A Novel HPLC-MS/MS Method for the Intracellular Quantification of the Active Triphosphate Metabolite of Remdesivir: GS-443902
Abstract
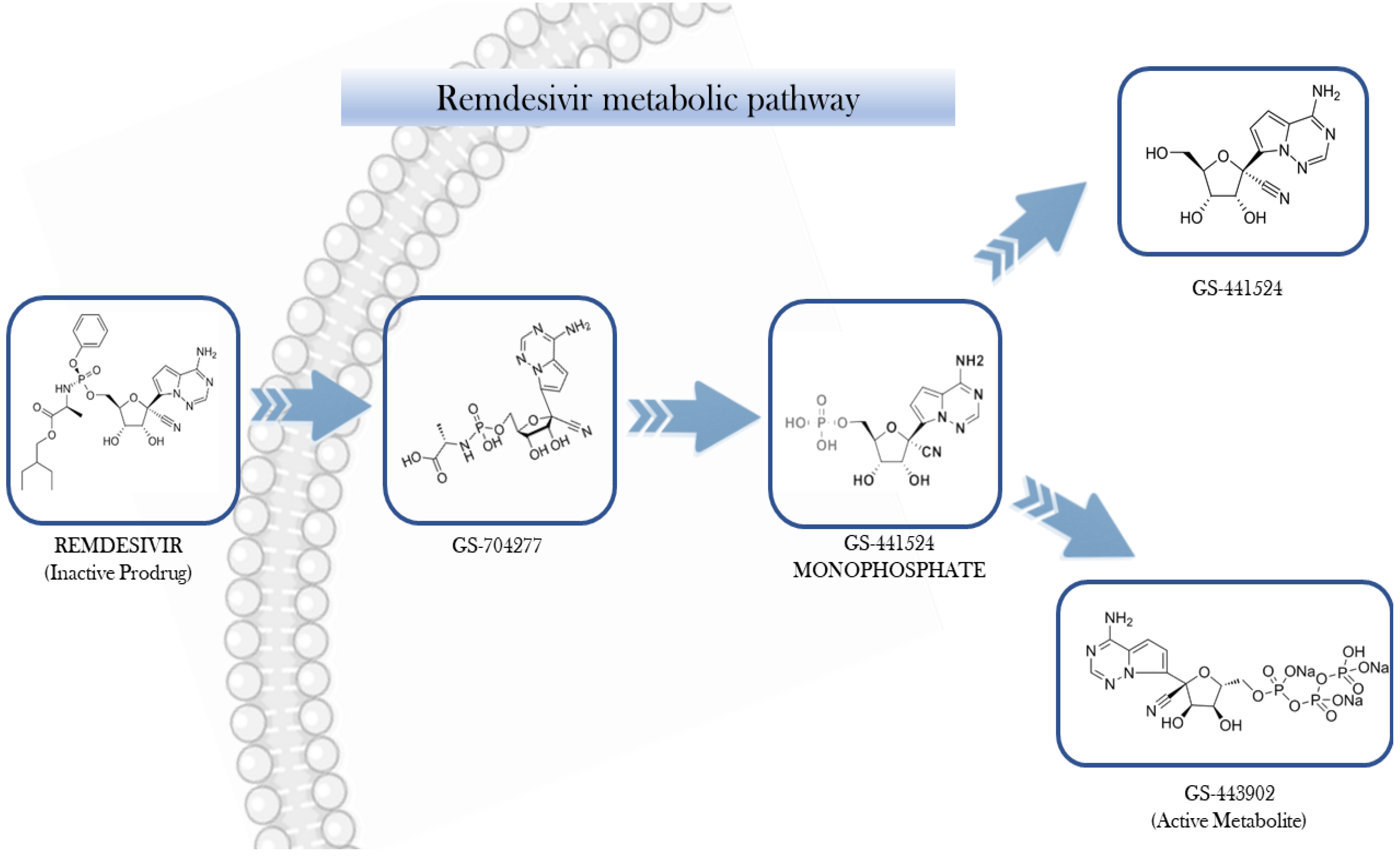
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standards and Internal Quality Control Samples
2.3. PBMCs Isolation
2.4. Sample Preparation
2.5. HPLC-MS/MS Settings
2.6. Method Validation
2.6.1. Specificity and Selectivity
2.6.2. Accuracy, Precision, Calibration, Sensitivity, Dilution Integrity
2.6.3. Recovery
2.6.4. Adsorption and Stability
2.6.5. Matrix Effect
2.6.6. Carry-Over
2.6.7. Application and Statistical Analysis
2.6.8. Incurred Sample Reanalysis
3. Results
3.1. Calibration Curve Linearity and Dilution Integrity
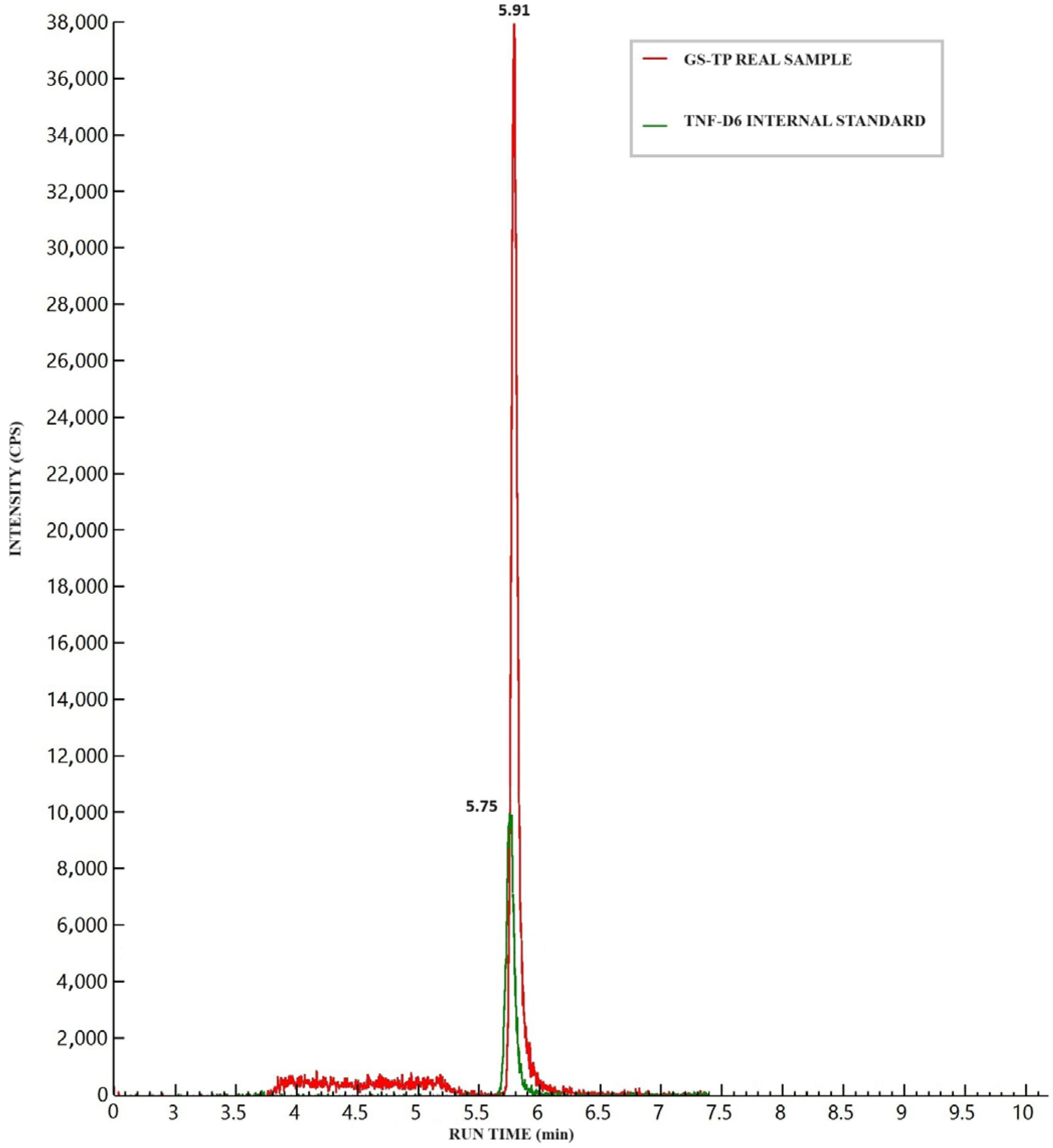
3.2. Specificity and Selectivity
3.3. LLOQ and LOD
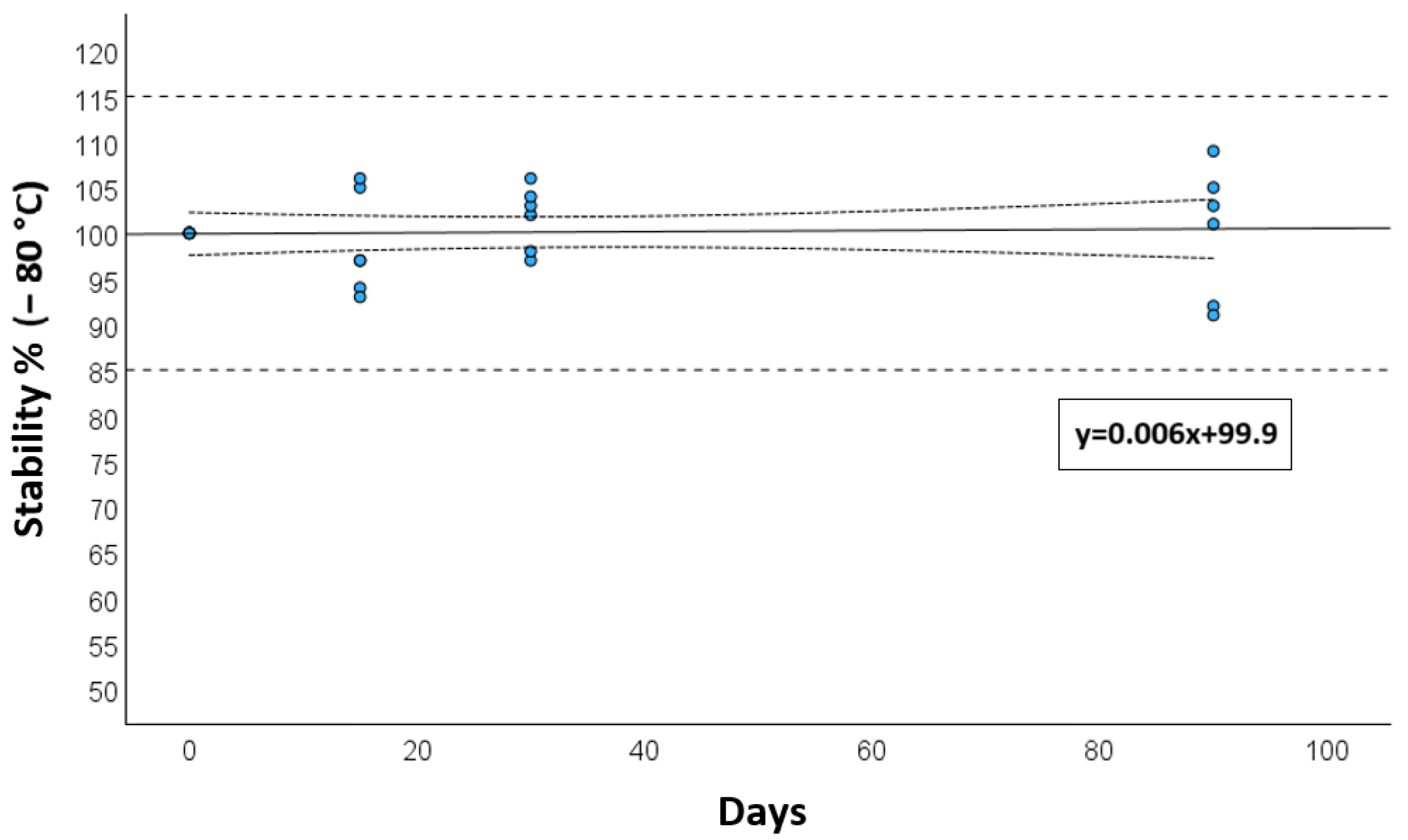
3.4. Adsorption and Stability
3.5. Recovery and Matrix Effect
3.6. Carry-Over
3.7. Testing of Patients’ Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus Type 2 |
| WHO | World Health Organization |
| RDV | Remdesivir |
| PK | Pharmacokinetics |
| PBMCs | peripheral blood mononuclear cells |
| HPLC-MS/MS | High-Performance Liquid Chromatography coupled with tandem Mass Spectrometry |
