]
- Crundwell, F. Process and Economic Considerations in Copper Metallurgy; CMS olutions Pvt.Ltd.: Corinth, Greece, 2006; pp. 1–53. [Google Scholar]
- Stange, W. The process design of gold leaching and carbon-in-pulp circuits. J. South Afr. Inst. Min. Metall. 1999, 13–26. Available online: https://www.saimm.co.za/Journal/v099n01p013.pdf (accessed on 6 June 2019).
- Mcewen Mining Inc. Preliminary Economic Assessment for the Fenix Project at Mcewen Mining Inc.’s Operations in Sinaloa, Mexico; Mcewen Mining Inc.: Belmont, Australia, 2018; pp. 1–330. [Google Scholar]
- Barr, G.; Defreyne, J.; Mayhew, K. CESLcopper process—An Economic Alternative to Smelting. CESL Engineering. 2005, pp. 1–13. Available online: https://www.teck.com/ media/ CESL- Publication- Copper- Process-Intermin-2005.pdf (accessed on 6 June 2019).
- Fleming, C.A. Platsoltm Process Provides a Viable Alternative to Smelting; SGS Minerals Services, Technical paper 2002-01; SGS A.G.: Geneva, Switzerland, 2002; pp. 1–5. [Google Scholar]
- Anderson, C.G. The optimization, design and economics of industrial NSC oxidative pressure leaching of complex sulfide concentrates. Int. J. Eng. Sci. 2013, 2, 1–16. [Google Scholar]
- Heinen, H.J.; Peterson, D.G.; Lindstrom, R.E. Processing Gold Ores Using Heap Leach Carbon Adsorption Methods; IC8770; U.S. Department of the Interior, Bureauof Mines: Reno, NV, USA, 1978; pp. 1–21.
- Hoye, R. Gold/Silver Heap Leaching and Management Practices that Minimize the Potential for Cyanide Releases; EPA/600/2-88/002; Final Report; U.S. Environmental Protection Agency: Washington, DC, USA, 1987; pp. 1–113.
- Technical Note, Mining Heap Leach Pads and Tailings Dams, Solmax International Technical Notes. Available online: https://www.solmax.com/en (accessed on 6 June 2019).
- Donald, I.B. Estimated Water Requirements for Gold Heap-Leach Operations. (ver.1.1,11 December2012): U.S. Geological Survey Open-File Report 2012–1085. pp. 1–17. Available online: http://pubs.usgs.gov/ of/ 2012/1085 (accessed on 6 June 2019).
- John, O.M.; House, C.I. The Chemistry of Gold Extraction; Society for Mining Metallurgy, and Exploration Inc.: Littleton, CO, USA, 2006. [Google Scholar]
- Leong Eugene, W.W.; Mujumdar, A.S. Gold Extraction and Recovery Processes; The National University of Singapore: Singapore, 2010; pp. 1–20. [Google Scholar]
- Padilla, G.A.; Cisternas, L.A.; Cueto, J.Y. On the optimization of heap leaching. Miner. Eng. 2008, 21, 673–678. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Neethling, S.J. The effect of particle porosity on liquid hold up in heap leaching. Miner. Eng. 2013, 45, 73–80. [Google Scholar] [CrossRef]
- Arias, J.A. Heap Leaching Copper ore Using Sodium Nitrate. U.S. Patent US6,569,391B1, 27 May 2003. [Google Scholar]
- Mbuyu, L.; Kasonde, M.; Kitala, K.; Lwambiyi, N.M.; Mukulu, D. Investigation in to the heap leaching of copper ore from the disele deposit. Hydrometallurgy 2009, 98, 177–180. [Google Scholar] [CrossRef]
- Matthias, V.; Andreas, L.; Bernd, F.; Hans-Peter, S.E. Improvements in copper heap leaching by use of wetting agents. Proc. EMC 2009, 1–12. [Google Scholar] [CrossRef]
- Tomina, V.N.; Khrennikov, A.A.; Lebed, A.B.; Naboichenko, S.S. Heap leaching of copper from the ores of Volkovskoe deposit. Russ. J. Non-Ferrous Met 2010, 51, 263–267. [Google Scholar] [CrossRef]
- Kimberly, M.; Frank, F. Conventional Heap Leaching of Uranium ore in the Western United States. Available online: https://www.korsika-traumurlaub.de/2017-Nov/heap- leaching-uranium.html; www.infomine.com. (accessed on 6 June 2019).
