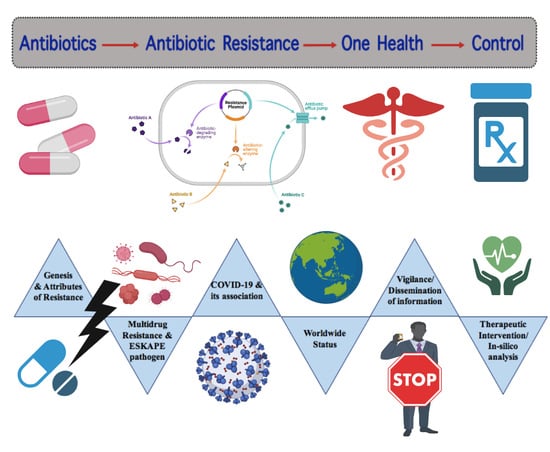
Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century
Abstract
:1. Introduction
2. Brief History and Background
3. Features of Different Resistant Bacteria
4. Origin and Development of Resistance
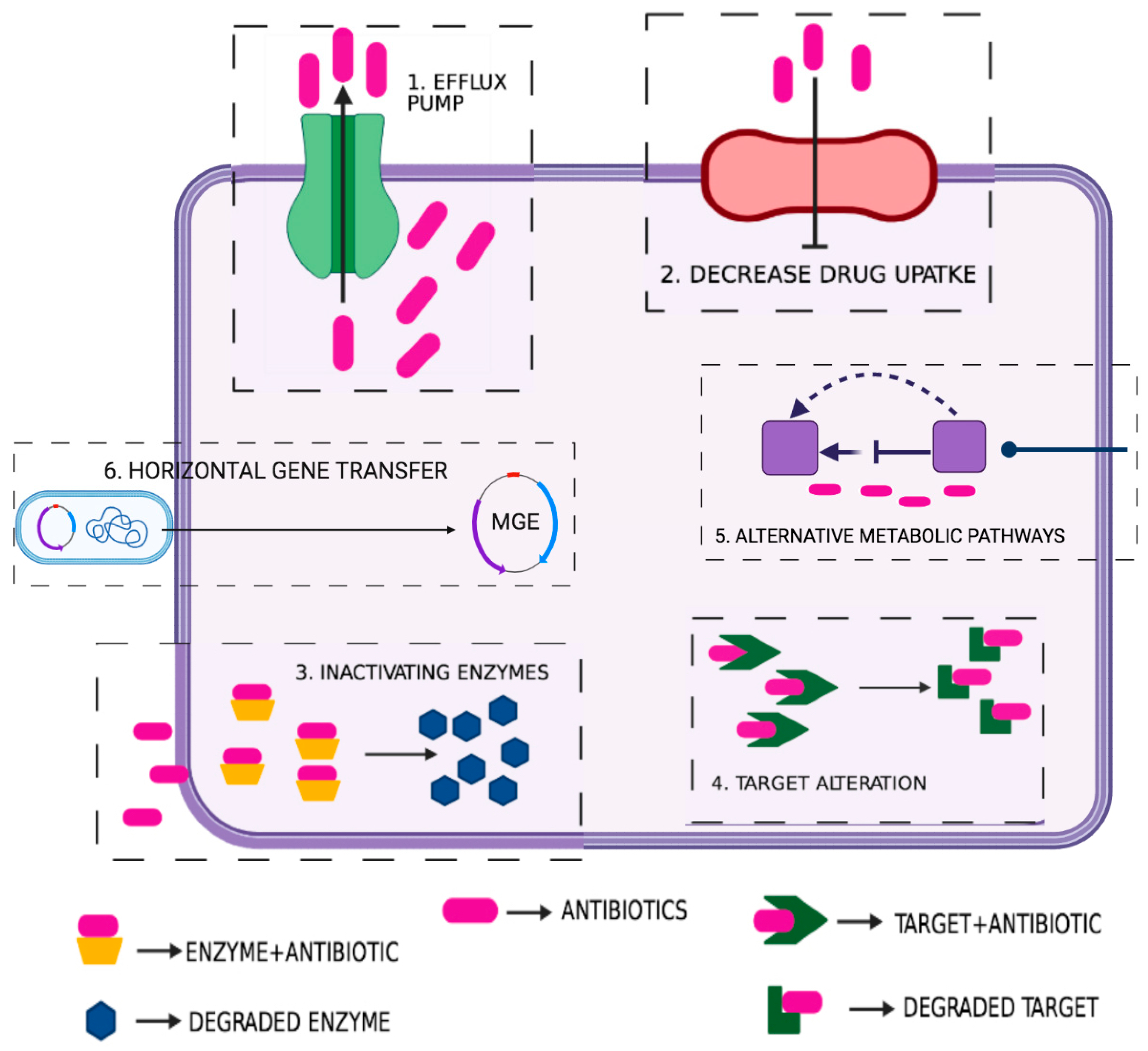
5. Characterization of Antibiotic Resistance Factors
5.1. Natural/Intrinsic Resistance
5.2. Acquired Resistance
5.2.1. Antibiotic Modification or Degradation
5.2.2. Antibiotic Efflux
5.2.3. Use of an Alternative Metabolic Pathway
5.2.4. Reduction of the Inner and Outer Membrane Permeability
6. Antibiotic Sequestration
7. ESKAPE Pathogens
8. Global and Developing Countries Scenario
9. Impact of COVID-19 Pandemic in AMR
10. Epidemiology and Surveillance
10.1. Control Strategy of Antibiotic Resistance
10.2. Therapeutic Interventions
11. In Silico Analysis
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of Mobile Genetic Elements Associated with Antibiotic Resistance in Salmonella Enterica Using a Newly Developed Web Tool: MobileElementFinder. J. Antimicrob. Chemother. 2020, 76, 101–109. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef] [PubMed]
- Ranjalkar, J.; Chandy, S. India’s National Action Plan for Antimicrobial Resistance—An Overview of the Context, Status, and Way Ahead. J. Fam. Med. Prim. Care 2019, 8, 1828. [Google Scholar] [CrossRef]
- World Health Organization Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-openingremarks-at-the-media-briefing-on-covid-19-11-march-2020 (accessed on 11 March 2020).
- Rezasoltani, S.; Yadegar, A.; Hatami, B.; Asadzadeh Aghdaei, H.; Zali, M.R. Antimicrobial Resistance as a Hidden Menace Lurking behind the COVID-19 Outbreak: The Global Impacts of Too Much Hygiene on AMR. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Bio-Med. Atenei Parm. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Adhanom Ghebreyesus, T. Addressing Mental Health Needs: An Integral Part of COVID-19 Response. World Psychiatry 2020, 19, 129–130. [Google Scholar] [CrossRef] [PubMed]
- World Antimicrobial Awareness Week. 2020. Available online: https://www.who.int/campaigns/world-antibiotic-awareness-week (accessed on 18 November 2020).
- Pelfrene, E.; Botgros, R.; Cavaleri, M. Antimicrobial Multidrug Resistance in the Era of COVID-19: A Forgotten Plight? Antimicrob. Resist. Infect. Control 2021, 10. [Google Scholar] [CrossRef]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg. Infect. Control 2017, 12. [Google Scholar] [CrossRef]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic Resistance in Vibrio Cholerae: Understanding the Ecology of Resistance Genes and Mechanisms. Vaccine 2019, 38 (Suppl. 1), A83–A92. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.J.; Payne, D.J.; Rappuoli, R.; De Gregorio, E. Technologies to Address Antimicrobial Resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 12887–12895. [Google Scholar] [CrossRef] [Green Version]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The Threat of Antimicrobial Resistance in Developing Countries: Causes and Control Strategies. Antimicrob. Resist. Infect. Control 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Gould, K. Antibiotics: From Prehistory to the Present Day. J. Antimicrob. Chemother. 2016, 71, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Shafer, W.M. Antimicrobial Resistance in Neisseria Gonorrhoeae in the 21st Century: Past, Evolution, and Future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [Green Version]
