Development and Validation of LC–MS/MS and IC–HRMS Methods for Highly Polar Pesticide Detection in Honeybees: A Multicenter Study for the Determination of Pesticides in Honeybees to Support Pollinators and Environmental Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents (LC + IC)
2.2. Samples (LC + IC)
2.3. Analytical Methods
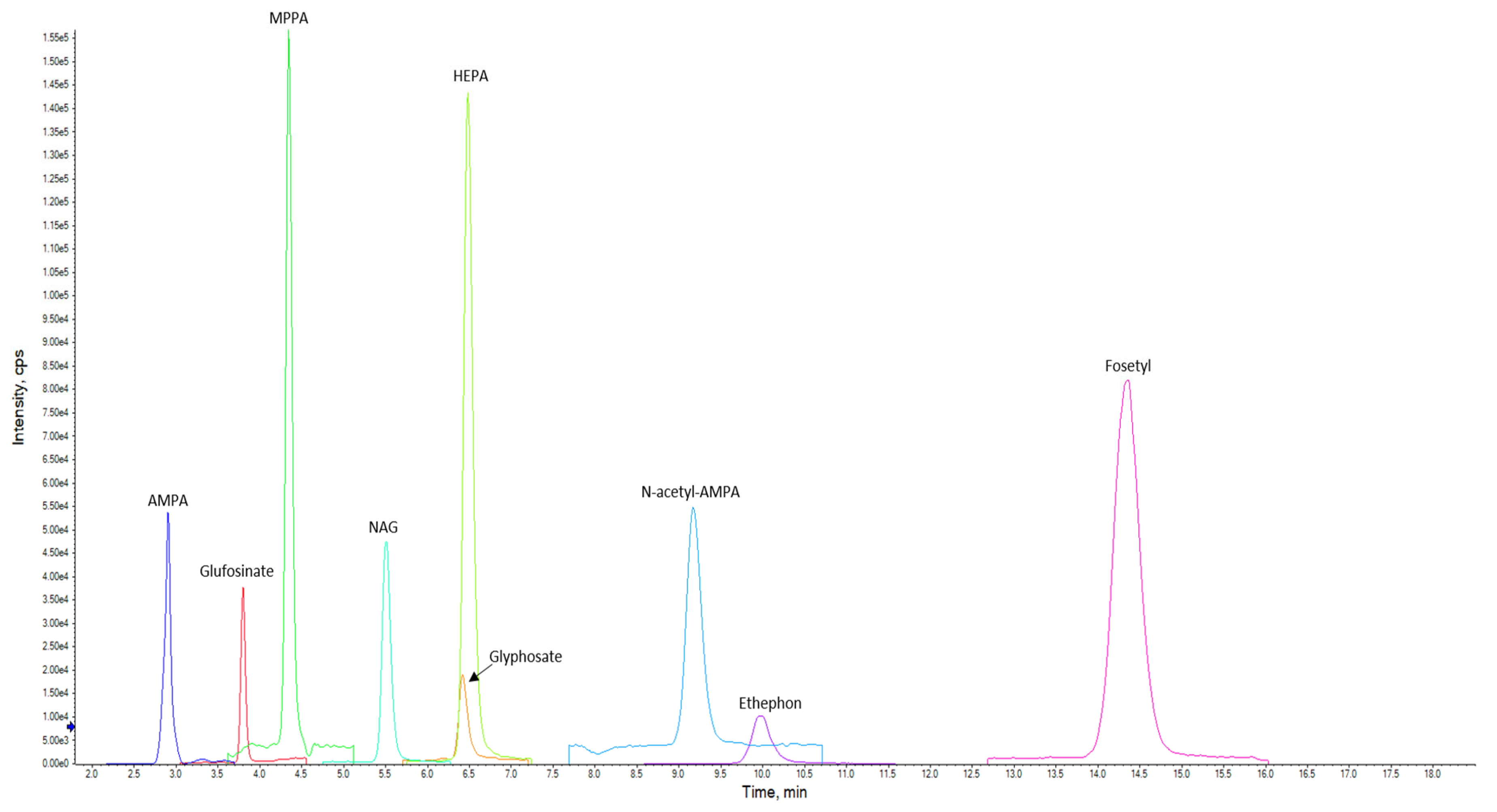
- Liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) for the detection of glyphosate, ethephon, glufosinate, fosetyl, and polar pesticide metabolites such as AMPA, N-acetyl-AMPA, HEPA, MPPA, and NAG in animal-derived products [6].
