Age-Stratified Spatial Radiological Risk Assessment of 226Ra 232Th and 40K in Water Surrounding the Geita Gold Mine in Tanzania
Abstract
1. Introduction
2. Materials and Methods
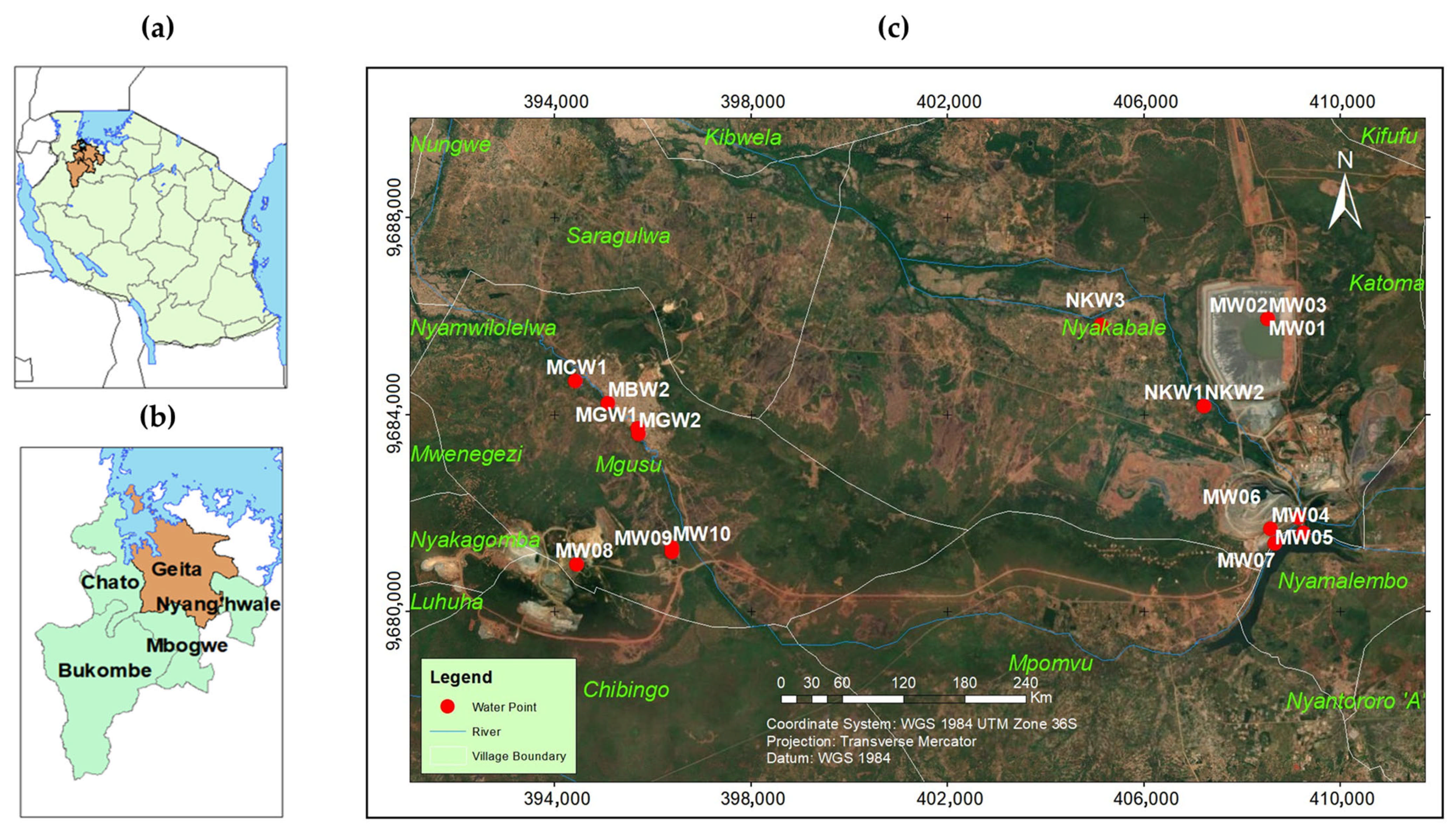
2.1. Description of Study Area
2.2. Sample Collection and Analysis
2.3. Radiometric Analysis
2.3.1. Energy Calibration of the System
2.3.2. Efficiency Calibration of the System
2.3.3. Activity Concentration Determination
2.4. Establishment of Annual Effective Ingestion Dose (AEID) and Total AEID (TAEID)
2.5. Statistical Analysis
3. Results and Discussions
3.1. Radionuclide Activity Concentration
3.2. Water Quality Parameters
3.3. Annual Effective Ingestion Dose (AEID) and Total Annual Effective Ingestion Dose (TAEID)
3.4. Cancer Risks and Hereditary Effects
3.5. Comparison of Cancer Risks and Hereditary Effects with Other Regions
| Country | Water Type | FCR | LFCR | SHE | ELHE | Reference |
|---|---|---|---|---|---|---|
| Tanzania | Spring, rivers and ponds | 2.0 × 10−6 | 1.3 × 10−4 | 7.1 × 10−8 | 4.8 × 10−6 | This study |
| Nigeria | Borehole | 1.9 × 10−4 | 1.3 × 10−2 | 6.8 × 10−6 | 4.8 × 10−6 | [71] |
| Nigeria | Salt lake | 6.3 × 10−7 | 4.4 × 10−5 | 2.3 × 10−8 | 2.7× 10−7 | [37] |
| Nigeria | Pond | 2.2 × 10−5 | 1.5 × 10−3 | 2.3 × 10−8 | 2.7× 10−7 | [75] |
| Nigeria | Rivers | 1.5 × 10−5 | 1.1 × 10−3 | 5.6 × 10−7 | 3.9× 10−4 | [75] |
| South Africa | Natural spring bottled drinking water | 1.8 × 10−4 | 1.2 × 10−2 | 6.4 × 10−6 | 4.5 × 10−4 | [39] |
| Egypt | Water from phosphate-polluted area | 5.6 × 10−5 | 4.0 × 10−3 | 2.0 × 10−6 | 1.4 × 10−4 | [72] |
| Ghana | Bottled drinking water | 7.3 × 10−7 | - | 1.8 × 10−8 | - | [73] |
| Spring borehole and well water | 3.2 × 10−9 | 6.9 × 10−11 | [74] |
3.6. Spatial Variability of Radionuclide Activity, Radiological Risk Indices and Water Parameters
3.7. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNSCEAR. UNSCEAR 2019 Report: Sources, Effects and Risks of Ionizing Radiation. Scientific Annexes A and B; UNSCEAR: Vienna, Austria, 2020; Volume 120, p. 312. [Google Scholar]
- ICRP. ICRP Approach for Radiological Protection from NORM in Industrial Processes; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2020; Volume 49, pp. 84–97. [Google Scholar]
- ICRP. ICRP Publication 142: Radiological Protection from Naturally Occurring Radioactive Material (NORM) in Industrial Processes; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2019; Volume 48, pp. 5–67. [Google Scholar]