- Mario, E.M.; Freddy, A.L.; Luis, A.C.; Edelmira, D.G.; Felipe, D.S. A posteriori analysis of analytical models for heap leaching using uncertainty and global sensitivity analyses. Minerals 2018, 8, 44. [Google Scholar] [CrossRef]
- Henry, S. International Atomic Energy Agency, Building uranium heap leach project. In Proceedings of the URAM Conference, Vienna, Austria, 23–27 June 2014; pp. 1–38. [Google Scholar]
- Vladimiros, G.P.; Georgiana, M. Recovery of rare earth elements from clay minerals. In Proceedings of the ERES 2014: 1st European Rare Earth Resources Conference, Milos Island, Greece, 4–7 April 2014; pp. 1–12. [Google Scholar]
- Chi, R.; Zhang, Z.; Xu, Z.; He, Z.; Ruan, Y. Novel solution injection technology for in-situ leaching of weathered crust elution-deposited rare earth ores. In Proceedings of the 53rd Conference of Metallurgists (COM2014), Metallurgical Society of the Canadian Institute of Mining, Metallurgy and Petroleum (MetSoc-CIM), Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- Vahidi, E.; Navarroc, J.; Zhao, F. An initial life cycle assessment of rare earth oxides production from ion-adsorption clays. Resour. Conserv. Recycl. 2016, 113, 1–11. [Google Scholar] [CrossRef]
- Rick, Q.H.; Wencai, Z.; Xinbo, Y.; Mohammad, R. Concept ion of an integrated flow sheet for rare earth elements recovery from coal coarse refuse. Miner. Eng. 2018, 122, 233–240. [Google Scholar] [CrossRef]
- Sierra, B. Impressive Heavy rare Earth Recoveries Increase Confidence in Heap Leach Processing Option; Texas Rare Earth Resources Corp.: Sierra Blanca, TX, USA, 2013. [Google Scholar]
- Free, M.; Sarswat, P.; Luttrell, G.; Noble, A.; Hu, X.; Allen, L.; McNeill, M.; Kim, D.J.; Arce, I. Economic extraction, recovery, and upgrading of rare earth elements from coal-based resources. In Proceedings of the 2018 AIChE Annual Meeting, Pittsburgh, PA, USA, 1 November 2018. [Google Scholar]

| Method | Extraction Metal | Summary | Reference |
|---|---|---|---|
| Heap leaching | Precious metals from mineral fines | Leaching has been used principally in connection with low-grade copper ores or pit wastes. | Michael Kerr et al., 1998 [10] |
| Heap leaching | Base metals from oxide ores | 75–82% of Nickel recovery was achieved in 160 days to 266 days, 90% Cobalt recovery was achieved in 14 days, Iron recovery (53.6%) was achieved in 198 days at ambient temperatures | Anthony et al., 2004 [11] |
| Heap leaching | Zn (Zinc) | The 95% of zinc recovery was possible in 16 days cycle at 25°C by column (heap) leaching. | Wen-qing et al., 2007 [12] |
| Heap leaching bio oxidation | Gold | 49–61% of gold was recovery by bio oxidation process at 81°C. The bio oxidation process was for gold recovery was taken 150 days. | Wes K. Sherlock 2010 [13] |
| Heap leaching with computation process | Copper | 71–73.5% of copper was recovery by developed a new heap leaching methodology with the combining analytical modelling at optimal flow rates. | Mario E. Mellado et al., 2011 [14] |
| Heap Leaching | Gold | 30–95% of gold was recovery by best available technology heap leaching compared to other techniques. | Caner Zanbak., 2012 [15] |
| Heap leaching | Platinum group metals (PGMs) and base metals (BMs) from a low grade flotation concentrate of PGM concentrator plants. | The extractions of 52% Cu, 95% Ni and 85% Co were achieved in 30 days (65°C) by heap bioleach. If cyanide leach process (23°C) can be operated in 21 days, 20.3% Pt, 87% Pd and 46% Rh, if 50 days or more to achieve 50% platinum. | Mwase et al., 2012 [16] |
