1. Introduction
The demand for rare earth elements (REEs) has grown due to their wide applications in the technologies of the 21st century. In 2017, the raw material supply group of the European Commission compiled a list of 41 elements or groups of elements considered critical [
1]. Among these elements, heavy and light REEs as well as scandium were highlighted because of the supply risk due to their rarity and their high impact on the global economy. The availability of technology to separate REEs from one another is also among the important factors accounting for the high cost of some REEs [
2]. Therefore, new sources of REEs need to be identified to enhance the global supply. Many researchers have identified and extracted REEs from low-grade REE materials such as power plant coal, hard coal fly ash, bauxite, coal deposits, and waste fly ash or by recycling electric and electronic equipment waste using various methods [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. Many processes such as subsequent solvent extraction, liquid-liquid extraction, acid leaching, etc. have been developed for rare earth extraction [
10,
15]. However, the presence of large amount of iron in the leached solution is still challenging for researchers [
15]. Moreover, finding a relatively low-cost process, which could selectively recover REEs, is of importance in the chemical engineering field.
In this study, a combination of sulfation, roasting, and leaching processes was thoroughly examined to recover REEs from bottom ash (BA) during the final stage by precipitation in order to find out a low-cost method for recovery of REEs in low concentrated secondary material. Ammonium oxalate was used as a leaching reagent; note that simultaneously, the affinity of this acid may induce the precipitation of REEs [
16,
17]. Roasting temperature, leaching time, and ammonium oxalate concentration are the main parameters, which were noted to study the selectivity of the process.
2. Materials and Methods
2.1. Materials
BA was collected from a recycling company in Japan (For confidential reason, the company will be unnamed). The change in structure with increasing temperature of the sulfated BA was investigated using a thermo-gravimetric differential thermal analyzer (TG-DTA) under ambient air flow, from room temperature up to 1000 °C, with a heating rate of 10 °C/min (Thermo plus EVO TG8120/Rigaku). Sulfated BA was roasted using an electric furnace (HPM-OG/AS ONE). The concentration of REEs as well as other metals in the solution was analyzed using an inductively coupled plasma-optimal emission spectrometer (ICP-OES; Perkin Elmer Optima 5300). Characterization of the powder samples was performed using X-ray diffraction (XRD) and X-ray fluorescence (XRF).
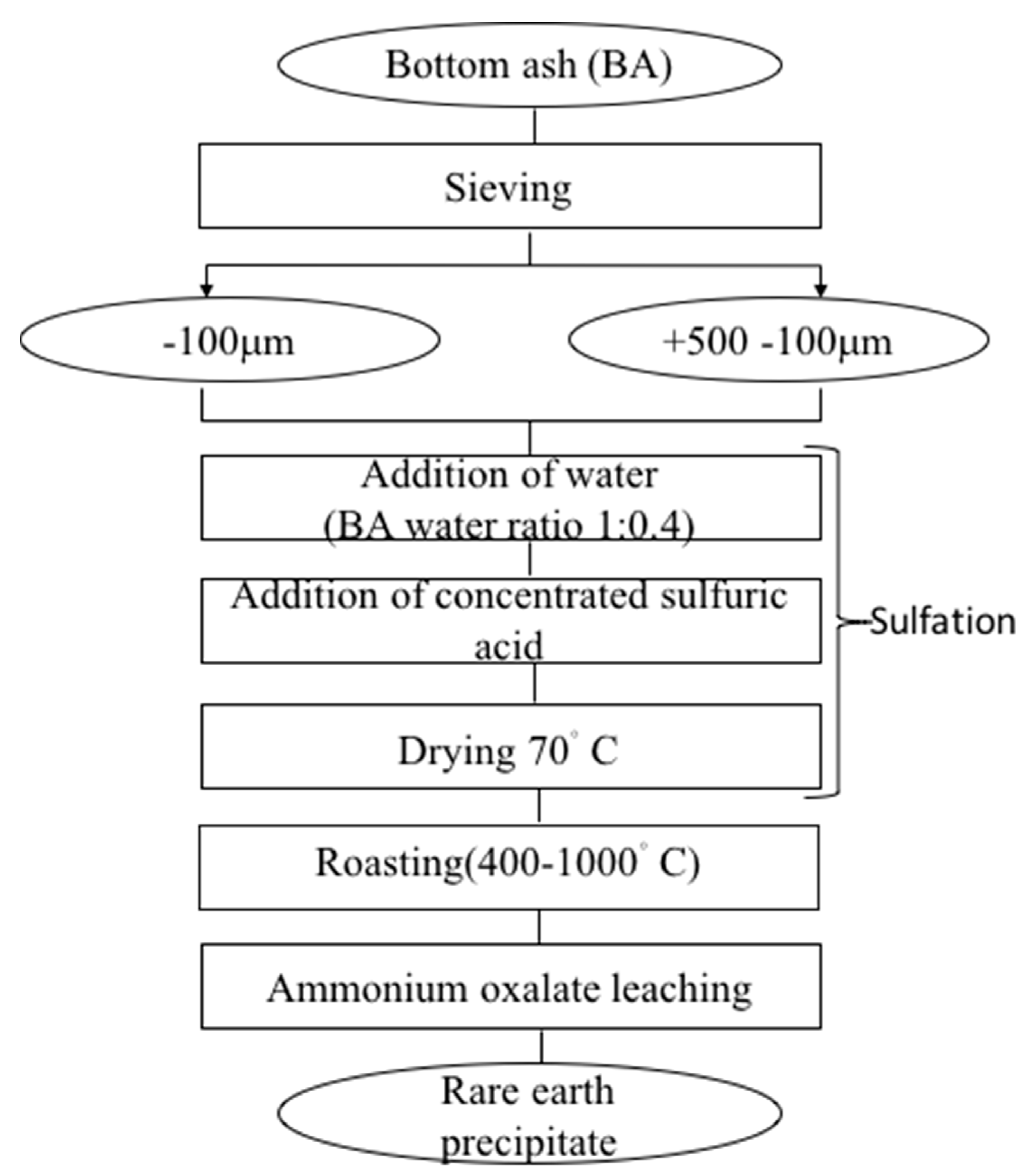
2.2. Sulfation–Roasting–Leaching–Precipitation
Sulfation–roasting–leaching–precipitation (SRLP) is a combination of four consecutive and complementary processes. The original sample was first crushed and passed through 500 μm and 100 μm sieves to obtain two samples (+100–500 μm and −100 μm). As described by Borra et al., both dried samples were moistened (the BA-to-deionized water mass ratio was 1:0.4) to ensure homogeneous mixing with acid, and thus aid in the conversion of metal oxides to sulfates [
5,
18]. After moistening, the samples were mixed with 64% sulfuric acid (sample-to-acid mass ratio was 1:1), as this ratio was found to ensure good dissolution of REEs while not excessively increasing the dissolution of iron [
5]. The mixtures were dried at 70 °C for 24 h to ensure complete sulfation. During this step, metals are converted into their respective sulfates. After drying, the samples were roasted at selected temperatures in an electric furnace. Some of these sulfates, the iron sulfates in particular [
19], are unstable at elevated temperatures, and consequently decompose during the roasting step into water-insoluble oxides [
5]. Rare earth sulfates, conversely, remain stable at high temperature and are water-soluble [
20]. After roasting, the samples were ground to obtain homogeneous powders. Ammonium oxalate leaching experiments were conducted at 25 °C and 300 rpm and the sample dosage was maintained at 67 g/L. Unless specified, the concentration of the ammonium oxalate was 50 g/L. After leaching, the solutions were filtered with 0.20 μm filter paper. The obtained filtrate was analyzed using an ICP-OES.
