Drought Stress in Cassava (Manihot esculenta): Management Strategies and Breeding Technologies
Abstract
1. Introduction
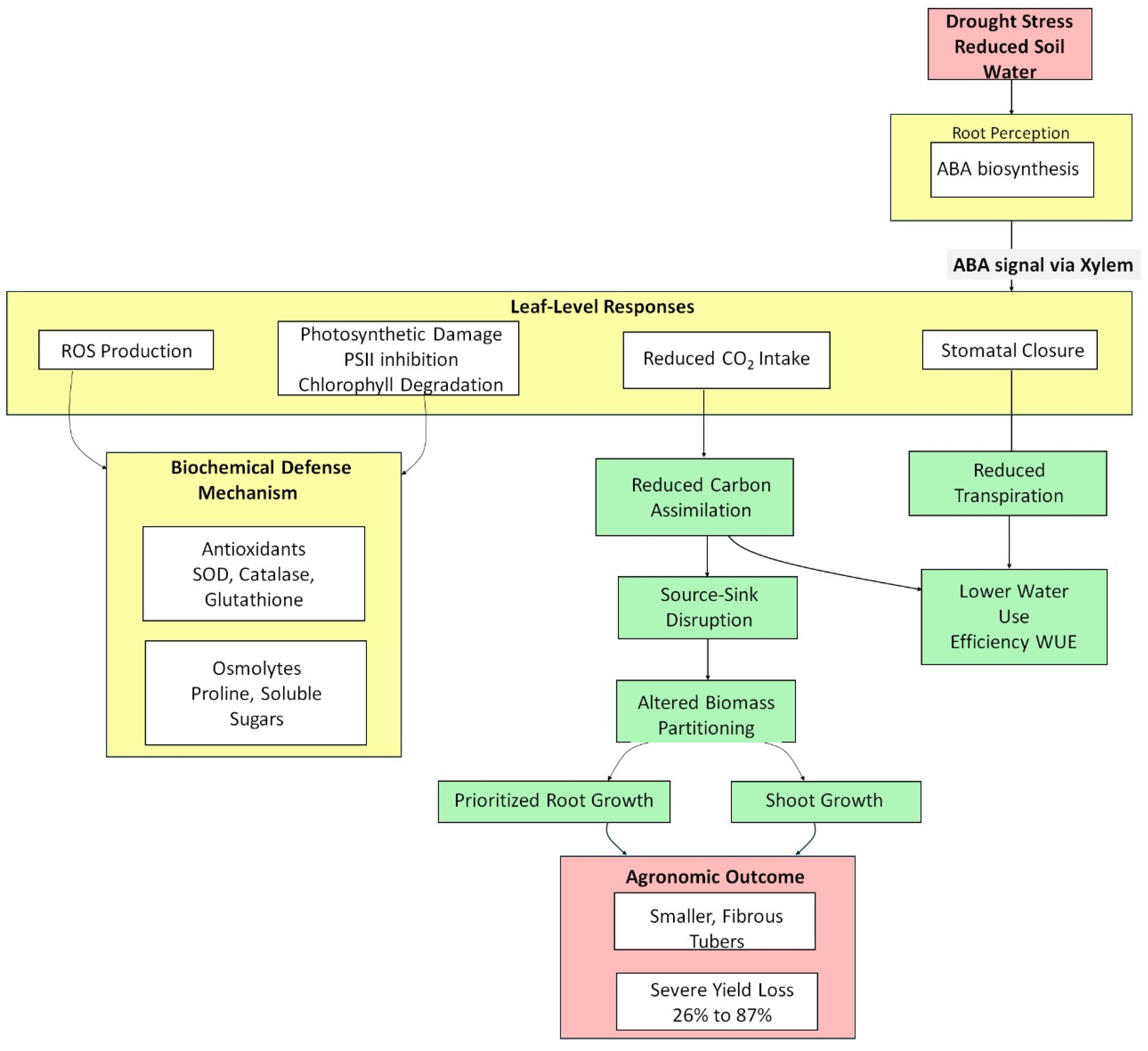
2. Effects of Drought Stress on Cassava Performance
2.1. Effects of Drought Stress on Physiological Traits
2.2. Effects of Drought Stress on Biochemical Traits
2.3. Effects of Drought Stress on Agronomic Traits
| Country | Genotype Names | Number of Cultivars | Yield Reduction | Reference |
|---|---|---|---|---|
| Kenya | 98/0002, TME-419, 95/0306, 95/0166, 14-2-1425, 96/1569, 98/2101, 98/0505, I91/02322, 98/2132, I91B/00462, I96/1439, M98/0068, 990/183, I92/0067, 92/0342, 94/0039, 97/4779, 99/0204, 96/0409, 98/0196, 94/0020, 01/0014, I91/02324, 92/0427, 95/0289, I92/0057, I92/0326, I92B/00061, 96/2132, I30/572, 98/0406, 96/2226, 94/0026, I91/02312, I91/02327, PYT, Ex-Mariakani | 37 | 59% | [21] |
| Brazil | IAC 576–70 | 1 | 45.7% | [50] |
| Brazil | 9624-09, BGM0089, BGM0096, BGM0116, BGM0163, BGM0279, BGM0331, BGM0360, BGM0541, BGM0598, BGM0785, BGM0815, BGM0818, BGM0856, BGM0876, BGM0908, BGM1171, BGM1195, BGM1482, BGM2020, Branquinha, BRS Amansa Burro, BRS Dourada, BRS Formosa, BRS Gema de Ovo, BRS Kiriris, Cacau, Cachimbo, Do Céu, Engana Ladrão, Eucalipto, GCP-001, GCP-009, GCP-014, GCP-020, GCP-025, GCP-043, GCP-046, GCP-095, GCP-128, GCP-179, GCP-190, GCP-194, GCP-227, GCP-374, Mani Branca, NG310, Paulo Rosa, Sacai | 49 | 73% | [61] |
| Nigeria | (30572, 96/0326, 91/02324, 95/0211, 96/0016, 96/0304, 96/0529, 96/0565 and 96/1632) | 9 | 87% | [62] |
| Uganda | Akena, AladoAlado, Bao, Buganda, Bukalasa, Ditu, Icilecili, Egabu, Guaranda, Kabwa, Kidimo, Kwatamumpare, Luderudu, Lugbara, Maburu, Magana, Mercury, MH96/0686, MH97/2961, MufumbaChai, Musita, Namukoni, Namulalu, NASE1, NASE11, NASE12, NASE2, NASE3, NASE9, Njule, Nyakakwa, Nyalanda, Nyamutukura, Nyapamitu, Nyaraboke, Nyarare, Omongole, Pilipili, Rugogoma, Rwaburaru, Ryahorore, TME14, TME204, Tongolo, Tongolo2 Yellow | 46 | 37% | [63] |
| Ghana | 00/0203, 96/1708, 96/409, ATR 002, ATR 007, Biabasse, CTSIA 110, CTSIA 112, CTSIA 230, CTSIA 45, CTSIA 48, CTSIA 65, CTSIA 72, I91934, MM 96/1751, NWA 004, Ponstisange, TME 419, TME 435, UCC 2001/449 | 20 | 39% | [64] |
| Nigeria | IBA120008, I098510, I010040, I070539, TMEB419, TMEB693, I011368, I980581, I070593, I920326 | 10 | 37% | [65] |
