The Impact of Endocrine-Disrupting Chemicals in Male Fertility: Focus on the Action of Obesogens
Abstract
1. Introduction
2. Brief History of Endocrine-Disrupting Chemicals
3. Endocrine Disruptors as Obesogens: Evidence from Basic and Clinical Studies
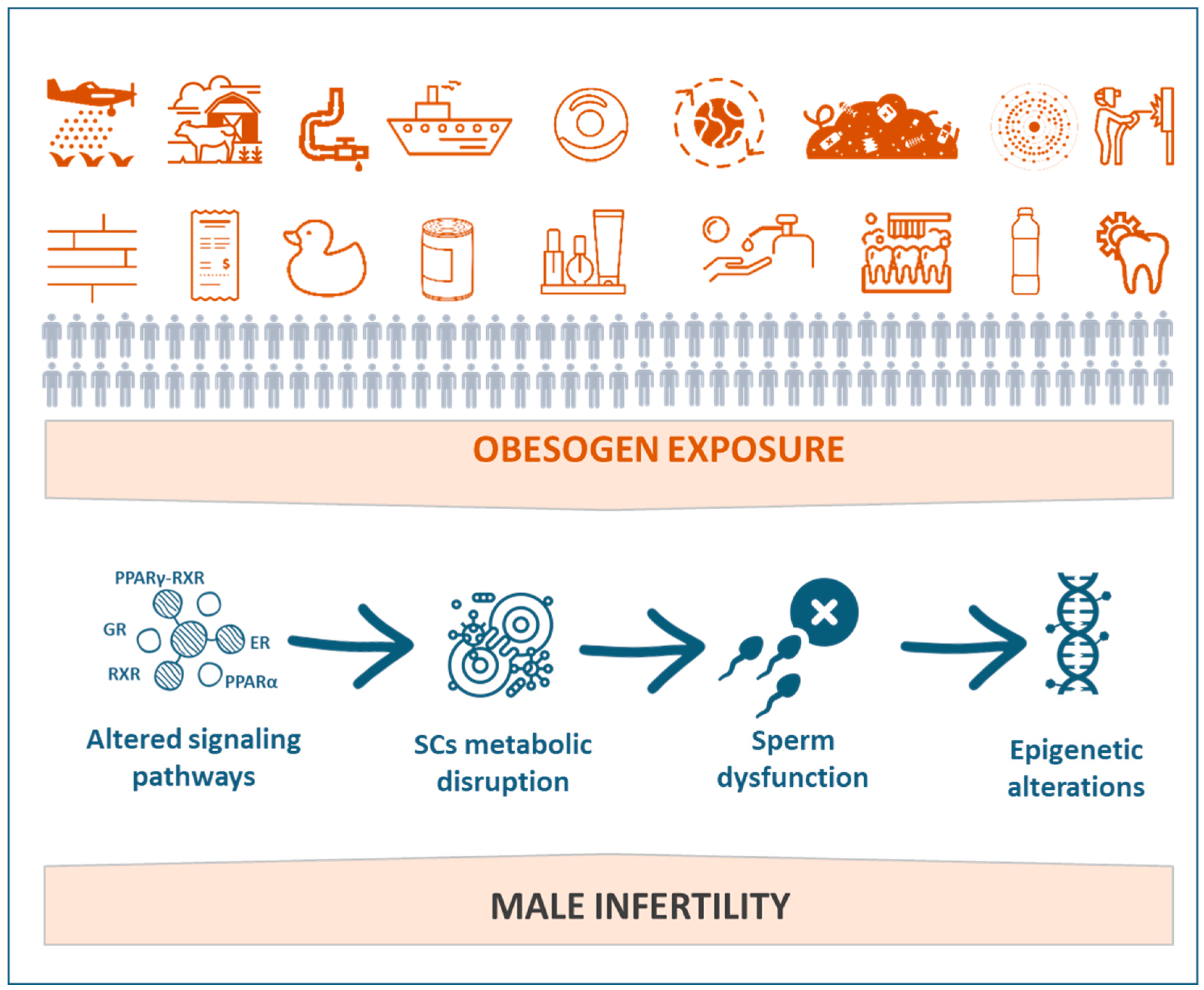
4. Obesogens as a Threat to Male Fertility
5. Reproductive Axis at the Interface with Environmental Obesogens
6. Leydig Cells Are a Sensitive Target of Obesogens
7. Mechanisms Mediating Obesogen-Related Sertoli Cell Dysfunction: A Metabolic Standpoint
8. How Can Germ Cells Be Affected by Obesogens?
9. Epigenetic Changes in Germ Cells
10. Obesogens Compromise Sperm Quality and Sperm Function-Related Events
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.; Soto, A.; Woodruff, T.; Vom Saal, F. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Bergman, A.; Heindel, J.J.; Kasten, T.; Kidd, K.A.; Jobling, S.; Neira, M.; Zoeller, R.T.; Becher, G.; Bjerregaard, P.; Bornman, R.; et al. The impact of endocrine disruption: A consensus statement on the state of the science. Environ. Health Perspect. 2013, 121, A104–A106. [Google Scholar] [CrossRef]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef]
- Grun, F.; Watanabe, H.; Zamanian, Z.; Maeda, L.; Arima, K.; Cubacha, R.; Gardiner, D.M.; Kanno, J.; Iguchi, T.; Blumberg, B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006, 20, 2141–2155. [Google Scholar] [CrossRef]
- Grün, F.; Blumberg, B. Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006, 147, s50–s55. [Google Scholar] [CrossRef]
- Trasande, L.; Zoeller, R.T.; Hass, U.; Kortenkamp, A.; Grandjean, P.; Myers, J.P.; DiGangi, J.; Bellanger, M.; Hauser, R.; Legler, J.; et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J. Clin. Endocrinol. Metab. 2015, 100, 1245–1255. [Google Scholar] [CrossRef]
- Trasande, L. Sicker, Fatter, Poorer: The Urgent Threat of Hormone-Disrupting Chemicals on Our Health and Future... and What We Can Do about It; Houghton Mifflin: Boston, MA, USA, 2019. [Google Scholar]
- Bruce, B.; Kristin, L. The Obesogen Effect, 1st ed.; Grand Central Life & Style: New York, NY, USA, 2018; p. 307. [Google Scholar]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Pu, Y.; Gingrich, J.; Padmanabhan, V. Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends Endocrinol. Metab. 2018, 29, 607–625. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Garcia, R.; Blumberg, B. Current Research Approaches and Challenges in the Obesogen Field. Front. Endocrinol. 2019, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J. History of the Obesogen Field: Looking Back to Look Forward. Front. Endocrinol. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Legler, J.; Fletcher, T.; Govarts, E.; Porta, M.; Blumberg, B.; Heindel, J.J.; Trasande, L. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1278–1288. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol 2011, 7, 346–353. [Google Scholar] [CrossRef]
- Kuo, C.C.; Moon, K.; Thayer, K.A.; Navas-Acien, A. Environmental chemicals and type 2 diabetes: An updated systematic review of the epidemiologic evidence. Curr. Diab. Rep. 2013, 13, 831–849. [Google Scholar] [CrossRef]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Fallara, G.; Cazzaniga, W.; Boeri, L.; Capogrosso, P.; Candela, L.; Pozzi, E.; Belladelli, F.; Schifano, N.; Ventimiglia, E.; Abbate, C. Male factor infertility trends throughout the last 10 years: Report from a tertiary-referral academic andrology centre. Andrology 2021, 9, 610–617. [Google Scholar] [CrossRef]
- Rodprasert, W.; Main, K.M.; Toppari, J.; Virtanen, H.E. Associations between male reproductive health and exposure to endocrine-disrupting chemicals. Curr. Opin. Endocr. Metab. Res. 2019, 7, 49–61. [Google Scholar] [CrossRef]
- Wong, E.W.; Cheng, C.Y. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol. Sci. 2011, 32, 290–299. [Google Scholar] [CrossRef]
- Mathur, P.P.; D’Cruz, S.C. The effect of environmental contaminants on testicular function. Asian J. Androl. 2011, 13, 585–591. [Google Scholar] [CrossRef]
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef] [PubMed]
- Kelce, W.R.; Wilson, E.M. Environmental antiandrogens: Developmental effects, molecular mechanisms, and clinical implications. J. Mol. Med. 1997, 75, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Sohoni, P.; Sumpter, J. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef]
- Phillips, K.P.; Foster, W.G. Key developments in endocrine disrupter research and human health. J. Toxicol. Environ. Health B 2008, 11, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.A.; Rice, S. Endocrine-disrupting chemicals as modulators of sex steroid synthesis. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.T.; Radwan, A.H.; Sorour, F.; El Okazy, A.; El-Agamy, E.-S.; El-Sebae, A.E.-K. Chlorpyrifos induced reproductive toxicity in male mice. Reprod. Toxicol. 2010, 29, 80–85. [Google Scholar] [CrossRef]
- Han, X.D.; Tu, Z.G.; Gong, Y.; Shen, S.N.; Wang, X.Y.; Kang, L.N.; Hou, Y.Y.; Chen, J.X. The toxic effects of nonylphenol on the reproductive system of male rats. Reprod. Toxicol. 2004, 19, 215–221. [Google Scholar] [CrossRef]
- Lee, B.-J.; Jung, E.-Y.; Yun, Y.-W.; Kang, J.-K.; Baek, I.-J.; Yon, J.-M.; Lee, Y.-B.; Sohn, H.-S.; Lee, J.-Y.; Kim, K.-S. Effects of exposure to genistein during pubertal development on the reproductive system of male mice. J. Reprod. Dev. 2004, 50, 399–409. [Google Scholar] [CrossRef][Green Version]
- McNutt, S.H.; Purwin, P.; Murray, C. Vulvo-vaginitis in swine: Preliminary report. J. Am. Vet. Med. Assoc. 1928, 73, 484. [Google Scholar]
- Bennetts, H.W.; Underwood, E.J.; Shier, F.L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. Vet. J. 1946, 22, 2–12. [Google Scholar] [CrossRef]
- Gassner, F.X.; Reifenstein, E.C., Jr.; Algeo, J.W.; Mattox, W.E. Effects of hormones on growth, fattening, and meat production potential of livestock. Recent Prog. Horm. Res. 1958, 14, 183–210; discussion 210–217. [Google Scholar] [PubMed]
- Department of Health, Environmental Assessment Worksheet. The Advancement of Medical Research and Education through the Department of Health, Education, and Welfare. Final Report of the Secretary’s Consultants of Medical Research Education; Department of Health: Washington, DC, USA, 1958. [Google Scholar]
- Aulerich, R.J.; Ringer, R.K.; Iwamoto, S. Reproductive failure and mortality in mink fed on Great Lakes fish. J. Reprod. Fertil. Suppl. 1973, 19, 365–376. [Google Scholar] [PubMed]
- Aulerich, R.J.; Ringer, R.K. Current status of PCB toxicity to mink, and effect on their reproduction. Arch. Environ. Contam. Toxicol. 1977, 6, 279–292. [Google Scholar] [CrossRef]
- Gilbertson, M.; Reynolds, L.M. Hexachlorobenzene (HCB) in the eggs of common terns in Hamilton Harbour, Ontario. Bull. Environ. Contam. Toxicol. 1972, 7, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Kioumourtzoglou, M.A.; Coull, B.A.; O’Reilly, E.J.; Ascherio, A.; Weisskopf, M.G. Association of Exposure to Diethylstilbestrol During Pregnancy with Multigenerational Neurodevelopmental Deficits. JAMA Pediatrics 2018, 172, 670–677. [Google Scholar] [CrossRef]
- Huo, D.; Anderson, D.; Herbst, A.L. Follow-up of Patients with Clear-Cell Adenocarcinoma of the Vagina and Cervix. N. Engl. J. Med. 2018, 378, 1746–1748. [Google Scholar] [CrossRef]
- McLachlan, J. Estrogens in the Environment; Elsevier Inc.: New York, NY, USA, 1980. [Google Scholar]
- Colborn, T.; Clement, C. Chemically-Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection; Princeton Scientific Pub. Co.: Princeton, NJ, USA, 1992. [Google Scholar]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ Br. Med. J. 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. The Impacts of Endocrine Disrupters on Wildlife, People and Their Environments. The Weybridge + 15 (1996–2011) Report; Publications Office of the European Union: Copenhagen, Denmark, 2012. [Google Scholar]
- Hotchkiss, A.K.; Rider, C.V.; Blystone, C.R.; Wilson, V.S.; Hartig, P.C.; Ankley, G.T.; Foster, P.M.; Gray, C.L.; Gray, L.E. Fifteen years after “Wingspread”—environmental endocrine disrupters and human and wildlife health: Where we are today and where we need to go. Toxicol. Sci. 2008, 105, 235–259. [Google Scholar] [CrossRef]
- Damstra, T.; Barlow, S.; Bergman, A.; Kavlock, R.; Van Der Kraak, G. Global Assessment of the State-of-the-Science of Endocrine Disruptors; World Health Organization: Geneva, Switzerland, 2002; pp. 11–32. [Google Scholar]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Bergman, Å.; Heindel, J.J.; Jobling, S.; Kidd, K.; Zoeller, T.R. State of the Science of Endocrine Disrupting Chemicals 2012: Summary for Decision-Makers; W.H.O.: Geneva, Switzerland, 2013. [Google Scholar]
