Oxidative Stress, Phytochemical Screening, and Antioxidant Activity on Microalgae (Arthrospira platensis) After Exposure to Glyphosate and Microplastics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microalgae Cultivation
2.3. Experimental Tests
2.4. Photosynthetic Pigment Content
2.5. Phytochemicals, Antioxidant, and Enzymatic Activities Analysis
2.5.1. Phytochemicals Analysis
2.5.2. Determination of Antioxidant Activity by Radical-Based Assays
2.5.3. Enzymatic Activities Determination
2.6. Statistical Analysis
3. Results
3.1. Cell Growth and Photosynthetic Pigment Contents
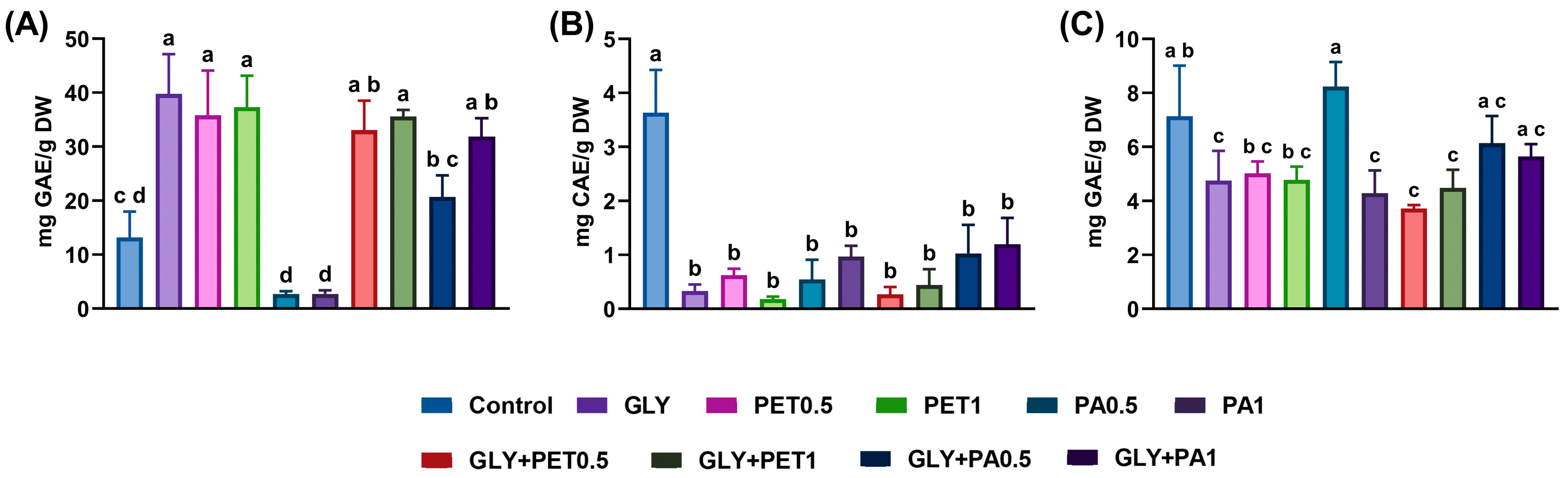
3.2. Effects on Phytochemicals and Antioxidant Activity
3.3. Effects on Enzymatic Biomarkers
3.4. Canonical Correspondence Analysis of Photosynthetic Pigment Profiles, Phytochemicals, Antioxidants, and Enzymatic Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podbielska, M.; Szpyrka, E. Microplastics—An Emerging Contaminants for Algae. Critical Review and Perspectives. Sci. Total Environ. 2023, 885, 163842. [Google Scholar] [CrossRef] [PubMed]
- Jolaosho, T.L.; Rasaq, M.F.; Omotoye, E.V.; Araomo, O.V.; Adekoya, O.S.; Abolaji, O.Y.; Hungbo, J.J. Microplastics in Freshwater and Marine Ecosystems: Occurrence, Characterization, Sources, Distribution Dynamics, Fate, Transport Processes, Potential Mitigation Strategies, and Policy Interventions. Ecotoxicol. Environ. Saf. 2025, 294, 118036. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Poirier, L.; Kamari, A.; Châtel, A.; Mouneyrac, C.; Lagarde, F. Is There Any Consistency between the Microplastics Found in the Field and Those Used in Laboratory Experiments? Environ. Pollut. 2016, 211, 111–123. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, J.-H.; Jo, A.-H.; Choi, Y.J.; Choi, C.Y.; Kang, J.-C.; Kim, J.-H. Microplastic Polyamide Toxicity: Neurotoxicity, Stress Indicators and Immune Responses in Crucian Carp, Carassius carassius. Ecotoxicol. Environ. Saf. 2023, 265, 115469. [Google Scholar] [CrossRef]
- Renzi, M.; Blašković, A.; Broccoli, A.; Bernardi, G.; Grazioli, E.; Russo, G. Chemical Composition of Microplastic in Sediments and Protected Detritivores from Different Marine Habitats (Salina Island). Mar. Pollut. Bull. 2020, 152, 110918. [Google Scholar] [CrossRef]
- Rajtar, N.; Starek, M.; Vincenti, L.; Dąbrowska, M.; Romek, M.; Rinaldi, R.; Lionetto, F.; Kepczynski, M. Effect of PET Micro/Nanoplastics on Model Freshwater Zooplankton. Polymers 2025, 17, 1256. [Google Scholar] [CrossRef] [PubMed]
- Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Role of Polyamide Microplastics as Vector of Parabens in the Environment: An Adsorption Study. Environ. Technol. Innov. 2023, 32, 103276. [Google Scholar] [CrossRef]
