Evaluating Weight Loss Efficacy in Obesity Treatment with Allurion’s Ingestible Gastric Balloon: A Retrospective Study Utilizing the Scale App Health Tracker
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Inclusion and Exclusion Criteria
2.3. Study Variables, Definitions, and Procedures
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Outcomes and Follow-Up
4. Discussion
4.1. Literature Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, N.S.; Catenacci, V.A.; Wyatt, H.R.; Hill, J.O. Obesity: Overview of an epidemic. Psychiatr. Clin. N. Am. 2011, 34, 717–732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fericean, R.M.; Citu, C.; Manolescu, D.; Rosca, O.; Bratosin, F.; Tudorache, E.; Oancea, C. Characterization and Outcomes of SARS-CoV-2 Infection in Overweight and Obese Patients: A Dynamic Comparison of COVID-19 Pandemic Waves. J. Clin. Med. 2022, 11, 2916. [Google Scholar] [CrossRef]
- Chioreanu, A.; Mot, I.C.; Horhat, D.I.; Balica, N.C.; Sarau, C.A.; Morar, R.; Domuta, E.M.; Dumitru, C.; Negrean, R.A.; Bumbu, B.A.; et al. Development and Preliminary Characterization of Polyester-Urethane Microparticles Used in Curcumin Drug Delivery System for Oropharyngeal Cancer. Medicina 2022, 58, 1689. [Google Scholar] [CrossRef]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Olateju, I.V.; Ogwu, D.; Owolabi, M.O.; Azode, U.; Osula, F.; Okeke, R.; Akabalu, I. Role of Behavioral Interventions in the Management of Obesity. Cureus 2021, 13, e18080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wadden, T.A.; Tronieri, J.S.; Butryn, M.L. Lifestyle modification approaches for the treatment of obesity in adults. Am. Psychol. 2020, 75, 235–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgescu, D.; Ionita, I.; Lascu, A.; Hut, E.F.; Dragan, S.; Ancusa, O.E.; Ionita, M.; Calamar-Popovici, D.; Georgescu, L.A.; Lighezan, D.F. Gallstone Disease and Bacterial Metabolic Performance of Gut Microbiota in Middle-Aged and Older Patients. Int. J. Gen. Med. 2022, 15, 5513–5531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kissler, H.J.; Settmacher, U. Bariatric surgery to treat obesity. Semin. Nephrol. 2013, 33, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.M.; Malhotra, S. Obesity Surgery Indications and Contraindications. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513285/ (accessed on 12 January 2024).