- Heindel, J.J.; Vom Saal, F.S.; Blumberg, B.; Bovolin, P.; Calamandrei, G.; Ceresini, G.; Cohn, B.A.; Fabbri, E.; Gioiosa, L.; Kassotis, C.; et al. Parma consensus statement on metabolic disruptors. Environ. Health 2015, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.S.; Schug, T.T.; Heindel, J.J.; Blumberg, B. Environmental Chemicals and Obesity. In Handbook of Obesity-Epidemiology, Etiology, and Physiopathology; Bray, G.A., Bouchard, C., Eds.; CRC Press: Boca Raton, FL, USA, 2014; Volume 1, pp. 471–488. [Google Scholar]
- Lee, D.H.; Porta, M.; Jacobs, D.R., Jr.; Vandenberg, L.N. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr. Rev. 2014, 35, 557–601. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.; Blumberg, B. Minireview: PPARγ as the target of obesogens. J. Steroid Biochem. Mol. Biol. 2011, 127, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.S.; Dimastrogiovanni, G.; Vanek, L.; Boulos, C.; Chamorro-García, R.; Tang, W.; Blumberg, B. On the utility of ToxCast™ and ToxPi as methods for identifying new obesogens. Environ. Health Perspect. 2016, 124, 1214. [Google Scholar] [CrossRef] [PubMed]
- Grun, F.; Blumberg, B. Minireview: The case for obesogens. Mol. Endocrinol. 2009, 23, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Le Maire, A.; Grimaldi, M.; Roecklin, D.; Dagnino, S.; Vivat-Hannah, V.; Balaguer, P.; Bourguet, W. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009, 10, 367–373. [Google Scholar] [CrossRef]
- Kanayama, T.; Kobayashi, N.; Mamiya, S.; Nakanishi, T.; Nishikawa, J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol. Pharmacol. 2005, 67, 766–774. [Google Scholar] [CrossRef]
- Fang, L.; Xu, C.; Li, J.; Borggaard, O.K.; Wang, D. The importance of environmental factors and matrices in the adsorption, desorption, and toxicity of butyltins: A review. Environ. Sci. Pollut. Res. Int. 2017, 24, 9159–9173. [Google Scholar] [CrossRef]
- Brtko, J.; Dvorak, Z. Triorganotin compounds--ligands for rexinoid inducible transcription factors: Biological effects. Toxicol. Lett. 2015, 234, 50–58. [Google Scholar] [CrossRef]
- Sousa, A.C.A.; Tanabe, S.; Pastorinho, M.R. Organotins: Sources and Impacts on Health and Environment A2. In Encyclopedia on Anthropocene; DellaSala, D.A., Goldstein, M.I., Eds.; Elsevier: Oxford, UK, 2018; Volume 1, pp. 133–139. [Google Scholar]
- Nielsen, J.B.; Strand, J. Butyltin compounds in human liver. Environ. Res. 2002, 88, 129–133. [Google Scholar] [CrossRef]
- Rantakokko, P.; Main, K.M.; Wohlfart-Veje, C.; Kiviranta, H.; Airaksinen, R.; Vartiainen, T.; Skakkebaek, N.E.; Toppari, J.; Virtanen, H.E. Association of placenta organotin concentrations with congenital cryptorchidism and reproductive hormone levels in 280 newborn boys from Denmark and Finland. Hum. Reprod. 2013, 28, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Rantakokko, P.; Main, K.M.; Wohlfart-Veje, C.; Kiviranta, H.; Airaksinen, R.; Vartiainen, T.; Skakkebaek, N.E.; Toppari, J.; Virtanen, H.E. Association of placenta organotin concentrations with growth and ponderal index in 110 newborn boys from Finland during the first 18 months of life: A cohort study. Environ. Health 2014, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Rantakokko, P.; Turunen, A.; Verkasalo, P.K.; Kiviranta, H.; Mannisto, S.; Vartiainen, T. Blood levels of organotin compounds and their relation to fish consumption in Finland. Sci. Total Environ. 2008, 399, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Mukai, H.; Tanabe, S.; Sakayama, K.; Miyazaki, T.; Masuno, H. Butyltin residues in livers of humans and wild terrestrial mammals and in plastic products. Environ. Pollut. 1999, 106, 213–218. [Google Scholar] [CrossRef]
- Sousa, A.C.; Pastorinho, M.R.; Takahashi, S.; Tanabe, S. History on organotin compounds, from snails to humans. Environ. Chem. Lett. 2014, 12, 117–137. [Google Scholar] [CrossRef]
- Shoucri, B.M.; Martinez, E.S.; Abreo, T.J.; Hung, V.T.; Moosova, Z.; Shioda, T.; Blumberg, B. Retinoid X Receptor Activation Alters the Chromatin Landscape to Commit Mesenchymal Stem Cells to the Adipose Lineage. Endocrinology 2017, 158, 3109–3125. [Google Scholar] [CrossRef]
- Asakawa, H.; Tsunoda, M.; Kaido, T.; Hosokawa, M.; Sugaya, C.; Inoue, Y.; Kudo, Y.; Satoh, T.; Katagiri, H.; Akita, H.; et al. Enhanced inhibitory effects of TBT chloride on the development of F1 rats. Arch. Environ. Contam. Toxicol. 2010, 58, 1065–1073. [Google Scholar] [CrossRef]
- Hao, C.; Cheng, X.; Xia, H.; Ma, X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci. Rep. 2012, 32, 619–629. [Google Scholar] [CrossRef]
- Biemann, R.; Navarrete Santos, A.; Navarrete Santos, A.; Riemann, D.; Knelangen, J.; Bluher, M.; Koch, H.; Fischer, B. Endocrine disrupting chemicals affect the adipogenic differentiation of mesenchymal stem cells in distinct ontogenetic windows. Biochem. Biophys. Res. Commun. 2012, 417, 747–752. [Google Scholar] [CrossRef]
- Thomas, P.; Dong, J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef]
- Watson, C.S.; Bulayeva, N.N.; Wozniak, A.L.; Alyea, R.A. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids 2007, 72, 124–134. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Masuno, H.; Iwanami, J.; Kidani, T.; Sakayama, K.; Honda, K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol. Sci. 2005, 84, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, Q.; Sakthivel, S.; Pavithran, P.V.; Vasukutty, J.R.; Kannan, K. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ. Res. 2015, 137, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Overby, H.; Heal, E.; Wang, S.; Chen, J.; Shen, C.L.; Zhao, L. Methylparaben and butylparaben alter multipotent mesenchymal stem cell fates towards adipocyte lineage. Toxicol. Appl. Pharmacol. 2017, 329, 48–57. [Google Scholar] [CrossRef]
- Kodani, S.D.; Overby, H.B.; Morisseau, C.; Chen, J.; Zhao, L.; Hammock, B.D. Parabens inhibit fatty acid amide hydrolase: A potential role in paraben-enhanced 3T3-L1 adipocyte differentiation. Toxicol. Lett. 2016, 262, 92–99. [Google Scholar] [CrossRef]
- Hu, P.; Chen, X.; Whitener, R.J.; Boder, E.T.; Jones, J.O.; Porollo, A.; Chen, J.; Zhao, L. Effects of parabens on adipocyte differentiation. Toxicol. Sci. 2013, 131, 56–70. [Google Scholar] [CrossRef]
- Ferrante, M.C.; Amero, P.; Santoro, A.; Monnolo, A.; Simeoli, R.; Di Guida, F.; Mattace Raso, G.; Meli, R. Polychlorinated biphenyls (PCB 101, PCB 153 and PCB 180) alter leptin signaling and lipid metabolism in differentiated 3T3-L1 adipocytes. Toxicol. Appl. Pharmacol. 2014, 279, 401–408. [Google Scholar] [CrossRef]
- Howell, G., 3rd; Mangum, L. Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol. Vitr. 2011, 25, 394–402. [Google Scholar] [CrossRef]
- Penza, M.; Montani, C.; Romani, A.; Vignolini, P.; Pampaloni, B.; Tanini, A.; Brandi, M.; Alonso-Magdalena, P.; Nadal, A.; Ottobrini, L. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology 2006, 147, 5740–5751. [Google Scholar] [CrossRef]
- Ruhlen, R.L.; Howdeshell, K.L.; Mao, J.; Taylor, J.A.; Bronson, F.H.; Newbold, R.R.; Welshons, W.V.; vom Saal, F.S. Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the fetal estrogenization syndrome and obesity in CD-1 mice. Environ. Health Perspect. 2008, 116, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Chen, S.; Wu, T.; Zhang, J.; Su, Y.; Chen, Y.; Wang, C. Tributyltin causes obesity and hepatic steatosis in male mice. Environ. Toxicol. 2011, 26, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Silha, J.V.; Krsek, M.; Skrha, J.V.; Sucharda, P.; Nyomba, B.L.; Murphy, L.J. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: Correlations with insulin resistance. Eur. J. Endocrinol. 2003, 149, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Degawa-Yamauchi, M.; Bovenkerk, J.E.; Juliar, B.E.; Watson, W.; Kerr, K.; Jones, R.; Zhu, Q.; Considine, R.V. Serum resistin (FIZZ3) protein is increased in obese humans. J. Clin. Endocrinol. Metab. 2003, 88, 5452–5455. [Google Scholar] [CrossRef]
- Penza, M.; Jeremic, M.; Marrazzo, E.; Maggi, A.; Ciana, P.; Rando, G.; Grigolato, P.G.; Di Lorenzo, D. The environmental chemical tributyltin chloride (TBT) shows both estrogenic and adipogenic activities in mice which might depend on the exposure dose. Toxicol. Appl. Pharmacol. 2011, 255, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Vitalone, A.; Catalani, A.; Cinque, C.; Fattori, V.; Matteucci, P.; Zuena, A.R.; Costa, L.G. Long-term effects of developmental exposure to low doses of PCB 126 and methylmercury. Toxicol. Lett. 2010, 197, 38–45. [Google Scholar] [CrossRef]
- Marth, P.C.; Mitchell, J.W. 2, 4-Dichlorophenoxyacetic acid as a differential herbicide. Botanical. Gazette. 1944, 106, 224–232. [Google Scholar] [CrossRef]
- Cook, J.; Hewett, C.; Hieger, I. The isolation ofa cancer-producing hydrocarbon from coal tar. Parts, I, II and III. J. Chem. Soc. 1933, 1, 395–405. [Google Scholar] [CrossRef]
- Conix, A. Thermoplastic polyesters from bisphenols. Ind. Eng. Chem. 1959, 51, 147–150. [Google Scholar] [CrossRef]
- Gray, H. DURSBAN—A new organo-phosphorus insecticide. Down Earth 1965, 21, 2. [Google Scholar]
