Adsorption and Desorption of Cd by Soil Amendment: Mechanisms and Environmental Implications in Field-Soil Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
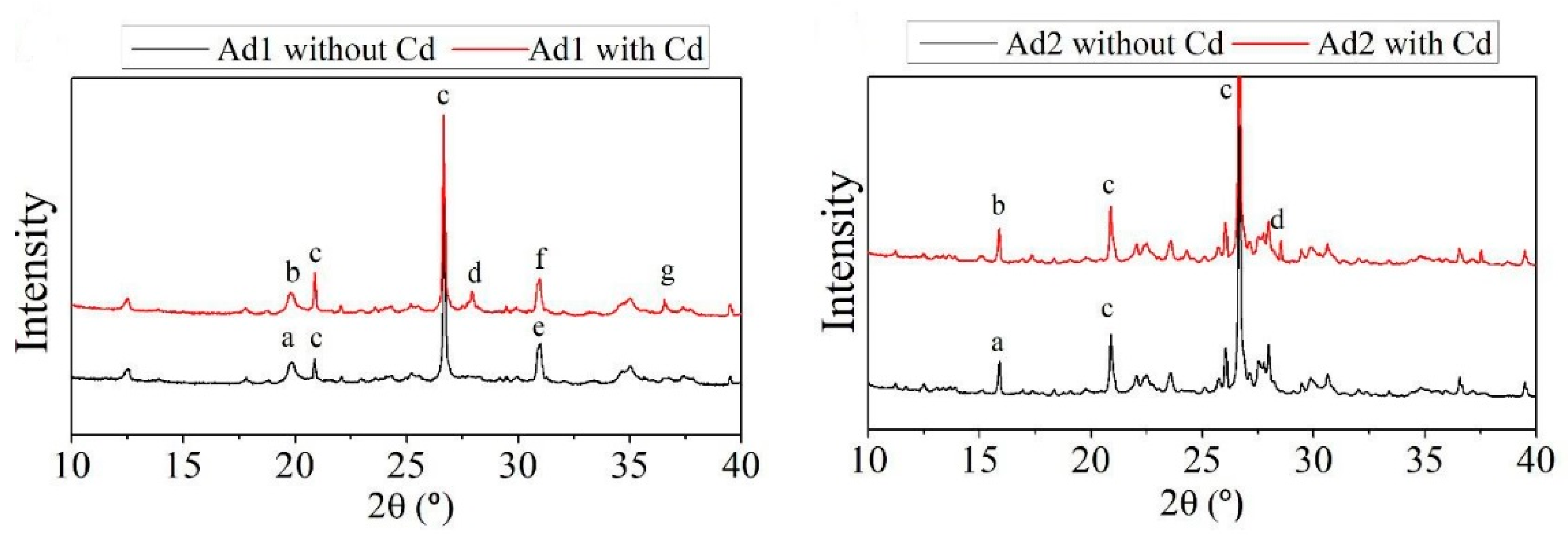
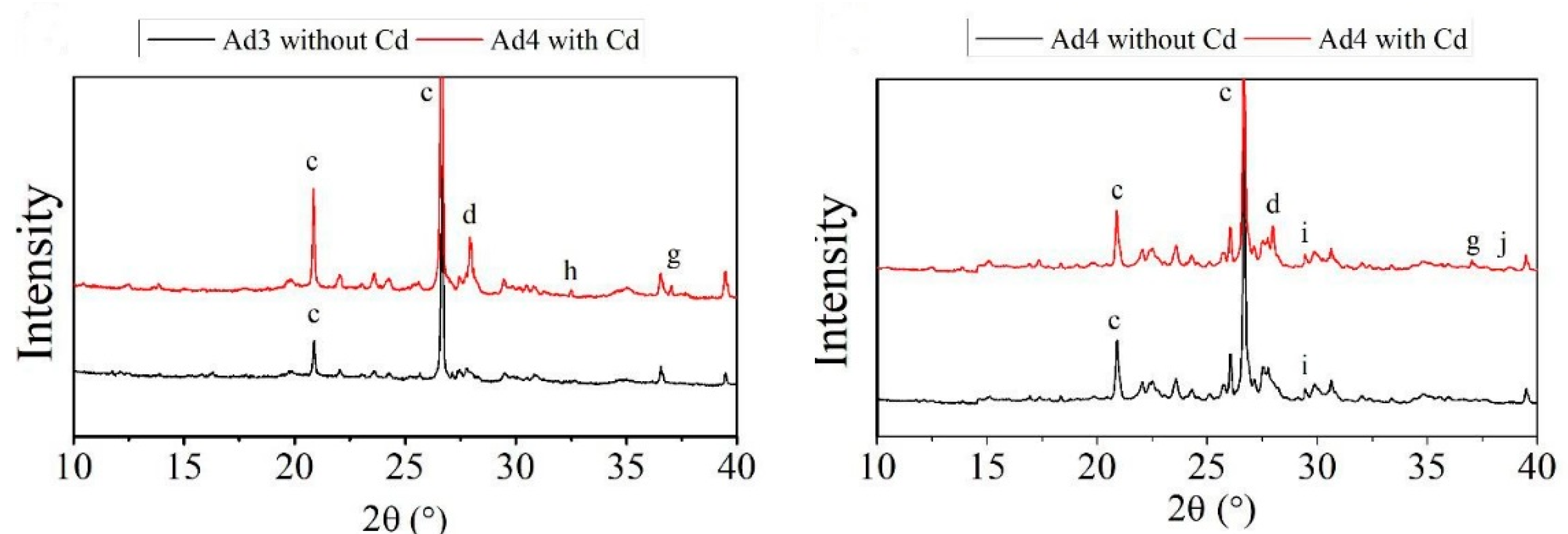
2.2. Characterization of Amendments
2.3. Adsorption and Desorption Experiments
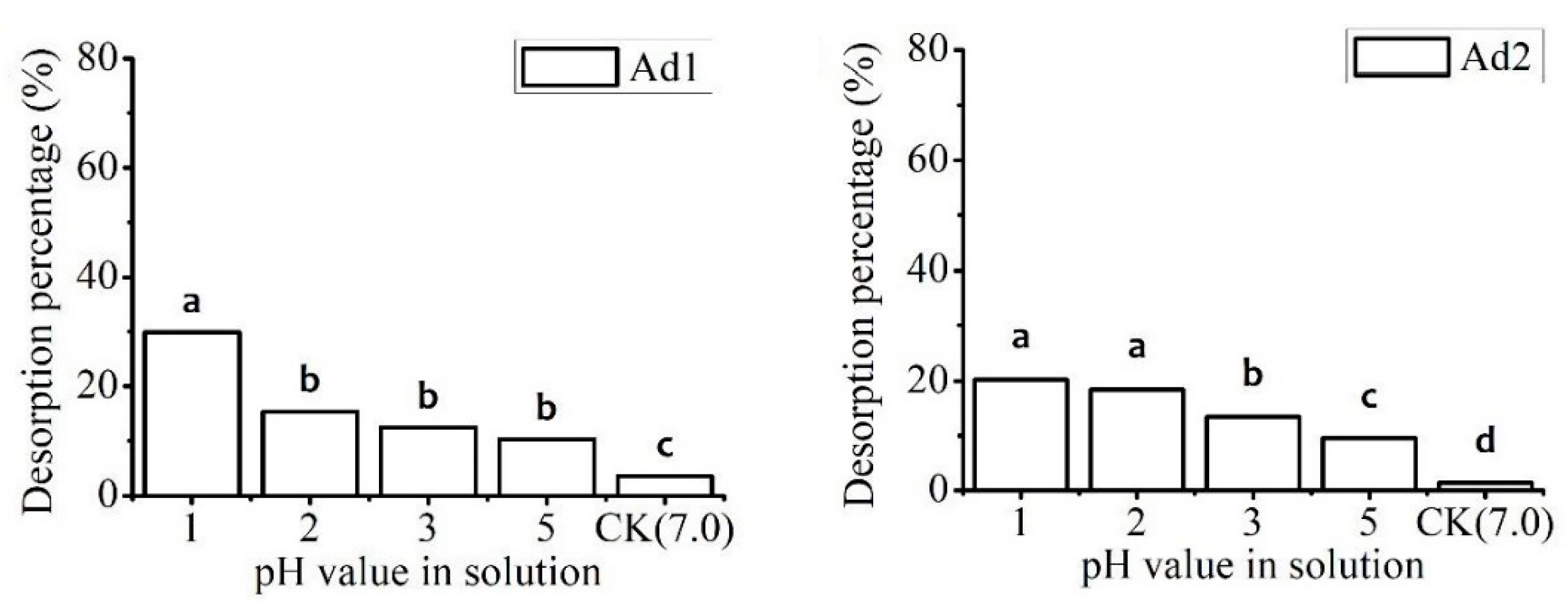
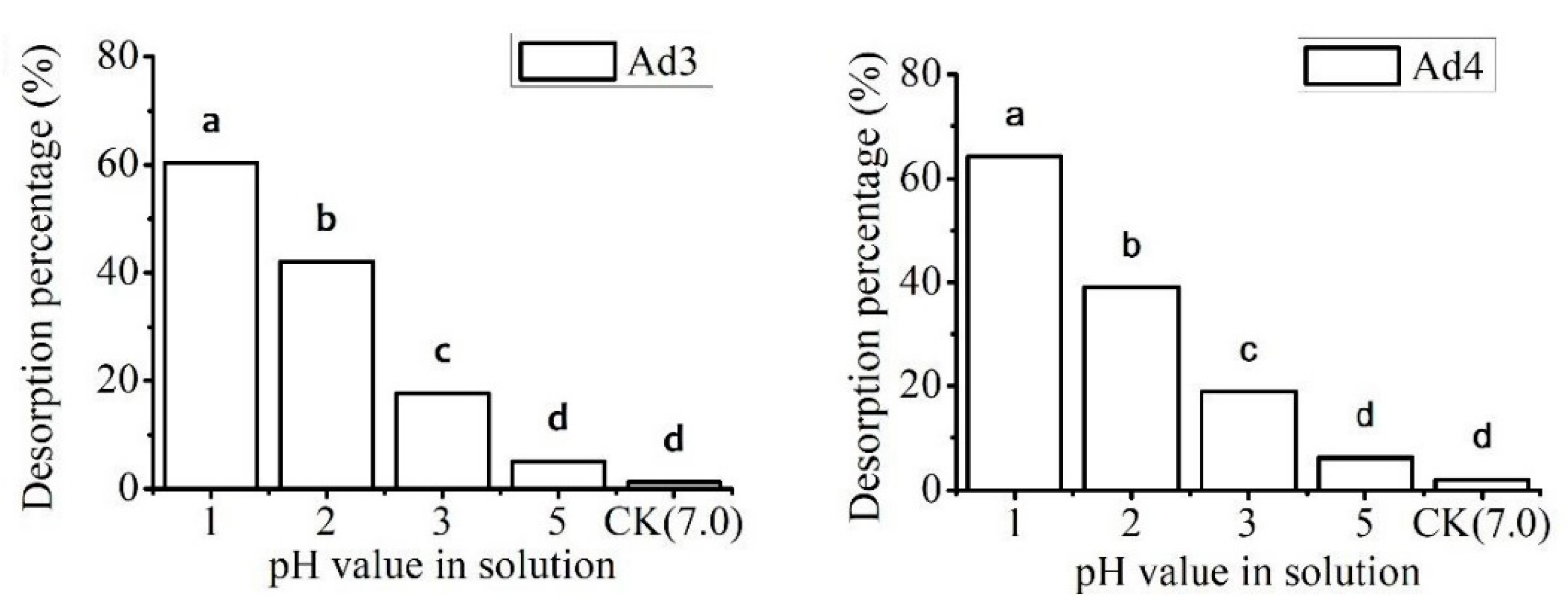
2.3.1. Effect of pH
2.3.2. Effect of Ion Strength
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Amendments
3.2. Cd Adsorption on Amendments
3.3. Cd Desorption from Amendment-Cd
3.3.1. Effect of pH
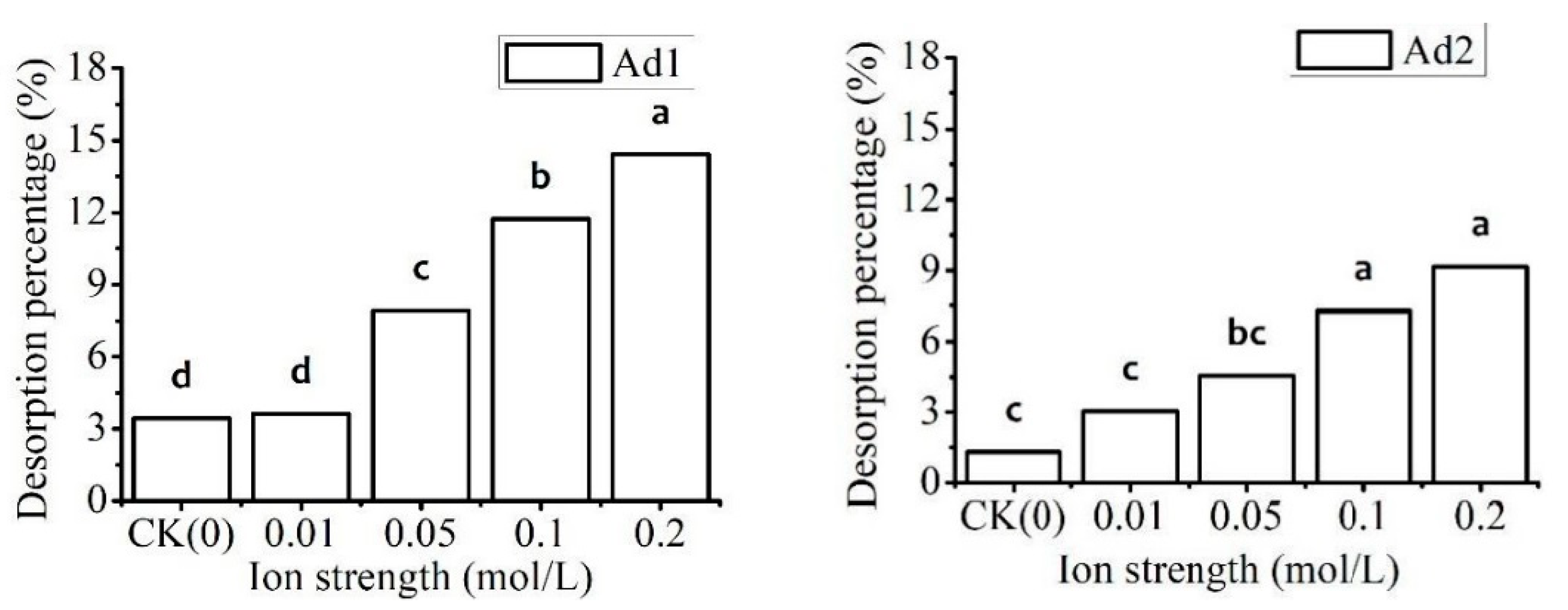
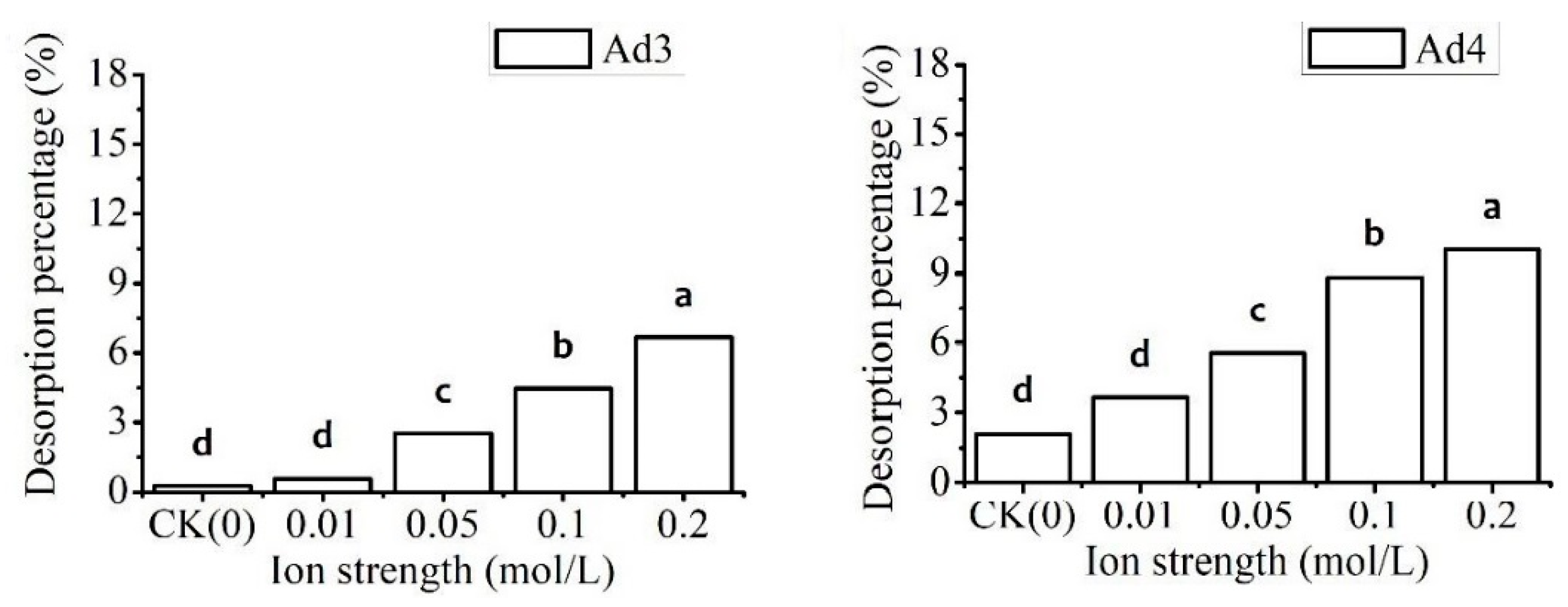
3.3.2. Effect of Ion Strength
3.4. Environmental Implications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Solgi, E.; Esmaili-Sari, A.; Riyahi-Bakhtiari, A.; Hadipour, M. Soil contamination of metals in the three industrial estates, Arak, Iran. Bull. Environ. Contam. Toxicol. 2012, 88, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Li, Z.Y.; Lu, X.N.; Duan, Q.N.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.T.; Aziz, R.; Yang, X.; Xiao, W.; Rafiq, M.K.; Ali, B.; Li, T. Cadmium phytoavailability to rice (Oryza sativa L.) grown in representative Chinese soils. A model to improve soil environmental quality guidelines for food safety. Ecotoxicol. Environ. Saf. 2014, 103, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Q.; Wu, M.; Lin, L.; Scholz, M. Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to china. J. Environ. Manag. 2016, 181, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils-to mobilize or to immobilize? J. Hazard Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Lei, M.; Sun, G.; Huang, Q.; Lu, Y.; Deacon, C.; Meharg, A.A.; Zhu, Y.G. Occurrence and partitioning of cadmium, arsenic and lead in mine impacted paddy rice: Hunan, China. Environ. Sci. Technol. 