- Pop, D.L.; Nodiţi, G.; Abu-Awwad, A.; Maliţa, D.C.; Zamfir, C.L.; Grigoraş, M.L.; Vermeşan, D.; Prejbeanu, R.; Hărăguş, H.G.; Boşcu, A.L.; et al. Alveolar rhabdomyosarcoma in an adolescent male patient—Case report and current perspectives. Rom. J. Morphol. Embryol. 2018, 9, 1247–1252. [Google Scholar]
- Teixeira, P.J.; Silva, M.N.; Mata, J.; Palmeira, A.L.; Markland, D. Motivation, self-determination, and long-term weight control. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Virzob, C.R.B.; Poenaru, M.; Morar, R.; Horhat, I.D.; Balica, N.C.; Prathipati, R.; Moleriu, R.D.; Toma, A.-O.; Juganaru, I.; Bloanca, V.; et al. Efficacy of Bilateral Cochlear Implantation in Pediatric and Adult Patients with Profound Sensorineural Hearing Loss: A Retrospective Analysis in a Developing European Country. J. Clin. Med. 2023, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- Mensinger, J.L.; Calogero, R.M.; Stranges, S.; Tylka, T.L. A weight-neutral versus weight-loss approach for health promotion in women with high BMI: A randomized-controlled trial. Appetite 2016, 105, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.; Caraba, A.; Ionita, I.; Lascu, A.; Hut, E.F.; Dragan, S.; Ancusa, O.E.; Suceava, I.; Lighezan, D. Dyspepsia and Gut Microbiota in Female Patients with Postcholecystectomy Syndrome. Int. J. Womens Health 2022, 14, 41–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinto, A.M.; Subak, L.L.; Nakagawa, S.; Vittinghoff, E.; Wing, R.R.; Kusek, J.W.; Herman, W.H.; West, D.S.; Kuppermann, M. The effect of weight loss on changes in health-related quality of life among overweight and obese women with urinary incontinence. Qual. Life Res. 2012, 21, 1685–1694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glass, J.; Chaudhry, A.; Zeeshan, M.S.; Ramzan, Z. New Era: Endoscopic treatment options in obesity-a paradigm shift. World J. Gastroenterol. 