During the subsequent leaching step, the metal oxides remain in the solid residue, whereas the rare earth sulfates dissolve in the solution. Typically used to precipitate REEs, ammonium oxalate is, in this study, used for both leaching and selective precipitation of REEs [
16,
17] (its affinity to rare earth elements may confer a property of good leaching reagent for REEs). Therefore, after reaching the optimal conditions to extract as many REEs as possible, the leached solution was kept for seven days until precipitation occurred, and the remaining solution was filtered and analyzed. In addition, to identify the optimal temperature at which the iron sulfate decomposition may be completed, sulfated samples were subjected to thermal decomposition from room temperature to 1000 °C using TG-DTA. All the experiments were repeated several times to avoid discrepancies in the results. The flowsheet of the experimental procedure is shown in
Figure 1.
3. Results and Discussion
3.1. Sample Characterization
As-received BA was ground using a planetary mill and sieved to produce two different size fractions, i.e., −100 μm and +100–500 μm. To compare the elemental composition of the samples, each size fraction was analyzed using XRF, and the REE composition was detected using an ICP-OES. The results of the present study were compared to previous studies as shown in
Table 1. Compared to previous low-grade materials, the BA used in the present work is more concentrated in REEs, particularly erbium (Er). Aluminum oxide, calcium oxide, iron oxide, and silica account for nearly 83% of the total mass of the +100–500 μm BA, and are therefore the dominant metals. Copper, chrome, and zinc oxide are less dominant. The REEs are more concentrated in the size fraction +100–500 μm with erbium as the dominant element. Therefore, analysis in the present work will consider aluminum, calcium, and iron oxides as the dominant metals, chromium and copper oxides as less dominant metals, and erbium (Er) as REEs. Furthermore, sample with the size fraction of +100–500 μm was used.