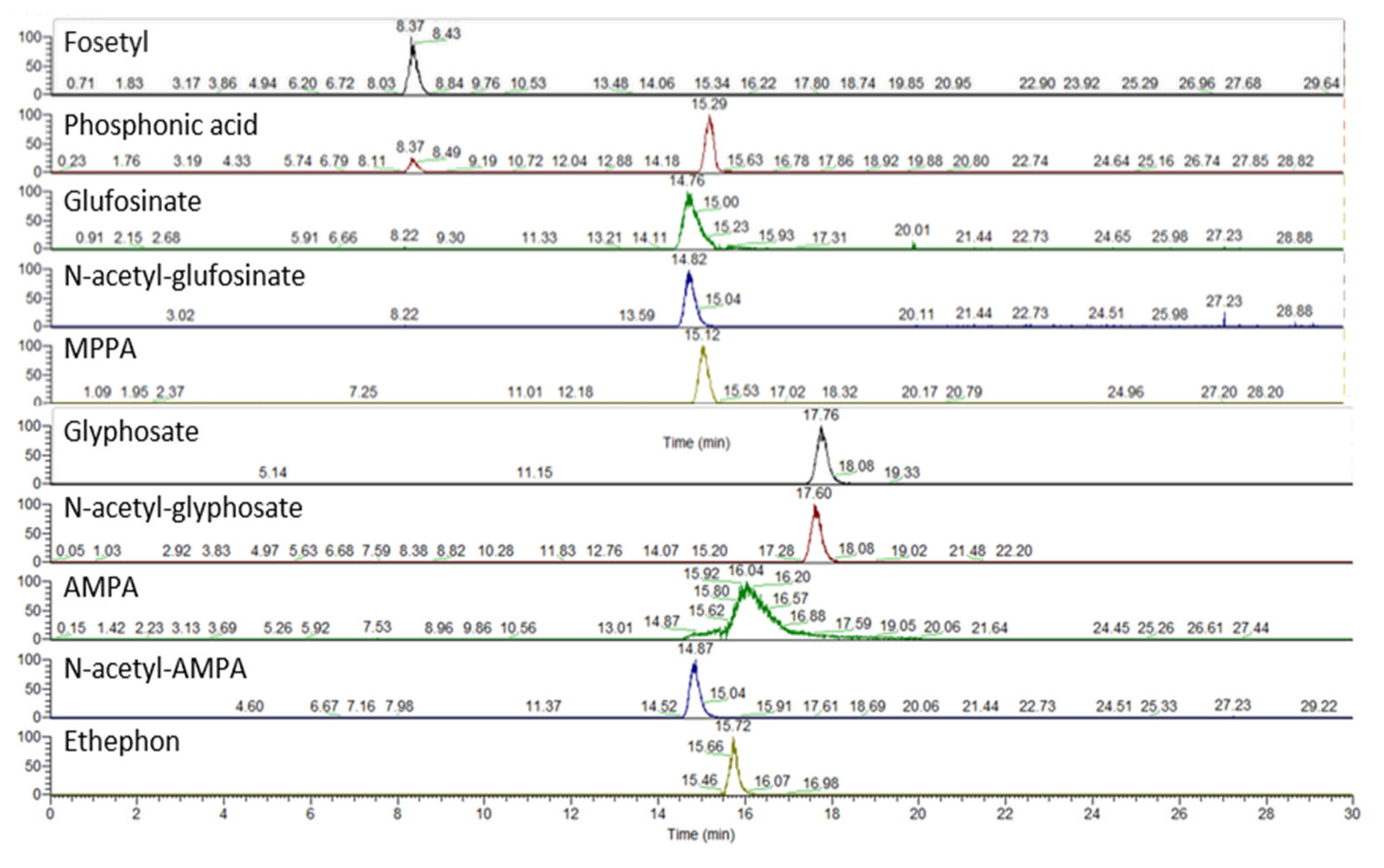
- Ion chromatography coupled with high-resolution mass spectrometry (IC–HRMS) for the detection of the abovementioned compounds plus N-acetyl-glyphosate and phosphonic acid in vegetables, fruit, and honey [5].
2.3.1. LC–MS/MS Method
Reference Materials
Sample Preparation Protocol
Chromatographic and Spectrometric Conditions
2.3.2. IC–HRMS Method
Reference Materials
- -
- LOQ = 0.01 mg/kg for glyphosate, N-acetyl-glyphosate, N-acetyl-AMPA, glufosinate, MPPA, and NAG.
- -
- LOQ = 0.05 mg/kg for ethephon.
- -
- LOQ = 0.10 mg/kg for fosetyl, phosphonic acid, and AMPA.
Sample Preparation Protocol
Chromatographic and Mass Spectrometric Conditions
2.4. Validation Protocol
2.4.1. LC–MS/MS Validation
Calibration Curves and Linearity Ranges
Recovery
- -
- RF = response factor (peak area ratio of the analyte/internal standard; peak area for N-acetyl-AMPA only).