| Sequential heap leaching | Platinum group metals and particularly for palladium | At 65 °C, 93% Copper, 75% Ni and 53% Co extracted by bio heap leaching in the 304 days. By cyanide leach experiment, 57.8% Pt, 99.7% Pd and 90.3% Au was extracted at 50°C in 60 days. | Mwase et al., 2014 [17] |
| Heap leaching with mathematical modeling | copper | By the Mellado et al., method for the optimal design of heap leaching, 53–56% copper recovery was possible in a61–67 days. | Jorcy Y. Trujillo et al., 2014 [18] |
| Heap bioleaching | Cu, metal extracted from reduced inorganic sulfur compounds. | Over 60% of Cu, extraction was possible by bio heap leaching at 45 °C during the 30 –48 days. | Watling et al., 2015 [19] |
| Heap leaching | Copper from ore | 73% of Cu recovery was achieved in 140 days at 25 °C. | Rautenbach, 2015 [22] |
| Heap leaching for rare earths extraction | Heavy rare earths and Yttrium | 91.3% and 87.2% of Yttrium and dysprosium achieved by heap leaching for60 days, respectively, at room temperatures. | Pingitore Nicholas et al., 2016 [23] |
| Heap leaching with increasing flux rate | Gold | 73–87% of gold extraction was achieved by using heap leach process with increasing flux rate in the 40 to60 days. | Ngantung, 2017 [24] |
| Heap leaching with the new model (MINLP) and GAMS software | Copper | 69.7–76.7% of copper recovery was obtained from 19.5–43.5 days with the new mathematical modeling named mixed integer nonlinear programming (MINLP) including GAMS software (general algebraic modeling system). | Isis F. Hernández et al., 2017 [27] |
| Heap bioleaching | Nickel | 60% recovery of nickel from the tailings for 110 days. | Anton Svetlov et al., 2017 [28] |
| Heap leaching with computational fluid dynamics model | Copper | 55% of copper was recovered in the 700 days cycle at the temperatures from 12–45°C. | Diane McBride et al., 2018 [29] |
| Metal Name | Heap Leaching Capital Expenditure (CAPEX)US$/t ore | Heap Leaching Operating Expenditure (OPEX) US$/t ore | Tank Leaching Capital Expenditure (CAPEX)US$/t ore | Tank Leaching Operating Expenditure (OPEX) US$/t ore | Autoclave Leaching Capital Expenditure (CAPEX)US$/t ore | Autoclave Leaching Operating Expenditure (OPEX)US$/t ore |
|---|---|---|---|---|---|---|
| Copper | 29.5 | 4.6 | 25 | 66 | 75 | 19 |
| Gold | 22 | 4.51 | 40.9 | 22.28 | 492 | 8.20 |
| Silver | 22.50 | 14.87 | 40.9 | 35.96 | 17.40 | 82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thenepalli, T.; Chilakala, R.; Habte, L.; Tuan, L.Q.; Kim, C.S. A Brief Note on the Heap Leaching Technologies for the Recovery of Valuable Metals. Sustainability 2019, 11, 3347. https://doi.org/10.3390/su11123347
Thenepalli T, Chilakala R, Habte L, Tuan LQ, Kim CS. A Brief Note on the Heap Leaching Technologies for the Recovery of Valuable Metals. Sustainability. 2019; 11(12):3347. https://doi.org/10.3390/su11123347
Chicago/Turabian StyleThenepalli, Thriveni, Ramakrishna Chilakala, Lulit Habte, Lai Quang Tuan, and Chun Sik Kim. 2019. "A Brief Note on the Heap Leaching Technologies for the Recovery of Valuable Metals" Sustainability 11, no. 12: 3347. https://doi.org/10.3390/su11123347
APA StyleThenepalli, T., Chilakala, R., Habte, L., Tuan, L. Q., & Kim, C. S. (2019). A Brief Note on the Heap Leaching Technologies for the Recovery of Valuable Metals. Sustainability, 11(12), 3347. https://doi.org/10.3390/su11123347