2019, 25, 4567–4579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tay, A.; Hoeksema, H.; Murphy, R. Uncovering Barriers and Facilitators of Weight Loss and Weight Loss Maintenance: Insights from Qualitative Research. Nutrients 2023, 15, 1297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.H.; Chun, H.J.; Choi, H.S.; Kim, E.S.; Keum, B.; Jeen, Y.T. Current status of intragastric balloon for obesity treatment. World J. Gastroenterol. 2016, 22, 5495–5504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mital, S.; Nguyen, H.V. Cost-effectiveness of procedure-less intragastric balloon therapy as substitute or complement to bariatric surgery. PLoS ONE 2021, 16, e0254063. [Google Scholar] [CrossRef] [PubMed]
- Crossan, K.; Sheer, A.J. Intragastric Balloon. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK578184/ (accessed on 12 January 2024).
- Ienca, R.; Al Jarallah, M.; Caballero, A.; Giardiello, C.; Rosa, M.; Kolmer, S.; Sebbag, H.; Hansoulle, J.; Quartararo, G.; Zouaghi, S.A.S.; et al. The Procedureless Elipse Gastric Balloon Program: Multicenter Experience in 1770 Consecutive Patients. Obes. Surg. 2020, 30, 3354–3362, Erratum in Obes. Surg. 2020, 30, 4691–4692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Auster-Gussman, L.A.; Rikhy, M.; Lockwood, K.G.; Branch, O.H.; Graham, S.A. The Effects of Providing a Connected Scale in an App-Based Digital Health Program: Cross-sectional Examination. JMIR mHealth uHealth 2023, 11, e40865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cox, C.E. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. A Publ. Am. Diabetes Assoc. 2017, 30, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Klingenberg, L.; Rosenkilde, M.; Gilbert, J.A.; Tremblay, A.; Sjödin, A. Physical activity plays an important role in body weight regulation. J. Obes. 2011, 2011, 360257. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Exercise, energy balance and body composition. Eur. J. Clin. Nutr. 2018, 72, 1246–1250. [Google Scholar] [CrossRef]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Garrido, N.; Santi-Cano, M.J. Motivation and Limiting Factors for Adherence to Weight Loss Interventions among Patients with Obesity in Primary Care. Nutrients 2022, 14, 2928. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, R.C.; Zhang, S.; Preacher, K.J.; Rucker, D.D. On the practice of dichotomization of quantitative variables. Psychol. Methods. 