- Santos, D.; Félix, L.; Luzio, A.; Parra, S.; Bellas, J.; Monteiro, S.M. Single and Combined Acute and Subchronic Toxic Effects of Microplastics and Copper in Zebrafish (Danio rerio) Early Life Stages. Chemosphere 2021, 277, 130262. [Google Scholar] [CrossRef]
- Santos, D.; Cabecinha, E.; Luzio, A.; Bellas, J.; Monteiro, S.M. Long-Term Effects of Individual and Combined Exposure to Microplastics and Copper in Zebrafish Hypothalamic-Pituitary-Gonadal Axis—A Multi-Biomarker Evaluation. J. Environ. Manag. 2025, 378, 124770. [Google Scholar] [CrossRef]
- Su, Y.; Cheng, Z.; Hou, Y.; Lin, S.; Gao, L.; Wang, Z.; Bao, R.; Peng, L. Biodegradable and Conventional Microplastics Posed Similar Toxicity to Marine Algae Chlorella vulgaris. Aquat. Toxicol. 2022, 244, 106097. [Google Scholar] [CrossRef]
- Zocchi, M.; Sommaruga, R. Microplastics Modify the Toxicity of Glyphosate on Daphnia magna. Sci. Total Environ. 2019, 697, 134194. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, C.R.; Searle, C.L.; Schaber, J.; Höök, T.O. Microplastics Impact Simple Aquatic Food Web Dynamics through Reduced Zooplankton Feeding and Potentially Releasing Algae from Consumer Control. Sci. Total Environ. 2023, 904, 166691. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wei, S.; Hu, M.; Wei, H.; Wang, X.; Shang, Y.; Li, L.; Shi, H.; Wang, Y. Microplastics Aggravate the Adverse Effects of BDE-47 on Physiological and Defense Performance in Mussels. J. Hazard. Mater. 2020, 398, 122909. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological Interactions of Microplastics/Nanoplastics and Environmental Contaminants: Current Knowledge and Future Perspectives. J. Hazard. Mater. 2021, 405, 123913. [Google Scholar] [CrossRef]
- Wołowicz, A.; Munir, H.M.S. Emerging Organic Micropollutants as Serious Environmental Problem: A Comprehensive Review. Sci. Total Environ. 2025, 958, 177948. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Gowda, G.B.; Adak, T.; Guru-Pirasanna-Pandi, G.; Patil, N.B.; Annamalai, M.; Rath, P.C. Pesticides Occurrence in Water Sources and Decontamination Techniques. In Pesticides—Updates on Toxicity, Efficacy and Risk Assessment; IntechOpen: London, UK, 2022; ISBN 978-1-80356-039-7. [Google Scholar]
- Hao, B.; Wu, H.; Zhang, S.; He, B. Individual and Combined Toxicity of Microplastics and Diuron Differs between Freshwater and Marine Diatoms. Sci. Total Environ. 2022, 853, 158334. [Google Scholar] [CrossRef]
- Verdú, I.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Leaching of Herbicides Mixtures from Pre-Exposed Agricultural Plastics Severely Impact Microalgae. Chemosphere 2023, 326, 138475. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, Z.; Chen, Y.; Zhang, Q.; Ke, M.; Lu, T.; Qian, H. The Mechanism of Different Cyanobacterial Responses to Glyphosate. J. Environ. Sci. 2023, 125, 258–265. [Google Scholar] [CrossRef]