| Kenya | 110(07/0621HS), 128(05/0099HS), 169(98/002HS), 183907/0751HS), 197(01/1797HS), 212(07/576HS), 29(06/1475HS), 29B29, 89(06/1475HS), 93(07/0756HS), 94(05/0741HS), 99(07/1313HS), CH05-203, CK9, COLICANANA, D31, Ex-Ndolo, EYOPE, F10-30R2, F19, FUMBA CHAI, I92/0427, I95/034095-N, KALAWE, KALESO, Karembo, KBH2002/066-3, KBH2006-026, KIBANDAMENO, KIZIMBANI, KME 1, LML/2008/363, M96/7151, MAGANA, MATUJA, MH95/0183, MIGYERA, MKUMBA-1, MKUMBA-2, MM06/0013, MM06/0046, MM06/0074, MM06/0082, MM06/0083, MM06/0138, MM06/0139, MM06/0143, MM08/2206, MM96/0669, MM96/0686, MM96/1871, MM96/1956, MM96/2480, MM96/3868, MM96/4684, MM96/5280, MM96/6966A, MM96/6966B, MM96/9308, MM97/0293, MM97/1744, MM98/1642, MM98/1669, MM98/2270, MM98/3567, Mucericer, NASE 14, NASE 18, NASE 3, ORERA, PWANI, SANGOJA, SAUTI, SERERE, TAJIRIKA, TME 14, TZ-130, UNKNOWN 4, YIZASO | 79 | 18% | [66] |
2.4. Effects of Drought Stress on WUE
2.5. Effects of Drought Stress on Carbon Assimilation Capacity
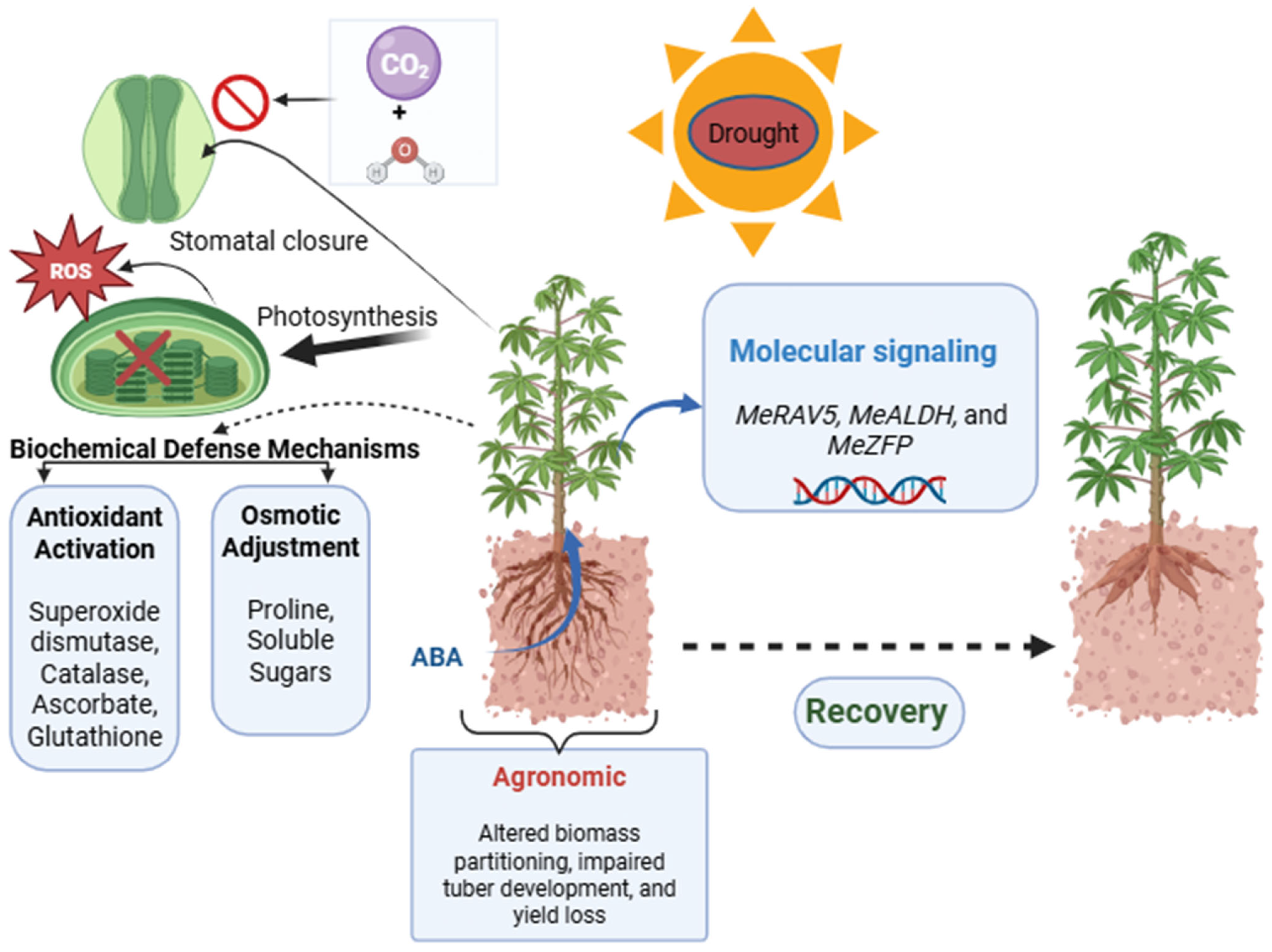
3. Drought Response and Tolerance
3.1. Physiological and Morphological Mechanisms
| Adaptive Trait | Type | Mechanism | Effect on Tuber Yield | References |
|---|---|---|---|---|
| Proline and soluble sugars | Molecular/metabolites | Osmotic adjustment | Maintains turgor, protects proteins/membranes | [50] |
| Stay green | Morphological | Allow continued photosynthesis | Improved tuber yield | [67] |
| MeALDH | Molecular | Detoxifies reactive aldehydes | Maintains membrane stability | [67] |
| MeZFP | Molecular | Regulates antioxidant genes | Maintains redox homeostasis | [67] |
| MeRAV5 | Molecular | The transcription Factor regulating H2O2 homeostasis and lignin biosynthesis | Strengthens cell walls, reduces damage | [83] |
| Reduced stomatal conductance | Physiological | Isohydric closure, limited CO2 uptake | Conserves water, maintains minimal assimilation | [84] |
| Leaf area modulation | Morphological | Reducing leaf area growth | Reduces water loss, maintains photosynthesis | [69] |
3.2. Molecular and Genetic Mechanisms
4. Mitigation Strategies of Drought Stress in Cassava Production
4.1. Mulching
4.2. Irrigation
4.3. The Use of Genotypes with Drought-Adaptive Traits
4.4. The Use of Early Bulking Genotypes
4.5. The Use of Drought-Tolerant and Water-Use-Efficient Germplasm
| Variety Name | Country | Reference |
|---|---|---|