| STD 9 | highest calibrator standard |
| RSD | relative standard deviation |
| IS | Internal Standard |
| QC | Quality Control |
| LLOQ | Lower Limit of Quantitation |
| RT | retention time |
| LOD | limit of detection |
| STD1 | lowest calibrator standard |
| REC | Recovery |
| ME | matrix effect |
| TFV-d6 | 2 H6-tenofovir diphosphate |
| SPE | solid phase extraction |
| TDF | Tenofovir disoproxil fumarate |
| TAF | Tenofovir alafenamide |
| ACN | Acetonitrile |
| MeOH | Methanol |
| DMSO | dimethyl sulfoxide |
| MCV | mean cell volume |
References
- Hao, Y.; Wang, Y.; Wang, M.; Zhou, L.; Shi, J.; Cao, J.; Wang, D. The origins of COVID-19 pandemic: A brief overview. Transbound. Emerg. Dis. 2022, 69, 3181–3197. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. 2020. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 18 September 2024).
- Salins, N.; Mani, R.K.; Gursahani, R.; Simha, S.; Bhatnagar, S. Symptom Management and Supportive Care of Serious COVID-19 Patients and their Families in India. Indian J. Crit. Care Med. 2020, 24, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Raghav, P.K.; Mann, Z.; Ahluwalia, S.K.; Rajalingam, R. Potential treatments