2002, 7, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Craig, C.L.; Thyfault, J.P.; Spence, J.C. A step-defined sedentary lifestyle index: <5000 steps/day. Appl. Physiol. Nutr. Metab. 2013, 38, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Salmi, A.; Greco, F.; Belleri, E. Ultrasound-guided insertion of the Elipse® gastric balloon: Technical details, learning curve, and perioperative outcome in 36 cases. J. Ultrasound 2020, 23, 593–597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tai, C.-M.; Lin, H.-Y.; Yen, Y.-C.; Huang, C.-K.; Hsu, W.-L.; Huang, Y.-W.; Chang, C.-Y.; Wang, H.-P.; Mo, L.-R. Effectiveness of intragastric balloon treatment for obese patients: One-year follow-up after balloon removal. Obes. Surg. 2013, 23, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Bilal, M.; Kim, M.C.; Cohen, J.; Study Group for Endoscopic Bariatric and Metabolic Therapies of the Korean Society of Gastrointestinal Endoscopy. The Clinical and Metabolic Effects of Intragastric Balloon on Morbid Obesity and Its Related Comorbidities. Clin. Endosc. 2021, 54, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Low, H.C.; Lim, L.G.; Dan, Y.Y.; Aung, M.O.; Cheng, C.L.; Wee, A.; Lim, S.G.; Ho, K.Y. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: A pilot study. Gastrointest. Endosc. 2012, 76, 756–760. [Google Scholar] [CrossRef]
- Mafort, T.T.; Madeira, E.; Madeira, M.; Guedes, E.P.; Moreira, R.O.; de Mendonça, L.M.C.; Farias, M.L.F.; Farias, A.J. Six-month intragastric balloon treatment for obesity improves lung function, body composition, and metabolic syndrome. Obes. Surg. 2014, 24, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M.R.; Fittipaldi-Fernandez, R.J.; Diestel, C.F.; Klein, M. Impact of intragastric balloon treatment on adipokines, cytokines, and metabolic profile in obese individuals. Obes. Surg. 2019, 29, 2600–2608. [Google Scholar] [CrossRef]
- Popov, V.B.; Ou, A.; Schulman, A.R.; Thompson, C.C. The impact of intragastric balloons on obesity-related co-morbidities: A systematic review and meta-analysis. Am. J. Gastroenterol. 2017, 112, 429–439. [Google Scholar] [CrossRef]