| BRS Formosa | Brazil | [61] |
| MH96/0686 and Magana | Uganda | [63] |
| I980581 | Nigeria | [65] |
| MM06/0013 | Kenya | [66] |
| M98/0068, 94/0039, 95/0306, 98/0002 and I92/0067 | Switzerland | [103] |
| Guajira, Guajira 3, Guajira 4, Concha Rosada, and MeVen 77-1 | Venezuela | [104] |
| OMM 1207-22 | Indonesia | [105] |
| MLG 10361 and MLG 10362 | Indonesia | [106] |
| 8S501 | India | [107] |
4.6. The Use of Genotypes with Enhanced Carbon Assimilation Capacity
5. Breeding Technologies for Cassava Drought Tolerance and WUE
5.1. Genes Associated with Drought Tolerance and WUE
| Genes | Coded Proteins | Function | References |
|---|---|---|---|
| WRKY | WRKY transcription factor family | Regulatory pathways for drought tolerance Drought stress responses. Water uptake from the soil. | [48] |
| MeALDH MeZFP MeMSD MeRD28 | Aldehyde dehydrogenase Zinc Finger Protein Manganese Superoxide Dismutase Responsive to Dehydration 28 | Drought stress tolerance Regulating metabolic pathways | [67] |
| MeABF, MeBADH1 | ABA-binding factor; betaine aldehyde dehydrogenase | Drought tolerance Water use efficiency | [116] |
| IPT | Isopentenyl transferase | Involved in cytokinin biosynthesis, affecting plant growth and drought stress responses. | [119] |
| MeERF | Ethylene-responsive factor | It coordinates ethylene signaling with drought-responsive pathways by modulating stomatal closure and leaf senescence. | [120] |
| MeKUP | Potassium transporter | Upregulation of metabolites that contribute to drought tolerance. | [121] |
| AREB1 | ABA-responsive element binding protein | Involved in drought response | [122] |
| ALDH7B4 | Aldehyde dehydrogenase | Drought tolerance | [123] |
| MeRSZ21b | Serine/arginine-rich splicing factor | Improved drought tolerance Modulating ABA-dependent signaling. | [124] |
| MeAMY MeBAM | α- and β-amylases | Drought tolerance | [125] |
5.2. Quantitative Trait Loci (QTL) Associated with Drought Tolerance and WUE
5.3. Proteins Associated with Drought Tolerance and WUE
5.4. Metabolites Associated with Drought Tolerance and WUE
5.5. Utilization of Drought Tolerance Indices
| Indices | Description | Formula | Reference |
|---|---|---|---|
| Drought susceptibility index | Measures the sensitivity of a genotype to drought based on tuber yield performance | [142] | |
