Validation of a UHPLC-MS/MS Method to Quantify Twelve Antiretroviral Drugs within Peripheral Blood Mononuclear Cells from People Living with HIV
Abstract
1. Introduction
2. Results and Discussion
2.1. Calibration Curve and Dilution Integrity
2.2. Specificity and Selectivity
2.3. Accuracy and Precision
2.4. Lowest Limit of Quantification (LLOQ) and Limit of Detection (LOD)
2.5. Recovery
2.6. Matrix Effect
2.7. Carry-Over
2.8. Stability
2.9. Testing of Patients’ Samples
3. Materials and Methods
3.1. Chemicals
3.2. Stock Solutions, Standards, and Quality Controls
3.3. Chromatographic Conditions
3.4. Mass Spectrometry Conditions
3.5. PBMC Isolation
3.6. STDs, QCs, and Patient Samples Extraction
3.7. Specificity and Selectivity
3.8. Accuracy, Precision, Calibration, and Limit of Quantification
3.9. Recovery
3.10. Stability
3.11. Matrix Effect
3.12. Carry-Over
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younai, F.S. Thirty years of the human immunodeficiency virus epidemic and beyond. Int. J. Oral Sci. 2013, 5, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, R.C.; Lockhart, S.M.; Miller, M.M.; Liedtke, M.D. Dolutegravir, a second-generation integrase inhibitor for the treatment of HIV-1 infection. Ann. Pharmacother. 2014, 48, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.M.; Schafer, J.J.; Desimone, J.A., Jr. Dolutegravir: A new integrase strand transfer inhibitor for the treatment of HIV. Pharmacotherapy 2014, 34, 506–520. [Google Scholar] [CrossRef] [PubMed]
- WHO. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidelines; Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Orkin, C.; Llibre, J.M.; Gallien, S.; Antinori, A.; Behrens, G.; Carr, A. Nucleoside reverse transcriptase inhibitor-reducing strategies in HIV treatment: Assessing the evidence. HIV Med. 2017, 19, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.M.; Emery, S.; Kelleher, A.D.; Law, M.; Chen, J.; Hazuda, D.J.; Nguyen, B.Y.; Teppler, H.; Cooper, D.A. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS 2007, 21, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available online: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (accessed on 11 June 2020).
- Rochat, M.A.; Schlaepfer, E.; Kuster, S.P.; Li, D.; Audige, A.; Ivic, S.; Fahrny, A.; Speck, R.F. Monitoring HIV DNA and cellular activation markers in HIV-infected humanized mice under cART. Virol. J. 2018, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.V.; Staskus, K.; Wietgrefe, S.W.; Rothenberger, M.; Reilly, C.; Chipman, J.G.; Beilman, G.J.; Khoruts, A.; Thorkelson, A.; Schmidt, T.E.; et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 2307–2312. [Google Scholar] [CrossRef]
- Lorenzo-Redondo, R.; Fryer, H.R.; Bedford, T.; Kim, E.Y.; Archer, J.; Pond, S.L.K.; Chung, Y.S.; Penugonda, S.; Chipman, J.; Fletcher, C.V.; et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016, 530, 51–56. [Google Scholar] [CrossRef]