- Klátyik, S.; Simon, G.; Oláh, M.; Takács, E.; Mesnage, R.; Antoniou, M.N.; Zaller, J.G.; Székács, A. Aquatic Ecotoxicity of Glyphosate, Its Formulations, and Co-Formulants: Evidence from 2010 to 2023. Environ. Sci. Eur. 2024, 36, 22. [Google Scholar] [CrossRef]
- Cao, G.; Liu, Y.; Zhang, S.; Yang, X.; Chen, R.; Zhang, Y.; Lu, W.; Liu, Y.; Wang, J.; Lin, M.; et al. A Novel 5-Enolpyruvylshikimate-3-Phosphate Synthase Shows High Glyphosate Tolerance in Escherichia Coli and Tobacco Plants. PLoS ONE 2012, 7, e38718. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Liu, X.; Zhang, L.; Zhang, S.; Li, Y.; Zhang, W.; Li, Q.; Zhao, Y.; Chen, X.; et al. Effect and Mechanism of Microplastics Exposure against Microalgae: Photosynthesis and Oxidative Stress. Sci. Total Environ. 2023, 905, 167017. [Google Scholar] [CrossRef]
- Yang, W.; Gao, X.; Wu, Y.; Wan, L.; Tan, L.; Yuan, S.; Ding, H.; Zhang, W. The Combined Toxicity Influence of Microplastics and Nonylphenol on Microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2020, 195, 110484. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Nanoplastic Affects Growth of S. Obliquus and Reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, P.; Zhang, X.; Zhang, Y.; Xie, S.; Deng, J. Effect of Microplastics Exposure on the Photosynthesis System of Freshwater Algae. J. Hazard. Mater. 2019, 374, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, S.; Liu, R.; George, S.; Bhaskaran, S. Polyethylene Terephthalate Nanoparticles Induce Oxidative Damage in Chlorella vulgaris. Plant Physiol. Biochem. 2024, 215, 108987. [Google Scholar] [CrossRef]
- Iummato, M.M.; Fassiano, A.; Graziano, M.; dos Santos Afonso, M.; Ríos de Molina, M.d.C.; Juárez, Á.B. Effect of Glyphosate on the Growth, Morphology, Ultrastructure and Metabolism of Scenedesmus vacuolatus. Ecotoxicol. Environ. Saf. 2019, 172, 471–479. [Google Scholar] [CrossRef]
- Romero, D.M.; Ríos de Molina, M.C.; Juárez, Á.B. Oxidative Stress Induced by a Commercial Glyphosate Formulation in a Tolerant Strain of Chlorella Kessleri. Ecotoxicol. Environ. Saf. 2011, 74, 741–747. [Google Scholar] [CrossRef]
- Hernández-García, C.I.; Martínez-Jerónimo, F. Multistressor Negative Effects on an Experimental Phytoplankton Community. The Case of Glyphosate and One Toxigenic Cyanobacterium on Chlorophycean Microalgae. Sci. Total Environ. 2020, 717, 137186. [Google Scholar] [CrossRef]
- Abbasi, S.; Amiranipour, S.; Karimi, J.; Mohsenzadeh, S.; Turner, A. Impacts of Polyethylene Microplastics on the Microalga, Spirulina (Arthrospira platensis). Environ. Pollut. 2023, 327, 121611. [Google Scholar] [CrossRef]
- Ismaiel, M.M.S.; Piercey-Normore, M.D.; Rampitsch, C. Proteomic Analyses of the Cyanobacterium arthrospira (Spirulina) Platensis under Iron and Salinity Stress. Environ. Exp. Bot. 2018, 147, 63–74. [Google Scholar] [CrossRef]