2009, 43, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Z.; Van, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Li, Z.A.; Mcbride, M.B.; Zou, B.; Wang, G. Health risk assessment for consumption of fish originating from ponds near dabaoshan mine, south China. Environ. Sci. Pollut. Res. Int. 2013, 20, 5844–5854. [Google Scholar] [CrossRef] [PubMed]
- Nabulo, G.; Young, S.D.; Black, C.R. Assessing risk to human health from tropical leafy vegetables grown on contaminated urban soils. Sci. Total Environ. 2010, 408, 5338–5351. [Google Scholar] [CrossRef] [PubMed]
- Żukowska, J.; Biziuk, M. Methodological evaluation of method for dietary heavy metal intake. J. Food Sci. 2008, 73, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Y. Food safety in China. J. Epidem. Commun. Health. 2013, 67, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Fu, W.; Zhang, M.; Zhao, K.; Tunney, H.; Guan, Y. Potentially hazardous metals contamination in soil-rice system and it’s spatial variation in shengzhou city, China. J. Geochem. Explor. 2016, 167, 62–69. [Google Scholar] [CrossRef]
- Porter, S.K.; Scheckel, K.G.; Impellitteri, C.A.; Ryan, J.A. Toxic metals in the environment: Thermodynamic considerations for possible immobilization strategies for Pb, Cd, As, and Hg. Crit. Rev. Environ. Sci. Technol. 2004, 34, 495–604. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Wang, Q.R.; Dong, Y.; Cui, Y.; Liu, X. Instances of soil and crop heavy metal contamination in China. Soil Sediment. Contam. 2001, 10, 497–510. [Google Scholar] [CrossRef]