- Louw, I. Radioanalytical Chemistry in Support of the Naturally Occurring Radioactive Material (NORM) Industries in South Africa. Ph.D. Thesis, University of the Witwatersrand, Faculty of Science, School of Chemistry, Johannesburg, South Africa, 2018. [Google Scholar]
- Altıkulaç, A.; Turhan, Ş.; Gümüş, H. The natural and artificial radionuclides in drinking water samples and consequent population doses. J. Radiat. Res. Appl. Sci. 2015, 8, 578–582. [Google Scholar] [CrossRef]
- WHO. Management of Radioactivity in Drinking Water; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Njinga, R.L.; Tshivhase, V.M. The impact of mine tailings on the Witwatersrand and the surrounding water bodies in Gauteng Province, South Africa. Mine Water Environ. 2017, 36, 638–645. [Google Scholar] [CrossRef]
- Mohuba, S.C.; Abiye, T.; Nhleko, S. Evaluation of radionuclide levels in drinking water from communities near active and abandoned gold mines and tailings in the West Rand Region, Gauteng, South Africa. Minerals 2022, 12, 1370. [Google Scholar] [CrossRef]
- IAEA Safety Standards Series No. GSR Part 3; Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards. IAEA: Vienna, Austria, 2014.
- Banzi, F.P.; Msaki, P.K.; Mohammed, N.K. Assessment of Natural Radioactivity in Soil and Its Contribution to Population Exposure in the Vicinity of Mkuju River Uranium Project in Tanzania. Expert Opin. Environ. Biol. 2017, 6, 4. [Google Scholar] [CrossRef]
- Kimaro, E.E.; Mohammed, N.K. Natural radioactivity levels in the area around the uranium deposit of the Bahi district in Dodoma region, Tanzania. Int. Res. J. Pure Appl. Chem. 2015, 9, 1–10. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Mazunga, M.S. Natural radioactivity in soil and water from Likuyu village in the neighbourhood of Mkuju uranium deposit. Int. J. Anal. Chem. 2013, 2013, 501856. [Google Scholar] [CrossRef]
- Panga, V.A.; Kumwenda, M.J.; Makundi, I.N. Assessment of heavy metals in fish and sediments from river Mtakuja in the vicinity of a gold mine in Tanzania. Bull. Nat. Sci. Res. 2023, 13, 10–18. [Google Scholar] [CrossRef]
- Rwiza, M.J.; Kim, K.W.; Kim, S.d. Geochemical Distribution of Trace Elements in Groundwater from the North Mara Large-Scale Gold Mining Area of Tanzania. Groundw. Monit. Remediat. 2016, 36, 83–93. [Google Scholar] [CrossRef]
- Bitala, M.F.; Kweyunga, C.; Manoko, M.L.K. Levels of Heavy Metals and Cyanide in Soil, Sediment and Water from the Vicinity of North Mara Gold Mine in Tarime District, Tanzania. Christian Council of Tanzania, Dodoma. 2009. Available online: http://www.protestbarrick.net/downloads/North%20Mara%20Pollution%20Report.pdf (accessed on 10 September 2025).