- Diaconu, M.; Soreanu, G.; Balan, C.D.; Buciscanu, I.I.; Maier, V.; Cretescu, I. Study of Spirulina platensis (Arthrospira) Development under the Heavy Metals Influence, as a Potential Promoter of Wastewater Remediation. Water 2023, 15, 3962. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, D.; Wu, W.; Li, F. Ecotoxicological Effect of Enrofloxacin on Spirulina Platensis and the Corresponding Detoxification Mechanism. Environ. Sci. Process. Impacts 2023, 25, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, S.; An, S.-A.; Khim, J.S. Methodological Advances and Future Directions of Microalgal Bioassays for Evaluation of Potential Toxicity in Environmental Samples: A Review. Environ. Int. 2023, 173, 107869. [Google Scholar] [CrossRef] [PubMed]
- Mörtl, M.; Németh, G.; Juracsek, J.; Darvas, B.; Kamp, L.; Rubio, F.; Székács, A. Determination of Glyphosate Residues in Hungarian Water Samples by Immunoassay. Microchem. J. 2013, 107, 143–151. [Google Scholar] [CrossRef]
- Poiger, T.; Buerge, I.J.; Bächli, A.; Müller, M.D.; Balmer, M.E. Occurrence of the Herbicide Glyphosate and Its Metabolite AMPA in Surface Waters in Switzerland Determined with On-Line Solid Phase Extraction LC-MS/MS. Environ. Sci. Pollut. Res. 2017, 24, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Schwientek, M.; Rügner, H.; Haderlein, S.B.; Schulz, W.; Wimmer, B.; Engelbart, L.; Bieger, S.; Huhn, C. Glyphosate Contamination in European Rivers Not from Herbicide Application? Water Res. 2024, 263, 122140. [Google Scholar] [CrossRef]
- Inês, S.; Ana, L.; Silva, E. Environmental Risk Assessment of Glyphosate and Aminomethylphosphonic Acid (AMPA) in Portuguese Groundwater Ecosystems. Environments 2024, 11, 258. [Google Scholar] [CrossRef]
- Tóth, G.; Háhn, J.; Szoboszlay, S.; Harkai, P.; Farkas, M.; Radó, J.; Göbölös, B.; Kaszab, E.; Szabó, I.; Urbányi, B.; et al. Spatiotemporal Analysis of Multi-Pesticide Residues in the Largest Central European Shallow Lake, Lake Balaton, and Its Sub-Catchment Area. Environ. Sci. Eur. 2022, 34, 50. [Google Scholar] [CrossRef]
- De Frond, H.; Rubinovitz, R.; Rochman, C.M. μATR-FTIR Spectral Libraries of Plastic Particles (FLOPP and FLOPP-e) for the Analysis of Microplastics. Anal. Chem. 2021, 93, 15878–15885. [Google Scholar] [CrossRef]
- Sun, T.; Zhan, J.; Li, F.; Ji, C.; Wu, H. Environmentally Relevant Concentrations of Microplastics Influence the Locomotor Activity of Aquatic Biota. J. Hazard. Mater. 2021, 414, 125581. [Google Scholar] [CrossRef]