3.2. Thermo-Gravimetric Differential Analysis of the Sulfurized Sample
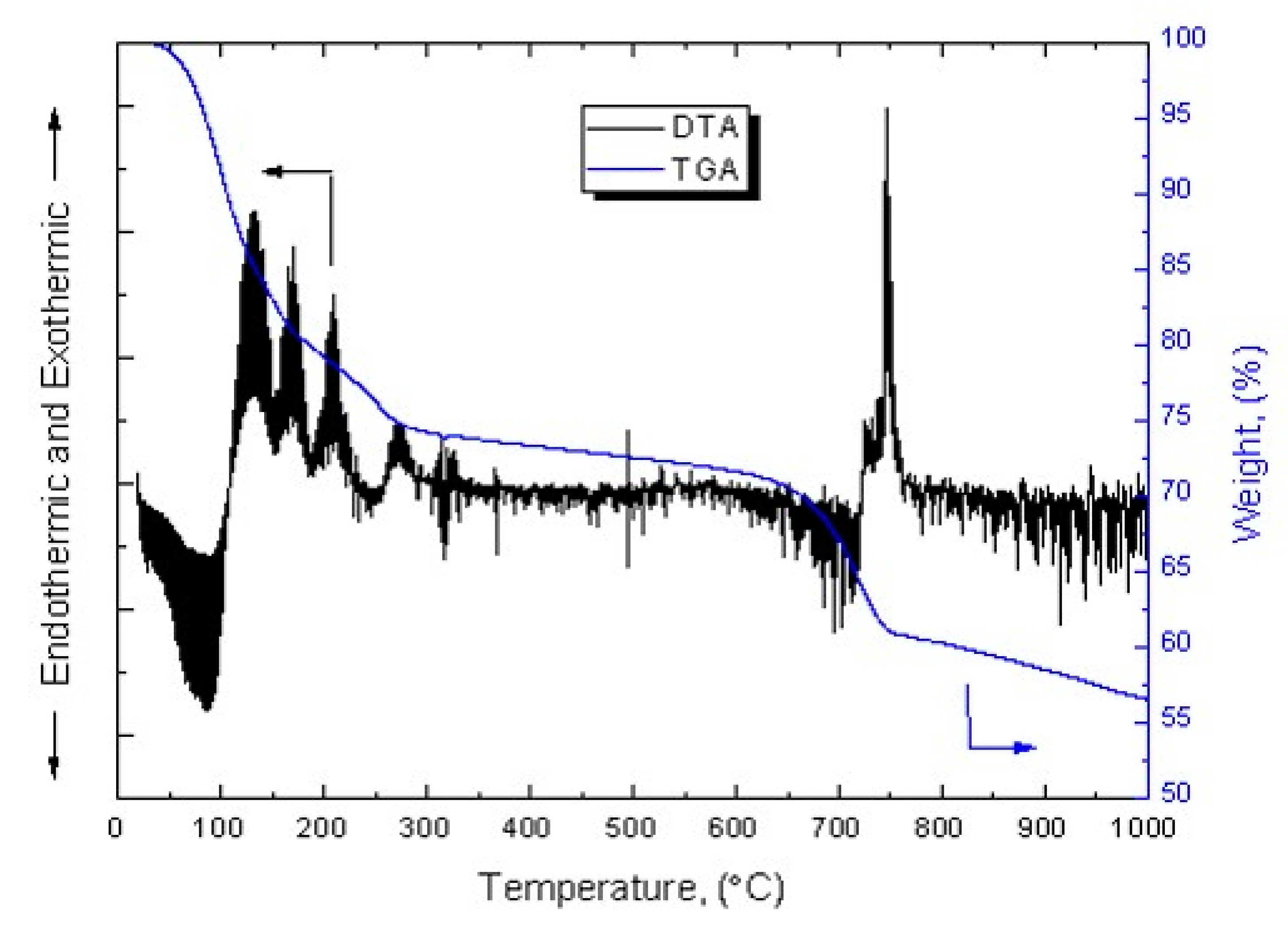
As suggested by Borra et al. (2016) [
5], +100–500 μm sieved BA was sulfurized with 64% sulfuric acid at a mass ratio of 1:1 and dried for one day. The obtained sample was termed “sulfurized sample” or “70 °C”. Prior to roasting, the sulfurized sample was subjected to thermal decomposition at a rate of 10 °C/ min using TG-DTA. The results are shown in
Figure 2. The TGA thermogram indicates the weight loss in the sample and that of the DTA shows the phase changes in the sample with increasing temperature. Until 300 °C, the sample loses approximately 25% of its original weight and some peaks appears in the DTA thermogram. Meaning that most sulfates lose their physical and chemical bound water molecules until 300 °C [
5]. From 300–650 °C, no significant changes were observed. From 650–750 °C, the sample once again lost 10% of its weight followed by the appearance of a significant peak at 750 °C. Borra et al. (2016) attributed these changes to the decomposition of iron sulfate into iron(III) oxide [
5].
3.3. Roasting Process
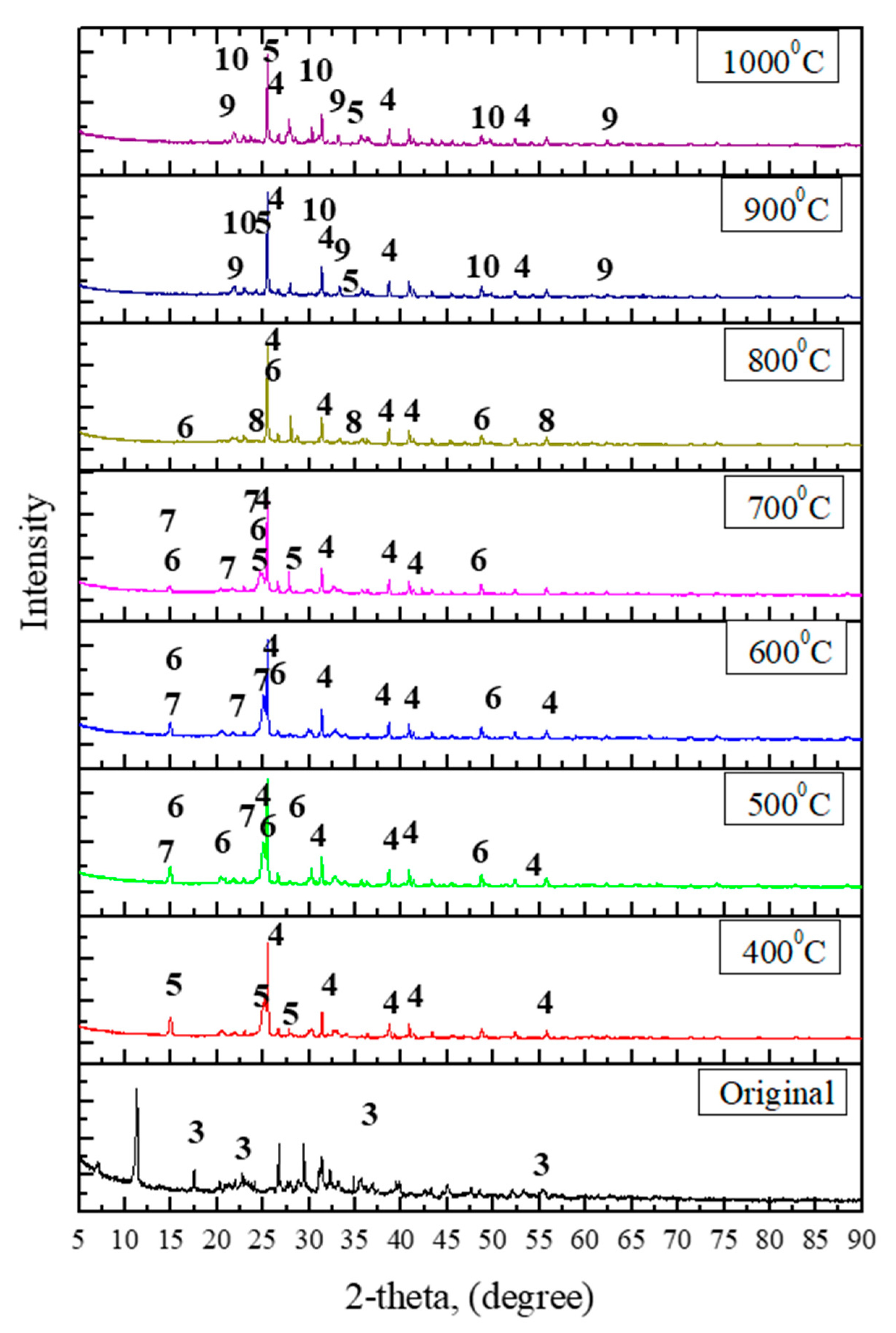
To understand the progressive changes occurring with increasing roasting temperature and their effect on the leaching process, sulfurized bottom ash was roasted at 400 °C, 500 °C, 600 °C, 700 °C, 800 °C, 900 °C, and 1000 °C, respectively, for an hour in the electric furnace. With increasing temperature, a progressive change in color is observed, which may indicate a phase changes in the material. The sample color shifts from white to brown from 600 °C to 700 °C, indicating the partial decomposition of iron sulfate into iron(III) oxide. As shown by the XRD patterns (
Figure 3), iron sulfate is totally decomposed into iron(III) oxide from 800 °C. These results are in accordance with those observed using TG-DTA. Calcium sulfate and aluminum sulfate were formed during the early stage and remained almost stable. REEs were not observed because their concentration was too low to be detected by the instrument.