- Rothenberger, M.K.; Keele, B.F.; Wietgrefe, S.W.; Fletcher, C.V.; Beilman, G.J.; Chipman, J.G.; Khoruts, A.; Estes, J.D.; Anderson, J.; Callisto, S.P.; et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc. Natl. Acad. Sci. USA 2015, 112, E1126–E1134. [Google Scholar] [CrossRef]
- Svicher, V.; Ceccherini-Silberstein, F.; Antinori, A.; Aquaro, S.; Perno, C.F. Understanding HIV compartments and reservoirs. Curr. HIV/AIDS Rep. 2014, 11, 186–194. [Google Scholar] [CrossRef]
- Bon, I.; Calza, L.; Musumeci, G.; Longo, S.; Bertoldi, A.; D’Urbano, V.; Gibellini, D.; Magistrelli, E.; Viale, P.L.; Re, M.C. Impact of Different Antiretroviral Strategies on Total HIV-DNA Level in Virologically Suppressed HIV-1 Infected Patients. Curr. HIV Res. 2017, 15, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Chargin, A.; Yin, F.; Song, M.; Subramaniam, S.; Knutson, G.; Patterson, B.K. Identification and characterization of HIV-1 latent viral reservoirs in peripheral blood. J. Clin. Microbiol. 2015, 53, 60–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moron-Lopez, S.; Puertas, M.C.; Galvez, C.; Navarro, J.; Carrasco, A.; Esteve, M.; Manye, J.; Crespo, M.; Salgado, M.; Martinez-Picado, J. Sensitive quantification of the HIV-1 reservoir in gut-associated lymphoid tissue. PLoS ONE 2017, 12, e0175899. [Google Scholar] [CrossRef] [PubMed]
- Nagano, D.; Araki, T.; Yanagisawa, K.; Ogawa, Y.; Gohda, F.; Uchiumi, H.; Handa, H.; Nakamura, T.; Yamamoto, K. Darunavir concentration in PBMCs may be a better indicator of drug exposure in HIV patients. Eur. J. Clin. Pharmacol. 2018, 74, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Dyavar, S.R.; Ye, Z.; Byrareddy, S.N.; Scarsi, K.K.; Winchester, L.C.; Weinhold, J.A.; Fletcher, C.V.; Podany, A.T. Normalization of cell associated antiretroviral drug concentrations with a novel RPP30 droplet digital PCR assay. Sci. Rep. 2018, 8, 3626. [Google Scholar] [CrossRef] [PubMed]
- De Nicolò, A.; Simiele, M.; Calcagno, A.; Abdi, A.M.; Bonora, S.; Di Perri, G.; D’Avolio, A. Intracellular antiviral activity of low-dose ritonavir in boosted protease inhibitor regimens. Antimicrob. Agents Chemother. 2014, 58, 4042–4047. [Google Scholar] [CrossRef] [PubMed]
- D’Avolio, A.; Simiele, M.; Calcagno, A.; Siccardi, M.; Larovere, G.; Agati, S.; Baietto, L.; Cusato, J.; Tettoni, M.; Sciandra, M.; et al. Intracellular accumulation of ritonavir combined with different protease inhibitors and correlations between concentrations in plasma and peripheral blood mononuclear cells. J. Antimicrob. Chemother. 2013, 68, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Roemer, E.; Nkwopara, E.; Robbins, B.; Cory, T.; Rue, T.; Fletcher, C.V.; Frenkel, L. Correlation between plasma, intracellular, and cervical tissue levels of raltegravir at steady-state dosing in healthy women. Antimicrob. Agents Chemother. 2014, 58, 3360–3365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Podany, A.T.; Bares, S.H.; Havens, J.; Dyavar, S.R.; O’Neill, J.; Lee, S.; Fletcher, C.V.; Swindells, S.; Scarsi, K.K. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018, 32, 761–765. [Google Scholar] [CrossRef]