- Jense, M.T.F.; Palm-Meinders, I.H.; Sanders, B.; Boerma, E.G.; Greve, J.W.M. The Swallowable Intragastric Balloon Combined with Lifestyle Coaching: Short-Term Results of a Safe and Effective Weight Loss Treatment for People Living with Overweight and Obesity. Obes. Surg. 2023, 33, 1668–1675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vantanasiri, K.; Matar, R.; Beran, A.; Jaruvongvanich, V. The Efficacy and Safety of a Procedureless Gastric Balloon for Weight Loss: A Systematic Review and Meta-Analysis. Obes. Surg. 2020, 30, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, G.; Shrewsbury, A.; Kotzampassi, K. Six intragastric balloons: Which to choose? World J. Gastrointest. Endosc. 2021, 13, 238–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciprian, G.; Khoury, J.; Ramirez, L.; Miskovsky, J. Endoscopy Management of Complete Gastric Outlet Obstruction Secondary to Elipse™ Intragastric Balloon. Cureus 2021, 13, e17542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
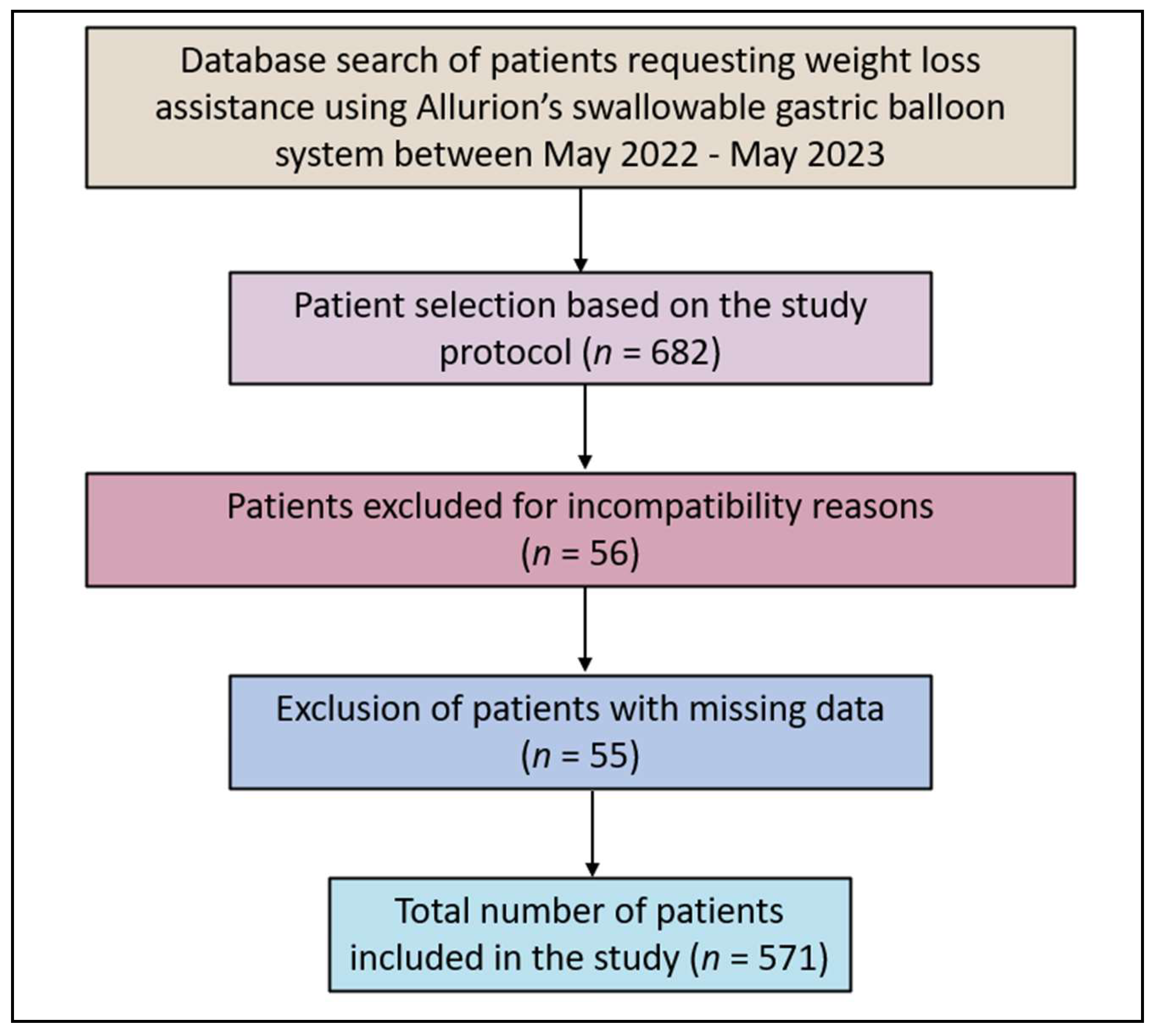
| Variables | Total (n = 571) | Less Active (n = 286) | More Active (n = 285) | p-Value |
|---|---|---|---|---|
| Age, years (median, IQR) | 41.0 (33.5–48.5) | 43.5 (38.7–47.3) | 40.3 (35.3–47.3) | <0.001 |
| Age range (years) | 20–71 | 24–71 | 20–68 | – |
| Age category, (n,%) | <0.001 | |||
| 20–40 years | 230 (40.3%) | 86 (30.1%) | 144 (50.5%) | |
| 40–60 years | 292 (51.1%) | 173 (60.5%) | 119 (41.8%) | |
| >60 years | 49 (8.6%) | 27 (9.4%) | 22 (7.7%) | |
| Starting weight, kg (mean ± SD) | 97.9 ± 22.3 | 98.2 ± 22.8 | 97.7 ± 21.0 | 0.842 |
| Starting BMI (kg/m2) (mean ± SD) | 34.5 ± 10.1 | 33.9 ± 10.3 | 35.2 ± 9.6 | 0.181 |
| BMI category (kg/m2), (n,%) | 0.106 | |||
| 27–30 | 118 (20.7%) | 54 (18.9%) | 64 (22.5%) | |
| 30–35 | 175 (30.6%) | 93 (32.5%) | 82 (28.8%) | |
| 35–40 | 97 (16.9%) | 57 (19.9%) | 40 (14.0%) | |
| >40 | 181 (31.8%) | 82 (28.7%) | 99 (34.7%) | |
| Activity level, number of steps (median, IQR) * | 8288 (3617–12,959) | 6629 (4438–12,120) | 10,944 (8361–13,527) | <0.001 |
| Symptoms after balloon ingestion, (n,%) | 0.479 | |||
| No symptoms | 547 (95.8%) | 257 (89.9%) | 257 (90.2%) | |
| Nausea | 21 (3.7%) | 9 (3.1%) | 12 (4.2%) | |
| Vomiting | 16 (2.8%) | 7 (2.4%) | 9 (3.2%) | |
| Abdominal discomfort | 20 (3.5%) | 13 (4.5%) | 7 (2.5%) |