- Su, P.-Y.; Huang, A.-H.; Lai, C.-H.; Lin, H.-F.; Lin, T.-M.; Ho, C.-H. Extensively Drug-Resistant Haemophilus Influenzae—Emergence, Epidemiology, Risk Factors, and Regimen. BMC Microbiol. 2020, 20. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Ndagi, U.; Falaki, A.A.; Abdullahi, M.; Lawal, M.M.; Soliman, M.E. Antibiotic Resistance: Bioinformatics-Based Understanding as a Functional Strategy for Drug Design. RSC Adv. 2020, 10, 18451–18468. [Google Scholar] [CrossRef]
- Doron, S.; Gorbach, S.L. Bacterial Infections: Overview. Int. Encycl. Public Health 2008, 273–282. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Kadri, S.S. Key Takeaways from the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter Baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Alkofide, H.; Alhammad, A.M.; Alruwaili, A.; Aldemerdash, A.; Almangour, T.A.; Alsuwayegh, A.; Almoqbel, D.; Albati, A.; Alsaud, A.; Enani, M. Multidrug-Resistant and Extensively Drug-Resistant Enterobacteriaceae: Prevalence, Treatments, and Outcomes—A Retrospective Cohort Study. Infect. Drug Resist. 2020, 13, 4653–4662. [Google Scholar] [CrossRef]
- Folgori, L.; Ellis, S.J.; Bielicki, J.A.; Heath, P.T.; Sharland, M.; Balasegaram, M. Tackling Antimicrobial Resistance in Neonatal Sepsis. Lancet Glob. Health 2017, 5, e1066–e1068. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Rajpara, N.; Kutar, B.M.R.N.S.; Sinha, R.; Nag, D.; Koley, H.; Ramamurthy, T.; Bhardwaj, A.K. Role of Integrons, Plasmids and SXT Elements in Multidrug Resistance of Vibrio Cholerae and Providencia Vermicola Obtained from a Clinical Isolate of Diarrhea. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, R.; Coles, M.; Linke, D.; Kulik, A.; Nega, M.; Wohlleben, W.; Stegmann, E. Distinct Mechanisms Contribute to Immunity in the Lantibiotic NAI-107 Producer StrainMicrobispora|ATCC PTA-5024. Environ. Microbiol. 2015, 18, 118–132. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Ogawara, H. Comparison of Antibiotic Resistance Mechanisms in Antibiotic-Producing and Pathogenic Bacteria. Molecules 2019, 24, 3430. [Google Scholar] [CrossRef] [Green Version]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidovic, N.; Vidovic, S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics 2020, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Rusic, D.; Vilovic, M.; Bukic, J.; Leskur, D.; Seselja Perisin, A.; Kumric, M.; Martinovic, D.; Petric, A.; Modun, D.; Bozic, J. Implications of COVID-19 Pandemic on the Emergence of Antimicrobial Resistance: Adjusting the Response to Future Outbreaks. Life 2021, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Poulin-Laprade, D.; Matteau, D.; Jacques, P.-É.; Rodrigue, S.; Burrus, V. Transfer Activation of SXT/R391 Integrative and Conjugative Elements: Unraveling the SetCD Regulon. Nucleic Acids Res. 2015, 43, 2045–2056. [Google Scholar] [CrossRef] [Green Version]