of COVID-19: Drug repurposing and therapeutic interventions. J. Pharmacol. Sci. 2023, 152, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Commissioner, Organization of the Food and Drug Administration (FDA). Approves First Treatment for COVID-19. FDA. 2024. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 18 September 2024).
- Kalenga, O.I.; Moeti, M.; Sparrow, A.; Nguyen, V.-K.; Lucey, D.; Ghebreyesus, T.A. The Ongoing Ebola Epidemic in the Democratic Republic of Congo, 2018–2019. N. Engl. J. Med. 2019, 381, 373–383. [Google Scholar] [CrossRef]
- Choy, K.-T.; Wong, A.Y.-L.; Kaewpreedee, P.; Sia, S.F.; Chen, D.; Hui, K.P.Y.; Chu, D.K.W.; Chan, M.C.W.; Cheung, P.P.-H.; Huang, X.; et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020, 178, 104786. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses 2019, 11, 326. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z. Can remdesivir and its parent nucleoside GS-441524 be potential oral drugs? An in vitro and in vivo DMPK assessment. Acta Pharm. Sin. B 2021, 11, 1607–1616. [Google Scholar] [CrossRef]
- Li, R.; Liclican, A.; Xu, Y.; Pitts, J.; Niu, C.; Zhang, J.; Kim, C.; Zhao, X.; Soohoo, D.; Babusis, D.; et al. Key Metabolic Enzymes Involved in Remdesivir Activation in Human Lung Cells. Antimicrob. Agents Chemother. 2021, 65, e0060221. Available online: https://journals.asm.org/doi/10.1128/aac.00602-21 (accessed on 14 May 2025). [CrossRef]
- Kanai, Y. Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol. Ther. 2022, 230, 107964. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9, e00221-18. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381. [Google Scholar] [CrossRef]
- Humeniuk, R.; Mathias, A.; Cao, H.; Osinusi, A.; Shen, G.; Chng, E.; Ling, J.; Vu, A.; German, P. Safety, Tolerability, and Pharmacokinetics of Remdesivir, An Antiviral for Treatment of COVID-19, in Healthy Subjects. Clin. Transl. Sci. 2020, 13, 896–906. [Google Scholar] [CrossRef]
- Tempestilli, M.; Caputi, P.; Avataneo, V.; Notari, S.; Forini, O.; Scorzolini, L.; Marchioni, L.; Ascoli Bartoli, T.; Castilletti, C.; Lalle, E.; et al. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J. Antimicrob. Chemother. 2020, 75, 2977–2980. [Google Scholar] [CrossRef]
- Gilead, E. Remdesivir. Summary on Compassionate Use. 2020. Available online: https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead_en.pdf (accessed on 22 May 2025).