- Almås, Å.R.; Manoko, M.L.K. Trace Element Concentrations in Soil, Sediments, and Waters in the Vicinity of Geita Gold Mines and North Mara Gold Mines in Northwest Tanzania. Soil Sediment Contam. 2012, 21, 135–159. [Google Scholar] [CrossRef]
- Maliganya, W.; Paul, R. The impact of Large-Scale Mining on the Livelihoods of Adjacent Communities: The Case of Geita Gold Mine, Tanzania; Research on Poverty Alleviation REPOA: Dar es Salaam, Tanzania, 2017. [Google Scholar]
- Sawe, S.F. Radiological characterization of phosphate rocks from Minjingu Tanzania and estimation of excess life time cancer risk due to gamma rays exposure. J. Rad. Res. Appl. Sci. 2025, 18, 101756. [Google Scholar] [CrossRef]
- Haneklaus, N.H.; Mwalongo, D.A.; Lisuma, J.B.; Amasi, A.I.; Mwimanzi, J.; Bituh, T.; Ćirić, J.; Nowak, J.; Ryszko, U.; Rusek, P.; et al. Rare earth elements and uranium in Minjingu phosphate fertilizer products: Plant food for thought. Resour. Conserv. Recycl. 2024, 207, 107694. [Google Scholar] [CrossRef]
- Mwalongo, D.A.; Haneklaus, N.H.; Lisuma, J.B.; Kivevele, T.T.; Mtei, K.M. Uranium in phosphate rocks and mineral fertilizers applied to agricultural soils in East Africa. Environ. Sci. Pollut. Res. 2023, 30, 33898–33906. [Google Scholar] [CrossRef] [PubMed]
- Kwelwa, S.D.; Dirks, P.H.G.M.; Sanislav, I.V.; Blenkinsop, T.; Kolling, S.L. Archaean gold mineralization in an extensional setting: The structural history of the Kukuluma and Matandani deposits, Geita Greenstone Belt, Tanzania. Minerals 2018, 8, 171. [Google Scholar] [CrossRef]
- Kileo, A.A.; Salama, A.; Chuma, F.; Pantaleo, P. Evaluation of Radioactivity Concentration and Radiological Impact for a Closed Open Pit Gold Mine. Braz. J. Radiat. Sci. 2025, 13, e2548. [Google Scholar] [CrossRef]
- Ameho, E.; Kpeglo, D.; Glover, E.; Adukpo, O.; Sulemana, A.; Agalga, R.; Kpordzro, R.; Quarshie, E.; Hogarh, J. Naturally occurring radioactive material in groundwater: Potential health risk to the inhabitants at Osino in the eastern region of Ghana. Int. J. Radiat. Res. 2023, 21, 779–787. [Google Scholar] [CrossRef]
- Ramadan, F.; Nour, H.E.; Wahed, N.A.; Rakha, A.; Amuda, A.K.; Faisal, M. Heavy metal contamination and environmental risk assessment: A case study of surface water in the Bahr Mouse stream, East Nile Delta, Egypt. Environ. Monit. Assess. 2024, 196, 429. [Google Scholar] [CrossRef]
- Ravisankar, R.; Chandramohan, J.; Chandrasekaran, A.; Prince Prakash Jebakumar, J.; Vijayalakshmi, I.; Vijayagopal, P.; Venkatraman, B. Assessments of radioactivity concentration of natural radionuclides and radiological hazard indices in sediment samples from the East coast of Tamil Nadu, India with statistical approach. Mar. Pollut. Bull. 2015, 97, 419–430. [Google Scholar] [CrossRef]
- Sarker, M.; Rahman, R.; Siraz, M.; Khandaker, M.U.; Yeasmin, S. The presence of primordial radionuclides in powdered milk and estimation of the concomitant ingestion dose. Radiat. Phys. Chem. 2021, 188, 109597. [Google Scholar] [CrossRef]
- Damla, N.; Cevik, U.; Kobya, A.I.; Ataksor, B.; Isık, U. Assessment of environmental radioactivity for Batman, Turkey. Environ. Monit. Assess. 2010, 160, 401–412. [Google Scholar] [CrossRef]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. An assessment of radiological hazards from gold mine tailings in the province of Gauteng in South Africa. Int. J. Environ. Res. Public Health 2016, 13, 138. [Google Scholar] [CrossRef]
- Long, A.; Zhang, Q.; Xiong, Z.; Xiao, P.; Xie, Q. Energy calibration method for an all-digital high resolution gamma-ray spectroscopy. In Proceedings of the 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Seattle, WA, USA, 8–15 November 2014; pp. 1620–1623. [Google Scholar]