- Hansens, E.J.; Bartley, C. Three new Insecticides for Housefly Control in Barns. J. Econ. Entomol. 1953, 46, 372–374. [Google Scholar] [CrossRef]
- Burroughs, W.; Culbertson, C.; Cheng, E.; Hale, W.; Homeyer, P. The influence of oral administration of diethylstilbestrol to beef cattle. J. Anim. Sci. 1955, 14, 1015–1024. [Google Scholar] [CrossRef]
- Dubrunfaut, A.P. Sur une propriété analytique des fermentations alcoolique et lactique, et sur leur application à l’étude des sucres. Ann. Chim. Phys. 1847, 21, 169–178. [Google Scholar]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.-I.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [CrossRef]
- Bacon, A.; Froome, K.; Gent, A.; Cooke, T.; Sowerby, P. Lead poisoning from drinking soft water. Lancet 1967, 289, 264–266. [Google Scholar] [CrossRef]
- Ikeda, K. On the taste of the salt of glutamic acid. Proc. 8th Int. Congr. Appl. Chem. 1912, 38, 147. [Google Scholar]
- Posselt, W.; Reimann, L. Chemische Untersuchungen des Tabaks und Darstellung des eigenhumlichen wirksamen Principes dieser Pflanze. Geigers Magazin der Pharmazie 1828, 24, 138–161. [Google Scholar]
- Leppert, B.; Strunz, S.; Seiwert, B.; Schlittenbauer, L.; Schlichting, R.; Pfeiffer, C.; Röder, S.; Bauer, M.; Borte, M.; Stangl, G.I.; et al. Maternal paraben exposure triggers childhood overweight development. Nat. Commun. 2020, 11, 561. [Google Scholar] [CrossRef]
- Creighton, J.T.; Gresham, W.B. Parathion for control of green peach aphid on shade-grown tobacco. J. Econ. Entomol. 1947, 40, 915–917. [Google Scholar] [CrossRef]
- De Kok, J.; De Kok, A.; Brinkman, U.T. Analysis of polybrominated aromatic ethers. J. Chromatogr. A 1979, 171, 269–278. [Google Scholar] [CrossRef]
- Gauger, K.J.; Kato, Y.; Haraguchi, K.; Lehmler, H.-J.; Robertson, L.W.; Bansal, R.; Zoeller, R.T. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ. Health Perspect. 2004, 112, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, F.D.; Mandel, J.S. Serum perfluorooctanoic acid and hepatic enzymes, lipoproteins, and cholesterol: A study of occupationally exposed men. Am. J. Ind. Med. 1996, 29, 560–568. [Google Scholar] [CrossRef]
- Hatch, E.E.; Nelson, J.W.; Qureshi, M.M.; Weinberg, J.; Moore, L.L.; Singer, M.; Webster, T.F. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: A cross-sectional study of NHANES data, 1999–2002. Environ. Health 2008, 7, 1–15. [Google Scholar] [CrossRef]
- Meneses, M.J.; Bernardino, R.L.; Sa, R.; Silva, J.; Barros, A.; Sousa, M.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Pioglitazone increases the glycolytic efficiency of human Sertoli cells with possible implications for spermatogenesis. Int. J. Biochem. Cell Biol. 2016, 79, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Kaneko, S.; Hayabuchi, H. Sex ratio in offspring of those affected by dioxin and dioxin-like compounds: The Yusho, Seveso, and Yucheng incidents. Occup. Environ. Med. 2001, 58, 540–541. [Google Scholar] [CrossRef]
- Caputo, R.; Monti, M.; Ermacora, E.; Carminati, G.; Gelmetti, C.; Gianotti, R.; Gianni, E.; Puccinelli, V. Cutaneous manifestations of tetrachlorodibenzo-p-dioxin in children and adolescents: Follow-up 10 years after the Seveso, Italy, accident. J. Am. Acad. Dermatol. 1988, 19, 812–819. [Google Scholar] [CrossRef]
- Carwile, J.L.; Michels, K.B. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. 2011, 111, 825–830. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Urinary bisphenol a levels and measures of obesity: Results from the national health and nutrition examination survey 2003–2008. ISRN Endocrinol. 2012, 2012, 965243. [Google Scholar] [CrossRef]
- Valvi, D.; Mendez, M.A.; Garcia-Esteban, R.; Ballester, F.; Ibarluzea, J.; Goni, F.; Grimalt, J.O.; Llop, S.; Marina, L.S.; Vizcaino, E.; et al. Prenatal exposure to persistent organic pollutants and rapid weight gain and overweight in infancy. Obesity Silver Spring 2014, 22, 488–496. [Google Scholar] [CrossRef]
- Mendez, M.A.; Garcia-Esteban, R.; Guxens, M.; Vrijheid, M.; Kogevinas, M.; Goni, F.; Fochs, S.; Sunyer, J. Prenatal organochlorine compound exposure, rapid weight gain, and overweight in infancy. Environ. Health Perspect. 2011, 119, 272–278. [Google Scholar] [CrossRef]
- Lind, P.M.; Roos, V.; Ronn, M.; Johansson, L.; Ahlstrom, H.; Kullberg, J.; Lind, L. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environ. Health 2012, 11, 21. [Google Scholar] [CrossRef]
- Tang-Péronard, J.L.; Heitmann, B.L.; Andersen, H.R.; Steuerwald, U.; Grandjean, P.; Weihe, P.; Jensen, T.K. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: A prospective cohort study of 656 children from the Faroe Islands. Am. Clin. Nutr. 2013, 99, 5–13. [Google Scholar] [CrossRef]
- Larsen, T.; Toubro, S.; Astrup, A. PPARgamma agonists in the treatment of type II diabetes: Is increased fatness commensurate with long-term efficacy? Int. J. Obes. 2003, 27, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Rubenstrunk, A.; Hanf, R.; Hum, D.W.; Fruchart, J.-C.; Staels, B. Safety issues and prospects for future generations of PPAR modulators. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 1065–1081. [Google Scholar] [CrossRef]
- Legler, J. An integrated approach to assess the role of chemical exposure in obesity. Obesity Silver Spring 2013, 21, 1084–1085. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, D.A. Decrease in eggshell weight in certain birds of prey. Nature 1967, 215, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Zoeller, R.T.; Hass, U.; Kortenkamp, A.; Grandjean, P.; Myers, J.P.; DiGangi, J.; Hunt, P.M.; Rudel, R.; Sathyanarayana, S.; et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: An updated analysis. Andrology 2016, 4, 565–572. [Google Scholar] [CrossRef]
- Attina, T.M.; Hauser, R.; Sathyanarayana, S.; Hunt, P.A.; Bourguignon, J.P.; Myers, J.P.; DiGangi, J.; Zoeller, R.T.; Trasande, L. Exposure to endocrine-disrupting chemicals in the USA: A population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016, 4, 996–1003. [Google Scholar] [CrossRef]
- Reame, V.; Pytlowanciv, E.Z.; Ribeiro, D.L.; Pissolato, T.F.; Taboga, S.R.; Góes, R.M.; Pinto-Fochi, M.E. Obesogenic environment by excess of dietary fats in different phases of development reduces spermatic efficiency of Wistar rats at adulthood: Correlations with metabolic status. Biol. Reprod. 2014, 91, 151–161. [Google Scholar] [CrossRef]
- Wan, H.-T.; Mruk, D.D.; Wong, C.K.; Cheng, C.Y. The apical ES–BTB–BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol. Med. 2013, 19, 396–405. [Google Scholar] [CrossRef][Green Version]
- Akingbemi, B.T.; Youker, R.T.; Sottas, C.M.; Ge, R.; Katz, E.; Klinefelter, G.R.; Zirkin, B.R.; Hardy, M.P. Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol. Reprod. 2001, 65, 1252–1259. [Google Scholar] [CrossRef]
- Alves, M.; Neuhaus-Oliveira, A.; Moreira, P.; Socorro, S.; Oliveira, P. Exposure to 2, 4-dichlorophenoxyacetic acid alters glucose metabolism in immature rat Sertoli cells. Reprod. Toxicol. 2013, 38, 81–88. [Google Scholar] [CrossRef]
- D’Cruz, S.C.; Jubendradass, R.; Jayakanthan, M.; Rani, S.J.A.; Mathur, P.P. Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: An in vivo and in silico study. Food Chem. Toxicol. 2012, 50, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.J.; Cook, M.W.; Thomas, L.V.; Gray, T.J. The effect of mono-(2-ethylhexyl) phthalate and other phthalate esters on lactate production by Sertoli cells in vitro. Toxicol. Lett. 1988, 40, 77–84. [Google Scholar] [CrossRef]
- D’Cruz, S.C.; Jubendradass, R.; Mathur, P.P. Bisphenol A induces oxidative stress and decreases levels of insulin receptor substrate 2 and glucose transporter 8 in rat testis. Reprod. Sci. 2012, 19, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Sai, L.; Li, X.; Liu, Y.; Guo, Q.; Xie, L.; Yu, G.; Bo, C.; Zhang, Z.; Li, L. Effects of chlorpyrifos on reproductive toxicology of male rats. Environ. Toxicol. 2014, 29, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, L.I.; Welsh, M.J.; Brabec, M.J. Effect of lead acetate on Sertoli cell lactate production and protein synthesis in vitro. Cell Biol. Toxicol. 1986, 2, 283–292. [Google Scholar] [CrossRef]
- Raychoudhury, S.S.; Flowers, A.F.; Millette, C.F.; Finlay, M.F. Toxic effects of polychlorinated biphenyls on cultured rat Sertoli cells. J. Androl. 2000, 21, 964–973. [Google Scholar]
- Itsuki-Yoneda, A.; Kimoto, M.; Tsuji, H.; Hiemori, M.; Yamashita, H. Effect of a hypolipidemic drug, di (2-ethylhexyl) phthalate, on mRNA-expression associated fatty acid and acetate metabolism in rat tissues. Biosci. Biotechnol. Biochem. 2007, 71, 414–420. [Google Scholar] [CrossRef]
- Mitra, S.; Srivastava, A.; Khandelwal, S. Long term impact of the endocrine disruptor tributyltin on male fertility following a single acute exposure. Environ. Toxicol. 2017, 32, 2295–2304. [Google Scholar] [CrossRef]
