Research on a Gas Index Reflecting the Sorption Process on Carbon Materials in Coal Mines
Abstract
:1. Introduction
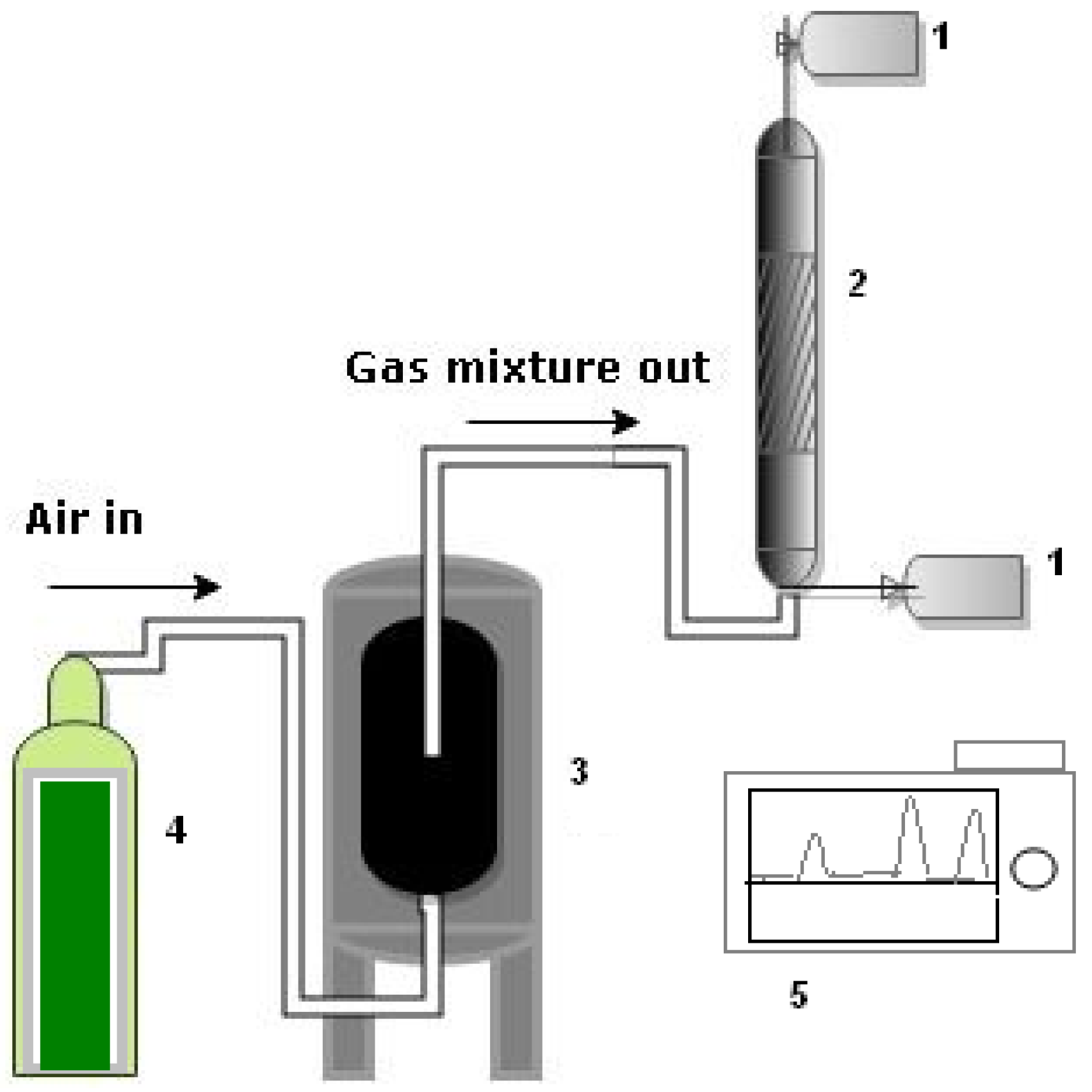
2. Materials and Methods
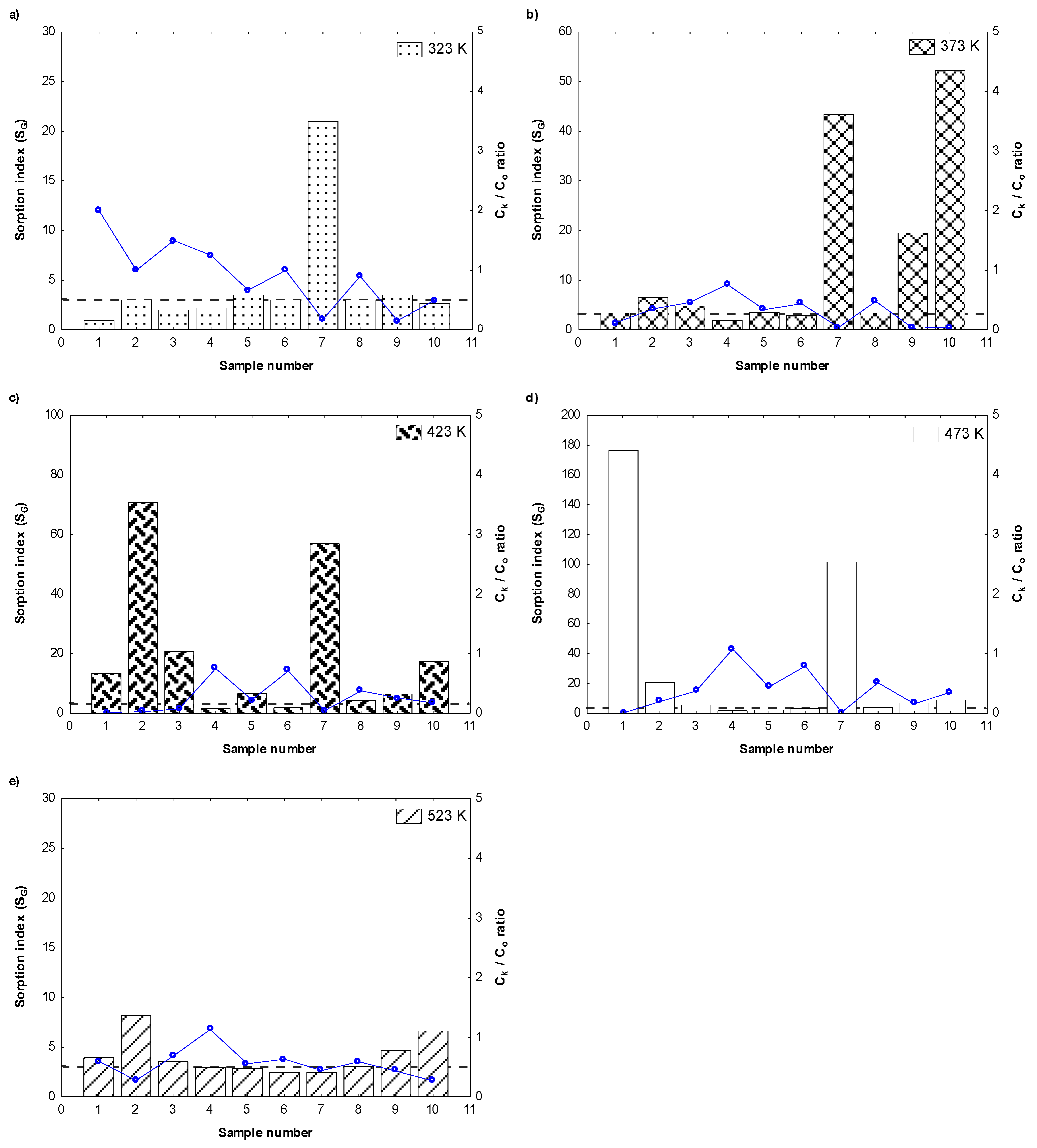
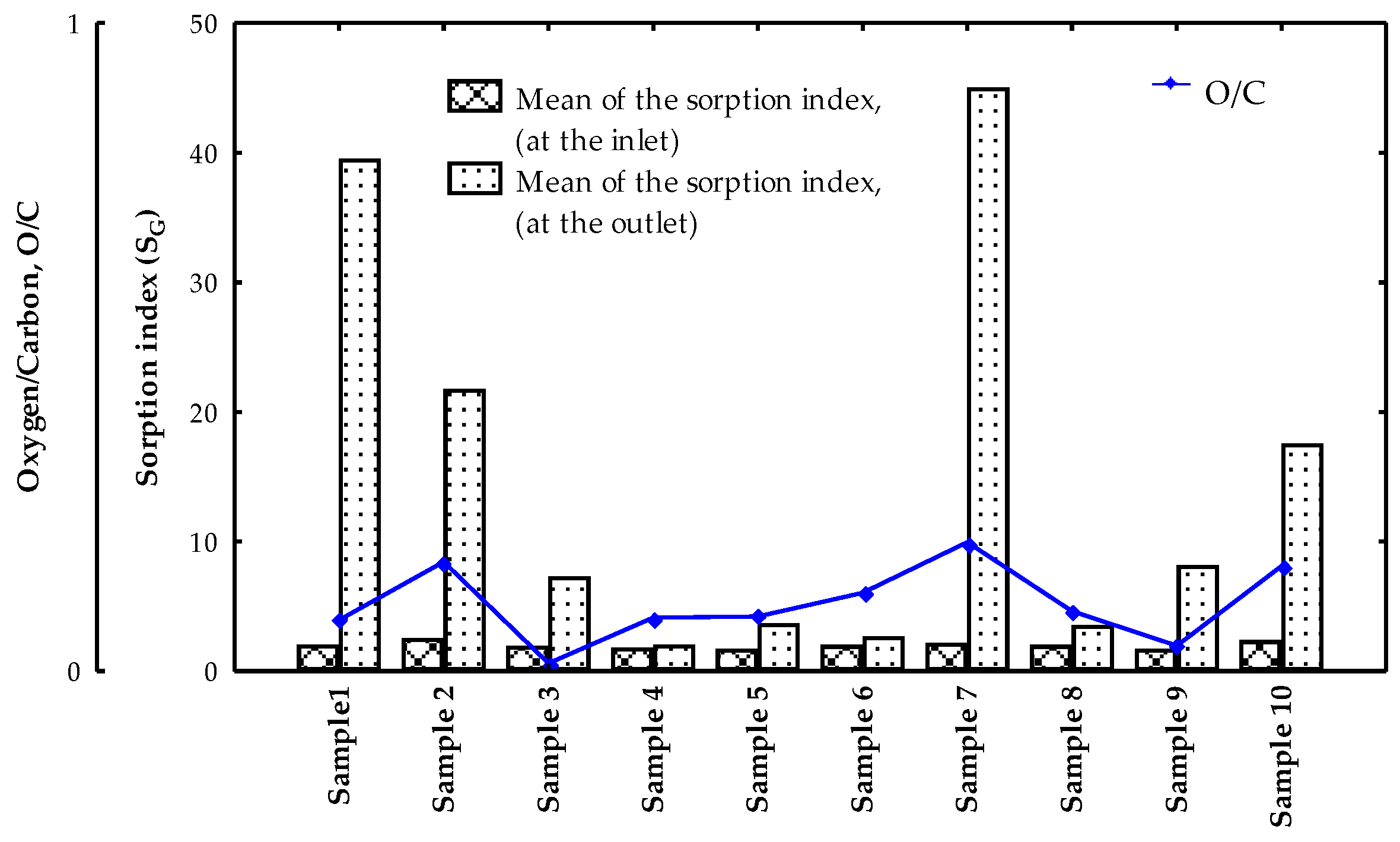
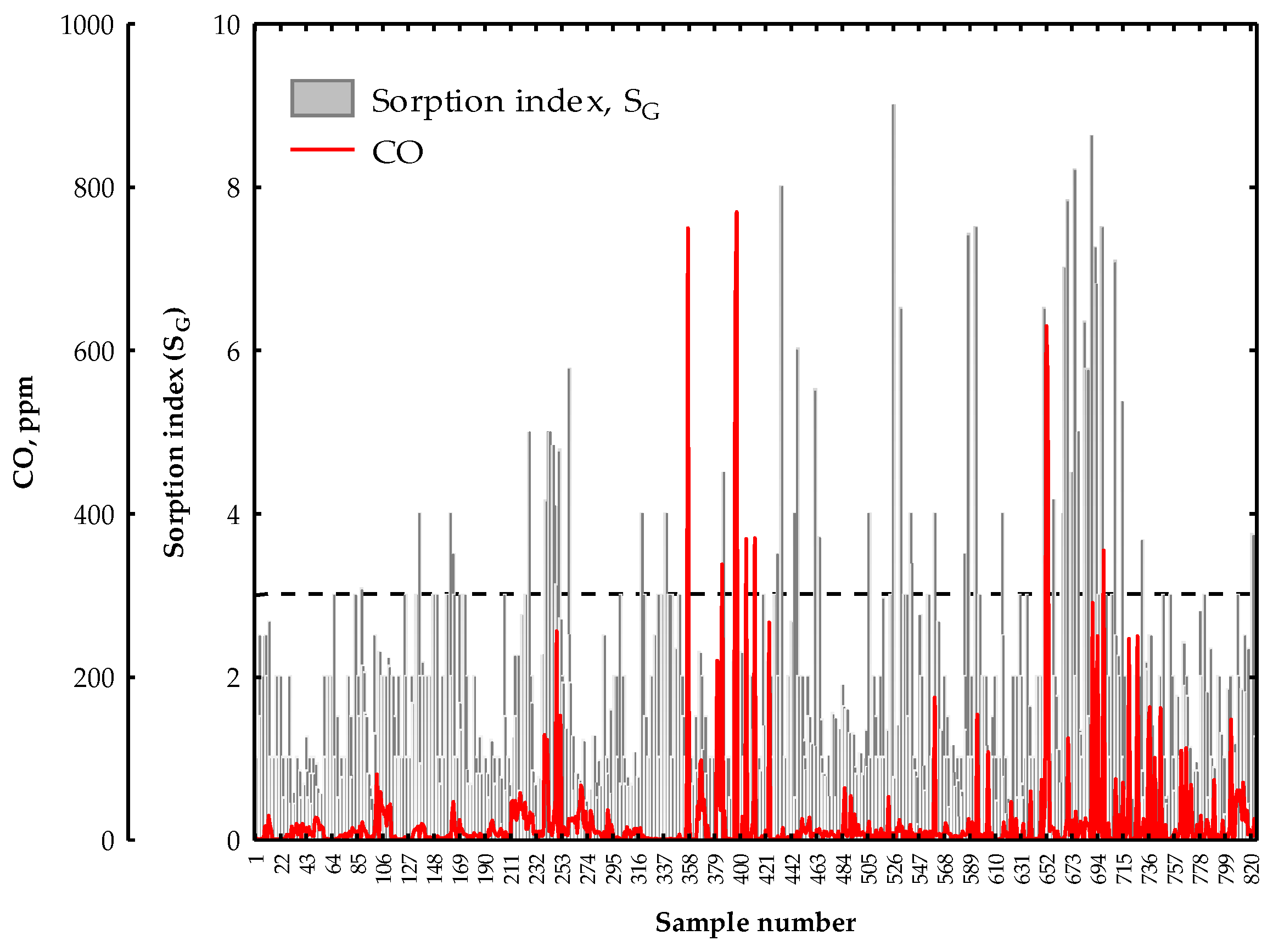
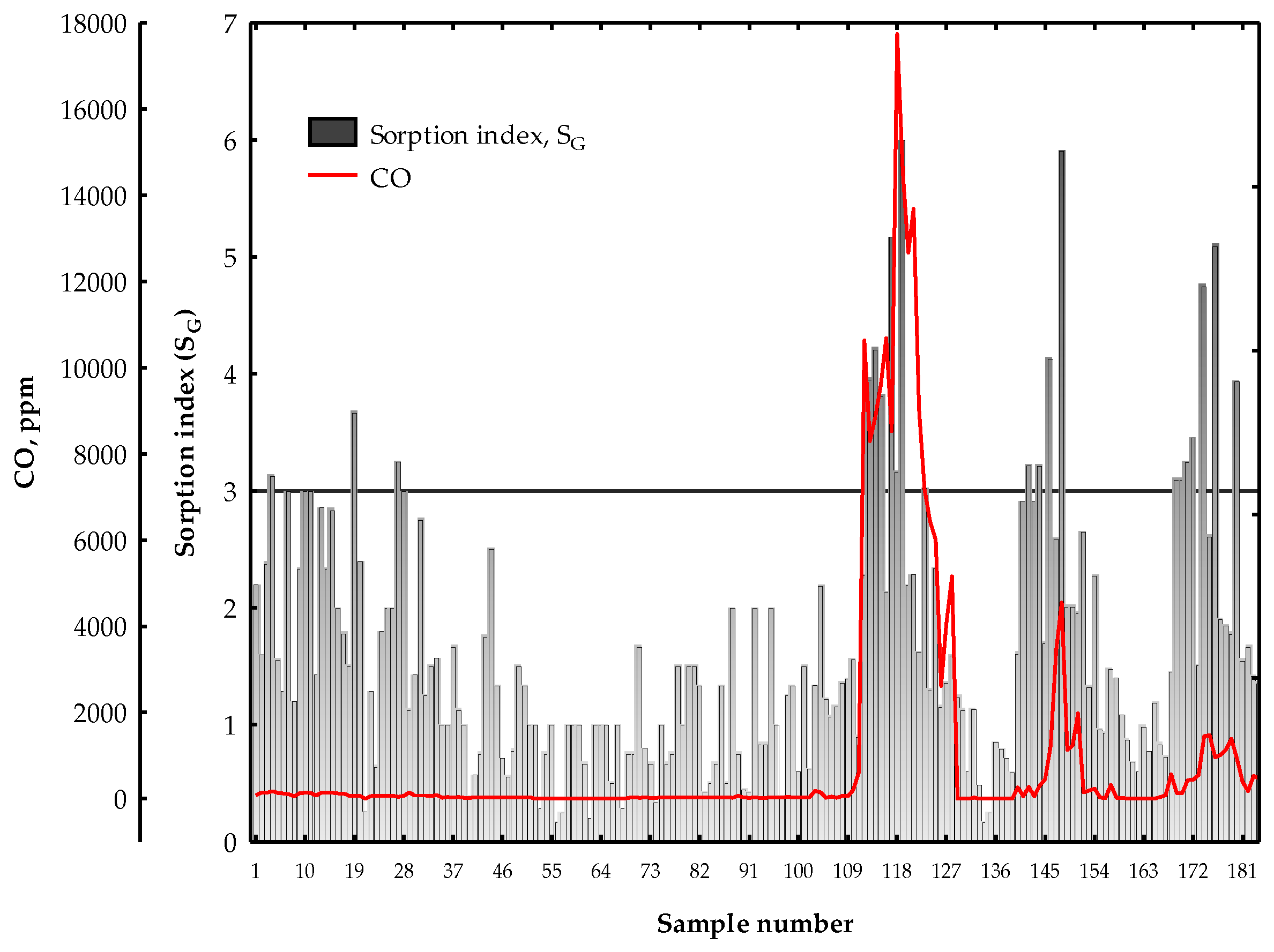