- Belgya, T.; Révay, Z. Gamma-ray spectrometry. In Handbook of Prompt Gamma Activation Analysis: With Neutron Beams; Springer: Berlin/Heidelberg, Germany, 2004; pp. 71–111. [Google Scholar]
- ORTEC. GammaVision ® Maestro-PRO ® Version 8.10.02; ORTEC: New York, NY, USA, 2017; pp. 23–34. [Google Scholar]
- Kovler, K.; Friedmann, H.; Michalik, B.; Schroeyers, W.; Tsapalov, A.; Antropov, S.; Bituh, T.; Nicolaides, D. Basic aspects of natural radioactivity. In Naturally Occurring Radioactive Materials in Construction: Integrating Radiation Protection in Reuse (COST Action Tu1301 NORM4BUILDING); Woodhead Publishing: Cambridge, UK, 2017; pp. 13–36. [Google Scholar] [CrossRef]
- Njuguna, S.M.; Makokha, V.A.; Yan, X.; Gituru, R.W.; Wang, Q.; Wang, J. Health risk assessment by consumption of vegetables irrigated with reclaimed waste water: A case study in Thika (Kenya). J. Environ. Manag. 2019, 231, 576–581. [Google Scholar] [CrossRef]
- Asaduzzaman, K.; Mannan, F.; Khandaker, M.; Farook, M.; Elkezza, A.; Amin, Y.; Sharma, S. Natural radioactivity levels in commercialized bottled drinking water and their radiological quality assessment. Desalination Water Treat. 2016, 57, 11999–12009. [Google Scholar] [CrossRef]
- Altıkulaç, A.; Kurnaz, A.; Turhan, S.; Kutucu, M. Natural radionuclides in bottled mineral waters consumed in Turkey and their contribution to radiation dose. ACS Omega 2022, 7, 34428–34435. [Google Scholar] [CrossRef] [PubMed]
- Caridi, F.; Paladini, G.; D’Agostino, M.; Marguccio, S.; Belvedere, A.; Belmusto, G.; Stilo, G.; Majolino, D.; Venuti, V. Radon-Specific Activity in Drinking Water and Radiological Health Risk Assessment: A Case Study. Appl. Sci. 2023, 13, 9660. [Google Scholar] [CrossRef]
- Ononugbo, C.; Nwaka, B. Natural radioactivity and radiological risk estimation of drinking water from Okposi and Uburu salt lake area, Ebonyi State, Nigeria. Phys. Sci. Int. J. 2017, 15, 1–15. [Google Scholar] [CrossRef][Green Version]
- Esi, O.E. Radiological impact of hydrocarbon waste release on drinking water of Ughievwen and Udu communities, Delta State, Nigeria. Nucl. Anal. 2024, 3, 100121. [Google Scholar] [CrossRef]
- John, S.O.O.; Olukotun, S.F.; Mathuthu, M. Assessment of Radioactivity Concentrations and Associated Radiological Health Risk in Natural Spring Mineral Bottled Drinking Water from South Africa. Water 2025, 17, 156. [Google Scholar] [CrossRef]
- NBS. Census Information Dissemination Platform; NBS: Dodoma, Tanzania, 2022. [Google Scholar]
- Mwimanzi, J.M.; Haneklaus, N.H.; Bituh, T.; Brink, H.; Kiegiel, K.; Lolila, F.; Marwa, J.J.; Rwiza, M.J.; Mtei, K.M. Radioactivity distribution in soil, rock and tailings at the Geita Gold Mine in Tanzania. J. Radiat. Res. Appl. Sci. 2025, 18, 101528. [Google Scholar] [CrossRef]
- Ahmad, N.; Ur Rehman, J.; Rehman, J.; Nasar, G. Effect of geochemical properties (pH, conductivity, TDS) on natural radioactivity and dose estimation in water samples in Kulim, Malaysia. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1688–1696. [Google Scholar] [CrossRef]
- Jia, G.; Torri, G. Determination of 210Pb and 212Pb in water and their radiological impact to the public via drinking water. Nucl. Sci. Tech. 2012, 23, 19–28. [Google Scholar]
- Burnett, J.; Croudace, I.; Warwick, P. Pre-concentration of naturally occurring radionuclides and the determination of 212Pb from fresh waters. J. Environ. Radioact. 2011, 102, 326–330. [Google Scholar] [CrossRef]
- Labidi, S.; Mahjoubi, H.; Essafi, F.; Salah, R.B. Natural radioactivity levels in mineral, therapeutic and spring waters in Tunisia. Radiat. Phys. Chem. 2010, 79, 1196–1202. [Google Scholar] [CrossRef]