- Perazzolo, S.; Shireman, L.M.; Koehn, J.; McConnachie, L.A.; Kraft, J.C.; Shen, D.D.; Ho, R.J.Y. Three HIV Drugs, Atazanavir, Ritonavir, and Tenofovir, Coformulated in Drug-Combination Nanoparticles Exhibit Long-Acting and Lymphocyte-Targeting Properties in Nonhuman Primates. J. Pharm. Sci. 2018, 107, 3153–3162. [Google Scholar] [CrossRef]
- Kraft, J.C.; McConnachie, L.A.; Koehn, J.; Kinman, L.; Collins, C.; Shen, D.D.; Collier, A.C.; Ho, R.J. Long-acting combination anti-HIV drug suspension enhances and sustains higher drug levels in lymph node cells than in blood cells and plasma. AIDS 2017, 31, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Ter Heine, R.; Hillebrand, M.J.; Rosing, H.; van Gorp, E.C.; Mulder, J.W.; Beijnen, J.H.; Huitema, A.D. Quantification of the HIV-integrase inhibitor raltegravir and detection of its main metabolite in human plasma, dried blood spots and peripheral blood mononuclear cell lysate by means of high-performance liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 451–458. [Google Scholar] [CrossRef] [PubMed]
- ter Heine, R.; Davids, M.; Rosing, H.; van Gorp, E.C.; Mulder, J.W.; van der Heide, Y.T.; Beijnen, J.H.; Huitema, A.D. Quantification of HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors in peripheral blood mononuclear cell lysate using liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Pelerin, H.; Compain, S.; Duval, X.; Gimenez, F.; Benech, H.; Mabondzo, A. Development of an assay method for the detection and quantification of protease and non-nucleoside reverse transcriptase inhibitors in plasma and in peripherical blood mononuclear cells by liquid chromatography coupled with ultraviolet or tandem mass spectrometry detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 819, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Nagano, D.; Araki, T.; Nakamura, T.; Yamamoto, K. Determination of intracellular darunavir by liquid chromatography coupled with fluorescence detection. J. Chromatogr. Sci. 2013, 52, 1021–1025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corkum, C.P.; Ings, D.P.; Burgess, C.; Karwowska, S.; Kroll, W.; Michalak, T.I. Immune cell subsets and their gene expression profiles from human PBMC isolated by Vacutainer Cell Preparation Tube (CPT) and standard density gradient. BMC Immunol. 2015, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Simiele, M.; D’Avolio, A.; Baietto, L.; Siccardi, M.; Sciandra, M.; Agati, S.; Cusato, J.; Bonora, S.; Di Perri, G. Evaluation of the mean corpuscular volume of peripheral blood mononuclear cells of HIV patients by a coulter counter to determine intracellular drug concentrations. Antimicrob. Agents Chemother. 2011, 55, 2976–2978. [Google Scholar] [CrossRef]