- Mitra, S.; Srivastava, A.; Khanna, S.; Khandelwal, S. Consequences of tributyltin chloride induced stress in Leydig cells: An ex-vivo approach. Environ. Toxicol. Pharmacol. 2014, 37, 850–860. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Alves, M.G.; Sousa, A.C.; Jarak, I.; Carvalho, R.A.; Oliveira, P.F.; Cavaco, J.E.; Rato, L. The effects of the obesogen tributyltin on the metabolism of Sertoli cells cultured ex vivo. Arch. Toxicol. 2018, 92, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Srivastava, A.; Khandelwal, S. Tributyltin chloride induced testicular toxicity by JNK and p38 activation, redox imbalance and cell death in sertoli-germ cell co-culture. Toxicology 2013, 314, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Mruk, D.D. A local autocrine axis in the testes that regulates spermatogenesis. Nat. Rev. Endocrinol. 2010, 6, 380–395. [Google Scholar] [CrossRef]
- Walker, W.H.; Cheng, J. FSH and testosterone signaling in Sertoli cells. Reproduction 2005, 130, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Rommerts, F.F.; de Jong, F.H.; Brinkmann, A.O.; van der Molen, H.J. Development and cellular localization of rat testicular aromatase activity. J. Reprod. Fertil. 1982, 65, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front. Neuroendocrinol. 2008, 29, 358–374. [Google Scholar] [CrossRef]
- Gore, A.C. Organochlorine pesticides directly regulate gonadotropin-releasing hormone gene expression and biosynthesis in the GT1-7 hypothalamic cell line. Mol. Cell. Endocrinol. 2002, 192, 157–170. [Google Scholar] [CrossRef]
- Stoker, T.E.; Parks, L.G.; Gray, L.E.; Cooper, R.L. Endocrine-disrupting chemicals: Prepubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC Recommendations. Crit. Rev. Toxicol. 2000, 30, 197–252. [Google Scholar] [CrossRef]
- Welshons, W.V.; Nagel, S.C.; vom Saal, F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006, 147, S56–S69. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid. Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Gould, J.C.; Leonard, L.S.; Maness, S.C.; Wagner, B.L.; Conner, K.; Zacharewski, T.; Safe, S.; McDonnell, D.P.; Gaido, K.W. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol. Cell Endocrinol. 1998, 142, 203–214. [Google Scholar] [CrossRef]
- Wozniak, A.L.; Bulayeva, N.N.; Watson, C.S. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-α-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ. Health Perspect. 2005, 113, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Lee, C.K.; Yeung, W.S.; Giesy, J.P.; Wong, M.H.; Zhang, X.; Hecker, M.; Wong, C.K. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod. Toxicol. 2011, 31, 409–417. [Google Scholar] [CrossRef]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-da-Silva, M.R.; Romano, M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic–pituitary–testicular axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Kaiser, U. Gonadotropin-releasing hormone regulation of gonadotropin biosynthesis and secretion. In Knobil and Neill’s Physiology of Reproduction; Neill, J.D., Ed.; Elsevier: San Diego, CA, USA, 2006; Volume 1. [Google Scholar]
- Arase, S.; Ishii, K.; Igarashi, K.; Aisaki, K.; Yoshio, Y.; Matsushima, A.; Shimohigashi, Y.; Arima, K.; Kanno, J.; Sugimura, Y. Endocrine disrupter bisphenol A increases in situ estrogen production in the mouse urogenital sinus. Biol. Reprod. 2011, 84, 734–742. [Google Scholar] [CrossRef]
- Stahlhut, R.W.; van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult US males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Havrankova, J.; Schmechel, D.; Roth, J.; Brownstein, M. Identification of insulin in rat brain. Proc. Natl. Acad. Sci. USA 1978, 75, 5737–5741. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, E.L.; Albanna, G.; Frolova, A.I.; Moley, K.H. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes 2012, 61, 1869–1878. [Google Scholar] [CrossRef]
- Harada, Y.; Tanaka, N.; Ichikawa, M.; Kamijo, Y.; Sugiyama, E.; Gonzalez, F.J.; Aoyama, T. PPARα-dependent cholesterol/testosterone disruption in Leydig cells mediates 2, 4-dichlorophenoxyacetic acid-induced testicular toxicity in mice. Arch. Toxicol. 2016, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Barlow, N.J.; Phillips, S.L.; Wallace, D.G.; Sar, M.; Gaido, K.W.; Foster, P.M. Quantitative changes in gene expression in fetal rat testes following exposure to di (n-butyl) phthalate. Toxicol. Sci. 2003, 73, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.; Shen, W.-J.; Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 1–25. [Google Scholar] [CrossRef]
- Desdoits-Lethimonier, C.; Albert, O.; Le Bizec, B.; Perdu, E.; Zalko, D.; Courant, F.; Lesne, L.; Guille, F.; Dejucq-Rainsford, N.; Jegou, B. Human testis steroidogenesis is inhibited by phthalates. Hum. Reprod. 2012, 27, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.-Y. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef]
- Jin, P.; Wang, X.; Chang, F.; Bai, Y.; Li, Y.; Zhou, R.; Chen, L. Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J. Biomed. Res. 2013, 27, 135–144. [Google Scholar]
- Wilson, V.S.; Lambright, C.R.; Furr, J.R.; Howdeshell, K.L.; Gray, L.E. The herbicide linuron reduces testosterone production from the fetal rat testis during both in utero and in vitro exposures. Toxicol. Lett. 2009, 186, 73–77. [Google Scholar] [CrossRef]
- Lambright, C.; Ostby, J.; Bobseine, K.; Wilson, V.; Hotchkiss, A.; Mann, P.; Gray, L. Cellular and molecular mechanisms of action of linuron: An antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicol. Sci. 2000, 56, 389–399. [Google Scholar] [CrossRef]
- Wolf, C.; Lambright, C.; Mann, P.; Price, M.; Cooper, R.L.; Ostby, J.; Gray, L.E. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p, p′-DDE, and ketoconazole) and toxic substances (dibutyl-and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol. Ind. Health 1999, 15, 94–118. [Google Scholar]
- Hotchkiss, A.; Parks-Saldutti, L.; Ostby, J.; Lambright, C.; Furr, J.; Vandenbergh, J.; Gray, L. A mixture of the “antiandrogens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol. Reprod. 2004, 71, 1852–1861. [Google Scholar] [CrossRef]
- Ostby, J.; Monosson, E.; Kelce, W.R.; Gray, L.E. Environmental antiandrogens: Low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol. Ind. Health 1999, 15, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Monosson, E.; Kelce, W.R.; Lambright, C.; Ostby, J.; Gray, L.E. Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicol. Ind. Health 1999, 15, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-i.; Kelce, W.R.; Sar, M.; Wilson, E.M. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J. Biol. Chem. 1995, 270, 19998–20003. [Google Scholar] [CrossRef]
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793. [Google Scholar] [CrossRef]
- Rato, L.; Meneses, M.J.; Silva, B.M.; Sousa, M.; Alves, M.G.; Oliveira, P.F. New insights on hormones and factors that modulate Sertoli cell metabolism. Histol. Histopathol. 2016, 31, 499–513. [Google Scholar]
- Cardoso, A.M.; Alves, M.G.; Mathur, P.P.; Oliveira, P.F.; Cavaco, J.E.; Rato, L. Obesogens and male fertility. Obes. Rev. 2017, 18, 109–125. [Google Scholar] [CrossRef]
- Foster, P.; Foster, J.R.; Cook, M.W.; Thomas, L.V.; Gangolli, S.D. Changes in ultrastructure and cytochemical localization of zinc in rat testis following the administration of Di-n-pentyl phthalate. Toxicol. Appl. Pharmacol. 1982, 63, 120–132. [Google Scholar] [CrossRef]
- Li, M.W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int. J. Biochem. Cell Biol. 2009, 41, 2302–2314. [Google Scholar] [CrossRef] [PubMed]
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef]
- Gao, Y.; Mruk, D.D.; Cheng, C.Y. Sertoli cells are the target of environmental toxicants in the testis–a mechanistic and therapeutic insight. Expert Opin. Ther. Targets 2015, 19, 1073–1090. [Google Scholar] [CrossRef]
- Cheng, C.Y. Toxicants target cell junctions in the testis: Insights from the indazole-carboxylic acid model. Spermatogenesis 2014, 4, e981485. [Google Scholar] [CrossRef]
- Su, L.; Mruk, D.D.; Cheng, C.Y. Regulation of drug transporters in the testis by environmental toxicant cadmium, steroids and cytokines. Spermatogenesis 2012, 2, 285–293. [Google Scholar] [CrossRef][Green Version]
- Cheng, C.Y.; Wong, E.W.; Lie, P.P.; Li, M.W.; Su, L.; Siu, E.R.; Yan, H.H.; Mannu, J.; Mathur, P.P.; Bonanomi, M. Environmental toxicants and male reproductive function. Spermatogenesis 2011, 1, 2–13. [Google Scholar] [CrossRef]