| Stress susceptibility index | Evaluates the relative susceptibility of a genotype to drought. | [142] | |
| Tolerance index | Measures the genotype’s drought tolerance based on tuber yield differences. | Yp − Ys | [143] |
| Yield stability index | Assesses stability by comparing tuber yields under stress to non-stress conditions | [144] | |
| Stress tolerance index | Quantify tuber yield performance and drought tolerance of cassava cultivars. | [145] | |
| Yield index | Compares each genotype’s performance relative to the average of all the evaluated genotypes | [146] | |
| Mean productivity | Calculates the average tuber yield of a genotype. | [147] | |
| Geometric mean productivity | It accounts for variability in tuber yield performance across environments (drought stress and non-stress). | [147] | |
| Harmonic mean | Measures cultivar performance based on mean yield response | [148] |
5.6. Multiple Traits Selection
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohidin, S.R.N.S.P.; Moshawih, S.; Hermansyah, A.; Asmuni, M.I.; Shafqat, N.; Ming, L.C. Cassava (Manihot esculenta Crantz): A Systematic Review for the Pharmacological Activities, Traditional Uses, Nutritional Values, and Phytochemistry. J. Evid. Based Integr. Med. 2023, 28, 2515690X231206227. [Google Scholar] [CrossRef]
- Parmar, A.; Sturm, B.; Hensel, O. Crops That Feed the World: Production and Improvement of Cassava for Food, Feed, and Industrial Uses. Food Secur. 2017, 9, 907–927. [Google Scholar] [CrossRef]
- Abirami, V.; Velmurugan, M.; Thangamani, C.; Natarajan, S.K.; Davamani, V.; Indu, R.C.; Venkatachalam, S.R. Cassava Intercropping Systems for Enhanced Land Productivity and Farmer Livelihoods: A Review. Plant Sci. Today 2025, 12, 8409. [Google Scholar] [CrossRef]
- Mutua, K.; Iddi, R. Promoting Cassava Value Addition among Smallholder Farmers: What Drivers Matter in Kenya? The Case of Cassava Farmers in Busia County, Kenya. Int. J. Food Agric. Econ. 2024, 12, 307–318. [Google Scholar]
- Amelework, A.B.; Bairu, M.W. Advances in Genetic Analysis and Breeding of Cassava (Manihot esculenta Crantz): A Review. Plants 2022, 11, 1617. [Google Scholar] [CrossRef]
- Orek, C. A Review of Management of Major Arthropod Pests Affecting Cassava Production in Sub-Saharan Africa. Crop Prot. 2024, 175, 106465. [Google Scholar] [CrossRef]