- Das, B.C.; Ramanan, P.A.; Gorakh, S.S.; Pillai, D.; Vattiringal Jayadradhan, R.K. Sub-Chronic Exposure of Oreochromis Niloticus to Environmentally Relevant Concentrations of Smaller Microplastics: Accumulation and Toxico-Physiological Responses. J. Hazard. Mater. 2023, 458, 131916. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution A L’etude D’une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques Sur La Croissance et La Photosynthese de Spirulina Mixima. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Luzio, A.; Bernardo, S.; Correia, C.; Moutinho-Pereira, J.; Dinis, L.-T. Phytochemical Screening and Antioxidant Activity on Berry, Skin, Pulp and Seed from Seven Red Mediterranean Grapevine Varieties (Vitis vinifera L.) Treated with Kaolin Foliar Sunscreen. Sci. Hortic. 2021, 281, 109962. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Soszynski, A.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the Antioxidant Potential and the Phenolic Composition of Different Anatomical Organs of the Marine Halophyte Limonium algarvense. Ind. Crop. Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]
- Granato, D.; Margraf, T.; Brotzakis, I.; Capuano, E.; van Ruth, S.M. Characterization of Conventional, Biodynamic, and Organic Purple Grape Juices by Chemical Markers, Antioxidant Capacity, and Instrumental Taste Profile. J. Food Sci. 2015, 80, C55–C65. [Google Scholar] [CrossRef] [PubMed]
- Durak, I.; Yurtarslanl, Z.; Canbolat, O.; Akyol, O. A Methodological Approach to Superoxide Dismutase (SOD) Activity Assay Based on Inhibition of Nitroblue Tetrazolium (NBT) Reduction. Clin. Chim. Acta Int. J. Clin. Chem. 1993, 214, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Massarsky, A.; Kozal, J.S.; Di Giulio, R.T. Glutathione and Zebrafish: Old Assays to Address a Current Issue. Chemosphere 2017, 168, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Jakoby, W.B. Assays for Differentiation of Glutathione S-Transferases. Methods Enzym. 1981, 77, 398–405. [Google Scholar] [CrossRef]
- Hosokawa, M.; Satoh, T. Measurement of Carboxylesterase (CES) Activities. Curr. Protoc. Toxicol. 2001, 10, 4.7.1–4.7.14. [Google Scholar] [CrossRef]
- Gartaganis, S.P.; Patsoukis, N.E.; Nikolopoulos, D.K.; Georgiou, C.D. Evidence for Oxidative Stress in Lens Epithelial Cells in Pseudoexfoliation Syndrome. Eye 2007, 21, 1406–1411. [Google Scholar] [CrossRef]
- Deng, J.; Yu, L.; Liu, C.; Yu, K.; Shi, X.; Yeung, L.W.; Lam, P.K.; Wu, R.S.; Zhou, B. Hexabromocyclododecane-Induced Developmental Toxicity and Apoptosis in Zebrafish Embryos. Aquat. Toxicol. 2009, 93, 29–36. [Google Scholar] [CrossRef]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-1-107-69440-8. [Google Scholar]
- Song, C.; Liu, Z.; Wang, C.; Li, S.; Kitamura, Y. Different Interaction Performance between Microplastics and Microalgae: The Bio-Elimination Potential of Chlorella sp. L38 and Phaeodactylum tricornutum MASCC-0025. Sci. Total Environ. 2020, 723, 138146. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; Kim, D.; An, Y.-J. Effects of Micro-Sized Polyethylene Spheres on the Marine Microalga Dunaliella salina: Focusing on the Algal Cell to Plastic Particle Size Ratio. Aquat. Toxicol. 2019, 216, 105296. [Google Scholar] [CrossRef]