- Xiao, X.; Mruk, D.D.; Tang, E.I.; Wong, C.K.; Lee, W.M.; John, C.M.; Turek, P.J.; Silvestrini, B.; Cheng, C.Y. Environmental toxicants perturb human Sertoli cell adhesive function via changes in F-actin organization mediated by actin regulatory proteins. Hum. Reprod. 2014, 29, 1279–1291. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Environmental contaminants: Is male reproductive health at risk? Spermatogenesis 2011, 1, 283–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliveira, P.F.; Alves, M.G. Sertoli Cell Metabolism and Spermatogenesis; Springer: Cham, Switzerland, 2015; p. 98. [Google Scholar]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Meroni, S.; Riera, M.; Pellizzari, E.; Cigorraga, S. Regulation of rat Sertoli cell function by FSH: Possible role of phosphatidylinositol 3-kinase/protein kinase B pathway. J. Endocrinol. 2002, 174, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Chehtane, M.; Khaled, A.R. Interleukin-7 mediates glucose utilization in lymphocytes through transcriptional regulation of the hexokinase II gene. Am. J. Physiol. Cell Physiol. 2010, 298, C1560–C1571. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Carvalho, R.A.; Cavaco, J.E.; Oliveira, P.F. Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci. Rep. 2012, 32, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Majors, B.S.; Betenbaugh, M.J.; Chiang, G.G. Links between metabolism and apoptosis in mammalian cells: Applications for anti-apoptosis engineering. Metab. Eng. 2007, 9, 317–326. [Google Scholar] [CrossRef]
- Yamada, S.; Kotake, Y.; Sekino, Y.; Kanda, Y. AMP-activated protein kinase-mediated glucose transport as a novel target of tributyltin in human embryonic carcinoma cells. Metallomics 2013, 5, 484–491. [Google Scholar] [CrossRef]
- Galardo, M.N.; Riera, M.F.; Pellizzari, E.H.; Cigorraga, S.B.; Meroni, S.B. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-bD-ribonucleoside, regulates lactate production in rat Sertoli cells. J. Mol. Endocrinol. 2007, 39, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.; Rajpert-De Meyts, E.; Main, K. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects: Opinion. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Weston, C.R.; Davis, R.J. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007, 19, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Urushibara, N.; Mitsuhashi, S.; Sasaki, T.; Kasai, H.; Yoshimizu, M.; Fujita, H.; Oda, A. JNK and p38 MAPK are independently involved in tributyltin-mediated cell death in rainbow trout (Oncorhynchus mykiss) RTG-2 cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, J.H.; Han, J.H.; Yoon, Y.D. Inhibitory effect of tributyltin on expression of steroidogenic enzymes in mouse testis. Int. J. Toxicol. 2008, 27, 175–182. [Google Scholar] [CrossRef]
- Fielden, M.R.; Halgren, R.G.; Fong, C.J.; Staub, C.; Johnson, L.; Chou, K.; Zacharewski, T.R. Gestational and lactational exposure of male mice to diethylstilbestrol causes long-term effects on the testis, sperm fertilizing ability in vitro, and testicular gene expression. Endocrinology 2002, 143, 3044–3059. [Google Scholar] [CrossRef]
- Koeberle, A.; Shindou, H.; Harayama, T.; Yuki, K.; Shimizu, T. Polyunsaturated fatty acids are incorporated into maturating male mouse germ cells by lysophosphatidic acid acyltransferase 3. FASEB J. 2012, 26, 169–180. [Google Scholar] [CrossRef]
- Sæther, T.; Tran, T.N.; Rootwelt, H.; Christophersen, B.O.; Haugen, T.B. Expression and regulation of Δ5-desaturase, Δ6-desaturase, stearoyl-coenzyme A (CoA) desaturase 1, and stearoyl-CoA desaturase 2 in rat testis. Biol. Reprod. 2003, 69, 117–124. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.; Dias, T.; Lopes, G.; Cavaco, J.; Socorro, S.; Oliveira, P. High-energy diets may induce a pre-diabetic state altering testicular glycolytic metabolic profile and male reproductive parameters. Andrology 2013, 1, 495–504. [Google Scholar] [CrossRef]
- Sasaki, H.; Matsui, Y. Epigenetic events in mammalian germ-cell development: Reprogramming and beyond. Nat. Rev. Genet. 2008, 9, 129–140. [Google Scholar] [CrossRef]
- Moore, D.S. The Developing Genome: An Introduction to Behavioral Epigenetics; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010, 21, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Piprek, R. Genetic mechanisms underlying male sex determination in mammals. J. Appl. Genet. 2009, 50, 347–360. [Google Scholar] [CrossRef]
- Cupp, A.S.; Uzumcu, M.; Suzuki, H.; Dirks, K.; Phillips, B.; Skinner, M.K. Effect of transient embryonic in vivo exposure to the endocrine disruptor methoxychlor on embryonic and postnatal testis development. J. Androl. 2003, 24, 736–745. [Google Scholar] [CrossRef]
- Uzumcu, M.; Suzuki, H.; Skinner, M.K. Effect of the anti-androgenic endocrine disruptor vinclozolin on embryonic testis cord formation and postnatal testis development and function. Reprod. Toxicol. 2004, 18, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Okano, M.; Hata, K.; Sado, T.; Tsujimoto, N.; Li, E.; Sasaki, H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 2004, 429, 900–903. [Google Scholar] [CrossRef]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef]
- Doyle, T.J.; Bowman, J.L.; Windell, V.L.; McLean, D.J.; Kim, K.H. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol. Reprod. 2013, 88, 112. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, E.; Leiter, E. Genital abnormalities and abnormal semen analyses in male patients exposed to diethylstilbestrol in utero. J. Urol. 1981, 125, 47–50. [Google Scholar] [CrossRef]
- Brouwers, M.; Feitz, W.; Roelofs, L.; Kiemeney, L.; De Gier, R.; Roeleveld, N. Hypospadias: A transgenerational effect of diethylstilbestrol? Hum. Reprod. 2006, 21, 666–669. [Google Scholar] [CrossRef]
- Kalfa, N.; Paris, F.; Soyer-Gobillard, M.-O.; Daures, J.-P.; Sultan, C. Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: A multigenerational national cohort study. Fertil. Steril. 2011, 95, 2574–2577. [Google Scholar] [CrossRef] [PubMed]
- Van de Werken, C.; van der Heijden, G.W.; Eleveld, C.; Teeuwssen, M.; Albert, M.; Baarends, W.M.; Laven, J.S.; Peters, A.H.; Baart, E.B. Paternal heterochromatin formation in human embryos is H3K9/HP1 directed and primed by sperm-derived histone modifications. Nat. Commun. 2014, 18, 1–15. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Collotta, M.; Bertazzi, P.; Bollati, V. Epigenetics and pesticides. Toxicology 2013, 307, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dansky, L.V.; Finnell, R.H. Parental epilepsy, anticonvulsant drugs, and reproductive outcome: Epidemiologic and experimental findings spanning three decades; 2: Human studies. Reprod. Toxicol. 1991, 5, 301–335. [Google Scholar] [CrossRef]
- Tabb, M.M.; Blumberg, B. New modes of action for endocrine-disrupting chemicals. Mol. Endocrinol. 2006, 20, 475–482. [Google Scholar] [CrossRef]
- Lambrot, R.; Xu, C.; Saint-Phar, S.; Chountalos, G.; Cohen, T.; Paquet, M.; Suderman, M.; Hallett, M.; Kimmins, S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Terashima, M.; Barbour, S.; Ren, J.; Yu, W.; Han, Y.; Muegge, K. Effect of high fat diet on paternal sperm histone distribution and male offspring liver gene expression. Epigenetics 2015, 10, 861–871. [Google Scholar] [CrossRef]
- Chen, L.-L.; Carmichael, G.G. Decoding the function of nuclear long non-coding RNAs. Curr. Opin. Cell Biol. 2010, 22, 357–364. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef]
- Bernstein, E.; Allis, C.D. RNA meets chromatin. Genes. Dev. 2005, 19, 1635–1655. [Google Scholar] [CrossRef]
- Whitehead, J.; Pandey, G.K.; Kanduri, C. Regulation of the mammalian epigenome by long noncoding RNAs. Biochim. Biophys. Acta 2009, 1790, 936–947. [Google Scholar] [CrossRef]
- Jolly, C.; Lakhotia, S.C. Human sat III and Drosophila hsrω transcripts: A common paradigm for regulation of nuclear RNA processing in stressed cells. Nucleic Acids Res. 2006, 34, 5508–5514. [Google Scholar] [CrossRef] [PubMed]
- Luke, B.; Lingner, J. TERRA: Telomeric repeat-containing RNA. EMBO J. 2009, 28, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Pezer, Ž.; Brajković, J.; Feliciello, I.; Ugarković, Đ. Transcription of satellite DNAs in insects. In Long Non-Coding RNAs; Springer: Berlin/Heidelberg, Germany, 2011; pp. 161–178. [Google Scholar]
- He, X.; Chen, X.; Zhang, X.; Duan, X.; Pan, T.; Hu, Q.; Zhang, Y.; Zhong, F.; Liu, J.; Zhang, H.; et al. An Lnc RNA (GAS5)/SnoRNA-derived piRNA induces activation of TRAIL gene by site-specifically recruiting MLL/COMPASS-like complexes. Nucleic. Acids. Res. 2015, 43, 3712–3725. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. Pesticides Sales. Available online: https://www.eea.europa.eu/airs/2017/environment-and-health/pesticides-sales (accessed on 24 October 2021).
- Alavanja, M.C. Introduction: Pesticides use and exposure, extensive worldwide. Rev. Environ. Health 2009, 24, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yu, G.; Zhang, Y.; Liu, X.; Du, C.; Wang, L.; Li, Z.; Wang, C. Heavy Metal Level in Human Semen with Different Fertility: A Meta-Analysis. Biol. Trace Elem. Res. 2017, 176, 27–36. [Google Scholar] [CrossRef]
- Younglai, E.V.; Foster, W.G.; Hughes, E.G.; Trim, K.; Jarrell, J.F. Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Arch. Environ. Contam. Toxicol. 2002, 43, 121–126. [Google Scholar] [CrossRef]