- De Nicolò, A.; Cantu, M.; D’Avolio, A. Matrix effect management in liquid chromatography mass spectrometry: The internal standard normalized matrix effect. Bioanalysis 2017, 9, 1093–1105. [Google Scholar] [CrossRef]
- Becher, F.; Pruvost, A.; Gale, J.; Couerbe, P.; Goujard, C.; Boutet, V.; Ezan, E.; Grassi, J.; Benech, H. A strategy for liquid chromatography/tandem mass spectrometric assays of intracellular drugs: Application to the validation of the triphosphorylated anabolite of antiretrovirals in peripheral blood mononuclear cells. J. Mass Spectrom. 2003, 38, 879–890. [Google Scholar] [CrossRef]
- Belkhir, L.; De Laveleye, M.; Vandercam, B.; Zech, F.; Delongie, K.A.; Capron, A.; Yombi, J.; Vincent, A.; Elens, L.; Haufroid, V. Quantification of darunavir and etravirine in human peripheral blood mononuclear cells using high performance liquid chromatography tandem mass spectrometry (LC-MS/MS), clinical application in a cohort of 110 HIV-1 infected patients and evidence of a potential drug-drug interaction. Clin. Biochem. 2015, 49, 580–586. [Google Scholar] [CrossRef]
- Patel, S.H.; Ismaiel, O.A.; Mylott, W.R., Jr.; Yuan, M.; Hauser, K.F.; McRae, M. Simultaneous determination of intracellular concentrations of tenofovir, emtricitabine, and dolutegravir in human brain microvascular endothelial cells using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Anal. Chim. Acta 2019, 1056, 79–87. [Google Scholar] [CrossRef] [PubMed]
- EMA. Guideline on Bioanalytical Method Validation. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf (accessed on 11 June 2020).
- FDA. Guidance for Industry: Bioanalytical Method Validation. Available online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf (accessed on 11 June 2020).
- Ford, N.; Lee, J.; Andrieux-Meyer, I.; Calmy, A. Safety, efficacy, and pharmacokinetics of rilpivirine: Systematic review with an emphasis on resource-limited settings. HIV AIDS (Auckl.) 2011, 3, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Simiele, M.; Ariaudo, A.; De Nicolo, A.; Favata, F.; Ferrante, M.; Carcieri, C.; Bonora, S.; Di Perri, G.; De Avolio, A. UPLC-MS/MS method for the simultaneous quantification of three new antiretroviral drugs, dolutegravir, elvitegravir and rilpivirine, and other thirteen antiretroviral agents plus cobicistat and ritonavir boosters in human plasma. J. Pharm. Biomed. Anal. 2017, 138, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Agnesod, D.; De Nicolò, A.; Simiele, M.; Mohamed Abdi, A.; Boglione, L.; Di Perri, G.; D’Avolio, A. Development and validation of a useful UPLC-MS/MS method for quantification of total and phosphorylated-ribavirin in peripheral blood mononuclear cells of HCV+ patients. J. Pharm. Biomed. Anal. 2013, 90, 119–126. [Google Scholar] [CrossRef] [PubMed]