- Rahman, I.M.M.; Begum, Z.A.; Sawai, H. Solidification/stabilization: A remedial option for metal contaminated soils. In Environmental Remediation Technologies for Metal-Contaminated Soils; Hasegawa, H., Rahman, I.M.M., Rahman, M.A., Eds.; Springer: Tokyo, Japan, 2016; pp. 125–146. [Google Scholar] [CrossRef]
- Koptsik, G.N. Modern approaches to remediation of heavy metal polluted soils: A review. Eurasian Soil Sci. 2014, 47, 707–722. [Google Scholar] [CrossRef]
- Priya, S.V.; Arulmozhi, M. Biosorbents for toxic heavy metals—A review. In Proceedings of the International Conference on Advances in Engineering, Science and Management, Nagapattinam, India, 30–31 March 2012; pp. 221–230. [Google Scholar]
- Sun, Y.; Sun, G.; Xu, Y.; Liu, W.; Liang, X.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J. Environ. Manag. 2005, 166, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Tlustos, P.; Szakova, J.; Korinek, K.; Pavlikova, D.; Hanc, A.; Balik, J. The effect of liming on cadmium, lead and zinc uptake reduction by spring wheat grown in contaminated soil. Plant Soil Environ. 2006, 52, 16–24. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Mitter, B. Organic amendments: Effects on cereals growth and cadmium remediation. Int. J. Environ. Sci. Technol. 2015, 12, 2919–2928. [Google Scholar] [CrossRef]
- Clemente, R.; Almela, C.; Bernal, M.P. A remediation strategy based on active phytoremediation followed by natural attenuation in a soil contaminated by pyrite waste. Environ. Pollut. 2006, 143, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Chen, D.; Liu, X.; Cui, L.; Li, L.; Pan, G.; Xie, D.; Zheng, J.W.; Zhang, X.H.; Zheng, J.F.; et al. Biochar soil amendment as a solution to prevent Cd-tainted rice from china: Results from a cross-site field experiment. Ecol. Eng. 2013, 58, 378–383. [Google Scholar] [CrossRef]