- -
- a = slope of the calibration curve (µg− 1).
- -
- b = intercept of the calibration curve.
- -
- DF = dilution factor (20).
2.4.2. IC–HRMS Validation
Calibration Curves and Linearity Ranges
LOQ, Repeatability, and Within-Laboratory Reproducibility
Measurement Uncertainty
3. Results and Discussion
3.1. Analytical Method for Determination of Polar Pesticides in Bees Compared to the QuPPe-AO Method
3.2. LC–MS/MS Method Performance Verification
3.2.1. Linearity Ranges
3.2.2. Limit of Quantification (LOQ)
3.2.3. Repeatability (RSDr)
3.2.4. Inter-Laboratory Precision (RSDR)
3.3. IC–HRMS Method Performance Verification
3.3.1. Linearity Ranges
- -
- for fosetyl and phosphonic acid, the linearity was confirmed between 0.002 to 0.500 mg/kg;
- -
- for ethephon and AMPA, the linearity was confirmed between 0.001 and 0.250 mg/kg;
- -
- for glufosinate, N-acetyl-glufosinate, MPPA, glyphosate, N-acetyl-glyphosate, and N-acetyl AMPA, the linearity was confirmed between 0.0002 and 0.050 mg/kg.
3.3.2. Limit of Quantification (LOQ)
3.3.3. Repeatability (RSDr)
3.3.4. Within-Laboratory Precision (RSDwR)
| Analyte (LOQ) (n = 18) | IZSLER | |
|---|---|---|
| Average Recovery % | RSDwR | |
| AMPA | 87 | 17.0 |
| Etephon | 103 | 18.0 |
| Fosetyl | 95 | 10.0 |
| Glufosinate | 94 | 11.0 |
| Glyphosate | 87 | 12.0 |
| MPPA | 97 | 10.0 |
| N-acetyl-AMPA | 119 | 16.0 |
| NAG | 93 | 10.0 |
| N-acetyl-glyphosate | 95 | 15.0 |
| Phosphonic acid | 98 | 12.0 |
3.3.5. Measurement Uncertainty
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission, 2024, Regulation (EU) 2024/1991 of the European Parliament and of the Council of 24 June 2024 on Nature Restoration and Amending Regulation (EU) 2022/869. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32024R1991&qid=1722240349976 (accessed on 8 April 2025).
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape Perspectives on Agricultural Intensification and Biodiversity—Ecosystem Service Management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Lehman, P.; Cady, N.; Ghimire, S.; Shahi, S.K.; Shrode, R.L.; Lehmler, H.; Mangalam, A.K. Low-Dose Glyphosate Exposure Alters Gut Microbiota Composition and Modulates Gut Homeostasis. Environ. Toxicol. Pharmacol. 2023, 100, 104149. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The Monitoring of Pesticides in Water Matrices and the Analytical Criticalities: A Review. TrAC Trends Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Gasparini, M.; Angelone, B.; Ferretti, E. Glyphosate and Other Highly Polar Pesticides in Fruit, Vegetables and Honey Using Ion Chromatography Coupled with High Resolution Mass Spectrometry: Method Validation and Its Applicability in an Official Laboratory. J. Mass Spectrom. 2020, 55, e4624. [Google Scholar] [CrossRef]
- Verdini, E.; Lattanzio, V.; Ciasca, B.; Fioroni, L.; Pecorelli, I. Improved Method for the Detection of Highly Polar Pesticides and Their Main Metabolites in Foods of Animal Origin: Method Validation and Application to Monitoring Programme. Separations 2023, 10, 44. [Google Scholar] [CrossRef]
- Rampazzo, G.; Gazzotti, T.; Pagliuca, G.; Nobile, M.; Chiesa, L.; Carpino, S.; Panseri, S. Determination of Glyphosate, Glufosinate, and Metabolites in Honey Based on Different Detection Approaches Supporting Food Safety and Official Controls. LWT Food Sci. Technol. 2024, 200, 116159. [Google Scholar] [CrossRef]
- Bargańska, Ż.; Lambropoulou, D.; Namieśnik, J. Problems and Challenges to Determine Pesticide Residues in Bumblebees. Critical Reviews in Analytical Chemistry 2018, 48, 447–458. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA), Review of the Existing Maximum Residue Levels for Glyphosate According to Article 12 of Regulation (EC) No 396/2005–Revised Version to Consider Omitted Data. 2019. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5862 (accessed on 8 April 2025).