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Timko, R.J.; Derick, R.L. Detection and Control of Spontaneous Heating in Coal Mine Pillars—A Case Study; Report of Investigations RI 9553; US Bureau of Mines: Pittsburgh, DC, USA, 1995.
- Qi, X.; Wang, D.; Hin, H.; Zhong, X. Environmental hazards of coal fire and their prevention in China. Environ. Eng. Manag. J. 2013, 12, 1915–1919. [Google Scholar]
- Kong, B.; Li, Z.; Yang, Y.; Liu, Z.; Yan, D. A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ. Sci. Pollut. Res. 2017, 24, 23453–23470. [Google Scholar] [CrossRef] [PubMed]
- Cioca, L.I.; Moraru, R.I.; Balan, L. Early detection of mine fires in a typical undermined bench face at mining division within Hunedoara energetic complex. In Proceedings of the 16th International Multidisciplinary Scientific GeoConference SGEM 2016, Albena, Bulgaria, 28 June–6 July 2016; pp. 349–356. [Google Scholar] [CrossRef]
- Feng, X.; Adamus, A. Overview of Research and use of Indicator Gases of Coal Spontaneous Combustion in China. GeoSci. Eng. 2014, 60, 55–65. [Google Scholar] [CrossRef]
- Brady, D. The role of gas monitoring in the prevention and treatment of mine fires. In Proceedings of the 2008 Coal Operators’ Conference, Wollongong, Australia, 14–15 February 2008; Aziz, N., Ed.; University of Wollongong & the Australasian Institute of Mining and Metallurgy: Wollongong, Australia; pp. 202–208. [Google Scholar]
- Smith, A.C.; Miron, Y.; Lazzara, C.P. Large-Scale Studies of Spontaneous Combustion of Coal; U.S. Department of the Interior, Bureau of Mines: Washington, DC, USA, 1991.