- Spagnoletti, M.; Ceccarelli, D.; Rieux, A.; Fondi, M.; Taviani, E.; Fani, R.; Colombo, M.M.; Colwell, R.R.; Balloux, F. Acquisition and Evolution of SXT-R391 Integrative Conjugative Elements in the Seventh-Pandemic Vibrio Cholerae Lineage. mBio 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, I.; Rahman, S.; Jan, A.T.; Siddiqui, M.T.; Mondal, A.H.; Haq, Q.M.R. Antibiotics, Resistome and Resistance Mechanisms: A Bacterial Perspective. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells Are Ringing. Cureus 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Lin, J.; Ma, J.; Cronan, J.E.; Wang, H. Triclosan Resistance of Pseudomonas Aeruginosa PAO1 Is due to FabV, a Triclosan-Resistant Enoyl-Acyl Carrier Protein Reductase. Antimicrob. Agents Chemother. 2010, 54, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Randall, C.P.; Mariner, K.R.; Chopra, I.; O’Neill, A.J. The Target of Daptomycin Is Absent from Escherichia Coli and Other Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2012, 57, 637–639. [Google Scholar] [CrossRef] [Green Version]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- Darwich, L.; Vidal, A.; Seminati, C.; Albamonte, A.; Casado, A.; López, F.; Molina-López, R.A.; Migura-Garcia, L. High Prevalence and Diversity of Extended-Spectrum β-Lactamase and Emergence of OXA-48 Producing Enterobacterales in Wildlife in Catalonia. PLoS ONE 2019, 14, e0210686. [Google Scholar] [CrossRef] [Green Version]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of Bacterial Efflux Pumps in Biofilm Formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [Green Version]
- Muktan, B.; Thapa Shrestha, U.; Dhungel, B.; Mishra, B.C.; Shrestha, N.; Adhikari, N.; Banjara, M.R.; Adhikari, B.; Rijal, K.R.; Ghimire, P. Plasmid Mediated Colistin Resistant Mcr-1 and Co-Existence of OXA-48 among Escherichia Coli from Clinical and Poultry Isolates: First Report from Nepal. Gut Pathog. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, M.U.; Jaja, I.F.; Nwobi, O.C. Occurrence and Characteristics of Mobile Colistin Resistance (Mcr) Gene-Containing Isolates from the Environment: A Review. Int. J. Environ. Res. Public Health 2020, 17, 1028. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Busó, L.; Harris, S.R. Using Genomics to Understand Antimicrobial Resistance and Transmission in Neisseria Gonorrhoeae. Microb. Genom. 2019, 5. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, N.; Banka, A. Multidrug-Resistant Tuberculosis/Rifampicin-Resistant Tuberculosis: Principles of Management. Lung India 2018, 35, 78. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laxminarayan, R.; Van Boeckel, T.; Frost, I.; Kariuki, S.; Khan, E.A.; Limmathurotsakul, D.; Larsson, D.G.J.; Levy-Hara, G.; Mendelson, M.; Outterson, K.; et al. The Lancet Infectious Diseases Commission on Antimicrobial Resistance: 6 Years Later. Lancet Infect. Dis. 2020, 20, e51–e60. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Huseby, D.L.; Brandis, G.; Hughes, D. Alternative Evolutionary Pathways for Drug-Resistant Small Colony Variant Mutants in Staphylococcus Aureus. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications. Int. J. Mol. Sci. 2019, 20, 4996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic Resistance in Pseudomonas Aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Hamed, S.M.; Elkhatib, W.F.; El-Mahallawy, H.A.; Helmy, M.M.; Ashour, M.S.; Aboshanab, K.M.A. Multiple Mechanisms Contributing to Ciprofloxacin Resistance among Gram Negative Bacteria Causing Infections to Cancer Patients. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Fernandez, L.; Hancock, R.E.W. Adaptive and Mutational Resistance: Role of Porins and Efflux Pumps in Drug Resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef] [Green Version]