- Spurgeon, D.J.; Stürzenbaum, S.R.; Svendsen, C.; Hankard, P.K.; Morgan, A.J.; Weeks, J.M.; Kille, P. Toxicological, cellular and gene expression responses in earthworms exposed to copper and cadmium. Comp. Biochem. Phys. C 2004, 138, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, L.; Mail, A.Z.; Pan, G. Biochar amendment greatly reduces rice cd uptake in a contaminated paddy soil: A two-year field experiment. Bioresources 2011, 6, 2605–2618. [Google Scholar] [CrossRef]
- Liang, X.; Han, J.; Xu, Y.; Sun, Y.; Wang, L.; Tan, X. In situ field-scale remediation of Cd polluted paddy soil using sepiolite and palygorskite. Geoderma 2014, 235–236, 9–18. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Hajabbasi, M.A.; Khademi, H.; Kazemian, H. Soil cadmium stabilization using an iranian natural zeolite. Geoderma 2007, 137, 388–393. [Google Scholar] [CrossRef]
- Najafi, S.; Jalali, M. Effects of organic acids on cadmium and copper sorption and desorption by two calcareous soils. Environ. Monit. Assess. 2015, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Riedel, G.S.; Riedel, G.; Kwon, S.; Landis, R.; Brown, S.S.; Menzie, C.A.; Ghosh, U. Activated carbon mitigates mercury and methylmercury bioavailability in contaminated sediments. Environ. Sci. Technol. 2013, 47, 13001–13011. [Google Scholar] [CrossRef] [PubMed]
- Van Poucke, R.; Ainsworth, J.; Maeseele, M.; Ok, Y.S.; Meers, E.; Tack, F.M.G. Chemical stabilization of Cd-contaminated soil using biochar. Appl. Geochem. 2018, 88, 122–130. [Google Scholar] [CrossRef]