- Lottermoser, B.; Ashley, P. Tailings dam seepage at the rehabilitated Mary Kathleen uranium mine, Australia. J. Geochem. Explor. 2005, 85, 119–137. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Emel, J.; Plisinski, J.; Geography, J.R.A. Monitoring geomorphic and hydrologic change at mine sites using satellite imagery: The Geita Gold Mine in Tanzania. Appl. Geogr. 2014, 54, 243–249. [Google Scholar] [CrossRef]
- Abdurabu, W.A.; Saleh, M.A.; Ramli, A.T.; Heryansyah, A. Occurrence of natural radioactivity and corresponding health risk in groundwater with an elevated radiation background in Juban District, Yemen. Environ. Earth Sci. 2016, 75, 1360. [Google Scholar] [CrossRef]
- Salih, N.; Aswood, M. Measuring the radioactivity concentration of 40K, 226Ra, 232Th with pH, conductivity and radiological risk in tap water. Int. J. Environ. Anal. Chem. 2024, 105, 1846–1866. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; El-Zohry, M.A.; Mehanni, A.E. Assessment of Radioactivity Levels and Annual Dose Intake from Water Consumption in Sohag Governorate, Egypt. Arab. J. Nucl. Sci. Appl. 2022, 55, 39–44. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking Water Quality: Small Water Supplies; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Ajayi, O.; Achuka, J. Radioactivity in drilled and dug well drinking water of Ogun State, Southwestern Nigeria, and consequent dose estimates. Radiat. Prot. Dosim. 2009, 135, 54–63. [Google Scholar] [CrossRef]
- Ajayi, O.; Owolabi, T. Determination of natural radioactivity in drinking water in private dug wells in Akure, Southwestern Nigeria. Radiat. Prot. Dosim. 2008, 128, 477–484. [Google Scholar] [CrossRef]
- Abd El-Mageed, A.I.; Abbady, A.E.-B.; Harb, S.; Saleh, I.I. Natural radioactivity of ground and hot spring water in some areas in Yemen. Desalination 2013, 321, 28–31. [Google Scholar] [CrossRef]
- Salih, N.F. Measurement of natural radioactivity levels in drinking water by gamma spectrometry. Arab. J. Geosci. 2022, 15, 1157. [Google Scholar] [CrossRef]
- Rakotondrabe, F.; Ngoupayou, J.R.N.; Mfonka, Z.; Rasolomanana, E.H.; Abolo, A.J.N.; Ako, A.A. Water quality assessment in the Bétaré-Oya gold mining area (East-Cameroon): Multivariate statistical analysis approach. Sci. Total Environ. 2018, 610, 831–844. [Google Scholar] [CrossRef]
- Rakotondrabe, F.; Ngoupayou, J.R.N.; Mfonka, Z.; Rasolomanana, E.H.; Abolo, A.J.N.; Asone, B.L.; Ako, A.A.; Rakotondrabe, M.H. Assessment of surface water quality of Bétaré-Oya gold mining area (East-Cameroon). J. Water Resour. Prot. 2017, 9, 960–984. [Google Scholar] [CrossRef]
- Aberikae, E.A.; Nyantakyi, E.K.; Kpeglo, D.O.; Abubakar, M.; Gbeddy, G.; Ackerson, N.O.B.; Yeboah, S.I.I.K.; Domfeh, M.K.; Atta-Darkwa, T.; Amankwah, E. Baseline assessment of naturally occurring radionuclides in borehole water of Asikam-gold mining community in Ghana. Sci. Afr. 2023, 20, e01730. [Google Scholar] [CrossRef]
- Mambou Ngueyep, L.; Takougang Kingni, S.; Ayiwouo Ngounouno, M.; Ndi, A. The impact of gold mining exploitation on the physicochemical quality of water: Case of Batouri (Cameroon) Effect of gold mine operations on water pollution. Int. J. Energy Water Resour. 2021, 5, 159–173. [Google Scholar] [CrossRef]
- Cobbina, S.; Myilla, M.; Michael, K. Small scale gold mining and heavy metal pollution: Assessment of drinking water sources in Datuku in the Talensi-Nabdam District. Int. J. Sci. Technol. Res 2013, 2, 96–100. [Google Scholar]
- 1011-4289; IAEA-TECDOC-2011 Exposure Due to Radionuclides in Food Other Than During a Nuclear or Radiological Emergency. Part 2: Considerations in Implementing Requirement 51 of IAEA General Safety Requirements Part 3 (International Basic Safety Standards). International Atomic Energy Agency (IAEA) Radiation Safety and Monitoring Section: Vienna, Austria, 2022.