- Xiao, D.; John Ling, K.H.; Tarnowski, T.; Humeniuk, R.; German, P.; Mathias, A.; Chu, J.; Chen, Y.-S.; van Ingen, E. Validation of LC-MS/MS methods for determination of remdesivir and its metabolites GS-441524 and GS-704277 in acidified human plasma and their application in COVID-19 related clinical studies. Anal. Biochem. 2021, 617, 114118. [Google Scholar] [CrossRef]
- De Nicolò, A.; Bonifacio, G.; Boglione, L.; Cusato, J.; Pensi, D.; Tomasello, C.; Di Perri, G.; D’Avolio, A. UHPLC–MS/MS method with automated on-line solid phase extraction for the quantification of entecavir in peripheral blood mononuclear cells of HBV+ patients. J. Pharm. Biomed. Anal. 2016, 118, 64–69. [Google Scholar] [CrossRef]
- De Nicolò, A.; Ianniello, A.; Ferrara, M.; Avataneo, V.; Cusato, J.; Antonucci, M.; De Vivo, E.; Waitt, C.; Calcagno, A.; Trentalange, A.; et al. Pharmaceuticals|Free Full-Text|Validation of a UHPLC-MS/MS Method to Quantify Twelve Antiretroviral Drugs within Peripheral Blood Mononuclear Cells from People Living with HIV. Pharmaceuticals 2020, 14, 12. Available online: https://www.mdpi.com/1424-8247/14/1/12 (accessed on 18 September 2024). [CrossRef]
- Humeniuk, R.; Mathias, A.; Kirby, B.J.; Lutz, J.D.; Cao, H.; Osinusi, A.; Babusis, D.; Porter, D.; Wei, X.; Ling, J.; et al. Pharmacokinetic, Pharmacodynamic, and Drug-Interaction Profile of Remdesivir, a SARS-CoV-2 Replication Inhibitor. Clin. Pharmacokinet. 2021, 60, 569–583. Available online: https://www.researchgate.net/publication/350493848_Pharmacokinetic_Pharmacodynamic_and_Drug-Interaction_Profile_of_Remdesivir_a_SARS-CoV-2_Replication_Inhibitor (accessed on 14 May 2025). [CrossRef] [PubMed]
- Pruijssers, A.J.; George, A.S.; Schäfer, A.; Leist, S.R.; Gralinksi, L.E.; Dinnon, K.H.; Yount, B.L.; Agostini, M.L.; Stevens, L.J.; Chappell, J.D.; et al. Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice. Cell Rep. 2020, 32, 107940. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Aldejohann, A.M.; Hoffmann, A.M.; Walther, G.; Kurzai, O.; Hamprecht, A.G. In Vitro Activity of Nitroxoline in Antifungal-Resistant Candida Species Isolated from the Urinary Tract. Antimicrob. Agents Chemother. 2022, 66, e0226521. [Google Scholar] [CrossRef] [PubMed]
- De Nicolò, A.; Palermiti, A.; Mugerwa, H.; Nakabuye, S.; Namusanje, J.; Kobusingye, J.; Odoch, D.; Lamorde, M.; Kengo, A.; Denti, P.; et al. Intracellular Penetration of Atazanavir, Ritonavir and Dolutegravir with Concomitant Rifampicin: A Dose Escalation Study. Clin. Pharmacol. Ther. 2025, 117, 1393–1402. [Google Scholar] [CrossRef]
- De Nicolò, A.; Palermiti, A.; Dispinseri, S.; Marchetti, G.; Trunfio, M.; De Vivo, E.; D’Avolio, A.; Muscatello, A.; Gori, A.; Rusconi, S.; et al. Plasma, Intracellular and Lymph Node Antiretroviral Concentrations and HIV DNA Change During Primary HIV Infection: Results from the INACTION P25 Study. Int. J. Antimicrob. Agents 2024, 64, 107200. [Google Scholar] [CrossRef]
- European Medicine Agency. M10 Bioanalytical Method Validation and Study Sample Analysis. 2025. Available online: https://www.ema.europa.eu/en/ich-m10-bioanalytical-method-validation-scientific-guideline (accessed on 25 May 2025).