| Variables | Less Active (n = 286) | More Active (n = 285) | p-Value |
|---|---|---|---|
| Type of exercise * (n,%) | <0.001 | ||
| Running | 93 (32.5%) | 159 (55.8%) | |
| Cycling | 35 (12.2%) | 27 (9.5%) | |
| Football | 57 (19.9%) | 62 (21.8%) | |
| Other | 101 (35.3%) | 37 (13.0%) | |
| Minutes of activity per session (median, IQR) | 40.5 (31.0–54.5) | 58.0 (50.5–66.0) | <0.001 |
| Number of daily steps (median, IQR) | 6629 (4438–12,120) | 10,944 (8361–13,527) | <0.001 |
| Minutes of moderate-intensity physical activity per week (median, IQR) | 110 (84–136) | 166 (121–184) | <0.001 |
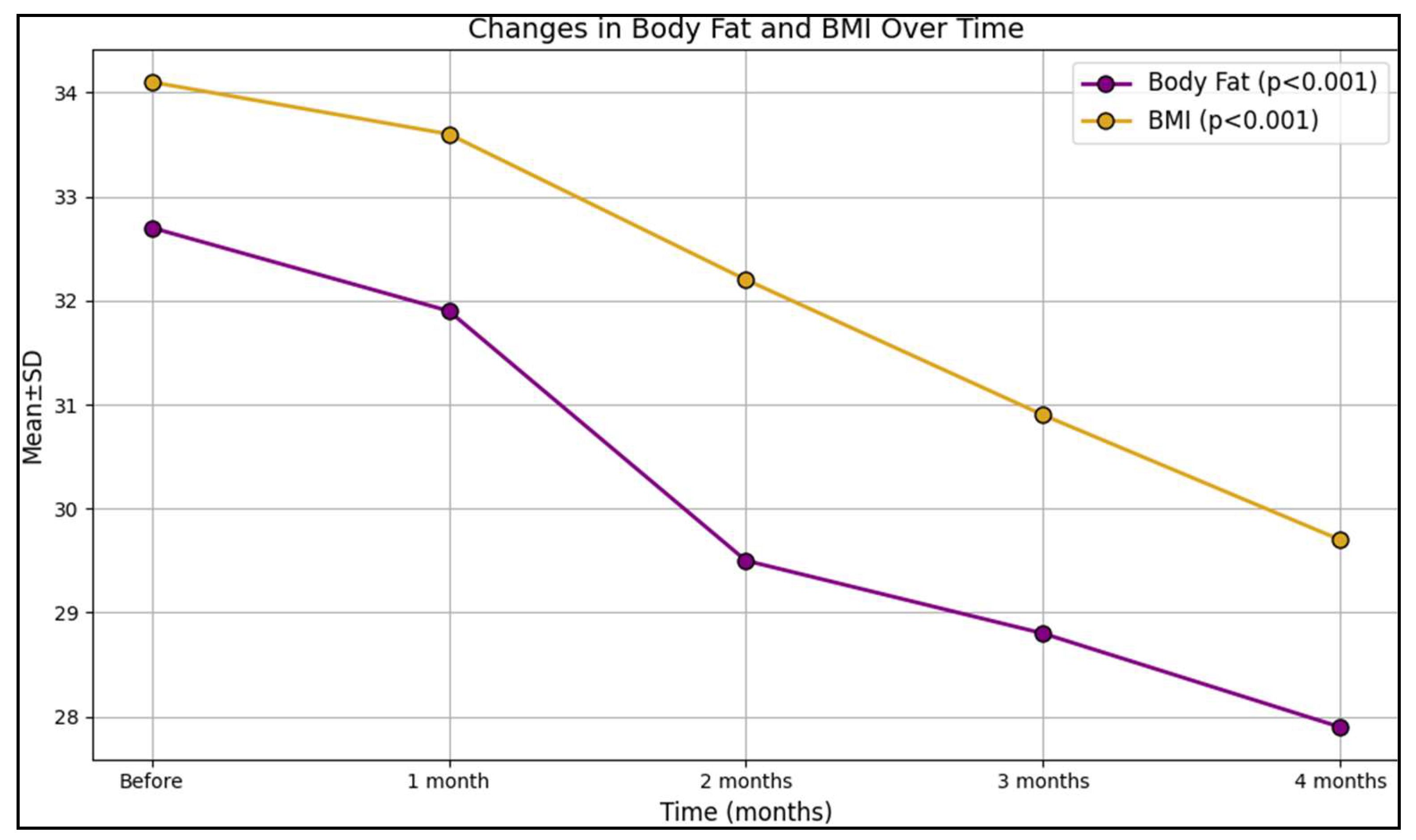
| Variables (Mean ± SD) | Before | 1 Month | 2 Months | 3 Months | 4 Months | p-Value * |
|---|---|---|---|---|---|---|
| Body fat | 32.7 ± 11.3 | 31.9 ± 12.0 | 29.5 ± 13.2 | 28.8 ± 10.8 | 27.9 ± 12.6 | <0.001 |
| Lean mass | 49.8 ± 14.9 | 50.1 ± 16.3 | 50.4 ± 13.8 | 51.5 ± 14.2 | 52.6 ± 15.4 | 0.008 |
| Bone mass | 3.1 ± 0.8 | 3.1 ± 0.8 | 3.0 ± 1.0 | 3.1 ± 1.2 | 3.2 ± 1.4 | 0.039 |
| Body water | 44.7 ± 11.9 | 44.9 ± 12.7 | 44.0 ± 13.3 | 43.1 ± 14.6 | 42.5 ± 13.8 | 0.009 |
| Visceral fat | 13.4 ± 5.9 | 13.0 ± 6.4 | 12.8 ± 6.1 | 12.3 ± 6.3 | 12.0 ± 6.8 | 0.001 |
| BMI (kg/m2) | 34.1 ± 7.8 | 33.6 ± 8.0 | 32.2 ± 9.5 | 30.9 ± 10.3 | 29.7 ± 12.5 | <0.001 |
| Weight (kg) | 97.9 ± 21.8 | 93.0 ± 19.6 | 89.6 ± 22.7 | 86.1 ± 23.7 | 84.0 ± 23.9 | <0.001 |
| Outcome | Group | Baseline (Mean ± SD) | Month 1 (Mean ± SD) | Month 2 (Mean ± SD) | Month 3 (Mean ± SD) | Month 4 (Mean ± SD) | p-Value for Time * Group Interaction | p-Value for Group Main Effect | p-Value for Time Main Effect |
|---|---|---|---|---|---|---|---|---|---|
| Body fat | Less Active | 33.1 ± 9.4 | 32.5 ± 9.2 | 31.8 ± 9.0 | 29.7 ± 9.8 | 28.3 ± 10.2 | 0.346 | 0.462 | <0.00001 |
| More Active | 32.2 ± 10.9 | 31.8 ± 10.7 | 31.2 ± 10.4 | 29.1 ± 10.0 | 27.5 ± 10.1 | ||||
| Lean mass | Less Active | 49.5 ± 14.8 | 50.0 ± 14.6 | 50.4 ± 14.3 | 50.9 ± 13.9 | 51.3 ± 13.6 | 0.045 | 0.309 | 0.022 |
| More Active | 50.9 ± 14.2 | 51.4 ± 14.0 | 51.9 ± 13.8 | 52.5 ± 13.5 | 53.7 ± 15.0 | ||||
| Bone mass | Less Active | 3.1 ± 0.8 | 3.0 ± 0.9 | 2.9 ± 0.9 | 2.9 ± 1.0 | 3.0 ± 1.0 | 0.001 | 0.374 | 0.039 |