- ICRP. Protection of the Public in Situations of Prolonged Radiation Exposure. Pergamon Press: Oxford, UK, 1999; Volume 82, Ann. ICRP 29 (1-2). [Google Scholar]
- Arabi, A.E.; Ahmed, N.; Salahel Din, K. Natural radionuclides and dose estimation in natural water resources from Elba protective area, Egypt. Radiat. Prot. Dosim. 2006, 121, 284–292. [Google Scholar] [CrossRef]
- Ahmad, N.; Rehman, J.u.; Rafique, M.; Nasir, T. Age-dependent annual effective dose estimations of 226Ra, 232Th, 40K and 222Rn from drinking water in Baling, Malaysia. Water Sci. Technol. Water Supply 2018, 18, 32–39. [Google Scholar] [CrossRef]
- Abbasi, A.; Mirekhtiary, F. Lifetime risk assessment of Radium-226 in drinking water samples. Int. J. Radiat. Res. 2019, 17, 163–169. [Google Scholar]
- Ahmad, N.; Khan, A.; Ahmad, I.; Hussain, J.; Ullah, N. Health implications of natural radioactivity in spring water used for drinking in Harnai, Balochistan. Int. J. Environ. Anal. Chem. 2021, 101, 1302–1309. [Google Scholar] [CrossRef]
- Bronzovic, M.; Marovic, G. Age-dependent dose assessment of 226Ra from bottled water intake. Health Phys. 2005, 88, 480–485. [Google Scholar] [CrossRef]
- Applegate, K.; Rühm, W.; Wojcik, A.; Bourguignon, M.; Brenner, A.; Hamasaki, K.; Imai, T.; Imaizumi, M.; Imaoka, T.; Kakinuma, S. Individual response of humans to ionising radiation: Governing factors and importance for radiological protection. Radiat. Environ. Biophys. 2020, 59, 185–209. [Google Scholar] [CrossRef]
- Narendran, N.; Luzhna, L.; Kovalchuk, O. Sex difference of radiation response in occupational and accidental exposure. Front. Genet. 2019, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Uzoekwe, S.; Anekwe, U.; Ibe, S. Estimation of Health Risks in Borehole Water Supply, Case Study in Ogbia, Nigeria. OSP J. Health Care Med. 2023, 4, 1–8. [Google Scholar]
- Mahmoud, M.; El-Zohry, M. The Natural Background Activity Concentration of (226Ra, 232Th and 40K) and the Annual Effective Dose from Different Water Sources Consumption in Phosphate Polluted Area. Egypt. J. Phys. 2020, 48, 19–26. [Google Scholar] [CrossRef]
- Portuphy, M.; Faanu, A.; Sawyerr, A. Radiological risk assessment due to ingestion of some bottled drinking water on the Ghanaian market. Ghana J. Sci. 2018, 59, 93–102. [Google Scholar] [CrossRef]
- Dangari, L.L.; Timtere, P.; Fwangle, I.I. Assessment of Natural Radioactivity in Spring, Borehole and Well Water in Hong Local Government Area, Adamawa State, Nigeria. Niger. J. Phys. 2025, 34, 1–12. [Google Scholar] [CrossRef]
- Adebayo, O.; Akinnawo, O. Assessment of radiological hazard of natural radioactivity in drinking water in Ondo, Nigeria. J. Sci. Res. Rep. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Faanu, A.; Lawluvi, H.; Kpeglo, D.O.; Darko, E.O.; Emi-Reynolds, G.; Awudu, A.R.; Adukpo, O.K.; Kansaana, C.; Ali, I.D.; Agyeman, B.; et al. Assessment of natural and anthropogenic radioactivity levels in soils, rocks and water in the vicinity of Chirano gold mine in Ghana. Radiat. Prot. Dosim. 2014, 158, 87–99. [Google Scholar] [CrossRef]
- Rabuku, A.T.W.; Malik, A.Q. Natural radioactivity measurement of gold mine tailings in Vatukoula, Fiji Islands. Renew. Energy Environ. Sustain. 2020, 5, 10. [Google Scholar] [CrossRef]
| Age Group (y) | Dose Conversion Factor (Sv Bq−1) | Annual Water Intake (L y−1) | |
|---|---|---|---|
| 226Ra | 40K | ||
| <1 | 4.7 × 10−6 | 6.2 × 10−8 | 150 |
| 7–12 | 8.0 × 10−7 | 1.3 × 10−8 | 350 |
| >17 | 2.8 × 10−7 | 6.2 × 10−9 | 730 |
| Sample ID and Location | UTM Northings | UTM Eastings | Activity Concentration | Water Quality Parameter | |||