- Amelework, A.B.; Bairu, M.W.; Maema, O.; Venter, S.L.; Laing, M. Adoption and Promotion of Resilient Crops for Climate Risk Mitigation and Import Substitution: A Case Analysis of Cassava for South African Agriculture. Front. Sustain. Food Syst. 2021, 5, 617783. [Google Scholar] [CrossRef]
- Borku, A.W. Cassava (Manihot esculenta Crantz): Its Nutritional Composition Insights for Future Research and Development in Ethiopia. Discov. Sustain. 2025, 6, 404. [Google Scholar] [CrossRef]
- Muiruri, S.K.; Ntui, V.O.; Tripathi, L.; Tripathi, J.N. Mechanisms and Approaches towards Enhanced Drought Tolerance in Cassava (Manihot esculenta). Curr. Plant Biol. 2021, 28, 100227. [Google Scholar] [CrossRef]
- Zhu, L.; Yi, H.; Su, H.; Guikema, S.; Liu, B. Impacts of Climate Change on Cassava Yield and Lifecycle Energy and Greenhouse Gas Performance of Cassava Ethanol Systems: An Example from Guangxi Province, China. J. Environ. Manag. 2023, 347, 119162. [Google Scholar] [CrossRef]
- Guo, Y. Closing Yield Gap of Cassava for Food Security in West Africa. Nat. Food 2020, 1, 325. [Google Scholar] [CrossRef]
- More, S.J.; Bardhan, K.; Ravi, V.; Pasala, R.; Chaturvedi, A.K.; Lal, M.K.; Siddique, K.H.M. Morphophysiological Responses and Tolerance Mechanisms in Cassava (Manihot esculenta Crantz) Under Drought Stress. J. Soil Sci. Plant Nutr. 2023, 23, 71–91. [Google Scholar] [CrossRef]
- Blum, A. Drought Resistance, Water-Use Efficiency, and Yield Potential—Are They Compatible, Dissonant, or Mutually Exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H. C3-C4 Intermediate Photosynthetic Characteristics of Cassava (Manihot esculenta Crantz). Photosynth. Res. 1987, 12, 219–235. [Google Scholar] [CrossRef]
- Okogbenin, E.; Setter, T.L.; Ferguson, M.; Mutegi, R.; Ceballos, H.; Olasanmi, B.; Fregene, M. Phenotypic Approaches to Drought in Cassava: Review. Front. Physiol. 2013, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Tofu, D.A.; Haile, F.; Tolossa, T. Livelihood Vulnerability and Socio-Economic Determinants of Households to Climate Change-Induced Recurrent Drought in Ethiopia. GeoJournal 2023, 88, 5043–5067. [Google Scholar] [CrossRef]
- Ayanlade, A.; Oluwaranti, A.; Ayanlade, O.S.; Borderon, M.; Sterly, H.; Sakdapolrak, P.; Jegede, M.O.; Weldemariam, L.F.; Ayinde, A.F.O. Extreme Climate Events in Sub-Saharan Africa: A Call for Improving Agricultural Technology Transfer to Enhance Adaptive Capacity. Clim. Serv. 2022, 27, 100311. [Google Scholar] [CrossRef]