- European Commission, 2024, Commission Implementing Regulation (EU) 2024/989 of 02 April 2024 Concerning a Coordinated Multiannual Control Programme of the Union for 2025, 2026 and 2027 to Ensure Compliance with Maximum Residue Levels of Pesticides and to Assess the Consumer Exposure to Pesticide Residues in and on Food of Plant and Animal Origin and Repealing Implementing Regulation (EU) 2023/731. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32024R0989 (accessed on 3 April 2025).
- Hofman-Caris, R.; Dingemans, M.; Reus, A.; Shaikh, S.M.; Sierra, J.M.; Karges, U.; der Beek, T.A.; Nogueiro, E.; Lythgo, C.; Parra Morte, J.M.; et al. Guidance Document on the Impact of Water Treatment Processes on Residues of Active Substances or Their Metabolites in Water Abstracted for the Production of Drinking Water. EFSA J. 2023, 21, 8194. [Google Scholar] [CrossRef]
- European Food Safety Authority Insect Pollinator Health—EFSA. Available online: https://www.efsa.europa.eu/en/topics/insect-pollinator-health (accessed on 8 April 2025).
- Adriaanse, P.I.; Arce, A.N.; Focks, A.; Ingels, B.; Jölli, D.; Lambin, S.; Rundlöf, M.; Süssenbach, D.; Del Aguila, M.; Ercolano, V.; et al. Revised Guidance on the Risk Assessment of Plant Protection Products on Bees (Apis Mellifera, Bombus spp. And Solitary Bees). EFSA J. 2023, 21, 7989. [Google Scholar] [CrossRef]
- Bokšová, A.; Kazda, J.; Bartoška, J.; Kamler, M. Effect of Glyphosate on the Foraging Activity of European Honey Bees (Apis Mellifera L.). Plant Soil Environ. 2023, 69, 195–201. [Google Scholar] [CrossRef]
- Balbuena, M.S.; Tison, L.; Hahn, M.L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of Sublethal Doses of Glyphosate on Honeybee Navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Rapisarda, P.; Grasso, A.; Favara, C.; Oliveri Conti, G. Glyphosate and Environmental Toxicity with “One Health” Approach, a Review. Environ. Res. 2023, 235, 116678. [Google Scholar] [CrossRef] [PubMed]
- European Commission, 2005, Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reg/2005/396/oj (accessed on 8 April 2025).
- European Commission, 2021, Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feedm, SANTE 11312/2021 V2, Implemented by 01/01/2024. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 8 April 2025).
- European Commission, Health & Consumer Protection Directorate-General Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues Analysis in Food and Feed, SANCO/12571/2013. Available online: https://www.eurl-pesticides.eu/library/docs/allcrl/AqcGuidance_Sanco_2013_12571.pdf (accessed on 8 April 2025).
- Vázquez, D.E.; Villegas Martínez, L.E.; Medina, V.; Latorre-Estivalis, J.M.; Zavala, J.A.; Farina, W.M. Glyphosate Affects Larval Gut Microbiota and Metamorphosis of Honey Bees with Differences between Rearing Procedures. Environ. Pollut. 2023, 334, 122200. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.J.; King, H.P.; Delenstarr, G.; Kumar, R.; Rubio, F.; Glaze, T. Glyphosate Residue Concentrations in Honey Attributed through Geospatial Analysis to Proximity of Large-Scale Agriculture and Transfer Off-Site by Bees. PLoS ONE 2018, 13, e0198876. [Google Scholar] [CrossRef]
- Weidenmüller, A.; Meltzer, A.; Neupert, S.; Schwarz, A.; Kleineidam, C. Glyphosate Impairs Collective Thermoregulation in Bumblebees. Science 2022, 376, 1122–1126. [Google Scholar] [CrossRef]
- Morzycka, B. Simple Method for the Determination of Trace Levels of Pesticides in Honeybees Using Matrix Solid-Phase Dispersion and Gas Chromatography. J. Chromatogr. A 2002, 982, 267–273. [Google Scholar] [CrossRef]
- Kamel, A. Refined Methodology for the Determination of Neonicotinoid Pesticides and Their Metabolites in Honey Bees and Bee Products by Liquid Chromatography−Tandem Mass Spectrometry (LC-MS/MS)†. J. Agric. Food Chem. 2010, 58, 5926–5931. [Google Scholar] [CrossRef]