3.4. Leaching Process
Ammonium oxalate is among the reagents that shows a high affinity to precipitate REEs. In this study, ammonium oxalate was used for the lixiviation of REEs during the first step. Leaching of sulfurized samples using deionized water was also tested to compare both results. During the second step, the leached solution was kept at room temperature for a period of time to ensure the precipitation of REEs, particularly erbium. Sulfurized samples are indicated in the graphs as 70 °C. The concentration of ammonium oxalate as well as the leaching time as a function of roasting temperature were the main parameters investigated. Since ammonium oxalate has particular affinity to REEs, REEs are selectively leached and precipitate as solid oxalate salts as shown in equation (1).
3.4.1. Effect of Roasting Temperature on the Leaching Process
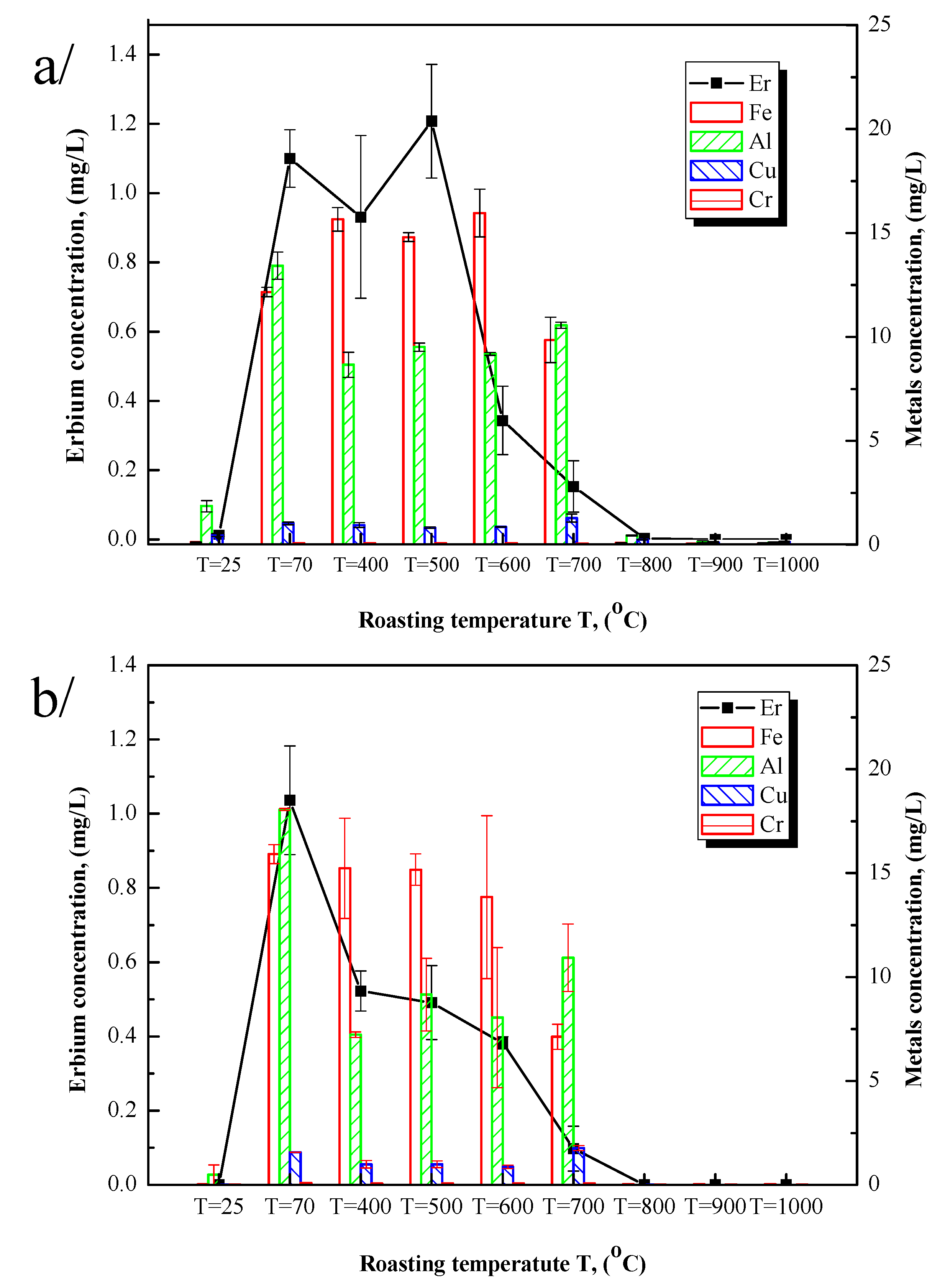
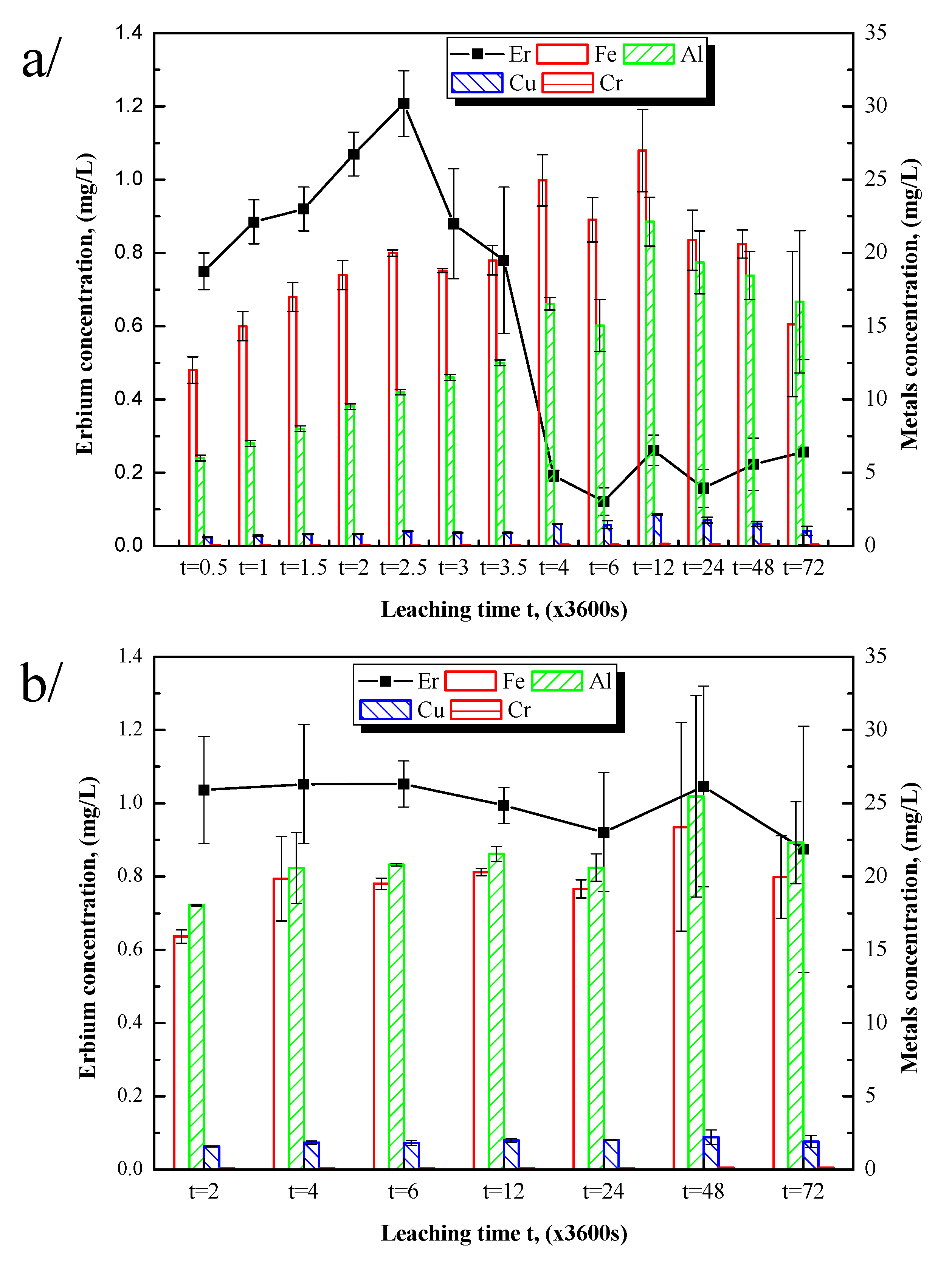
To note the optimal roasting temperature at which Er (the most abundant rare earth element in the material) and other metals will reach their optimal leaching conditions, samples were roasted at 400 °C, 500 °C, 600 °C, 700 °C, 800 °C, 900 °C, and 1000 °C, respectively, and the sulfurized sample (70 °C) and the original were used. The ideal conditions for selectivity were to obtain a high concentration of Er and a low concentration of metals in the solution. Experiments were conducted for 2 h. Ammonium oxalate leaching as well as water leaching were both done. Er and metal concentrations in one gram of the sample were reported in
Figure 4.
Figure 4a shows the leaching results using ammonium oxalate, and
Figure 4b indicates the leaching results using the deionized water function of roasting temperatures compared to the original sample.