- He, H.; Nfy, T.; Yao, A.; Qiu, R.; Li, W.C.; Ye, Z. Growth and cd uptake by rice (Oryza sativa) in acidic and Cd-contaminated paddy soils amended with steel slag. Chemosphere 2017, 189, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, M.; Zhao, Z.; Li, X.; Han, Y.; Chen, S. Alleviation of cadmium phytotoxicity to wheat is associated with cd re-distribution in soil aggregates as affected by amendments. RSC Adv. 2018, 8, 17426–17434. [Google Scholar] [CrossRef]
- Castaldi, P.; Santona, L.; Enzo, S.; Melis, P. Sorption processes and xrd analysis of a natural zeolite exchanged with Pb(2+), Cd(2+) and Zn(2+) cations. J. Hazard. Mater. 2008, 156, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Zama, E.F.; Zhu, Y.G.; Reid, B.J.; Sun, G.X. The role of biochar properties in influencing the sorption and desorption of Pb(II), Cd(II) and As(III) in aqueous solution. J. Clean. Prod. 2017, 148, 127–136. [Google Scholar] [CrossRef]
- Ren, X.; Shao, D.; Yang, S.; Hu, J.; Sheng, G.; Tan, X.; Wang, X.K. Comparative study of Pb(II) sorption on XC-72 carbon and multi-walled carbon nanotubes from aqueous solutions. Chem. Eng. J. 2011, 170, 170–177. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.C.C.; dos Santos, L.B.O.; Abate, G.; Cosentino, I.C.; Fantini, M.C.A.; Masini, J.C.; Matos, J.R. Adsorption of Pb2+, Cu2+, and Cd2+, in FDU-1 silica and FDU-1 silica modified with humic acid. Micropor. Mesopor. Mat. 2008, 110, 250–259. [Google Scholar] [CrossRef]
- Yan, G.Y.; Viraraghavan, T. Heavy-metal removal from aqueous solutionby fungus Mucor rouxii. Water Res. 2003, 37, 4486–4496. [Google Scholar] [CrossRef]
- Tabaraki, R.; Ahmady-Asbchin, S.; Abdi, O. Biosorption of Zn(II) from aqueous solutions by Acinetobacter sp. isolated from petroleum spilled soil. J. Environ. Chem. Eng. 2013, 1, 604–608. [Google Scholar] [CrossRef]