- Kiljanek, T.; Niewiadowska, A.; Semeniuk, S.; Gaweł, M.; Borzęcka, M.; Posyniak, A. Multi-Residue Method for the Determination of Pesticides and Pesticide Metabolites in Honeybees by Liquid and Gas Chromatography Coupled with Tandem Mass Spectrometry—Honeybee Poisoning Incidents. J. Chromatogr. A 2016, 1435, 100–114. [Google Scholar] [CrossRef]
- Balsebre, A.; Báez, M.E.; Martínez, J.; Fuentes, E. Matrix Solid-Phase Dispersion Associated to Gas Chromatography for the Assessment in Honey Bee of a Group of Pesticides of Concern in the Apicultural Field. J. Chromatogr. A 2018, 1567, 47–54. [Google Scholar] [CrossRef]
- Tosi, S.; Costa, C.; Vesco, U.; Quaglia, G.; Guido, G. A 3-Year Survey of Italian Honey Bee-Collected Pollen Reveals Widespread Contamination by Agricultural Pesticides. Sci. Total Environ. 2018, 615, 208–218. [Google Scholar] [CrossRef]
- Valverde, S.; Ares, A.M.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Determination of Neonicotinoid Insecticides in Bee Products by Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Pestic. Toxicol. 2021, 27–52. [Google Scholar] [CrossRef]
- Butovskaya, E.; Gasparini, M.; Angelone, B.; Cancemi, G.; Tranquillo, V.; Prestini, G.; Bosi, F.; Menotta, S. Occurrence of Glyphosate and Other Polar Pesticides in Honey from Lombardy and Emilia-Romagna Regions in Italy: Three-Year Monitoring Results. Foods 2023, 12, 4448. [Google Scholar] [CrossRef] [PubMed]
- Jesús, F.; Rosa García, A.; Stecconi, T.; Cutillas, V.; Rodríguez Fernández-Alba, A. Determination of Highly Polar Anionic Pesticides in Beehive Products by Hydrophilic Interaction Liquid Chromatography Coupled to Mass Spectrometry. Anal. Bioanal. Chem. 2023, 416, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Murcia-Morales, M.; Vejsnæs, F.; Brodschneider, R.; Hatjina, F.; Van der Steen, J.J.M.; Oller-Serrano, J.L.; Fernández-Alba, A.R. Enhancing the Environmental Monitoring of Pesticide Residues through Apis Mellifera Colonies: Honey Bees versus Passive Sampling. Sci. Total Environ. 2023, 884, 163847. [Google Scholar] [CrossRef]
- Ozols, N.; Brusbārdis, V.; Filipovičs, M.; Gailis, J.; Radenkovs, V.; Rubene, B.; Zagorska, V. Pesticide Contamination of Honey-Bee-Collected Pollen in the Context of the Landscape Composition in Latvia. Toxics 2024, 12, 862. [Google Scholar] [CrossRef]
- Rampazzo, G.; Nobile, M.; Carpino, S.; Chiesa, L.; Ghidini, S.; Gazzotti, T.; Panseri, S. Detection of Glyphosate, Glufosinate, and Their Metabolites in Multi-Floral Honey for Food Safety. Food Addit. Contam. Part A 2025, 42, 213–222. [Google Scholar] [CrossRef]
- Schäfer, A.-K.; Vetter, W.; Anastassiades, M. Analysis of Highly Polar Anionic Pesticides in Food of Plant and Animal Origin by Ion Chromatography and Tandem Mass Spectrometry with Emphasis on Addressing Adverse Effects Caused by Matrix Co-Extractives. Anal. Bioanal. Chem. 2024, 416, 4503–4517. [Google Scholar] [CrossRef]
- Tóth, E.; Tölgyesi, Á.; Bálint, M.; Ma, X.; Sharma, V.K. Separation of Fosetyl and Phosphonic Acid in Food Matrices with Mixed-Mode HPLC Column Coupled with Tandem Mass Spectrometric Detection and Method Application to Other Highly Polar Pesticides. J. Chromatogr. B 2021, 1189, 123083. [Google Scholar] [CrossRef]
- Verdini, E.; Pacini, T.; Orsini, S.; Sdogati, S.; Pecorelli, I. Chromatographic Comparison of Commercially Available Columns for Liquid Chromatography in Polar Pesticide Detection and Quantification Using a Score-Based Methodology. Foods 2024, 13, 3131. [Google Scholar] [CrossRef]
- European Parliament and Council, Regulation 2017/625, Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products. Available online: https://data.europa.eu/eli/reg/2017/625/oj (accessed on 15 March 2017).
- Cantwell, H. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 3rd ed.; European Cooperation for Accreditation: Paris, France, 2025; Available online: http://www.eurachem.org (accessed on 8 April 2025).
- UNI CEN EN ISO/IEC 17025:2018; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization, ISO: Geneva, Switzerland, 2018.