- European Medicines Agency. Q2 (R1) Validation of Analytical Procedures: Text and Methodology. 2006. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 25 May 2025).
- Avataneo, V.; De Nicolò, A.; Cusato, J.; Antonucci, M.; Manca, A.; Palermiti, A.; Waitt, C.; Walimbwa, S.; Lamorde, M.; Di Perri, G.; et al. Development and validation of a UHPLC-MS/MS method for quantification of the prodrug remdesivir and its metabolite GS-441524: A tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease. J. Antimicrob. Chemother. 2020, 75, 1772–1777. [Google Scholar] [CrossRef]
- De Nicolò, A.; Cantu, M.; D’Avolio, A. Matrix effect management in liquid chromatography mass spectrometry: The internal standard normalized matrix effect. Bioanalysis 2017, 9, 1093–1105. [Google Scholar] [CrossRef]
- Yan, V.C.; Pham, C.-D.; Yan, M.J.; Yan, A.J.; Khadka, S.; Arthur, K.; Ackroyd, J.J.; Georgiou, D.K.; Roon, L.E.; Bushman, L.R.; et al. Pharmacokinetics of Orally Administered GS-441524 in Dogs. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kuklenyik, Z.; Martin, A.; Pau, C.-P.; Holder, A.; Youngpairoj, A.S.; Zheng, Q.; Cong, M.-E.; Garcia-Lerma, J.G.; Heneine, W.; Pirkle, J.L.; et al. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. J. Chromatogr. B 2009, 877, 3659–3666. [Google Scholar] [CrossRef]
- Martinez, D.R.; Moreira, F.R.; Zweigart, M.R.; Gully, K.L.; la Cruz, G.D.; Brown, A.J.; Adams, L.E.; Catanzaro, N.; Yount, B.; Baric, T.J.; et al. Efficacy of the oral nucleoside prodrug GS-5245 (Obeldesivir) against SARS-CoV-2 and coronaviruses with pandemic potential. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, W.; Bao, H.; Feng, H.; Mei, S.; Chen, P.; Gao, Y.; Cui, Z.; Zhang, Q.; Meng, X.; et al. VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of COVID-19. N. Engl. J. Med. 2023, 388, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Podany, A.T.; Bares, S.H.; Havens, J.; Dyavar, S.R.; O’Neill, J.; Lee, S.; Fletcher, C.V.; Swindells, S.; Scarsi, K.K. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018, 32, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Corcione, S.; De Nicolò, A.; Montrucchio, G.; Scabini, S.; Avataneo, V.; Bonetto, C.; Mornese Pinna, S.; Cusato, J.; Canta, F.; Urbino, R.; et al. Real-life study on the pharmacokinetic of remdesivir in ICU patients admitted for severe COVID-19 pneumonia. Br. J. Clin. Pharmacol. 2021, 87, 4861–4867. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.V.; Staskus, K.; Wietgrefe, S.W.; Rothenberger, M.; Reilly, C.; Chipman, J.G.; Beilman, G.J.; Khoruts, A.; Thorkelson, A.; Schmidt, T.E.; et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 2307–2312. [Google Scholar] [CrossRef]
| GS-443902 Concentration in the Calibrating Solution [ng/mL] | GS-443902 Amount in the PBMC Sample [ng] | |
|---|---|---|
| STD 1 | 3.125 | 0.3125 |
| STD 2 | 6.25 | 0.625 |