- De Nicolò, A.; Abdi, A.M.; Boglione, L.; Baiett, L.; Allegra, S.; Di Perri, G.; D’Avolio, A. UPLC-MS/MS method with automated on-line SPE for the isomer-specific quantification of the first-generation anti-HCV protease inhibitors in peripheral blood mononuclear cells. J. Pharm. Biomed. Anal. 2015, 115, 443–449. [Google Scholar] [CrossRef]
- De Nicolò, A.; Agnesod, D.; Simiele, M.; Rigano, D.; Adriani, A.; Canaparo, R.; Astegiano, M.; Rizzetto, M.; Di Perri, G.; D’Avolio, A. UPLC-MS/MS method for quantification of the azathioprine metabolites 6-mercaptoguanosine and 6-methylmercaptopurine riboside in peripheral blood mononuclear cells. J. Pharm. Biomed. Anal. 2014, 98, 271–278. [Google Scholar] [CrossRef][Green Version]
- De Nicolò, A.; Bonifacio, G.; Boglione, L.; Cusato, J.; Pensi, D.; Tomasello, C.; Di Perri, G.; D’Avolio, A. UHPLC-MS/MS method with automated on-line solid phase extraction for the quantification of entecavir in peripheral blood mononuclear cells of HBV+ patients. J. Pharm. Biomed. Anal. 2015, 118, 64–69. [Google Scholar] [CrossRef]
- Aouri, M.; Calmy, A.; Hirschel, B.; Telenti, A.; Buclin, T.; Cavassini, M.; Rauch, A.; Decosterd, L.A. A validated assay by liquid chromatography-tandem mass spectrometry for the simultaneous quantification of elvitegravir and rilpivirine in HIV positive patients. J. Mass Spectrom. 2013, 48, 616–625. [Google Scholar] [CrossRef]
- Bennetto-Hood, C.; Tabolt, G.; Savina, P.; Acosta, E.P. A sensitive HPLC-MS/MS method for the determination of dolutegravir in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 945, 225–232. [Google Scholar] [CrossRef]
- D’Avolio, A.; Baietto, L.; Siccardi, M.; Sciandra, M.; Simiele, M.; Oddone, V.; Bonora, S.; Di Perri, G. An HPLC-PDA method for the simultaneous quantification of the HIV integrase inhibitor raltegravir, the new nonnucleoside reverse transcriptase inhibitor etravirine, and 11 other antiretroviral agents in the plasma of HIV-infected patients. Ther. Drug Monit. 2008, 30, 662–669. [Google Scholar] [CrossRef]
- D’Avolio, A.; Sciandra, M.; Siccardi, M.; Baietto, L.; de Requena, D.G.; Bonora, S.; Di Perri, G. A simple and sensitive assay for determining plasma tipranavir concentration in the clinical setting by new HPLC method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 848, 374–378. [Google Scholar] [CrossRef] [PubMed]
- DeJesus, E.; Rockstroh, J.K.; Henry, K.; Molina, J.M.; Gathe, J.; Ramanathan, S.; Wei, X.; Yale, K.; Szwarcberg, J.; White, K.; et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet 2012, 379, 2429–2438. [Google Scholar] [CrossRef] [PubMed]