- Vitku, J.; Sosvorova, L.; Chlupacova, T.; Hampl, R.; Hill, M.; Sobotka, V.; Heracek, J.; Bicikova, M.; Starka, L. Differences in bisphenol A and estrogen levels in the plasma and seminal plasma of men with different degrees of infertility. Physiol. Res. 2015, 64, S303–S311. [Google Scholar] [CrossRef]
- Chang, W.-H.; Wu, M.-H.; Pan, H.-A.; Guo, P.-L.; Lee, C.-C. Semen quality and insulin-like factor 3: Associations with urinary and seminal levels of phthalate metabolites in adult males. Chemosphere 2017, 173, 594–602. [Google Scholar] [CrossRef]
- La Rocca, C.; Tait, S.; Guerranti, C.; Busani, L.; Ciardo, F.; Bergamasco, B.; Perra, G.; Mancini, F.R.; Marci, R.; Bordi, G.; et al. Exposure to Endocrine Disruptors and Nuclear Receptors Gene Expression in Infertile and Fertile Men from Italian Areas with Different Environmental Features. Int. J. Environ. Res. Public Health 2015, 12, 12426–12445. [Google Scholar] [CrossRef]
- Foldesy, R.G.; Bedford, J.M. Biology of the scrotum. I. Temperature and androgen as determinants of the sperm storage capacity of the rat cauda epididymidis. Biol. Reprod. 1982, 26, 673–682. [Google Scholar] [CrossRef]
- Klinefelter, G.R.; Suarez, J.D. Toxicant-induced acceleration of epididymal sperm transit: Androgen-dependent proteins may be involved. Reprod. Toxicol. 1997, 11, 511–519. [Google Scholar] [CrossRef]
- Lubicz-Nawrocki, C.M. Effects of castration and testosterone replacement on the number of spermatozoa in the cauda epididymidis of hamsters. J. Reprod. Fertil. 1974, 39, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Din-Udom, A.; Sujarit, S.; Pholpramool, C. Short-term effect of androgen deprivation on intraluminal pressure and contractility of the rat epididymis. J. Reprod. Fertil. 1985, 73, 405–410. [Google Scholar] [CrossRef]
- Yan, F.; Chen, Y.; Zuo, Z.; Chen, Y.; Yang, Z.; Wang, C. Effects of tributyltin on epididymal function and sperm maturation in mice. Environ. Toxicol. Pharmacol. 2009, 28, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Ito, C.; Natsume, Y.; Sugitani, Y.; Yamanaka, H.; Kuretake, S.; Yanagida, K.; Sato, A.; Toshimori, K.; Noda, T. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc. Natl. Acad. Sci. USA 2002, 99, 11211–11216. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Sousa, A.C.; Oliveira, P.F.; Alves, M.G.; Mathur, P.P. Sperm Maturation as a Possible Target of Obesogens. Immunol. Endocr. Metab. Agents Med. Chem. Former. Curr. Med. Chem. Immunol. Endocr. Metab. Agents 2017, 17, 15–31. [Google Scholar] [CrossRef]
- Sinowatz, F.; Wrobel, K.H.; Sinowatz, S.; Kugler, P. Ultrastructural evidence for phagocytosis of spermatozoa in the bovine rete testis and testicular straight tubules. J. Reprod. Fertil. 1979, 57, 1–4. [Google Scholar] [CrossRef]
- Wysocki, P.; Strzezek, J. Isolation and biochemical characteristics of a molecular form of epididymal acid phosphatase of boar seminal plasma. Theriogenology 2006, 66, 2152–2159. [Google Scholar] [CrossRef]
- Kumi-Diaka, J.; Townsend, J. Effects of Genistein Isoflavone (4′,5′,7-Trihydroxyisoflavone) and Dexamethasone on Functional Characteristics of Spermatozoa. J. Med. Food 2001, 4, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Jung, E.Y.; Baek, I.J.; Lee, Y.B.; Lee, J.Y.; Nam, S.Y. Exposure to genistein does not adversely affect the reproductive system in adult male mice adapted to a soy-based commercial diet. J. Vet. Sci. 2004, 5, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.; Afeiche, M.; Gaskins, A.; Williams, P.; Petrozza, J.; Tanrikut, C.; Hauser, R.; Chavarro, J. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum. Reprod. 2015, 30, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Morán-Martínez, J.; Carranza-Rosales, P.; Morales-Vallarta, M.; Heredia-Rojas, J.A.; Bassol-Mayagoitia, S.; Betancourt-Martínez, N.D.; Cerda-Flores, R.M. Chronic environmental exposure to lead affects semen quality in a Mexican men population. Iran. J. Reprod. Med. 2013, 11, 267–274. [Google Scholar]
- Wang, C.; Yang, L.; Wang, S.; Zhang, Z.; Yu, Y.; Wang, M.; Cromie, M.; Gao, W.; Wang, S.L. The classic EDCs, phthalate esters and organochlorines, in relation to abnormal sperm quality: A systematic review with meta-analysis. Sci. Rep. 2016, 6, 19982. [Google Scholar] [CrossRef] [PubMed]
- Lassen, T.H.; Frederiksen, H.; Jensen, T.K.; Petersen, J.H.; Joensen, U.N.; Main, K.M.; Skakkebaek, N.E.; Juul, A.; Jorgensen, N.; Andersson, A.M. Urinary bisphenol A levels in young men: Association with reproductive hormones and semen quality. Environ. Health Perspect. 2014, 122, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Vilela, J.; Hartmann, A.; Silva, E.F.; Cardoso, T.; Corcini, C.D.; Varela-Junior, A.S.; Martinez, P.E.; Colares, E.P. Sperm impairments in adult vesper mice (Calomys laucha) caused by in utero exposure to bisphenol A. Andrologia 2014, 46, 971–978. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Lee, J.S.; Yoon, S.J.; Ryu, B.Y.; Pang, M.G. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci. Rep. 2015, 5, 9169. [Google Scholar] [CrossRef]
- Miki, K.; Qu, W.; Goulding, E.H.; Willis, W.D.; Bunch, D.O.; Strader, L.F.; Perreault, S.D.; Eddy, E.M.; O’Brien, D.A. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl. Acad. Sci. USA. 2004, 101, 16501–16506. [Google Scholar] [CrossRef] [PubMed]
- Nakada, K.; Sato, A.; Yoshida, K.; Morita, T.; Tanaka, H.; Inoue, S.; Yonekawa, H.; Hayashi, J. Mitochondria-related male infertility. Proc. Natl. Acad. Sci. USA 2006, 103, 15148–15153. [Google Scholar] [CrossRef]
- Sutovsky, P.; Manandhar, G. Mammalian spermatogenesis and sperm structure: Anatomical and compartmental analysis. Sperm Cell Prod. Maturation Fertil. Regen. 2006, 1–30. [Google Scholar]
- Ramalho-Santos, J.; Varum, S.; Amaral, S.; Mota, P.C.; Sousa, A.P.; Amaral, A. Mitochondrial functionality in reproduction: From gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Update 2009, 15, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Barroso, G.; Taylor, S.; Morshedi, M.; Manzur, F.; Gavino, F.; Oehninger, S. Mitochondrial membrane potential integrity and plasma membrane translocation of phosphatidylserine as early apoptotic markers: A comparison of two different sperm subpopulations. Fertil. Steril. 2006, 85, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef]
- Troiano, L.; Granata, A.R.; Cossarizza, A.; Kalashnikova, G.; Bianchi, R.; Pini, G.; Tropea, F.; Carani, C.; Franceschi, C. Mitochondrial membrane potential and DNA stainability in human sperm cells: A flow cytometry analysis with implications for male infertility. Exp. Cell Res. 1998, 241, 384–393. [Google Scholar] [CrossRef]
- Wang, M.-J.; Ou, J.-X.; Chen, G.-W.; Wu, J.-P.; Shi, H.-J.; O, W.-S.; Martin-DeLeon, P.A.; Chen, H. Does Prohibitin Expression Regulate Sperm Mitochondrial Membrane Potential, Sperm Motility, and Male Fertility? Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2012. [Google Scholar]
- Gallon, F.; Marchetti, C.; Jouy, N.; Marchetti, P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil. Steril. 2006, 86, 1526–1530. [Google Scholar] [CrossRef]
- Marchetti, C.; Obert, G.; Deffosez, A.; Formstecher, P.; Marchetti, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002, 17, 1257–1265. [Google Scholar] [CrossRef]
- Mayorga-Torres, B.J.; Cardona-Maya, W.; Cadavid, A.; Camargo, M. Evaluation of sperm functional parameters in normozoospermic infertile individuals. Actas. Urol. Esp. 2013, 37, 221–227. [Google Scholar] [CrossRef]
- Wang, X.; Sharma, R.K.; Gupta, A.; George, V.; Thomas, A.J.; Falcone, T.; Agarwal, A. Alterations in mitochondria membrane potential and oxidative stress in infertile men: A prospective observational study. Fertil. Steril. 2003, 80, 844–850. [Google Scholar] [CrossRef]
- Caito, S.W.; Aschner, M. Mitochondrial Redox Dysfunction and Environmental Exposures. Antioxid Redox Signal 2015, 23, 578–595. [Google Scholar] [CrossRef]
- Skibinska, I.; Jendraszak, M.; Borysiak, K.; Jedrzejczak, P.; Kotwicka, M. 17beta-estradiol and xenoestrogens reveal synergistic effect on mitochondria of human sperm. Ginekol. Pol. 2016, 87, 360–366. [Google Scholar] [CrossRef]
- Aly, H.A. Aroclor 1254 induced oxidative stress and mitochondria mediated apoptosis in adult rat sperm in vitro. Environ. Toxicol. Pharmacol. 2013, 36, 274–283. [Google Scholar] [CrossRef]