- Prata, J.C.; Venâncio, C.; Girão, A.V.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Effects of Virgin and Weathered Polystyrene and Polypropylene Microplastics on Raphidocelis subcapitata and Embryos of Danio Rerio under Environmental Concentrations. Sci. Total Environ. 2022, 816, 151642. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, T.; Wang, J.; Jiang, L.; Yin, Y.; Guo, H. Size-Dependent Toxic Effects of Polystyrene Microplastic Exposure on Microcystis aeruginosa Growth and Microcystin Production. Sci. Total Environ. 2021, 761, 143265. [Google Scholar] [CrossRef]
- Qu, M.; Wang, L.; Xu, Q.; An, J.; Mei, Y.; Liu, G. Influence of Glyphosate and Its Metabolite Aminomethylphosphonic Acid on Aquatic Plants in Different Ecological Niches. Ecotoxicol. Environ. Saf. 2022, 246, 114155. [Google Scholar] [CrossRef]
- Wang, C.; Lin, X.; Li, L.; Lin, S. Differential Growth Responses of Marine Phytoplankton to Herbicide Glyphosate. PLoS ONE 2016, 11, e0151633. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Ratha, S.K.; Renuka, N.; Ramanna, L.; Gupta, S.K.; Rawat, I.; Bux, F. Effect of Microplastics on Growth and Biochemical Composition of Microalga Acutodesmus obliquus. Algal Res. 2021, 56, 102296. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of Polystyrene Microplastics on the Growth, Photosynthetic Efficiency and Aggregation of Freshwater Microalgae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 714, 136767. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Liang, J.; Luo, Y.; Tang, N.; Ye, S.; Zhu, Z.; Xing, W.; Guo, J.; Zhang, H. Microcystis aeruginosa’s Exposure to an Antagonism of Nanoplastics and MWCNTs: The Disorders in Cellular and Metabolic Processes. Chemosphere 2022, 288, 132516. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Ahmadmahmoodi, R.; Malekabadi, M.B.; Rahimi, R.; Johari, S.A. Aquatic Pollution Caused by Mercury, Lead, and Cadmium Affects Cell Growth and Pigment Content of Marine Microalga, Nannochloropsis oculata. Environ. Monit. Assess. 2020, 192, 330. [Google Scholar] [CrossRef]
- Davarpanah, E.; Guilhermino, L. Single and Combined Effects of Microplastics and Copper on the Population Growth of the Marine Microalgae Tetraselmis chuii. Estuar. Coast. Shelf Sci. 2015, 167, 269–275. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Martel Quintana, A.; Roberti, R. Cyanobacteria: A Natural Source for Controlling Agricultural Plant Diseases Caused by Fungi and Oomycetes and Improving Plant Growth. Horticulturae 2022, 8, 58. [Google Scholar] [CrossRef]