- Djerada, Z.; Feliu, C.; Tournois, C.; Vautier, D.; Binet, L.; Robinet, A.; Marty, H.; Gozalo, C.; Lamiable, D.; Millart, H. Validation of a fast method for quantitative analysis of elvitegravir, raltegravir, maraviroc, etravirine, tenofovir, boceprevir and 10 other antiretroviral agents in human plasma samples with a new UPLC-MS/MS technology. J. Pharm. Biomed. Anal. 2013, 86, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Beguin, A.; Telenti, A.; Biollaz, J.; Buclin, T.; Rochat, B.; Decosterd, L.A. Intracellular measurements of anti-HIV drugs indinavir, amprenavir, saquinavir, ritonavir, nelfinavir, lopinavir, atazanavir, efavirenz and nevirapine in peripheral blood mononuclear cells by liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 819, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Prathipati, P.K.; Mandal, S.; Pon, G.; Vivekanandan, R.; Destache, C.J. Pharmacokinetic and Tissue Distribution Profile of Long Acting Tenofovir Alafenamide and Elvitegravir Loaded Nanoparticles in Humanized Mice Model. Pharm. Res. 2017, 34, 2749–2755. [Google Scholar] [CrossRef]
- Dyavar, S.R.; Gautam, N.; Podany, A.T.; Winchester, L.C.; Weinhold, J.A.; Mykris, T.M.; Campbell, K.M.; Alnouti, Y.; Fletcher, C.V. Assessing the lymphoid tissue bioavailability of antiretrovirals in human primary lymphoid endothelial cells and in mice. J. Antimicrob. Chemother. 2019, 74, 2974–2978. [Google Scholar] [CrossRef]
- Jones, K.; Bray, P.G.; Khoo, S.H.; Davey, R.A.; Meaden, E.R.; Ward, S.A.; Back, D.J. P-Glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: Potential for accelerated viral drug resistance? AIDS 2001, 15, 1353–1358. [Google Scholar] [CrossRef]
- Khoo, S.H.; Hoggard, P.G.; Williams, I.; Meaden, E.R.; Newton, P.; Wilkins, E.G.; Smith, A.; Tjia, J.F.; Lloyd, J.; Jones, K.; et al. Intracellular accumulation of human immunodeficiency virus protease inhibitors. Antimicrob. Agents Chemother. 2002, 46, 3228–3235. [Google Scholar] [CrossRef][Green Version]
- Lee, C.G.; Gottesman, M.M.; Cardarelli, C.O.; Ramachandra, M.; Jeang, K.T.; Ambudkar, S.V.; Pastan, I.; Dey, S. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry 1998, 37, 3594–3601. [Google Scholar] [CrossRef]
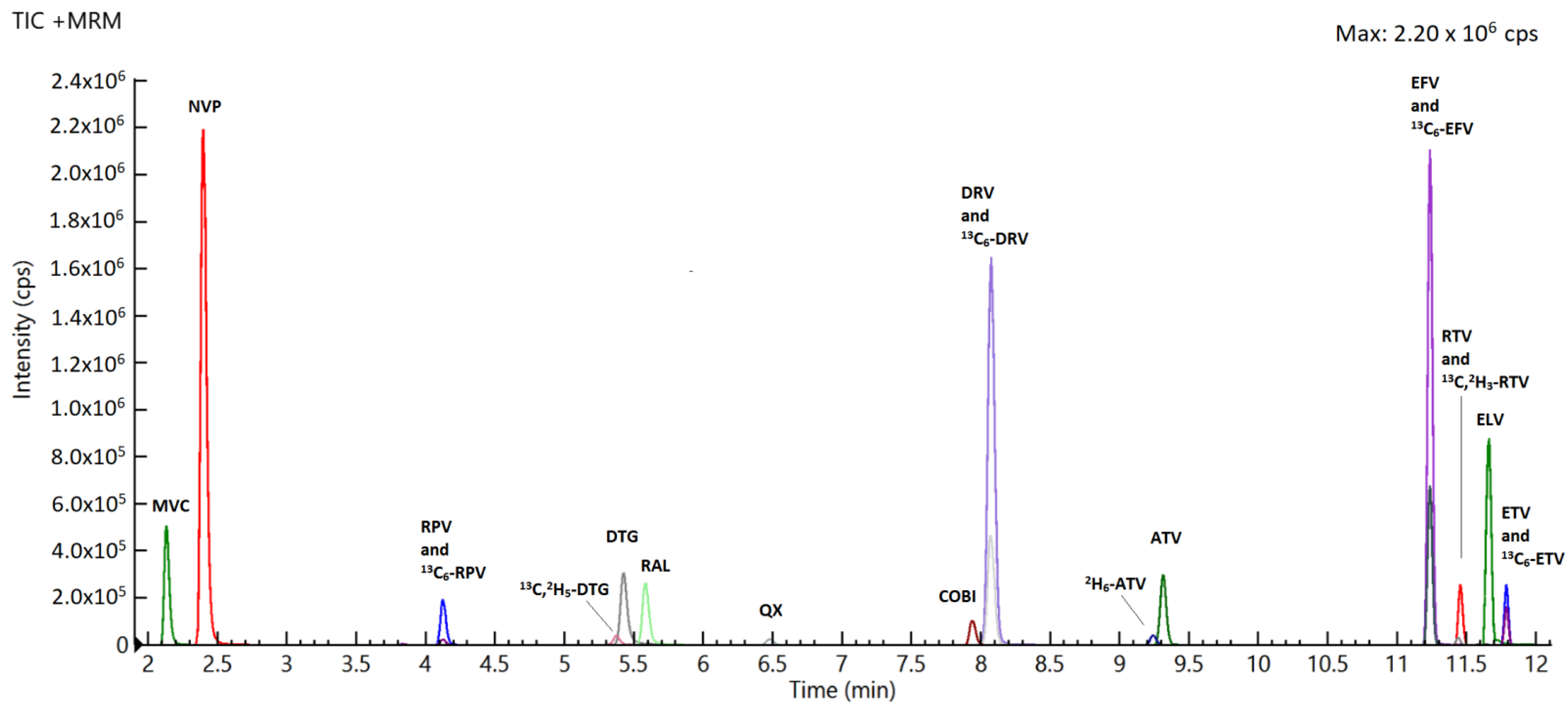
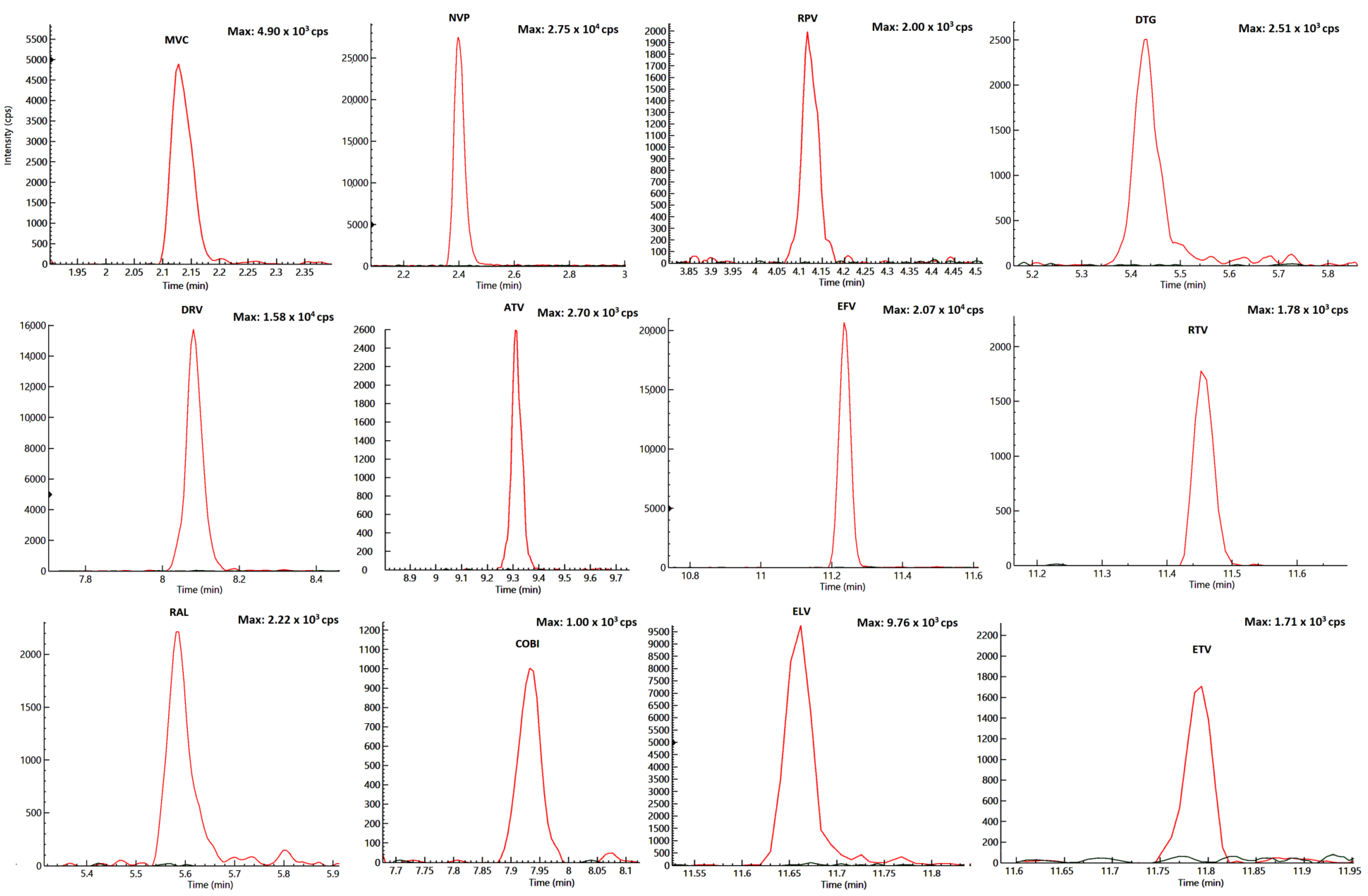
| Drugs | RT (min) | Highest Spiking Solution (ng/mL) | STD9/ULOQ (ng/Sample) | STD1/LLOQ (ng/Sample) | LOD (ng/Sample) | R2 | [M-H] + (m/z) | Dwell (ms) | Entrance Voltage (V) | FIRST Trace (m/z) | Collision Energy First Ion Trace (eV) | Second Trace (m/z) | Collision Energy Second Ion Trace (eV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MVC | 2.15 | 100 | 10 | 0.039 | 0.019 | 0.996 | 514.4 | 30 | 33 | 117.2 | −69 | 389.3 | −26 |
| NVP | 2.35 | 1000 | 100 | 0.390 | 0.195 | 0.997 | 267.1 | 30 | 25 | 80.2 | −56 | 226.1 | −32 |
| RPV | 4.08 | 100 | 10 | 0.039 | 0.019 | 0.998 | 367.3 | 30 | 45 | 128.2 | −83 | 195.2 | −48 |
| DTG | 5.32 | 100 | 10 | 0.039 | 0.019 | 0.998 | 420.3 | 25 | 30 | 127.1 | −46 | 277.1 | −34 |
| RAL | 5.60 | 100 | 10 | 0.039 | 0.019 | 0.996 | 445.2 | 25 | 26 | 109.1 | −41 | 361.1 | −25 |
| COBI | 7.90 | 100 | 10 | 0.039 | 0.019 | 0.996 | 776.4 | 25 | 15 | 98.1 | −82 | 606.2 | −34 |
| DRV | 8.15 | 1000 | 100 | 0.390 | 0.195 | 0.998 | 548.3 | 25 | 15 | 69.1 | −66 | 392.2 | −18 |