- EU Reference Laboratories for Residues of Pesticides. Quick Method for the Analysis of Numerous Highly Polar Pesticides in Food Involving Extraction with Acidified Methanol and LC-MS/MS Measurement. In II Food of Animal Origin (QuPPE-AO-Method), Version 3.3.; Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlSRM/EurlSrm_meth_QuPPe_AO_V3_3.pdf (accessed on 8 April 2025).
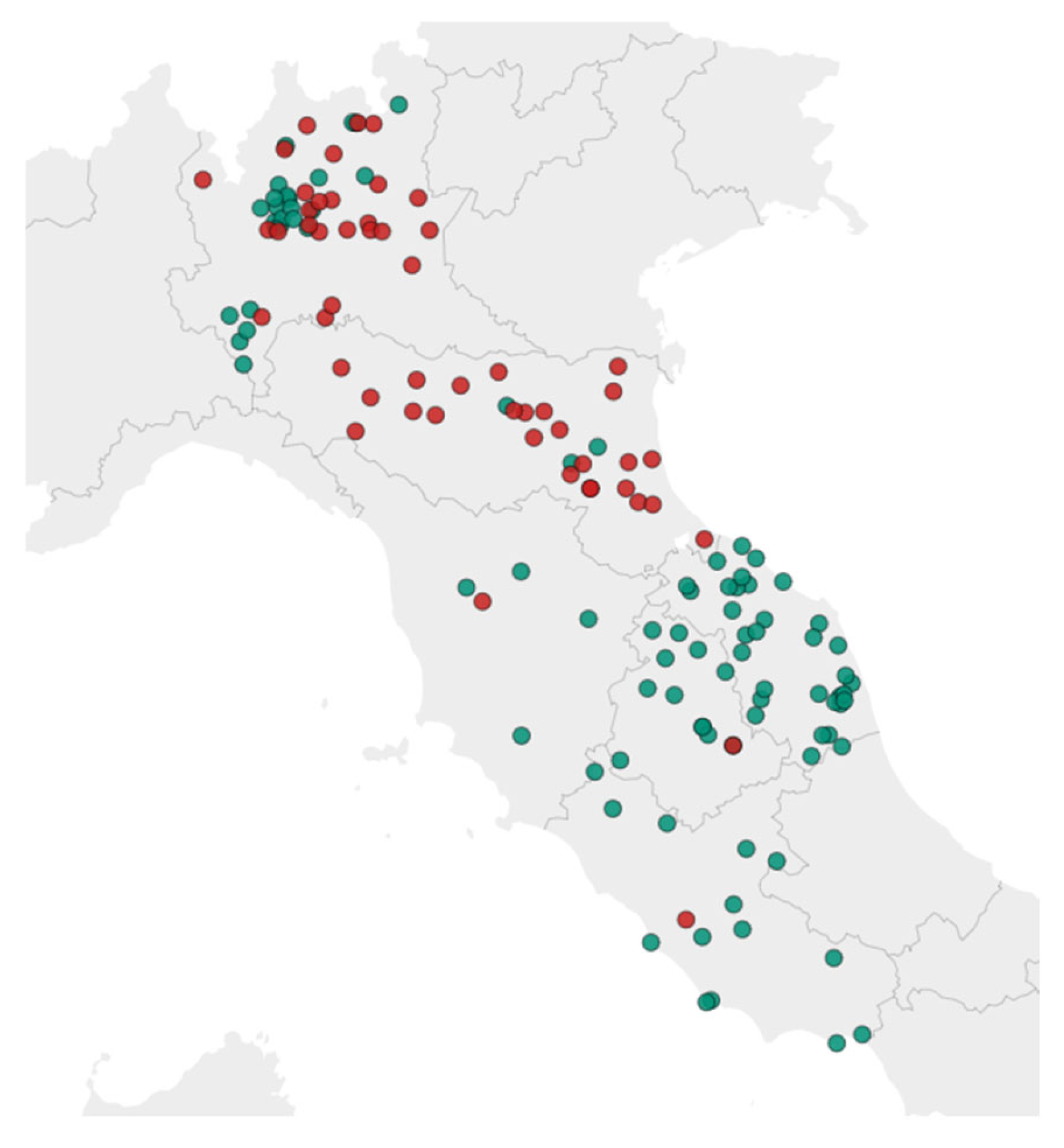
| Analyte | Level (mg/kg) (n = 6) | IZSUM | IZSLT | ||
|---|---|---|---|---|---|
| Average Recovery % | RSDr | Average Recovery % | RSDr | ||
| AMPA | 0.005 | 93 | 4.0 | 102 | 10.6 |
| 0.010 | 97 | 2.5 | 92 | 3.6 | |
| 0.020 | 101 | 5.0 | 88 | 6.4 | |
| 0.100 | 101 | 3.5 | 114 | 4.1 | |
| Ethephon | 0.005 | 91 | 10.1 | 99 | 7.8 |
| 0.010 | 93 | 3.4 | 110 | 3.0 | |
| 0.020 | 95 | 5.8 | 97 | 1.8 | |
| 0.100 | 95 | 4.3 | 115 | 1.6 | |
| Fosetyl | 0.005 | 97 | 6.9 | 92 | 3.5 |
| 0.010 | 87 | 5.2 | 98 | 6.0 | |
| 0.020 | 105 | 5.3 | 106 | 7.1 | |
| 0.100 | 101 | 3.3 | 106 | 3.8 | |
| Glufosinate | 0.005 | 104 | 6.0 | 98 | 10.5 |
| 0.010 | 96 | 8.8 | 92 | 6.6 | |
| 0.020 | 105 | 13.8 | 85 | 3.3 | |
| 0.100 | 99 | 4.3 | 98 | 6.3 | |
| Glyphosate | 0.005 | 114 | 4.7 | 102 | 3.5 |
| 0.010 | 102 | 4.6 | 115 | 5.7 | |
| 0.020 | 119 | 11.0 | 101 | 10.0 | |
| 0.100 | 98 | 10.8 | 108 | 3.4 | |
| HEPA | 0.005 | 95 | 5.7 | 100 | 3.6 |
| 0.010 | 84 | 6.8 | 111 | 4.2 | |
| 0.020 | 87 | 7.3 | 106 | 6.0 | |
| 0.100 | 102 | 4.4 | 117 | 1.8 | |
| MPPA | 0.005 | 95 | 19.2 | 103 | 7.3 |
| 0.010 | 99 | 3.6 | 94 | 3.1 | |
| 0.020 | 109 | 12.0 | 105 | 3.7 | |
| 0.100 | 104 | 6.6 | 111 | 2.3 | |
| N-acetyl-glufosinate | 0.005 | 79 | 10.4 | 97 | 10.3 |
| 0.010 | 95 | 6.7 | 74 | 14.8 | |
| 0.020 | 104 | 7.7 | 81 | 6.4 | |
| 0.100 | 104 | 3.4 | 99 | 3.0 | |
| N-acetyl-AMPA | 0.005 | 79 | 7.7 | 93 | 19.7 |
| 0.010 | 70 | 3.7 | 112 | 12.7 | |
| 0.020 | 91 | 3.9 | 108 | 4.2 | |
| 0.100 | 102 | 2.6 | 118 | 2.6 | |
| Analyte (0.020 mg/kg) | Day | IZSUM | IZSLT | |||
|---|---|---|---|---|---|---|
| Operator 1 | Operator 2 | Operator 3 | Mean | |||
| Recovery % | Recovery % | Recovery % | Average Recovery % | RSDR | ||