- Rudolf, J.D.; Bigelow, L.; Chang, C.; Cuff, M.E.; Lohman, J.R.; Chang, C.-Y.; Ma, M.; Yang, D.; Clancy, S.; Babnigg, G.; et al. Crystal Structure of the Zorbamycin-Binding Protein ZbmA, the Primary Self-Resistance Element in Streptomyces Flavoviridis ATCC21892. Biochemistry 2015, 54, 6842–6851. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, M.; Kumagai, T.; Matsuo, H.; Alam Bhuiyan, Z.; Ueda, K.; Mochizuki, H.; Nakamura, N.; Davies, J.E. Overproduction of the Bleomycin-Binding Proteins from Bleomycin-Producing Streptomyces Verticillus and a Methicillin-Resistant Staphylococcus Aureus in Escherichia Coli and Their Immunological Characterisation. FEBS Lett. 1995, 362, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Durán-Manuel, E.M.; Cruz-Cruz, C.; Ibáñez-Cervantes, G.; Bravata-Alcantará, J.C.; Sosa-Hernández, O.; Delgado-Balbuena, L.; León-García, G.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Castro-Escarpulli, G.; et al. Clonal Dispersion of Acinetobacter Baumannii in an Intensive Care Unit Designed to Patients COVID-19. J. Infect. Dev. Ctries. 2021, 15, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.X.; Wang, C.Y.; Li, Y.Y.; Li, J.; Wan, Q.Q.; Chen, J.H.; Tay, F.R.; Niu, L.N. Considerations and Caveats in Combating ESKAPE Pathogens against Nosocomial Infections. Adv. Sci. 2019, 7, 1901872. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Yam, E.L.Y.; Hsu, L.Y.; Yap, E.P.-H.; Yeo, T.W.; Lee, V.; Schlundt, J.; Lwin, M.O.; Limmathurotsakul, D.; Jit, M.; Dedon, P.; et al. Antimicrobial Resistance in the Asia Pacific Region: A Meeting Report. Antimicrob. Resist. Infect. Control 2019, 8. [Google Scholar] [CrossRef]
- Muhammed, M.T.; Aki-Yalcin, E. Homology Modeling in Drug Discovery: Overview, Current Applications, and Future Perspectives. Chem. Biol. Drug Des. 2018, 93, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S.; et al. Surveillance of Antimicrobial Resistance in Low- and Middle-Income Countries: A Scattered Picture. Antimicrob. Resist. Infect. Control 2021, 10. [Google Scholar] [CrossRef]
- Kållberg, C.; Årdal, C.; Salvesen Blix, H.; Klein, E.; Martinez, E.M.; Lindbæk, M.; Outterson, K.; Røttingen, J.-A.; Laxminarayan, R. Introduction and Geographic Availability of New Antibiotics Approved between 1999 and 2014. PLoS ONE 2018, 13, e0205166. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71. [Google Scholar] [CrossRef] [PubMed]
- Afshinnekoo, E.; Bhattacharya, C.; Burguete-García, A.; Castro-Nallar, E.; Deng, Y.; Desnues, C.; Dias-Neto, E.; Elhaik, E.; Iraola, G.; Jang, S.; et al. COVID-19 Drug Practices Risk Antimicrobial Resistance Evolution. Lancet Microbe 2021, 2, e135–e136. [Google Scholar] [CrossRef]
- World Health Organization Record Number of Countries Contribute Data Revealing Disturbing Rates of Antimicrobial Resistance. World Health Organization, Geneva. 2020. Available online: https://www.who.int/news-room/detail/01-06-2020-record-number-of-countries-contribute-data-revealing-disturbing-rates-of-antimicrobial-resistance (accessed on 1 June 2020).