- Marjanović, B.; Benković, M.; Jurina, T.; Sokač Cvetnić, T.; Valinger, D.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Bioactive Compounds from Spirulina spp.—Nutritional Value, Extraction, and Application in Food Industry. Separations 2024, 11, 257. [Google Scholar] [CrossRef]
- Bashandy, S.A.E.; Awdan, S.A.E.; Ebaid, H.; Alhazza, I.M. Antioxidant Potential of Spirulina platensis Mitigates Oxidative Stress and Reprotoxicity Induced by Sodium Arsenite in Male Rats. Oxid. Med. Cell Longev. 2016, 7174351. [Google Scholar] [CrossRef]
- Inwongwan, S.; Sriwari, S.; Pumas, C. Metabolomic Insights into the Adaptations and Biotechnological Potential of Euglena Gracilis Under Different Trophic Conditions. Plants 2025, 14, 1580. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Del Mondo, A.; Sansone, C.; Brunet, C. Insights into the Biosynthesis Pathway of Phenolic Compounds in Microalgae. Comput. Struct. Biotechnol. J. 2022, 20, 1901–1913. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of alga species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abuzead, S.M.M.; Halawa, S.M. Protective Role of Spirulina platensis against Acute Deltamethrin-Induced Toxicity in Rats. PLoS ONE 2013, 8, e72991. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Yue, Z.; Qian, J.; Li, W.; Liu, X.; Dai, H.; Liu, X.; Pi, F.; Wang, J. Spotlight on the Long-Term Effects of Micro/Nanoplastics Exposure on Spirulina platensis: Algal Cells, Extracellular Polymeric Substances, and Phycocyanin. Food Chem. 2025, 472, 142940. [Google Scholar] [CrossRef] [PubMed]
- Khoshnamvand, M.; You, D.; Xie, Y.; Feng, Y.; Sultan, M.; Pei, D.-S.; Fu, A. Alleviating Binary Toxicity of Polystyrene Nanoplastics and Atrazine to Chlorella vulgaris through Humic Acid Interaction: Long-Term Toxicity Using Environmentally Relevant Concentrations. Chemosphere 2024, 358, 142111. [Google Scholar] [CrossRef] [PubMed]
- Mofeed, J.; Mosleh, Y.Y. Toxic Responses and Antioxidative Enzymes Activity of Scenedesmus obliquus Exposed to Fenhexamid and Atrazine, Alone and in Mixture. Ecotoxicol. Environ. Saf. 2013, 95, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Talib, M.; Singh, N.; Darbha, G.K. Toxic Effects of Polystyrene Nanoplastics and Polycyclic Aromatic Hydrocarbons (Chrysene and Fluoranthene) on the Growth and Physiological Characteristics of Chlamydomonas reinhardtii. Aquat. Toxicol. 2024, 268, 106838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qu, Q.; Lu, T.; Ke, M.; Zhu, Y.; Zhang, M.; Zhang, Z.; Du, B.; Pan, X.; Sun, L.; et al. The Combined Toxicity Effect of Nanoplastics and Glyphosate on Microcystis aeruginosa Growth. Environ. Pollut. 2018, 243, 1106–1112. [Google Scholar] [CrossRef]