| AMPA | Day 1 | 107 | 89 | 77 | 93 | 7.2 |
| Day 2 | 87 | 100 | 87 | |||
| Day 3 | 100 | 92 | 90 | |||
| Day 4 | 102 | 102 | 93 | |||
| Ethephon | Day 1 | 84 | 100 | 98 | 91 | 6.9 |
| Day 2 | 88 | 95 | 94 | |||
| Day 3 | 90 | 96 | 97 | |||
| Day 4 | 71 | 80 | 98 | |||
| Fosetyl | Day 1 | 109 | 96 | 112 | 101 | 5.0 |
| Day 2 | 92 | 96 | 93 | |||
| Day 3 | 97 | 100 | 106 | |||
| Day 4 | 99 | 94 | 114 | |||
| Glufosinate | Day 1 | 101 | 92 | 85 | 93 | 7.5 |
| Day 2 | 93 | 101 | 89 | |||
| Day 3 | 91 | 107 | 87 | |||
| Day 4 | 99 | 90 | 80 | |||
| Glyphosate | Day 1 | 89 | 97 | 98 | 98 | 5.5 |
| Day 2 | 91 | 91 | 87 | |||
| Day 3 | 102 | 103 | 116 | |||
| Day 4 | 88 | 109 | 107 | |||
| HEPA | Day 1 | 99 | 107 | 111 | 103 | 6.6 |
| Day 2 | 90 | 80 | 99 | |||
| Day 3 | 102 | 117 | 114 | |||
| Day 4 | 96 | 114 | 109 | |||
| MPPA | Day 1 | 101 | 107 | 104 | 103 | 7.9 |
| Day 2 | 102 | 80 | 110 | |||
| Day 3 | 102 | 117 | 109 | |||
| Day 4 | 87 | 114 | 104 | |||
| N-acetyl-glufosinate | Day 1 | 104 | 96 | 82 | 91 | 9.8 |
| Day 2 | 90 | 94 | 76 | |||
| Day 3 | 98 | 91 | 77 | |||
| Day 4 | 97 | 105 | 81 | |||
| N-acetyl-AMPA | Day 1 | 92 | 95 | 115 | 94 | 13.6 |
| Day 2 | 67 | 86 | 105 | |||
| Day 3 | 99 | 91 | 109 | |||
| Day 4 | 78 | 76 | 113 | |||
| Analyte | Level (mg/kg) (n = 6) | IZSLER | |
|---|---|---|---|
| Average Recovery % | RSDr | ||
| AMPA | 0.10 | 93 | 6.0 |
| 0.50 | 90 | 6.0 | |
| Ethephon | 0.05 | 106 | 14.0 |
| 0.50 | 98 | 10.0 | |
| Fosetyl | 0.10 | 103 | 4.0 |
| 1.00 | 108 | 3.0 | |
| Glufosinate | 0.01 | 93 | 8.0 |
| 0.10 | 98 | 3.0 | |
| Glyphosate | 0.01 | 84 | 5.0 |
| 0.10 | 93 | 4.0 | |
| MPPA | 0.01 | 109 | 3.0 |
| 0.10 | 114 | 4.0 | |
| N-acetyl-AMPA | 0.01 | 100 | 7.0 |
| 0.10 | 109 | 3.0 | |
| NAG | 0.01 | 96 | 6.0 |
| 0.10 | 110 | 2.0 | |
| N-acetyl-glyphosate | 0.01 | 107 | 14.0 |
| 0.10 | 85 | 6.0 | |
| Phosphonic acid | 0.10 | 102 | 3.0 |
| 1.00 | 100 | 2.0 | |
| U’ (Expanded MU) (%) | |||
|---|---|---|---|
| IZSUM | IZSLT | IZSLER | |
| AMPA | 20 | 22 | 38 |
| Ethephon | 35 | 15 | 46 |
| Fosetyl | 15 | 18 | 49 |