| ATV | 9.30 | 100 | 10 | 0.039 | 0.019 | 0.999 | 705.4 | 30 | 10 | 144.2 | −59 | 168.15 | −58 |
| EFV * | 11.21 | 1000 | 100 | 0.390 | 0.195 | 0.997 | 314.1 | 25 | −29 | 68.9 | 48 | 244.1 | 22 |
| RTV | 11.44 | 100 | 10 | 0.039 | 0.019 | 0.996 | 721.4 | 25 | 27 | 140.1 | −95 | 296.15 | −29 |
| ELV | 11.64 | 100 | 10 | 0.039 | 0.019 | 0.996 | 448.2 | 25 | 30 | 143.0 | −61 | 344.0 | −42 |
| ETV | 11.76 | 100 | 10 | 0.039 | 0.019 | 0.997 | 435.1 | 25 | 51 | 77.1 | −110 | 144.1 | −55 |
| 13C6-RPV | 3.67 | - | 1 | 1 | 1 | - | 373.3 | 30 | 39 | 182.2 | −50 | 195.2 | −50 |
| 13C,2H5-DTG | 5.32 | - | 1 | 1 | 1 | - | 426.3 | 25 | 40 | 133.2 | −50 | 277.1 | −35 |
| QX | 6.48 | - | 0.8 | 0.8 | 0.8 | - | 313.1 | 25 | 44 | 78.1 | −72 | 246.2 | −43 |
| 13C6-DRV | 8.15 | - | 10 | 10 | 10 | - | 554.3 | 25 | 16 | 113.1 | −31 | 398.2 | −20 |
| 2H6-ATV | 9.21 | - | 1 | 1 | 1 | - | 711.5 | 30 | 39 | 147.2 | −61 | 338.2 | −41 |
| 13C6-EFV * | 11.17 | - | 10 | 10 | 10 | - | 320.1 | 25 | −24 | 68.9 | 61 | 250.1 | 23 |
| 13C,2H3-RTV | 11.35 | - | 0.4 | 0.4 | 0.4 | - | 725.3 | 25 | 8 | 201.1 | −57 | 272.2 | −41 |
| 13C6-ETV | 11.72 | - | 1 | 1 | 1 | - | 441.2 | 25 | 45 | 77.1 | −110 | 150.2 | −55 |
| Drugs | QC High | QC Medium | QC Low | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiking Sol. Conc. (ng/mL) | Final Amount per Sample (ng) | Accuracy % | Imprecision RSD% | Spiking Sol. Conc. (ng/mL) | Final Amount per Sample (ng) | Accuracy % | Imprecision RSD% | Spiking Sol. Conc. (ng/mL) | Final Amount per Sample (ng) | Accuracy % | Imprecision RSD% | ||||
| Intra-Day % | Inter-Day % | Intra-Day % | Inter-Day % | Intra-Day % | Inter-Day % | ||||||||||
| MVC | 80 | 8 | 95.6 | 5.3 | 11.5 | 10 | 1 | 95.5 | 3.4 | 14.7 | 1 | 0.1 | 92.5 | 6.0 | 7.6 |
| NVP | 800 | 80 | 96.1 | 4.0 | 10.0 | 100 | 10 | 104.8 | 1.9 | 12.0 | 10 | 1 | 87.7 | 5.8 | 9.2 |
| RPV | 80 | 8 | 103.7 | 2.8 | 5.2 | 10 | 1 | 87.4 | 4.4 | 3.2 | 1 | 0.1 | 89.7 | 4.5 | 10.6 |
| DTG | 80 | 8 | 98.6 | 2.9 | 7.6 | 10 | 1 | 85.1 | 3.4 | 13.8 | 1 | 0.1 | 93.4 | 7.2 | 6.8 |
| RAL | 80 | 8 | 106.3 | 4.1 | 6.0 | 10 | 1 | 90.8 | 4.3 | 9.9 | 1 | 0.1 | 92.8 | 5.9 | 9.5 |
| COBI | 80 | 8 | 94.7 | 7.8 | 8.5 | 10 | 1 | 86.9 | 7.7 | 12.0 | 1 | 0.1 | 95.2 | 1.5 | 9.3 |
| DRV | 800 | 80 | 101.4 | 1.8 | 3.7 | 100 | 10 | 87.4 | 3.0 | 9.5 | 10 | 1 | 88.1 | 3.3 | 12.0 |
| ATV | 80 | 8 | 103.7 | 2.7 | 5.2 | 10 | 1 | 92.7 | 3.4 | 12.9 | 1 | 0.1 | 94.4 | 6.1 | 9.3 |
| EFV | 800 | 80 | 103.1 | 1.1 | 3.7 | 100 | 10 | 90.8 | 3.1 | 5.7 | 10 | 1 | 88.1 | 4.4 | 4.0 |
| RTV | 80 | 8 | 111.5 | 12.6 | 9.5 | 10 | 1 | 93.5 | 12.1 | 9.7 | 1 | 0.1 | 94.4 | 9.9 | 8.2 |
| ELV | 80 | 8 | 100.4 | 4.7 | 8.4 | 10 | 1 | 95.5 | 3.0 | 5.3 | 1 | 0.1 | 98.9 | 8.7 | 7.9 |
| ETV | 80 | 8 | 104.1 | 1.8 | 4.0 | 10 | 1 | 90.1 | 4.5 | 9.5 | 1 | 0.1 | 94.3 | 5.5 | 5.9 |
| Drugs | Recovery (RSD) | Matrix Effect (RSD) | IS-Normalized Matrix Effect (RSD) |
|---|---|---|---|