| STD 3 | 12.5 | 1.25 |
| STD 4 | 25 | 2.5 |
| STD 5 | 50 | 5 |
| STD 6 | 100 | 10 |
| STD 7 | 200 | 20 |
| STD 8 | 400 | 40 |
| STD 9 | 800 | 80 |
| QC H | 640 | 64 |
| QC M | 160 | 16 |
| QC L | 40 | 4 |
| General Detector Settings | ||||
| Drying Gas Temperature [°C] | 130 | |||
| HSID Temperature [°C] | 300 | |||
| Nebulizer Gas [L/h] | 350 | |||
| ElectroSpray V1 Negative [kV] | −4.8 | |||
| Source Temperature [°C] | 350 | |||
| Multipole 1 RF | 370 | |||
| Collision Pressure | 420 | |||
| Ionization | ESI- | |||
| Analyte-Specific Parameters | ||||
| GS-443902 | 2H6-TNF-DP (IS) | |||
| Primary | Secondary | Primary | Secondary | |
| Ion Trace (m/z) | 529.9 > 158.9 | 529.9 > 431.9 | 452.0 > 176.9 | 452.0 > 354.9 |
| Collision Energy | 43 | 30 | 31 | 28 |
| Entrance voltage | −29 | −39 | −23 | −23 |
| Collision Cell Lens 2 | 100 | 120 | 96 | 92 |
| Recovery Mean% (RSD%) | Matrix Effect Mean% (RSD%) | Extraction Efficiency Mean% (RSD%) | IS-nREC Mean% (RSD%) | IS-nME Mean% (RSD%) | IS-nEE Mean% (RSD%) | ||
|---|---|---|---|---|---|---|---|
| GS-443902 | H | 51.2 (21.3) | 53.7 (17.) | 95.0 (8.1) | 119.0 (3.7) | 113.3 (7.1) | 101.2 (5.1) |
| M | 85.8 (14.6) | 67.3 (15.4) | 101.9 (11.2) | 114.0 (11.4) | 112.9 (9.2) | 98.8 (1.7) | |
| L | 119.5 (13.7) | 114.6 (15.3) | 105.8 (18.2) | 114.0 (3.4) | 111.7 (11.9) | 98.9 (2.9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palermiti, A.; De Nicolò, A.; Antonucci, M.; Soloperto, S.; Billi, M.; Manca, A.; Cusato, J.; Menegatti, G.; Lamorde, M.; Calcagno, A.; et al. A Novel HPLC-MS/MS Method for the Intracellular Quantification of the Active Triphosphate Metabolite of Remdesivir: GS-443902. J. Xenobiot. 2025, 15, 107. https://doi.org/10.3390/jox15040107
Palermiti A, De Nicolò A, Antonucci M, Soloperto S, Billi M, Manca A, Cusato J, Menegatti G, Lamorde M, Calcagno A, et al. A Novel HPLC-MS/MS Method for the Intracellular Quantification of the Active Triphosphate Metabolite of Remdesivir: GS-443902. Journal of Xenobiotics. 2025; 15(4):107. https://doi.org/10.3390/jox15040107
Chicago/Turabian StylePalermiti, Alice, Amedeo De Nicolò, Miriam Antonucci, Sara Soloperto, Martina Billi, Alessandra Manca, Jessica Cusato, Giorgia Menegatti, Mohammed Lamorde, Andrea Calcagno, and et al. 2025. "A Novel HPLC-MS/MS Method for the Intracellular Quantification of the Active Triphosphate Metabolite of Remdesivir: GS-443902" Journal of Xenobiotics 15, no. 4: 107. https://doi.org/10.3390/jox15040107
APA StylePalermiti, A., De Nicolò, A., Antonucci, M., Soloperto, S., Billi, M., Manca, A., Cusato, J., Menegatti, G., Lamorde, M., Calcagno, A., Waitt, C., & D’Avolio, A. (2025). A Novel HPLC-MS/MS Method for the Intracellular Quantification of the Active Triphosphate Metabolite of Remdesivir: GS-443902. Journal of Xenobiotics, 15(4), 107. https://doi.org/10.3390/jox15040107