- Grizard, G.; Ouchchane, L.; Roddier, H.; Artonne, C.; Sion, B.; Vasson, M.P.; Janny, L. In vitro alachlor effects on reactive oxygen species generation, motility patterns and apoptosis markers in human spermatozoa. Reprod. Toxicol. 2007, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Shukla, M.; Kumar Patel, D.; Shukla, Y.; Mathur, N.; Kumar Gupta, Y.; Saxena, D.K. Correlation of phthalate exposures with semen quality. Toxicol. Appl. Pharmacol. 2008, 231, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Vanage, G. Mutagenic effect of Bisphenol A on adult rat male germ cells and their fertility. Reprod. Toxicol. 2013, 40, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hulak, M.; Gazo, I.; Shaliutina, A.; Linhartova, P. In vitro effects of bisphenol A on the quality parameters, oxidative stress, DNA integrity and adenosine triphosphate content in sterlet (Acipenser ruthenus) spermatozoa. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 64–71. [Google Scholar] [CrossRef]
- Chitra, K.C.; Sujatha, R.; Latchoumycandane, C.; Mathur, P.P. Effect of lindane on antioxidant enzymes in epididymis and epididymal sperm of adult rats. Asian. J. Androl. 2001, 3, 205–208. [Google Scholar]
- Latchoumycandane, C.; Chitra, C.; Mathur, P. Induction of oxidative stress in rat epididymal sperm after exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch. Toxicol. 2002, 76, 113–118. [Google Scholar] [CrossRef]
- Chitra, K.C.; Latchoumycandane, C.; Mathur, P.P. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology 2003, 185, 119–127. [Google Scholar] [CrossRef]
- Palleschi, S.; Rossi, B.; Diana, L.; Silvestroni, L. Di (2-ethylhexyl) phthalate stimulates Ca2+ entry, chemotaxis and ROS production in human granulocytes. Toxicol. Lett. 2009, 187, 52–57. [Google Scholar] [CrossRef]
- Drbohlav, P.; Jirsová, S.; Masata, J.; Jech, L.; Bencko, V.; Omelka, M.; Zvárová, J. Relationship between the levels of toxic polychlorinated biphenyls in blood and follicular fluid of sterile women. Ceska Gynekol. 2005, 70, 377–383. [Google Scholar]
- Tsutsumi, O.; Uechi, H.; Sone, H.; Yonemoto, J.; Takai, Y.; Momoeda, M.; Tohyama, C.; Hashimoto, S.; Morita, M.; Taketani, Y. Presence of dioxins in human follicular fluid: Their possible stage-specific action on the development of preimplantation mouse embryos. Biochem. Biophys. Res. Commun. 1998, 250, 498–501. [Google Scholar] [CrossRef]
- Sofikitis, N.V.; Miyagawa, I.; Zavos, P.M.; Toda, T.; Iino, A.; Terakawa, N. Confocal scanning laser microscopy of morphometric human sperm parameters: Correlation with acrosin profiles and fertilizing capacity. Fertil. Steril. 1994, 62, 376–386. [Google Scholar] [CrossRef]
- Langlois, M.R.; Oorlynck, L.; Vandekerckhove, F.; Criel, A.; Bernard, D.; Blaton, V. Discrepancy between sperm acrosin activity and sperm morphology: Significance for fertilization in vitro. Clin. Chim. Acta 2005, 351, 121–129. [Google Scholar] [CrossRef]
- Reichart, M.; Lederman, H.; Har-Even, D.; Kedem, P.; Bartoov, B. Human sperm acrosin activity with relation to semen parameters and acrosomal ultrastructure. Andrologia 1993, 25, 59–66. [Google Scholar] [CrossRef]
- Breitbart, H.; Cohen, G.; Rubinstein, S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction 2005, 129, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Sperm function tests and fertility. Int. J. Androl. 2006, 29, 69–75. [Google Scholar] [CrossRef]
- Rijsselaere, T.; Van Soom, A.; Tanghe, S.; Coryn, M.; Maes, D.; de Kruif, A. New techniques for the assessment of canine semen quality: A review. Theriogenology 2005, 64, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H. Laboratory semen assessment and prediction of fertility: Still utopia? Reprod. Domest. Anim. 2003, 38, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Knez, J.; Kranvogl, R.; Breznik, B.P.; Vončina, E.; Vlaisavljević, V. Are urinary bisphenol A levels in men related to semen quality and embryo development after medically assisted reproduction? Fertil. Steril. 2014, 101, 215–221.e215. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-K.; Zhou, Z.; Miao, M.; He, Y.; Wang, J.; Ferber, J.; Herrinton, L.J.; Gao, E.; Yuan, W. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil. Steril. 2011, 95, 625–630.e624. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Kwon, W.; Lee, J.; Yoon, S.; Ryu, B.; Pang, M.; Rahman, M.; Kwon, W.; Lee, J.; Yoon, S.; et al. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci. Rep. 2015, 5, 9169. [Google Scholar] [CrossRef]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Jönsson, B.A.; Lindh, C.H.; Giwercman, A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ. Int. 2015, 85, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Duty, S.M.; Silva, M.J.; Barr, D.B.; Brock, J.W.; Ryan, L.; Chen, Z.; Herrick, R.F.; Christiani, D.C.; Hauser, R. Phthalate exposure and human semen parameters. Epidemiology 2003, 269–277. [Google Scholar] [CrossRef]
- Lenters, V.; Portengen, L.; Smit, L.A.; Jönsson, B.A.; Giwercman, A.; Rylander, L.; Lindh, C.H.; Spanò, M.; Pedersen, H.S.; Ludwicki, J.K. Phthalates, perfluoroalkyl acids, metals and organochlorines and reproductive function: A multipollutant assessment in Greenlandic, Polish and Ukrainian men. Occup. Environ. Med. 2015, 72, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Altshul, L.; Chen, Z.; Ryan, L.; Overstreet, J.; Schiff, I.; Christiani, D.C. Environmental organochlorines and semen quality: Results of a pilot study. Environ. Health Perspect. 2002, 110, 229. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Chen, Z.; Pothier, L.; Ryan, L.; Altshul, L. The relationship between human semen parameters and environmental exposure to polychlorinated biphenyls and p, p’-DDE. Environ. Health Perspect. 2003, 111, 1505. [Google Scholar] [CrossRef]
- Hauser, R.; Williams, P.; Altshul, L.; Calafat, A.M. Evidence of interaction between polychlorinated biphenyls and phthalates in relation to human sperm motility. Environ. Health Perspect. 2005, 113, 425. [Google Scholar] [CrossRef]
- Richthoff, J.; Rylander, L.; Jönsson, B.A.; Akesson, H.; Hagmar, L.; Nilsson-Ehle, P.; Stridsberg, M.; Giwercman, A. Serum levels of 2, 2′, 4, 4′, 5, 5′-hexachlorobiphenyl (CB-153) in relation to markers of reproductive function in young males from the general Swedish population. Environ. Health Perspect. 2003, 111, 409. [Google Scholar] [CrossRef]
- Toft, G.; Rignell-Hydbom, A.; Tyrkiel, E.; Shvets, M.; Giwercman, A.; Lindh, C.H.; Pedersen, H.S.; Ludwicki, J.K.; Lesovoy, V.; Hagmar, L. Semen quality and exposure to persistent organochlorine pollutants. Epidemiology 2006, 450–458. [Google Scholar] [CrossRef]
- Aneck-Hahn, N.; Schulenburg, G.; Bornman, M.; Farias, P.; De Jager, C.; Aneck-Hahn, N.; Schulenburg, G.; Bornman, M.; Farias, P.; De Jager, C. Impaired semen quality associated with environmental DDT exposure in young men living in a malaria area in the Limpopo Province, South Africa. J. Androl. 2007, 28, 423–434. [Google Scholar] [CrossRef]
- De Jager, C.; Farias, P.; Barraza-Villarreal, A.; Avila, M.H.; Ayotte, P.; Dewailly, E.; Dombrowski, C.; Rousseau, F.; Sanchez, V.D.; Bailey, J.L. Reduced seminal parameters associated with environmental DDT exposure and p, p′-DDE concentrations in men in Chiapas, Mexico: A cross-sectional study. J. Androl. 2006, 27, 16–27. [Google Scholar] [CrossRef]
- Pant, N.; Pant, A.; Chaturvedi, P.; Shukla, M.; Mathur, N.; Gupta, Y.; Saxena, D.; Pant, N.; Pant, A.; Chaturvedi, P.; et al. Semen quality of environmentally exposed human population: The toxicological consequence. Environ. Sci. Pollut. Res. Int. 2013, 20, 8274–8281. [Google Scholar] [CrossRef]
- Tavares, R.; Amaral, S.; Paiva, C.; Baptista, M.; Ramalho-Santos, J.; Tavares, R.; Amaral, S.; Paiva, C.; Baptista, M.; Ramalho-Santos, J. In vitro exposure to the organochlorine p,p’-DDE affects functional human sperm parameters. Chemosphere 2015, 120, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; You, L.; Zeng, Q.; Sun, Y.; Huang, Y.-H.; Wang, C.; Wang, P.; Cao, W.-C.; Yang, P.; Li, Y.-F. Phthalate exposure and human semen quality: Results from an infertility clinic in China. Environ. Res. 2015, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Joensen, U.N.; Bossi, R.; Leffers, H.; Jensen, A.A.; Skakkebæk, N.E.; Jørgensen, N. Do perfluoroalkyl compounds impair human semen quality? Environ. Health Perspect. 2009, 117, 923. [Google Scholar] [CrossRef] [PubMed]
- Vested, A.; Ramlau-Hansen, C.H.; Olsen, S.F.; Bonde, J.P.; Kristensen, S.L.; Halldorsson, T.I.; Becher, G.; Haug, L.S.; Ernst, E.H.; Toft, G. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ. Health Perspect. 2013, 121, 453. [Google Scholar] [CrossRef]
- Dallinga, J.W.; Moonen, E.J.; Dumoulin, J.C.; Evers, J.L.; Geraedts, J.P.; Kleinjans, J.C. Decreased human semen quality and organochlorine compounds in blood. Hum. Reprod. 2002, 17, 1973–1979. [Google Scholar] [CrossRef]
- Padungtod, C.; Savitz, D.A.; Overstreet, J.W.; Christiani, D.C.; Ryan, L.M.; Xu, X. Occupational pesticide exposure and semen quality among Chinese workers. J. Occup. Environ. Med. 2000, 42, 982–992. [Google Scholar] [CrossRef]