- Bian, R.; Joseph, S.; Cui, L.; Pan, G.; Li, L.; Liu, X.; Zhang, A.; Rutidge, H.; Wong, S.; Chia, C.; et al. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J. Hazard. Mater. 2014, 272, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, S.; Na, Z.; Lu, D.; Liu, Z. Adsorption, concentration, and recovery of aqueous heavy metal ions with the root powder of Eichhornia crassipes. Ecol. Eng. 2013, 60, 160–166. [Google Scholar] [CrossRef]
- Khan, S.; Chao, C.; Waqas, M.; Arp, H.P.; Zhu, Y.G. Sewage sludge biochar influence upon rice (Oryza sativa) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environ. Sci. Technol. 2013, 47, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.B.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2016, 142, 3809–3821. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, X.F.; Xu, Y.M.; Qin, X.; Huang, Q.Q.; Wang, L.; Sun, Y.B. Remediation of Heavy Metal-Polluted Agricultural Soils Using Clay Minerals: A Review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Meitei, M.D.; Prasad, M.N.V. Lead(II) and cadmium(II) biosorption on Spirodela polyrhiza, (L.) schleiden biomass. J. Environ. Chem. Eng. 2013, 1, 200–207. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Suchithra, P.S. Heavy metals uptake from aqueous solutions and industrial wastewaters by humic acid-immobilized polymer/bentonite composite: Kinetics and equilibrium modeling. Chem. Eng. J. 2010, 156, 146–156. [Google Scholar] [CrossRef]
- Horká, M.; Růžička, F.; Holá, V.; Šlais, K. Capillary isoelectric focusing of microorganisms in the pH range 2–5 in a dynamically modified FS capillary with UV detection. Anal. Bioanal. Chem. 2006, 385, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Martinsa, R.J.E.; Pardob, R.; Boaventura, R.A.R. Cadmium(II) and zinc(II) adsorption