- Piro, A.; Nisticò, D.M.; Oliva, D.; Mazzuca, S. Microplastics Mitigate the Effects of Glyphosate on the Shikimic Acid Pathway Enzymes in Cyanobacteria. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2024, 158, 746–753. [Google Scholar] [CrossRef]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Microplastics and Associated Emerging Contaminants in the Environment: Analysis, Sorption Mechanisms and Effects of Co-Exposure. Trends Environ. Anal. Chem. 2022, 35, e00170. [Google Scholar] [CrossRef]
- Concha-Graña, E.; Moscoso-Pérez, C.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Adsorption of Pesticides and Personal Care Products on Pristine and Weathered Microplastics in the Marine Environment. Comparison between Bio-Based and Conventional Plastics. Sci. Total Environ. 2022, 848, 157703. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of Antibiotics on Microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Yu, H.; Peng, J.; Cao, X.; Wang, Y.; Zhang, Z.; Xu, Y.; Qi, W. Effects of Microplastics and Glyphosate on Growth Rate, Morphological Plasticity, Photosynthesis, and Oxidative Stress in the Aquatic Species Salvinia cucullata. Environ. Pollut. 2021, 279, 116900. [Google Scholar] [CrossRef]





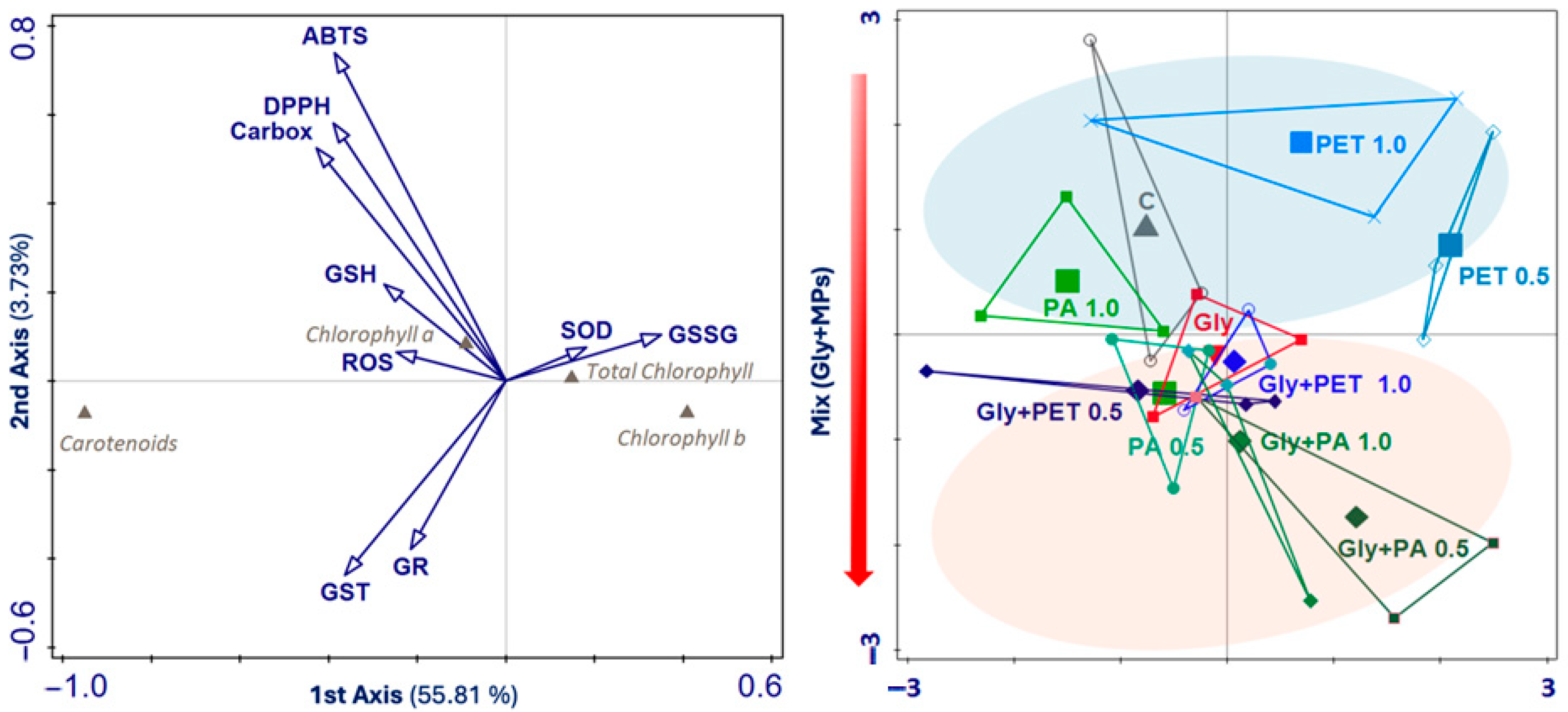
| All Variables | ||
|---|---|---|
| Axis 1 | Axis 2 | |
| Total variation | 180.000 | |
| Explanatory variables account for | 59.7% | |
| Explained fitted variation (cumulative) | 55.8% | 13.71% |
| Permutation Results | ||
| On the first axis | F = 27.8, p = 0.007 | |
| On all axes | F = 3.6, p = 0.006 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.; Cabecinha, E.; Gago, J.; Monteiro, S.M.; Luzio, A. Oxidative Stress, Phytochemical Screening, and Antioxidant Activity on Microalgae (Arthrospira platensis) After Exposure to Glyphosate and Microplastics. J. Xenobiot. 2025, 15, 106. https://doi.org/10.3390/jox15040106
Santos D, Cabecinha E, Gago J, Monteiro SM, Luzio A. Oxidative Stress, Phytochemical Screening, and Antioxidant Activity on Microalgae (Arthrospira platensis) After Exposure to Glyphosate and Microplastics. Journal of Xenobiotics. 2025; 15(4):106. https://doi.org/10.3390/jox15040106
Chicago/Turabian StyleSantos, Dércia, Edna Cabecinha, Jesús Gago, Sandra Mariza Monteiro, and Ana Luzio. 2025. "Oxidative Stress, Phytochemical Screening, and Antioxidant Activity on Microalgae (Arthrospira platensis) After Exposure to Glyphosate and Microplastics" Journal of Xenobiotics 15, no. 4: 106. https://doi.org/10.3390/jox15040106
APA StyleSantos, D., Cabecinha, E., Gago, J., Monteiro, S. M., & Luzio, A. (2025). Oxidative Stress, Phytochemical Screening, and Antioxidant Activity on Microalgae (Arthrospira platensis) After Exposure to Glyphosate and Microplastics. Journal of Xenobiotics, 15(4), 106. https://doi.org/10.3390/jox15040106