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial Co-Infection and Secondary Infection in Patients with COVID-19: A Living Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2020, 26. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-Infections in People with COVID-19: A Systematic Review and Meta-Analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- WHO. Clinical Management of COVID-19. 2020. Available online: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed on 27 May 2020).
- WHO. Solidarity Clinical Trial for COVID-19 Treatments. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (accessed on 11 August 2021).
- Bork, J.T.; Leekha, S.; Claeys, K.; Seung, H.; Tripoli, M.; Amoroso, A.; Heil, E.L. Change in Hospital Antibiotic Use and Acquisition of Multidrug-Resistant Gram-Negative Organisms after the Onset of Coronavirus Disease 2019. Infect. Control Hosp. Epidemiol. 2020, 1–3. [Google Scholar] [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial Resistance and COVID-19: Intersections and Implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Huttner, B.D.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t Neglect Antimicrobial Stewardship Principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Cave, E. COVID-19 Super-Spreaders: Definitional Quandaries and Implications. Asian Bioeth. Rev. 2020, 12, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Taneja, N.; Sharma, M. Antimicrobial Resistance in the Environment: The Indian Scenario. Indian J. Med. Res. 2019, 149, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Nwaiwu, O.; Aduba, C.C. An in silico analysis of acquired antimicrobial resistance genes in Aeromonas plasmids. AIMS Microbiol. 2020, 6, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Panja, A.S.; Sarkar, A.; Biswas, R.; Bandyopadhyay, B.; Bandopadhyay, R. Modification of Drug-Binding Proteins Associated with the Efflux Pump in MDR-MTB in Course of Evolution: An Unraveled Clue Based on in Silico Approach. J. Antibiot. 2019, 72, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Feldgarden, M.; Brover, V.; Gonzalez-Escalona, N.; Frye, J.G.; Haendiges, J.; Haft, D.H.; Hoffmann, M.; Pettengill, J.B.; Prasad, A.B.; Tillman, G.E.; et al. AMRFinderPlus and the Reference Gene Catalog Facilitate Examination of the Genomic Links among Antimicrobial Resistance, Stress Response, and Virulence. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Rodrigues, G.L.; Panzenhagen, P.; Ferrari, R.G.; dos Santos, A.; Paschoalin, V.M.F.; Conte-Junior, C.A. Frequency of Antimicrobial Resistance Genes in Salmonella from Brazil by in Silico Whole-Genome Sequencing Analysis: An Overview of the Last Four Decades. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]

| Antibiotics | Mode of Action | Mechanism of Resistance | Examples | Reference |
|---|---|---|---|---|
| ß-Lactams (Cephalosporin, Carbapenems, etc.) | Peptidoglycan biosynthesis Cell wall synthesis Inhibition | Hydrolysis, efflux, altered target, reduced permeability, inactivation of antibiotics via ß-lactamase (extended spectrum ß-lactamase; carbapenem-hydrolyzing ß-lactamase) | Staphylococcus aureus, Pseudomonas aeruginosa, Enteric bacteria, Streptococcus pneumoniae, Vibrio cholerae, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii | [12,30,42,43,44,45] |