|---|---|---|---|---|---|---|---|
| 226Ra (mBq L−1) | 40K (mBq L−1) | pH | TDS (mg L−1) | EC (µS cm−1) | |||
| MW01 (3), Mining area | 408,530 | 968,5973 | 130 | 6130 | 8.00 | 1330 | 2070 |
| MW02 (3), Mining area | 408,530 | 9,685,973 | 28 | 7230 | 7.26 | 1300 | 2040 |
| MW03 (3), Mining area | 408,530 | 9,685,973 | 96 | 7880 | 7.71 | 1320 | 2060 |
| MW04 (3), Mining area | 408,530 | 9,685,973 | 25 | 2370 | 7.45 | 660 | 1020 |
| MW05 (3), Mining area | 409,253 | 9,681,623 | 14 | 2710 | 7.09 | 530 | 840 |
| MW06 (3), Mining area | 409,146 | 9,681,936 | 20 | 2500 | 6.15 | 350 | 600 |
| MW07 (3), Mining area | 408,663 | 9,681,412 | 60 | 3100 | 7.06 | 120 | 190 |
| MW08 (3), Mining area | 408,582 | 9,681,719 | 48 | 6920 | 7.45 | 140 | 220 |
| MW09 (3), Mining area | 396,385 | 9,681,250 | 32 | 6110 | 7.59 | 60 | 100 |
| MW10 (3), Mining area | 396,385 | 9,681,397 | 19 | 7260 | 7.14 | 70 | 40 |
| NKW1 (3), Nyakabale village | 407,222 | 9,684,202 | 70 | 3890 | 6.95 | 70 | 110 |
| NKW2 (3), Nyakabale village | 407,302 | 9,683,900 | 67 | 3890 | 6.89 | 590 | 150 |
| NKW3 (3), Nyakabale village | 405,135 | 9,685,853 | 80 | 4230 | 7.31 | 40 | 50 |
| MGW1 (3), Mgusu village | 395,693 | 9,683,748 | 50 | 5340 | 7.19 | 30 | 50 |
| MGW2 (3), Mgusu village | 395,713 | 9,683,639 | 72 | 4440 | 7.40 | 30 | 490 |
| MCW1 (3), Machinjioni village | 394,428 | 9,684,716 | 92 | 5740 | 7.16 | 20 | 40 |
| MBW1 (3), Mabubi River | 395,088 | 9,684,266 | 67 | 5710 | 7.16 | 90 | 130 |
| Average ± SD a | 57 ± 32 | 5026 ± 1787 | 7.23 ± 0.4 | 397 ± 485 | 597 ± 746 | ||
| Range | 14–130 | 2370–7880 | 6.15–8 | 20–1330 | 40–2070 | ||
| Source/Type of Water | Country | Activity Concentration (mBq L−1) | References | |
|---|---|---|---|---|
| 226Ra | 40K | |||
| Tailings, spring water and ponds | Tanzania | 57 | 5026 | This study |
| River water (Kilowoko River) | Tanzania | 2500 | 11,000 | [12] |
| Spring water | Tanzania | - | 2820 | [11] |
| Bottled mineral water | Turkey | 380 | 4260 | [35] |
| Tap water | Iraq | 121 | 1091 | [50] |
| Wells | Egypt | 270 | 1610 | [51] |
| Groundwater | Yemen | 2950 | 34,900 | [55] |
| Spring water | Yemen | 3480 | 16,500 | [55] |
| Dug wells | Yemen | 94 | 3306 | [49] |
| Dug and drilled a well for water | Nigeria | 4540 | 2940 | [53] |
| Private dug wells | Nigeria | 7150 | 13,540 | [54] |
| Drinking water | Iraq | 76,000 | 447,000 | [56] |
| Guidance level | 1000 | - | [47] | |
| Sample Code | 226Ra-AEID (µSv y−1) | 40K-AEID (µSv y−1) | TAEID (µSv y−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Infants | Children | Adults | Infants | Children | Adults | Infants | Children | Adults | |
| MW01 (3) | 91.7 | 36.4 | 27.3 | 57.0 | 27.9 | 28.5 | 148.7 | 64.3 | 55.8 |
| MW02 (3) | 19.7 | 7.8 | 5.9 | 67.2 | 32.9 | 33.6 | 87.0 | 40.7 | 39.5 |
| MW03 (3) | 67.7 | 26.9 | 20.2 | 73.3 | 35.9 | 36.6 | 141.0 | 62.7 | 56.8 |
| MW04 (3) | 17.6 | 7.0 | 5.3 | 22.0 | 10.8 | 11.0 | 39.7 | 17.8 | 16.3 |
| MW05 (3) | 9.9 | 3.9 | 2.9 | 25.2 | 12.3 | 12.6 | 35.1 | 16.3 | 15.5 |
| MW06 (3) | 14.1 | 5.6 | 4.2 | 23.3 | 11.4 | 11.6 | 37.4 | 17.0 | 15.8 |
| MW07 (3) | 42.3 | 16.8 | 12.6 | 28.8 | 14.1 | 14.4 | 71.1 | 30.9 | 27.0 |
| MW08 (3) | 33.8 | 13.4 | 10.1 | 64.4 | 31.5 | 32.2 | 98.2 | 44.9 | 42.3 |
| MW09 (3) | 22.6 | 9.0 | 6.7 | 56.8 | 27.8 | 28.4 | 79.4 | 36.8 | 35.1 |
| MW10 (3) | 13.4 | 5.3 | 4.0 | 67.5 | 33.0 | 33.8 | 80.9 | 38.4 | 37.8 |
| NKW1 (3) | 49.4 | 19.6 | 14.7 | 36.2 | 17.7 | 18.1 | 85.5 | 37.3 | 32.8 |
| NKW2 (3) | 47.2 | 18.8 | 14.1 | 36.2 | 17.7 | 18.1 | 83.4 | 36.5 | 32.2 |
| NKW3 (3) | 56.4 | 22.4 | 16.8 | 39.3 | 19.3 | 19.7 | 95.7 | 41.7 | 36.5 |
| MGW1 (3) | 35.3 | 14.0 | 10.5 | 49.7 | 24.3 | 24.8 | 84.9 | 38.3 | 35.3 |