- Singh, A.K.; Singh, R.V.K.; Singh, M.P.; Chandra, H.; Shukla, N.K. Mine fire gas indices and their application to Indian underground coal mine fires. Int. J. Coal Geol. 2007, 69, 192–204. [Google Scholar] [CrossRef]
- Xie, J.; Xue, S.; Cheng, W.; Wang, G. Early detection of spontaneous combustion of coal in underground coal mines with development of an ethylene enriching system. Int. J. Coal Geol. 2011, 85, 123–127. [Google Scholar] [CrossRef]
- Tang, Y. Sources of underground CO: Crushing and ambient temperature oxidation of coal. J. Loss Prev. Process Ind. 2015, 38, 50–57. [Google Scholar] [CrossRef]
- Dudzińska, A. Sorption properties of hard coals with regard to gases present in the mine atmosphere. J. Earth Sci. 2017, 28, 124–130. [Google Scholar] [CrossRef]
- Dudzińska, A.; Howaniec, N.; Smoliński, A. Experimental study on sorption and desorption of propylene on Polish hard coals. Energy Fuels 2015, 29, 4850–4854. [Google Scholar] [CrossRef]
- Zarębska, K.; Baran, P.; Cygankiewicz, J.; Dudzińska, A. Prognosticating fire hazards in goafs in Polish collieries. AGH Drill. Oil Gas 2012, 29, 463–478. [Google Scholar] [CrossRef]
- Zhang, Q. Development and Current Situation of Study on Theory of Methane Adsorption on Coal. Procedia Earth Planet. Sci. 2011, 3, 318–324. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. Modern state of the theory of gas and vapour adsorption by microporous adsorbents. Pure Appl. Chem. 1965, 10, 309–322. [Google Scholar] [CrossRef]
- Dubinin, M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X. New Insights from Supercritical Methane Adsorption in Coal: Gas Resource Estimation, Thermodynamics, and Engineering Application. Energy Fuels 2018, 32, 5001–5009. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Smoliński, A. The interaction between coal and multi-component gas mixtures in the process of coal heating at various temperatures: An experimental study. Fuel 2018, 213, 150–157. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Smoliński, A. Sorption characteristic of coal as regards of gas mixtures emitted in the process of the self-heating of coal. In Proceedings of the International Conference Energy, Environment and Material Systems (EEMS), Polanica Zdrój, Poland, 13–15 September 2017; Wzorek, M., Królczyk, G., Król, A., Eds.; EDP Sciences: Les Ulis, France, 2017; Volume 19. [Google Scholar] [PubMed]
- Wojtacha-Rychter, K.; Smoliński, A. Multi-component gas mixture transport through porous structure of coal. Fuel 2018, 233, 37–44. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Faiz, M.M.; Aziz, N.I.; Hutton, A.C.; Jones, B.G. Porosity and gas sorption capacity of some eastern Australian coals in relation to coal rank and composition. In Proceedings of the Coalbed Methane Symposium, Townsville, Australia, 19–21 November 1992; Beamish, B.B., Gamson, P.D., Eds.; James Cook University of North Queensland: Townsville, Australia; Volume 4, pp. 9–20. [Google Scholar]
- Gensterblum, Y.; Busch, A.; Krooss, B.M. Molecular concept and experimental evidence of competitive adsorption of H2O, CO2 and CH4 on organic material. Fuel 2014, 115, 581–588. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, C.; Cheng, W.; Zhang, Q.; Nie, W. Effects of Oxygen Element and Oxygen-Containing Functional Groups on Surface Wettability of Coal Dust with Various Metamorphic Degrees Based on XPS Experiment. J. Anal. Methods Chem. 2015, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]

| Sample | Carbon, % w/w | Oxygen, % w/w |
|---|---|---|
| 1 | 61.39 | 4.84 |
| 2 | 67.70 | 11.42 |
| 3 | 89.06 | 1.12 |
| 4 | 76.93 | 6.37 |
| 5 | 81.01 | 6.83 |
| 6 | 74.66 | 9.02 |
| 7 | 64.18 | 12.78 |
| 8 | 79.55 | 7.39 |
| 9 | 85.58 | 3.38 |
| 10 | 63.69 | 10.32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtacha-Rychter, K.; Smoliński, A. Research on a Gas Index Reflecting the Sorption Process on Carbon Materials in Coal Mines. Sustainability 2018, 10, 2468. https://doi.org/10.3390/su10072468
Wojtacha-Rychter K, Smoliński A. Research on a Gas Index Reflecting the Sorption Process on Carbon Materials in Coal Mines. Sustainability. 2018; 10(7):2468. https://doi.org/10.3390/su10072468
Chicago/Turabian StyleWojtacha-Rychter, Karolina, and Adam Smoliński. 2018. "Research on a Gas Index Reflecting the Sorption Process on Carbon Materials in Coal Mines" Sustainability 10, no. 7: 2468. https://doi.org/10.3390/su10072468
APA StyleWojtacha-Rychter, K., & Smoliński, A. (2018). Research on a Gas Index Reflecting the Sorption Process on Carbon Materials in Coal Mines. Sustainability, 10(7), 2468. https://doi.org/10.3390/su10072468