| Aminoglycosides (Gentamicin, Streptomycin, Spectinomycin, Amikacin, Tobramycin, etc.) | Inhibition of Translation and cell membrane synthesis | Modifying enzyme inactivation by Phosphorylation (phosphorylase), acetylation (acetylase), nucleotidylation, efflux, altered target ribosomal binding site, decrease uptake by reducing permeability, other modifying enzymes includes acetyltransferases, adenyl transferases, phosphotransferases. | Enteric bacteria, Staphylococci, Streptococci, Bacteriodes, Pseudomonas aeruginosa, Vibrio cholerae, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, etc. | [12,30,42,43,44,45] |
| Glycopeptides (Vancomycin, Teicoplanin) | Peptidoglycan biosynthesis | Altered target | Enterococci, Lactobacilli, Staphylococcus haemolyticus, Enterococcus faecium, Enterococcus faecalis, etc. | [30,42,43,44,45] |
| Tetracyclines (Tigecycline, Minocycline, Doxycycline) | 30S ribosomal subunit | Monooxygenation, ABC efflux pump, ribosomal modification, tetracycline inactivating enzyme | Staphylococci, Streptococci, Enterococci, Enterobacteriaceae, Haemophilus, Listeria, Acinetobacter baumannii, etc. | [30,42,43,44,45] |
| Macrolides (Erythromycin, azithromycin) | Translation | Glycosylation, efflux, methylation of rRNA target | Streptococci, Enterococci, Staphylococci, Acinetobacter baumannii, etc. | [12,43,45] |
| Phenicols (Chloramphenicol, Azidamphenicol, Thiamphenicol) | Translation inhibitors | Acetylation by chloramphenicol acetyltransferase, efflux pump, target site alteration | Bacillus subtilis, Streptococcus pneumoniae, Enterobacteriaceae, Haemophilus influenzae, Vibrio cholerae, Escherichia coli etc. | [12,42,43,44,45] |
| Folate inhibitors (Trimethoprim, Sulfamethoxazole) | Inhibit folate synthesis pathways | Efflux, altered target | Staphylococci, Streptococci, Enterobacteriaceae, Neisseria, Acinetobacter baumannii, etc. | [30,45] |
| Rifamycins (Rifampin) | Transcription | ADP-ribosylation, efflux, altered DNA-dependent RNA target | Enteric bacteria, Staphylococci, Streptococci, Mycobacterium tuberculosis, Vibrio cholerae, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, etc. | [12,30,45] |
| Quinolone (nalidixic acid, ciprofloxacin, levofloxacin, ofloxacin, norfloxacin) | Inhibitors of DNA synthesis Topoisomerase I and II | Altered DNA gyrase or DNA topoisomerase IV subunit A (parC) efflux or reduced permeability | Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus epidermis, Streptococcus pneumoniae Acinetobacter baumannii etc. | [12,30,42,43,44,45] |
| Cationic peptides (Colistin, Polymyxin-B) | Cell membrane Lipopolysaccahride layer of bacteria | Altered target, efflux | Escherichia coli, Salmonella Typhimurium, Acinetobacter baumannii, etc. | [30,42,45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, M.; Sarkar, A. Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. J. Xenobiot. 2021, 11, 197-214. https://doi.org/10.3390/jox11040013
Saha M, Sarkar A. Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. Journal of Xenobiotics. 2021; 11(4):197-214. https://doi.org/10.3390/jox11040013
Chicago/Turabian StyleSaha, Mousumi, and Agniswar Sarkar. 2021. "Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century" Journal of Xenobiotics 11, no. 4: 197-214. https://doi.org/10.3390/jox11040013
APA StyleSaha, M., & Sarkar, A. (2021). Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. Journal of Xenobiotics, 11(4), 197-214. https://doi.org/10.3390/jox11040013