by the aquatic moss Fontinalis antipyretica: Effect of temperature, pH and water hardness. Water Res. 2004, 38, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, J.; Li, Z.T.; Liu, X.M.; Xia, F.; Xu, J.M. First “charosphere” view towards the transport and transformation of Cd with addition of manure derived biochar. Environ. Poll. 2017, 227, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, W.; Xiong, L.; Xu, N.; Ni, J.R. Influence of pH, ionic strength and humic acid on competitive adsorption of Pb(II), Cd(II) and Cr(III) onto titanate nanotubes. Chem. Eng. J. 2013, 215–216, 366–374. [Google Scholar] [CrossRef]
- Zhu, Q.; Vries, W.D.; Liu, X.; Zeng, M.; Hao, T.; Du, E.; Zhang, F.; Shen, J. The contribution of atmospheric deposition and forest harvesting to forest soil acidification in china since 1980. Atmos. Environ. 2016, 146, 215–222. [Google Scholar] [CrossRef]
- Jia, W.L.; Wang, B.L.; Wang, C.P.; Sun, H.W. Tourmaline and biochar for the remediation of acid soil polluted with heavy metals. J. Environ. Chem. Eng. 2017, 5, 2107–2114. [Google Scholar] [CrossRef]
- Ledin, M.; Pedersen, K.; Allard, B. Effects of pH, ionic strength on the adsorption of Cs, Sr, Eu, Zn, Cd and Hg by pseudomonas putida. Water Air Soil Poll. 1997, 93, 367–381. [Google Scholar] [CrossRef]
| Amendments | pH | CEC | Organic | Cd |
|---|---|---|---|---|
| (water/Soil = 2.5:1) | (cmol+/kg) | Carbon (g kg−1) | (mg kg−1) | |
| Ad1 | 7.95 ± 0.06 | 125.60 ± 8.19 | 58.62 ± 3.12 | ND |
| Ad2 | 8.13 ± 0.09 | 210.30 ± 10.23 | 36.73 ± 1.95 | ND |
| Ad3 | 7.92 ± 0.04 | 176.70 ± 7.65 | 420.21 ± 23.14 | ND |
| Ad4 | 9.28 ± 0.06 | 115.10 ± 1.88 | 67.38 ± 25.89 | ND |
| Amendments | Particle Mean Size (µm) | BET Surface Area (m2 g−1) | ||