| More Active | 3.2 ± 1.0 | 3.1 ± 1.1 | 3.0 ± 1.2 | 3.2 ± 1.1 | 3.3 ± 1.2 | ||||
| Body water | Less Active | 43.9 ± 10.5 | 44.1 ± 10.2 | 44.3 ± 9.9 | 43.2 ± 10.8 | 42.7 ± 11.9 | 0.523 | 0.289 | 0.009 |
| More Active | 44.6 ± 9.3 | 44.8 ± 9.0 | 45.0 ± 8.7 | 43.7 ± 9.6 | 42.1 ± 10.5 | ||||
| Visceral fat | Less Active | 13.0 ± 5.4 | 12.7 ± 5.1 | 12.4 ± 4.9 | 12.1 ± 5.3 | 11.9 ± 6.6 | 0.711 | 0.254 | 0.001 |
| More Active | 13.8 ± 6.0 | 13.4 ± 5.7 | 12.9 ± 5.5 | 12.3 ± 5.1 | 12.1 ± 6.3 | ||||
| BMI (kg/m2) | Less Active | 33.9 ± 10.3 | 33.5 ± 10.1 | 33.1 ± 9.9 | 30.6 ± 11.2 | 29.2 ± 11.8 | 0.229 | 0.181 | <0.00001 |
| More Active | 35.2 ± 9.6 | 34.8 ± 9.4 | 34.4 ± 9.1 | 31.7 ± 11.5 | 30.1 ± 12.0 | ||||
| Weight (kg) | Less Active | 98.2 ± 22.8 | 96.9 ± 22.1 | 95.6 ± 21.4 | 92.8 ± 20.6 | 84.6 ± 19.3 | 0.158 | 0.842 | <0.00001 |
| More Active | 97.7 ± 21.0 | 96.3 ± 20.3 | 95.0 ± 19.6 | 90.4 ± 21.1 | 82.1 ± 22.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dejeu, D.; Dejeu, P.; Bradea, P.; Muresan, A.; Dejeu, V. Evaluating Weight Loss Efficacy in Obesity Treatment with Allurion’s Ingestible Gastric Balloon: A Retrospective Study Utilizing the Scale App Health Tracker. Clin. Pract. 2024, 14, 765-778. https://doi.org/10.3390/clinpract14030061
Dejeu D, Dejeu P, Bradea P, Muresan A, Dejeu V. Evaluating Weight Loss Efficacy in Obesity Treatment with Allurion’s Ingestible Gastric Balloon: A Retrospective Study Utilizing the Scale App Health Tracker. Clinics and Practice. 2024; 14(3):765-778. https://doi.org/10.3390/clinpract14030061
Chicago/Turabian StyleDejeu, Danut, Paula Dejeu, Paula Bradea, Anita Muresan, and Viorel Dejeu. 2024. "Evaluating Weight Loss Efficacy in Obesity Treatment with Allurion’s Ingestible Gastric Balloon: A Retrospective Study Utilizing the Scale App Health Tracker" Clinics and Practice 14, no. 3: 765-778. https://doi.org/10.3390/clinpract14030061
APA StyleDejeu, D., Dejeu, P., Bradea, P., Muresan, A., & Dejeu, V. (2024). Evaluating Weight Loss Efficacy in Obesity Treatment with Allurion’s Ingestible Gastric Balloon: A Retrospective Study Utilizing the Scale App Health Tracker. Clinics and Practice, 14(3), 765-778. https://doi.org/10.3390/clinpract14030061