| MGW2 (3) | 50.8 | 20.2 | 15.1 | 41.3 | 20.2 | 20.7 | 92.1 | 40.4 | 35.8 |
| MCW1 (3) | 64.9 | 25.8 | 19.3 | 53.4 | 26.1 | 26.7 | 118.2 | 51.9 | 46.0 |
| MBW1 (3) | 47.2 | 18.8 | 14.1 | 53.1 | 26.0 | 26.6 | 100.3 | 44.7 | 40.6 |
| Mean ± SD a | 40.2 ± 22.6 | 16.0 ± 9.0 | 12.0 ± 6.7 | 46.7 ± 16.6 | 22.9 ± 8.1 | 23.4 ± 8.3 | 87.0 ± 31.5 | 38.8 ± 13.7 | 35.4 ± 12.0 |
| Range | 9.9–91.7 | 3.9–36.4 | 2.9–27.3 | 22.0–73.3 | 10.8–35.9 | 11.0–36.6 | 35.1–148.7 | 16.3–64.3 | 15.5–56.8 |
| Sample ID | FCR × 10−6 | LFCR × 10–4 | SHE × 10−8 | ELHE × 10–6 | ||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| MW01 (3) | 3.1 | 2.1 | 2.2 | 11.2 | 7.1 | 7.6 |
| MW02 (3) | 2.2 | 1.4 | 1.5 | 7.9 | 5.1 | 5.4 |
| MW03 (3) | 3.1 | 2.0 | 2.1 | 11.4 | 7.3 | 7.7 |
| MW04 (3) | 0.9 | 0.6 | 0.6 | 3.3 | 2.1 | 2.2 |
| MW05 (3) | 0.9 | 0.5 | 0.6 | 3.1 | 2.0 | 2.1 |
| MW06 (3) | 0.9 | 0.6 | 0.6 | 3.2 | 2.0 | 2.2 |
| MW07 (3) | 1.5 | 1.0 | 1.1 | 5.4 | 3.5 | 3.7 |
| MWS08 (3) | 2.3 | 1.5 | 1.6 | 8.5 | 5.4 | 5.8 |
| MW09 (3) | 2.0 | 1.2 | 1.3 | 7.0 | 4.5 | 4.8 |
| MW10 (3) | 2.1 | 1.3 | 1.4 | 7.6 | 4.8 | 5.1 |
| NKW1 (3) | 1.8 | 1.2 | 1.2 | 6.6 | 4.2 | 4.5 |
| NKW2 (3) | 1.8 | 1.1 | 1.2 | 6.4 | 4.1 | 4.4 |
| NKW3 (3) | 2.0 | 1.3 | 1.4 | 7.3 | 4.7 | 5.0 |
| MGW1 (3) | 2.0 | 1.2 | 1.3 | 7.1 | 4.5 | 4.8 |
| MGW2 (3) | 2.0 | 1.3 | 1.3 | 7.2 | 4.6 | 4.9 |
| MCW1 (3) | 2.5 | 1.6 | 1.7 | 9.2 | 5.9 | 6.3 |
| MBW1 (3) | 2.2 | 1.4 | 1.5 | 8.1 | 5.2 | 5.5 |
| Mean ± SD a | 2.0 ± 0.7 | 1.3 ± 0.4 | 1.3 ± 0.5 | 7.1 ± 2.4 | 4.5 ± 1.5 | 4.8 ± 1.6 |
| Range | 0.9–3.1 | 0.5–2.1 | 0.6–2.2 | 3.1–11.4 | 2.0–7.3 | 2.1–7.7 |
| Parameter | 226Ra | 40K | pH | TDS | EC | TAEID | FCR | LCFR | SHE | ELHE |
|---|---|---|---|---|---|---|---|---|---|---|
| Ra-226 | 1.000 | |||||||||
| K-40 | 0.490 ** | 1.000 | ||||||||
| pH | 0.320 * | 0.496 ** | 1.000 | |||||||
| TDS | −0.147 | 0.025 | 0.103 | 1.000 | ||||||
| EC | 0.080 | −0.065 | 0.297 * | 0.843 ** | 1.000 | |||||
| TAEID | 0.653 ** | 0.872 ** | 0.546 ** | −0.015 | 0.007 | 1.000 | ||||
| FCR | 0.653 ** | 0.871 ** | 0.540 ** | −0.002 | 0.026 | 0.997 ** | 1.000 | |||
| LFCR | 0.997 ** | 0.865 ** | 0.544 ** | −0.009 | 0.017 | 0.999 ** | 0.998 ** | 1.000 | ||
| SHE (Adults) | 0.507 ** | 0.870 ** | 0.546 ** | −0.005 | 0.011 | 0.987 ** | 0.987 ** | 0.987 ** | 1.000 | |
| ELHE (Adults) | 0.662 ** | 0.865 ** | 0.544 ** | −0.010 | 0.016 | 0.999 ** | 0.998 ** | 1.000 ** | 0.986 ** | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwimanzi, J.M.; Haneklaus, N.H.; Lolila, F.; Marwa, J.J.; Rwiza, M.J.; Mtei, K.M. Age-Stratified Spatial Radiological Risk Assessment of 226Ra 232Th and 40K in Water Surrounding the Geita Gold Mine in Tanzania. J. Xenobiot. 2025, 15, 152. https://doi.org/10.3390/jox15050152
Mwimanzi JM, Haneklaus NH, Lolila F, Marwa JJ, Rwiza MJ, Mtei KM. Age-Stratified Spatial Radiological Risk Assessment of 226Ra 232Th and 40K in Water Surrounding the Geita Gold Mine in Tanzania. Journal of Xenobiotics. 2025; 15(5):152. https://doi.org/10.3390/jox15050152
Chicago/Turabian StyleMwimanzi, Jerome M., Nils H. Haneklaus, Farida Lolila, Janeth J. Marwa, Mwemezi J. Rwiza, and Kelvin M. Mtei. 2025. "Age-Stratified Spatial Radiological Risk Assessment of 226Ra 232Th and 40K in Water Surrounding the Geita Gold Mine in Tanzania" Journal of Xenobiotics 15, no. 5: 152. https://doi.org/10.3390/jox15050152
APA StyleMwimanzi, J. M., Haneklaus, N. H., Lolila, F., Marwa, J. J., Rwiza, M. J., & Mtei, K. M. (2025). Age-Stratified Spatial Radiological Risk Assessment of 226Ra 232Th and 40K in Water Surrounding the Geita Gold Mine in Tanzania. Journal of Xenobiotics, 15(5), 152. https://doi.org/10.3390/jox15050152