Figure 4a displays the results suggesting that without sulfuric acid pre-treatment, the leaching ratio is very low, which highlights the importance of the pre-treatment process. The concentration of Er as well as other metals considerably increased during the sulfation process. Sulfation itself increases the dissolution rate of the chemical elements without selectivity. Roasting at 400 °C or 500 °C slightly decreases the metal rate in solution and increases the Er concentration with temperature, showing the affinity of ammonium oxalate with REEs. The dissolution of Er decreases from 600 °C to 700 °C, temperatures at which the decomposition of iron sulfate into iron oxide is ongoing. The dissolution of both REEs and metals is basically non-existent above 700 °C. Below 700 °C, water-insoluble forms of Fe, Al, and Cu are formed, explaining the observed low yield. It was concluded that the entire stability of the metals by roasting, which addresses the water-insoluble forms, is not favorable for the leaching process. These results are in accordance with those reported by Borra et al., (2016) which showed that a higher roasting temperature over a longer duration has a negative effect on the dissolution of REEs because of the increasing number of sulfates that are decomposed to water-insoluble oxy-sulfates and oxides [
5,
18]. Hence, the decrease in the Er yield from 600 °C may be related to the oxidization of rare earth oxides. In conclusion, 500 °C may be the optimal temperature for REE leaching.
Furthermore, the water dissolution results of Er and metals are shown in
Figure 4b. Er leaching decreased with increasing roasting temperature. The sulfation process increased the yield of Er in water, but the roasting temperature had a negative effect on water leaching compared to ammonium oxalate leaching, even when the roasting temperature was less than 700 °C. This result may be due to the sulfates losing their chemical water and becoming increasingly insoluble in water. Therefore, an unroasted sulfurized sample (70 °C) shows the optimal dissolution yield in water.