- Pant, N.; Kumar, G.; Upadhyay, A.; Patel, D.; Gupta, Y.; Chaturvedi, P. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ. Sci. Pollut. Res. 2014, 21, 11066–11074. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.; Mansell, S.; Barratt, C.; Wilson, S.; Publicover, S.; RamalhoSantos, J.; Tavares, R.; Mansell, S.; Barratt, C.; Wilson, S.; et al. p’-DDE activates CatSper and compromises human sperm function at environmentally relevant concentrations. Hum. Reprod. 2013, 28, 3167–3177. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Pant, A.; Shukla, M.; Mathur, N.; Gupta, Y.; Saxena, D.; Pant, N.; Pant, A.; Shukla, M.; Mathur, N.; et al. Environmental and experimental exposure of phthalate esters: The toxicological consequence on human sperm. Hum. Exp. Toxicol. 2011, 30, 507–514. [Google Scholar] [CrossRef]
- Russo, A.; Troncoso, N.; Sanchez, F.; Garbarino, J.; Vanella, A.; Russo, A.; Troncoso, N.; Sanchez, F.; Garbarino, J.; Vanella, A. Propolis protects human spermatozoa from DNA damage caused by benzo[a]pyrene and exogenous reactive oxygen species. Life Sci. 2006, 78, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Nandi, P.; Varghese, A.; Gutgutia, R.; Banerjee, S.; Bhattacharyya, A.; Mukhopadhyay, D.; Nandi, P.; Varghese, A.; Gutgutia, R.; et al. The in vitro effect of benzo[a]pyrene on human sperm hyperactivation and acrosome reaction. Fertil. Steril. 2010, 94, 595–598. [Google Scholar] [CrossRef]
- Fraser, L.; Beyret, E.; Milligan, S.; Adeoya-Osiguwa, S.; Fraser, L.; Beyret, E.; Milligan, S.; Adeoya-Osiguwa, S. Effects of estrogenic xenobiotics on human and mouse spermatozoa. Hum. Reprod. 2006, 21, 1184–1193. [Google Scholar] [CrossRef]
- Mohamed, E.-S.A.; Park, Y.-J.; Song, W.-H.; Shin, D.-H.; You, Y.-A.; Ryu, B.-Y.; Pang, M.-G. Xenoestrogenic compounds promote capacitation and an acrosome reaction in porcine sperm. Theriogenology 2011, 75, 1161–1169. [Google Scholar] [CrossRef]
- Adeoya-Osiguwa, S.; Markoulaki, S.; Pocock, V.; Milligan, S.; Fraser, L.; Adeoya-Osiguwa, S.; Markoulaki, S.; Pocock, V.; Milligan, S.; Fraser, L. 17β-Estradiol and environmental estrogens significantly affect mammalian sperm function. Hum. Reprod. 2003, 18, 100–107. [Google Scholar] [CrossRef]
- Kholkute, S.; Rodriguez, J.; Dukelow, W.; Kholkute, S.; Rodriguez, J.; Dukelow, W. Effects of polychlorinated biphenyls (PCBs) on in vitro fertilization in the mouse. Reprod. Toxicol. 1994, 8, 69–73. [Google Scholar] [CrossRef]

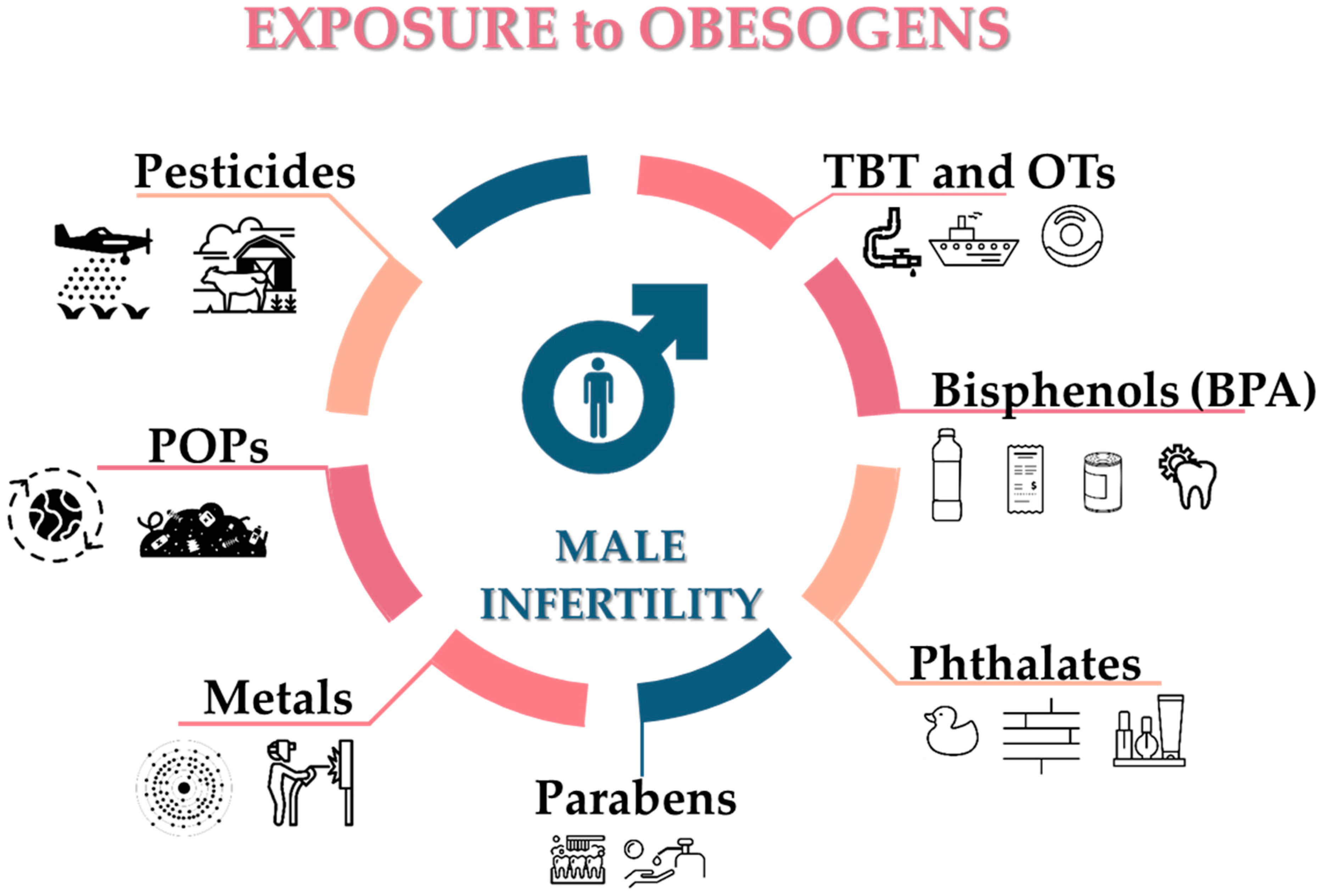
| Obesogens | Main Sources | References |
|---|---|---|
| 2,4-D | Herbicides | [87] |
| B[α]P | Residential wood burnings, cigarette smoke, charbroiled food, coal tar, and automobile fume emissions | [88] |
| BPA | Food and drink packaging plastics, medical devices, and thermal paper | [89] |
| Chlorpyrifos | Insecticides | [90] |
| Diazinon | Insecticides | [91] |
| Diethylstilbestrol | Cattle feed and medical treatments for breast and prostate cancers | [92] |
| Fructose | Fruit, vegetables, and honey | [93] |
| Genistein | Soybeans and soy products, fava beans, and coffee | [94] |
| Lead | Diet, dust, ceramics, paints, and infant toys | [95] |
| MSG | Food additives and natural foods such as tomatoes and cheese | [96] |
| Nicotine | Tobacco, insecticides, and nightshade plants | [97] |
| Parabens | Preservatives in personal care products | [98] |
| Parathion | Insecticides and acaricides | [99] |
| PBDEs | Flame retardant in building materials, electronics, furnishings, plasticizers, and textiles | [100] |
| PCBs | Electric equipment, transistors, plasticizers, surface coatings, paints, and carbonless copy paper | [101] |
| PFOA | Crawl and stain repellent on carpets, furniture, waterproof clothing, mattresses, and microwavable food items, non-stick kitchen utensils | [102] |
| Phthalates | Plastics, PVC products, infant toys, detergents, and personal care products | [103] |
| TBT | Antifouling paints, plastic products, silicones, and polyurethanes | [6] |
| TZD | Antidiabetic drugs | [104] |
| Obesogens | Specie(s)/ Tissue(s)/Cells | Doses | Glycolytic Metabolism Effects | Lipid Metabolism Effects | Toxic Effects | |
|---|---|---|---|---|---|---|
| 2,4-D | Rat SCs | 100 nM, 10 µM, 1 mM | ↓GLUT3, PFK1 LDH mRNA, ↓Lactate production [122] | n.d. | n.d. | |
| BPA | Rat testis | 0.005, 0.5, 50, 500 µg/Kg body wg/day | ↓IRS-1, ↓GLUT2 [123] ↓HEX, ↓PFK [125] | n.d. | n.d. | |
| CPYF | Rat testis | 0, 2.7, 5.4, 12.8 mg/Kg body wg | ↑LDH [126] | n.d. | n.d. | |
| Lead | Rat SCs | 0.01, 0.05, 0.1 mM | ↑Lactate production [127] | n.d. | ↑Lipid peroxidation, ↑CAT activity, ↑GSH, ↓SOD activity [127] | |
| PCBs | Rat SCs | 10−7 M (PCB22) 10−8 M (PCB77) | ↑Lactate production [128] | n.d. | n.d. | |
| PIO | Rat SCs | 1, 10, 100 µM | ↑Glucose uptake ↓GLUT3 ↑Lactate production ↑LDH ↑MCT4[104] | n.d. | n.d. | |
| PTLs | Rat | Testis | CE-2 diet with 2%(mass) of DEHP | n.d. | ↓ACC ↑LCAD ↑3KACT [129] | n.d. |
| SCs | 0.1–200 µM | ↑Pyruvate production ↑Lactate production [124] | n.d. | n.d. | ||
| TBT | Rat | Testis | 10, 20, 30 mg/Kg of body wg | n.d. | n.d. | ↓BTB ↑TBARS ↑ROS ↓Steroidogenesis [130] |
| LCs | 300–3000 nM | n.d. | n.d. | ↓MMP ↓Steroidogenesis ↑Apoptosis [131] | ||
| SCs | 0.1 nM, 10 nM | ↓Glucose uptake ↓Pyruvate uptake ↓GLUT1 ↓Lactate production [132] | n.d. | n.d. | ||
| SCs/GCs co-culture | 300, 600 and 1000 nM | n.d. | n.d. | ↑Apoptosis [133] | ||
| Sperm Parameters | Species | Obesogens | Doses/Concentrations | Outcomes | References |
|---|---|---|---|---|---|
| Motility | Human | BPA | n.a. | ↓ | [277,278] |
| Rat | 0.0001, 0.01, 1, and 100 mM | ↓ | [279] | ||
| Human | PTLs | n.a. | ↓ | [261,280,281] | |
| Human | POPs | n.a. | ↓ | [282] | |
| Human | PCBs | n.a. | ↓ | [283,284,285] | |
| Rat | Aroclor 1254 | 10−9, 10−8, and 10−7 M | ↓ | [259] | |
| Human | CB153 | n.a. | ↓ | [286,287] | |
| Human | DDT | n.a. | ↓ | [287,288,289] | |
| Human | p,p’-DDE | n.a. | ↓ | [97] | |
| 53.89, 269.45, and 538.9 mg/L | ↓ | [290] | |||
| 10, 25, 50, and 100 mM | ↓ | [291] | |||
| Human | Alachlor | 0.18, 0.37, 0.90, and 1.85 mM | ↓ | [260] | |
| Concentration | Human | BPA | n.a. | ↓ | [278] |
| Human | PTLs | n.a. | ↓ | [261,281,292] | |
| Human | PFOS, PFOA | n.a. | ↓ | [293,294] | |
| Human | PCBs | n.a. | ↓ | [283,295] | |
| Human | Pesticides | n.a. | ↓ | [296] | |
| Human | DDT | n.a. | ↓ | [288] | |
| Morphology | Human | BPA | n.a. | ↓ | [278] |
| Human | PTLs | n.a. | ↓ | [261,297] | |
| Human | PCBs | n.a. | ↓ | [283,284] | |
| Human | DDT | n.a. | ↓ | [288,289] | |
| Human | p,p’-DDE | n.a. | ↓ | [289] | |
| Viability | Human | BPA | n.a. | ↓ | [277,278] |
| Human | Alachlor | 0.18, 0.37, 0.90, and 1.85 mM | ↓ | [260] | |
| p,p’-DDE | 1, 10, 25, 50, and 100 µM | ↓ | [291,298] | ||
| PTLs | 5.73, 28.65, and 57.3 mg/mL | ↓ | [299] | ||
| Mitochondrial function | Human | 0.18, 0.37, 0.90, and 1.85 mM | ↓ | [260] | |
| p,p’-DDE | 1, 10, 25, 50, and 100 µM | ↓ | [291,298] | ||
| Rat | Aroclor 1254 | 10−9, 10−8, and 10−7 M | ↓ | [259] | |
| Oxidative stress | Human | Alachlor | 0.37 and 1.85 mM | ↑ | [260] |
| Human | B[α]P | 500 µM | ↑ | [300] | |
| Rat | Aroclor 1254 | 10−9, 10−8, and 10−7 M | ↑ | [259] | |
| Lipid peroxidation | Rat | Aroclor 1254 | 10−9, 10−8, and 10−7 M | ↑ | [259] |
| Capacitation | Human | p,p’-DDE | 25, 50, and 100 µM | ↓ | [291] |
| Mouse | BPA | 0.0001, 0.01, 1, and 100 µM | ↓ | [279] | |
| Human | B[α]P | 12.5, 25, 50, and 100 µg/mL | ↑ | [301] | |
| Human | Genistein | 1, 10, and 100 nM | ↑ | [302] | |
| Boar | 0.001, 0.01, 0.1, 1, 10, and 100 µM | ↑ | [303] | ||
| Mouse | 1, 10, 100, and 1000 nM | ↑ | [304] | ||
| Acrosome integrity | Human | p,p’-DDE | 1, 10, 25, and 50 µM | ↓ | [298] |
| Rat | Aroclor 1254 | 10−9, 10−8, and 10−7 M | ↑ | [259] | |
| Human | B[α]P | 12.5, 25, 50, and 100 µg/mL | ↓ | [301] | |
| Mouse | BPA | 0.0001, 0.01, 1, and 100 µM | ↓ | [279] | |
| Human | Genistein | 1, 10, and 100 nM | ↑ | [302] | |
| Boar | 0.001, 0.01, 0.1, 1, 10, and 100 µM | ↑ | [303] | ||
| Mouse | 1, 10, 100, and 1000 nM | ↑ | [304] | ||
| Sperm-oocyte interaction | Mouse | BPA | 0.0001, 0.01, 1, and 100 µM | ↓ | [279] |
| Rat | PCB77 | 0.01, 0.1, 1, and 10 µg/mL | ↓ | [305] | |
| Mouse | Genistein | 100 nM | ↑ | [304] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rato, L.; Sousa, A.C.A. The Impact of Endocrine-Disrupting Chemicals in Male Fertility: Focus on the Action of Obesogens. J. Xenobiot. 2021, 11, 163-196. https://doi.org/10.3390/jox11040012
Rato L, Sousa ACA. The Impact of Endocrine-Disrupting Chemicals in Male Fertility: Focus on the Action of Obesogens. Journal of Xenobiotics. 2021; 11(4):163-196. https://doi.org/10.3390/jox11040012
Chicago/Turabian StyleRato, Luís, and Ana C. A. Sousa. 2021. "The Impact of Endocrine-Disrupting Chemicals in Male Fertility: Focus on the Action of Obesogens" Journal of Xenobiotics 11, no. 4: 163-196. https://doi.org/10.3390/jox11040012
APA StyleRato, L., & Sousa, A. C. A. (2021). The Impact of Endocrine-Disrupting Chemicals in Male Fertility: Focus on the Action of Obesogens. Journal of Xenobiotics, 11(4), 163-196. https://doi.org/10.3390/jox11040012