|---|---|---|---|---|
| Ads | Ads-Cd | Ads | Ads-Cd | |
| Ad1 | 7.293 ± 1.274 d | 9.108 ± 1.541 c | 58.704 ± 2.755 a | 47.171 ± 2.222 a |
| Ad2 | 14.596 ± 1.713 c | 17.033 ± 1.437 b | 21.271 ± 1.897 c | 5.220 ± 0.637 c |
| Ad3 | 35.902 ± 3.799 a | 37.117 ± 3.215 a | 4.632 ± 0.515 d | 2.634 ± 0.506 c |
| Ad4 | 29.599 ± 2.271 b | 33.304 ± 2.434 a | 36.512 ± 1.205 b | 10.124 ± 1.557 b |
| Amendments | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| b | K1 | R2 | lgKF | 1/n | R2 | |
| Ad1 | 8.711 | 0.095 | 0.875 | 1.6144 | 0.6042 | 0.960 |
| Ad2 | 7.474 | 0.131 | 0.942 | 1.5788 | 0.5823 | 0.978 |
| Ad3 | 17.668 | 0.832 | 0.973 | 2.8511 | 0.7192 | 0.958 |
| Ad4 | 12.821 | 0.204 | 0.931 | 2.3442 | 0.731 | 0.907 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, M.; Zhao, Z.; Ma, C.; Chen, S. Adsorption and Desorption of Cd by Soil Amendment: Mechanisms and Environmental Implications in Field-Soil Remediation. Sustainability 2018, 10, 2337. https://doi.org/10.3390/su10072337
Li S, Wang M, Zhao Z, Ma C, Chen S. Adsorption and Desorption of Cd by Soil Amendment: Mechanisms and Environmental Implications in Field-Soil Remediation. Sustainability. 2018; 10(7):2337. https://doi.org/10.3390/su10072337
Chicago/Turabian StyleLi, Shanshan, Meng Wang, Zhongqiu Zhao, Changbao Ma, and Shibao Chen. 2018. "Adsorption and Desorption of Cd by Soil Amendment: Mechanisms and Environmental Implications in Field-Soil Remediation" Sustainability 10, no. 7: 2337. https://doi.org/10.3390/su10072337
APA StyleLi, S., Wang, M., Zhao, Z., Ma, C., & Chen, S. (2018). Adsorption and Desorption of Cd by Soil Amendment: Mechanisms and Environmental Implications in Field-Soil Remediation. Sustainability, 10(7), 2337. https://doi.org/10.3390/su10072337