3.4.2. Effect of Leaching Time on REE Recovery
The leaching time for a 500 °C roasted sample was evaluated every 30 min. The results are shown in
Figure 5a. Er concentration started decreasing after 2 h 30 min of leaching while the metal concentration was still increasing. This phenomenon may be explained by lixiviation followed by precipitation of erbium after 2 h 30 min. Ammonium oxalate is known to show a strong affinity to REEs and is used for REE recovery via precipitation [
16]. Therefore, 2 h 30 min may be the optimal time to leach a 500 °C roasted sample to recover as much as possible REEs by precipitation.
Comparatively, the water dissolution results (
Figure 5b) indicated that the concentration of Er in solution does not vary with time. However, the lowest metal yield was observed after 2 h of contact time. Ammonium oxalate has strong lixiviation and precipitation forces vs. REEs.
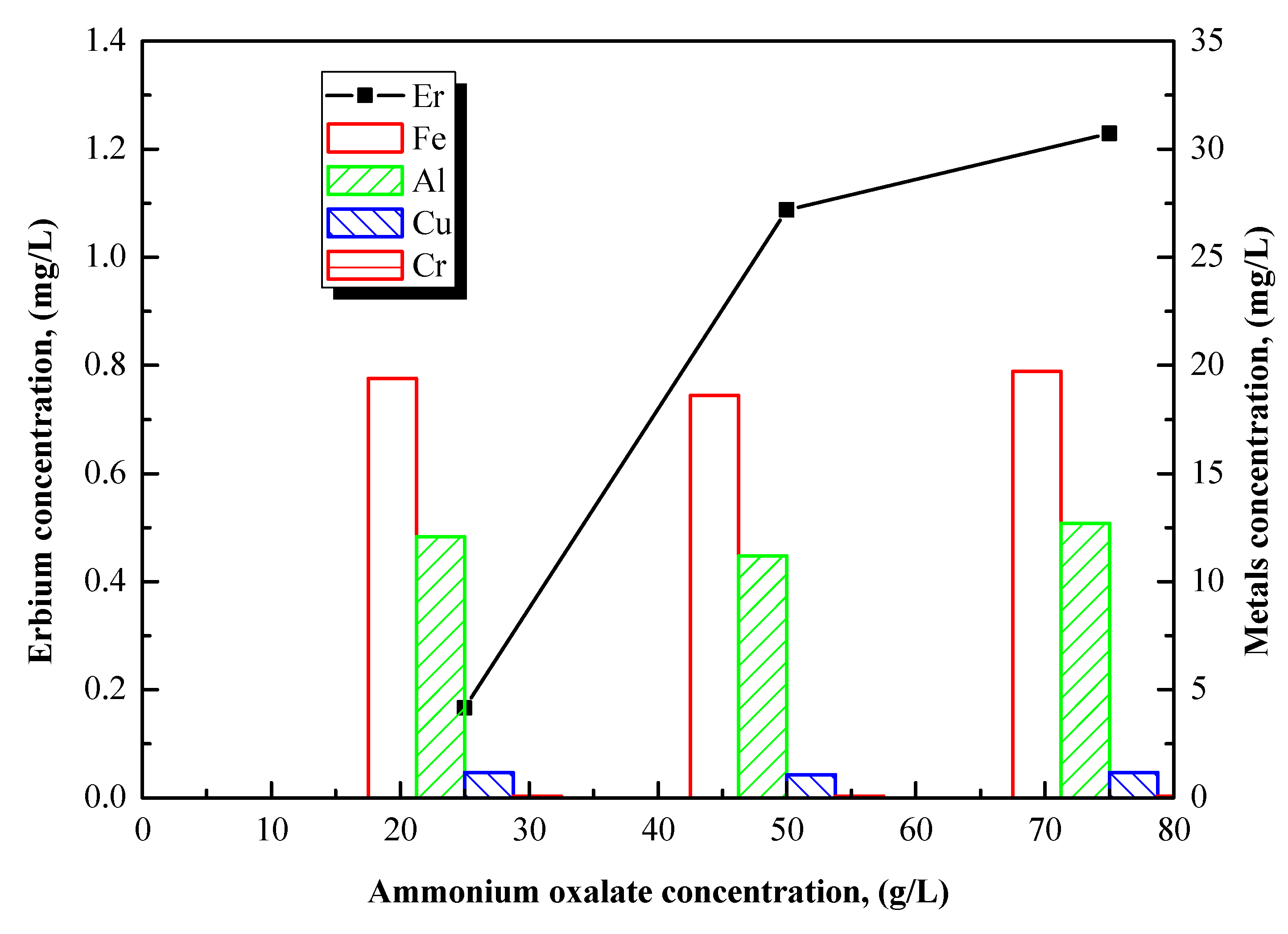
3.4.3. Effect of Leaching Acid Concentration
Leaching experiments were conducted at various concentrations of ammonium oxalate under the optimal conditions previously found: a +100–500 μm particle size fraction, a roasting temperature of 500 °C, and 2.5 h of leaching time.
Figure 6 shows the effect of the ammonium oxalate concentration on Er or other metal recoveries. The ammonium oxalate concentration shows no significant effect on the metal concentration in the solution, but highly affects Er leaching. Er concentrations in solution using 50 g/L or 75 g/L of acid are quite similar, indicating an equilibrium at approximately 50 g/L.