| MVC | 89.2% (6.1%) | 4.6% (6.7%) | 13.9% (9.1%) |
| NVP | 89.5% (2.6%) | −10.1% (5.9%) | −2.4% (10.5%) |
| RPV | 83.2% (3.0%) | −5.0% (11.6%) | 2.8% (4.7%) |
| DTG | 92.8% (3.8%) | −11.1% (7.2%) | 0.1% (4.7%) |
| RAL | 91.2% (2.2%) | −15.7% (5.4%) | −5.3% (4.9%) |
| COBI | 77.6% (3.0%) | 31.2% (9.1%) | 19.8% (7.5%) |
| DRV | 84.6% (2.9%) | −15.0% (4.3%) | −1.0% (12.9%) |
| ATV | 80.3% (2.9%) | 9.3% (6.0%) | −0.1% (4.2%) |
| EFV | 86.6% (2.4%) | −4.0% (4.8%) | −0.6% (1.7%) |
| RTV | 85.3% (2.7%) | −8.1% (7.7%) | −7.2% (8.0%) |
| ELV | 87.3% (4.3%) | −27.4% (10.3%) | −10.1% (10.5) |
| ETV | 79.7% (3.1%) | −17.6% (6.0%) | 4.5% (6.6%) |
| Median Trough Concentrations (IQR) | ||||
|---|---|---|---|---|
| Drugs | n | Plasma (ng/mL) | PBMC (ng/mL) | PBMC/Plasma Ratio |
| MVC | 1 | 68 (na *) | 163 (na) | 2.40 (na) |
| RPV | 1 | 80 (na) | 399 (na) | 4.98 (na) |
| DTG | 34 | 913 (616–1392) | 270 (156–450) | 0.26 (0.14–0.53) |
| RAL | 2 | 138 (136–na) | 43 (40–na) | 0.31 (0.29–na) |
| COBI | 17 | 49 (6–142) | 204 (63–686) | 3.52 (3.15–4.48) |
| DRV | 31 | 2389 (1523–3963) | 935 (581–1642) | 0.34 (0.23–0.57) |
| ATV | 21 | 778 (424–1547) | 1429 (552–3255) | 1.53 (1.01–2.72) |
| RTV | 29 | 82 (47–389) | 497 (278–845) | 3.81 (2.07–6.70) |
| ELV | 1 | 228 (na) | 1641 (na) | 7.20 (na) |
| ETV | 5 | 514 (78–763) | 2137 (500–3126) | 5.51 (3.01–6.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Nicolò, A.; Ianniello, A.; Ferrara, M.; Avataneo, V.; Cusato, J.; Antonucci, M.; De Vivo, E.; Waitt, C.; Calcagno, A.; Trentalange, A.; et al. Validation of a UHPLC-MS/MS Method to Quantify Twelve Antiretroviral Drugs within Peripheral Blood Mononuclear Cells from People Living with HIV. Pharmaceuticals 2021, 14, 12. https://doi.org/10.3390/ph14010012
De Nicolò A, Ianniello A, Ferrara M, Avataneo V, Cusato J, Antonucci M, De Vivo E, Waitt C, Calcagno A, Trentalange A, et al. Validation of a UHPLC-MS/MS Method to Quantify Twelve Antiretroviral Drugs within Peripheral Blood Mononuclear Cells from People Living with HIV. Pharmaceuticals. 2021; 14(1):12. https://doi.org/10.3390/ph14010012
Chicago/Turabian StyleDe Nicolò, Amedeo, Alice Ianniello, Micol Ferrara, Valeria Avataneo, Jessica Cusato, Miriam Antonucci, Elisa De Vivo, Catriona Waitt, Andrea Calcagno, Alice Trentalange, and et al. 2021. "Validation of a UHPLC-MS/MS Method to Quantify Twelve Antiretroviral Drugs within Peripheral Blood Mononuclear Cells from People Living with HIV" Pharmaceuticals 14, no. 1: 12. https://doi.org/10.3390/ph14010012
APA StyleDe Nicolò, A., Ianniello, A., Ferrara, M., Avataneo, V., Cusato, J., Antonucci, M., De Vivo, E., Waitt, C., Calcagno, A., Trentalange, A., Muccioli, G., Bonora, S., Di Perri, G., & D'Avolio, A. (2021). Validation of a UHPLC-MS/MS Method to Quantify Twelve Antiretroviral Drugs within Peripheral Blood Mononuclear Cells from People Living with HIV. Pharmaceuticals, 14(1), 12. https://doi.org/10.3390/ph14010012