| Glufosinate | 20 | 21 | 36 |
| Glyphosate | 22 | 9 | 37 |
| HEPA | 17 | 7 | N.D. |
| MPPA | 34 | 16 | 42 |
| N-acetyl-AMPA | 39 | 39 | 48 |
| NAG | 19 | 22 | 41 |
| N-acetyl-glyphosate | N.D. | N.D. | 35 |
| Phosphonic Acid | N.D. | N.D. | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacini, T.; Verdini, E.; Orsini, S.; Russo, K.; Mauti, T.; Gasparini, M.; Borgia, M.; Angelone, B.; D’Amore, T.; Pecorelli, I. Development and Validation of LC–MS/MS and IC–HRMS Methods for Highly Polar Pesticide Detection in Honeybees: A Multicenter Study for the Determination of Pesticides in Honeybees to Support Pollinators and Environmental Protection. J. Xenobiot. 2025, 15, 95. https://doi.org/10.3390/jox15040095
Pacini T, Verdini E, Orsini S, Russo K, Mauti T, Gasparini M, Borgia M, Angelone B, D’Amore T, Pecorelli I. Development and Validation of LC–MS/MS and IC–HRMS Methods for Highly Polar Pesticide Detection in Honeybees: A Multicenter Study for the Determination of Pesticides in Honeybees to Support Pollinators and Environmental Protection. Journal of Xenobiotics. 2025; 15(4):95. https://doi.org/10.3390/jox15040095
Chicago/Turabian StylePacini, Tommaso, Emanuela Verdini, Serenella Orsini, Katia Russo, Tabita Mauti, Mara Gasparini, Marialuisa Borgia, Barbara Angelone, Teresa D’Amore, and Ivan Pecorelli. 2025. "Development and Validation of LC–MS/MS and IC–HRMS Methods for Highly Polar Pesticide Detection in Honeybees: A Multicenter Study for the Determination of Pesticides in Honeybees to Support Pollinators and Environmental Protection" Journal of Xenobiotics 15, no. 4: 95. https://doi.org/10.3390/jox15040095
APA StylePacini, T., Verdini, E., Orsini, S., Russo, K., Mauti, T., Gasparini, M., Borgia, M., Angelone, B., D’Amore, T., & Pecorelli, I. (2025). Development and Validation of LC–MS/MS and IC–HRMS Methods for Highly Polar Pesticide Detection in Honeybees: A Multicenter Study for the Determination of Pesticides in Honeybees to Support Pollinators and Environmental Protection. Journal of Xenobiotics, 15(4), 95. https://doi.org/10.3390/jox15040095