3.5. Precipitation and Recovery of REEs
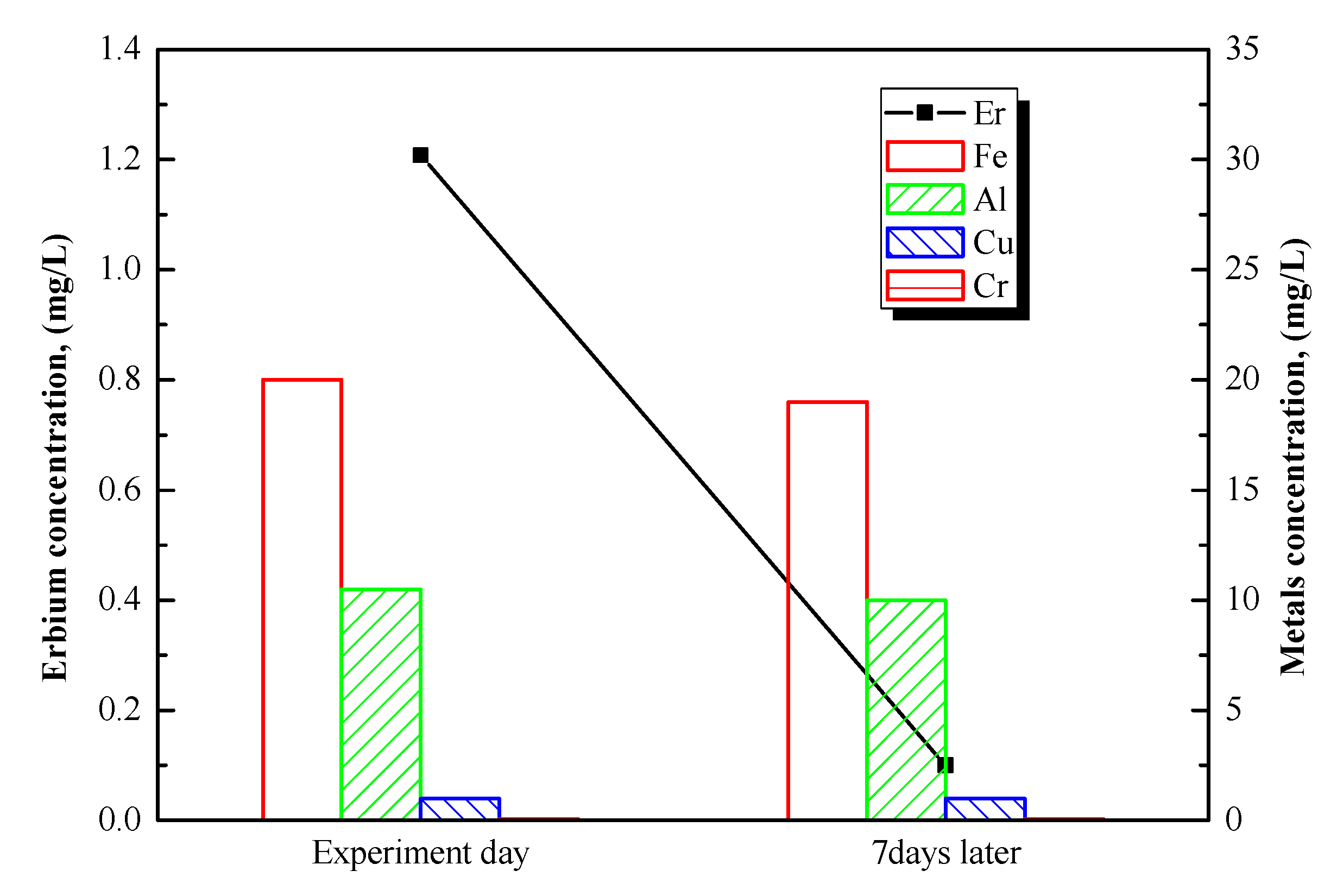
The prepared sample, under the obtained optimal conditions for ammonium oxalate was maintained, at room temperature, to investigate the precipitation design of erbium as well as other metals in the solution.
Figure 7 shows the concentration of erbium and metals in solution on the day of the experiment and after 7 days. As shown previously in
Figure 5a, Er remained in the solution after 3 days of leaching. Therefore, a longer time is required to completely precipitate Er. According to
Figure 7, the original concentration of Er in solution is approximately 1198 ppm, which represents 36.15% of the total erbium present in the 500 °C roasted sulfurized BA (referring to aqua regia leaching). When 94.24% of the Er in the solution precipitated, 97.21% of Fe, 94.13% of Al, and 70.74% of Cu remained in the solution. Therefore, a high purity of Er sulfate precipitate could be separated from Fe, Al, and Cu. A longer maintenance time may increase the concentration of Fe in the precipitate. The selective recovery of Er was effective by sulfation–roasting–leaching–precipitation process.
4. Application, Limitation, and Perspectives
Bottom ash is an important secondary source of metals. In Japan, 80% of bottom ash is still landfilled without any recycling process [
21] due to the fact that the ash contains a high concentration of heavy and other metals, which do not fit the Japanese industrial standards to be reuse as cement kiln, etc. Therefore, in addition to previous authors’ works [
22,
23] concerning the removal of heavy metals from ashes, erbium recovery from bottom ash gives hope to the REE industry as well as the waste management field. Erbium which finds its main application in optical fiber fabrication is really on high demand in REE market. The present work findings could be an alternative to pre-concentrate erbium from bottom ash. However, only 36.15% of the erbium containing, in the bottom ash could be leached. Repeating experiments could enhance the leaching rate. 7 days are enough to recover REE precipitates with fewer metals content. Beyond 7 days, metals especially iron in solution precipitates progressively and a complex of rare earth iron may be formed. To avoid this phenomenon, the precipitation duration should be strictly respected. Furthermore, more purification processes of the rare earth precipitate need to be undergone to obtain a high purity erbium powder which could be reused in high technologies.
5. Conclusions
Sulfation, roasting leaching, and precipitation were applied to BA to selectively recover rare REEs, particularly Er. XRF analysis of the BA showed a high concentration of aluminum oxide, iron oxide, and calcium oxide. Further analysis indicated some trace of REEs, of which Er was abundant. The sulfation process induced the formation of sulfate molecules of Al, Fe, and Cu, which were transformed into their most stable forms with an increasing roasting temperature up to 600 °C. Iron(III) oxide in 800 °C roasted sample was observed in the XRD patterns.
Analysis of the effect of Er leaching using ammonium oxalate on the roasting temperature pointed out 500 °C as the optimal temperature to selectively recover Er. Roasting above 600 °C considerably decreased the leaching rate due to the formation of oxy-sulfates or oxide forms of the metals, which are insoluble in water and almost insoluble in ammonium oxalate.
Moreover, the leaching of the 500 °C roasted sample showed a 36.15% Er yield compared to aqua regia leaching of the same sample. The leaching time of 2 h 30 min with ammonium oxalate showed a progressive precipitation of Er without a pronounced effect on other metals in the solution. When 94.24% of Er in the solution precipitated, 97.21% of Fe, 94.13% of Al, and 70.74% of Cu remained in the solution. Therefore, a high purity of concentrated Er sulfate could be obtained by precipitation. The present study could be an alternative for a secondary source of erbium.
Author Contributions
Conceptualization, J.P.; investigation, M.G., J.P.; writing-original draft preparation, J.P.; writing-review and editing, G.D.; supervision, T.F., J.A.
Funding
This research was funded by the Carbon Mineralization Flagship Center, the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, and the Ministry of Environment of the Korea.
Acknowledgments
This work was joint research between the University of Tokyo and the Korean Institute of Geoscience and Mineral Resources (KIGAM). The financial support and assistance of the Sponsor, the Carbon Mineralization Flagship Center, the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, and the Ministry of Environment of the Korea are gratefully acknowledged. Special thanks to the smart lab of Tokyo University for conducted the XRD analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European commission. Communication from the commission to the European parliament. In Proceedings of the Council, the European economic and Social Committee and the Committee of the Regions, Brussels, Belgium, 13 September 2017; pp. 1–8. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52017DC0490 (accessed on 21 May 2018).
- Mineral Prices. Available online: http://mineralprices.com (accessed on 21 May 2018).
- Singh, S.; Ram, L.C.; Mastro, R.E.; Verma, S.K. A comparative evaluation of minerals and trace elements in the ashes from lignite, coal refuse and biomass fired power plants. Int. J. Coal Geol. 2011, 87, 112–120. [Google Scholar] [CrossRef]
- Ponou, J.; Wang, L.P.; Dodbiba, G.; Okaya, K.; Noda, M.; Fujita, T. Recovery of rare earth elements from aqueous solution obtained from Vietnamese clay minerals using dried and carbonized parachlorella. J. Environ. Chem. Eng. 2014, 2, 1070–1081. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Pontikes, Y.; Binnemans, K.; Gerven, T.V. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Ponou, J.; Dodbiba, G.; Anh, J.W.; Fujita, T. Selective recovery of rare earth elements from aqueous solution obtained from coal power plant ash. J. Environ. Chem. Eng. 2016, 4, 3761–3766. [Google Scholar] [CrossRef]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Gerven, T.V. Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Koller, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar]
- Huang, Z.; Fan, M.; Tiand, H. Coal and coal byproducts: A large and developable unconventional resource for critical materials-Rare earth elements. J. Rare Earth 2018, 36, 337–338. [Google Scholar] [CrossRef]
- Fulford, G.D.; Lever, G.; Sato, T. Recovery of Rare Earth Elements from Bayer Process Red Mud. U.S. Patent 5030424, 9 July 1991. [Google Scholar]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Ochsenkühn, K.M.; Parissakis, G. Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Qu, Y.; Lian, B. Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour. Technol. 2013, 136, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, D.I.; Molchanova, T.V. The investigation of sulphuric acid sorption recovery of scandium and uranium from the red mud of alumina production. Hydrometallurgy 1997, 45, 249–259. [Google Scholar] [CrossRef]
- Yatsenko, S.P.; Pyagai, I.N. Red mud pulp carbonization with scandium extraction during alumina production. Theor. Found. Chem. Eng. 2010, 44, 563–568. [Google Scholar] [CrossRef]
- Logomerac, V.G. Complex utilisation of red mud by smelting and solvent extraction. Trav. Com. Int. Etude Bauxites Alum. Alum. 1979, 15, 279–285. [Google Scholar]
- Taran, M.; Aghaie, E. Designing and optimization of separation process of iron impurities from kaolin by oxalic acid in bench-scale stirred-tank reactor. Appl. Clay Sci. 2015, 107, 109–116. [Google Scholar] [CrossRef]
- Jorjani, E.; Shahbazi, M. The production of rare earth elements group via tributyl phosphate extraction and precipitation stipping using oxalic acid. Arab. J. Chem. 2012. [Google Scholar] [CrossRef]
- Guo, X.; Li, D.; Park, K.H.; Tian, Q.; Wu, Z. Leaching behavior of metals from a limonitic nickel laterite using a sulfation-roasting-leaching process. Hydrometallurgy 2009, 99, 144–150. [Google Scholar] [CrossRef]
- Onal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; Gerven, T.V. Recycling of NdFeB magnets using sulfation, selective roasting and water leaching. J. Sustain. Metall. 2015, 1, 199–215. [Google Scholar] [CrossRef]
- Bergman, A.; Gardestrom, P.; Ericson, I. Release and refixation of ammonium during photorespiration. Int. J. Plant Biol. 1981, 53, 528–532. [Google Scholar]
- Tanigaki, N. Latest Results of Bottom Ash Handling in Japan, Japan Environmental Facilities Manufacturers Association (JEFMA); Nippon Steel & Sumikin Engineering Co., Ltd.: Chiyoda-ku, Japan, 2015. [Google Scholar]
- Pöykiö, R.; Mäkelä, M.; Watkins, G.; Nurmesniemi, H.; Dahl, O. Heavy metals leaching in bottom ash and fly ash fractions from industrial-scale BFB-boiler for environmental risks assessment. Trans. Nonferrous Met. Soc. China 2016, 26, 256–264. [Google Scholar] [CrossRef]
- Patra, S.; Whaung, S.T.; Kwan, W.L. Analysis of heavy metals in incineration bottom ash in Singapore and potential impact of pre-sorting on ash quality. Energy Procedia 2017, 143, 454–459. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).