- FSC Food Security Cluster. Available online: https://fscluster.org/sites/default/files/2024-05/ROSEA_SADC_El-Nino_Southern-Africa_Response_Plan_17052024-ENG%201_0.pdf (accessed on 9 November 2024).
- Affoh, R.; Zheng, H.; Dangui, K.; Dissani, B.M. The Impact of Climate Variability and Change on Food Security in Sub-Saharan Africa: Perspective from Panel Data Analysis. Sustainability 2022, 14, 759. [Google Scholar] [CrossRef]
- Shan, Z.; Luo, X.; Wei, M.; Huang, T.; Khan, A.; Zhu, Y. Physiological and Proteomic Analysis on Long-Term Drought Resistance of Cassava (Manihot esculenta Crantz). Sci. Rep. 2018, 8, 17982. [Google Scholar] [CrossRef]
- Orek, C.; Gruissem, W.; Ferguson, M.; Vanderschuren, H. Morpho-Physiological and Molecular Evaluation of Drought Tolerance in Cassava (Manihot esculenta Crantz). Field Crops Res. 2020, 255, 107861. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, F.; Zhang, N.; Wu, C.; Lin, J.; Lin, S.; Pan, Z.; Lei, X.; Zheng, J.; Guo, J.; et al. Overexpression of the Cassava (Manihot esculenta) PP2C26 Gene Decreases Drought Tolerance and Abscisic Acid Responses in Transgenic Arabidopsis Thaliana. Biochem. Biophys. Res. Commun. 2025, 760, 151715. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2009; pp. 153–188. [Google Scholar]
- Bayram, S.; Soylu, S.; Elif, Y. Drought and Oxidative Stress. Afr. J. Biotechnol. 2011, 10, 11102–11109. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Ibrahim, O.R.; Opabode, J.T. Pre-Treatment of Two Contrasting Water-Stressed Genotypes of Cassava (Manihot esculenta Crantz) with Ascorbic Acid. I. Growth, Physiological and Antioxidant Responses. Physiol. Mol. Biol. Plants 2019, 25, 1385–1394. [Google Scholar] [CrossRef]
- Charles, O. A Review of Drought-Stress Responsive Genes and Their Applications for Drought Stress Tolerance in Cassava (Manihot esculenta Crantz). Discov. Biotechnol. 2024, 1, 5. [Google Scholar] [CrossRef]
- Van Laere, J.; Willemen, A.; De Bauw, P.; Hood-Nowotny, R.; Merckx, R.; Dercon, G. Carbon Allocation in Cassava Is Affected by Water Deficit and Potassium Application—A 13C-CO2 Pulse Labelling Assessment. Rapid Commun. Mass Spectrom. 2022, 37, e9426. [Google Scholar] [CrossRef]
- Ma, M.; Gu, J.; Wang, Z.-Y. An Optimization Method for Measuring the Stomata in Cassava (Manihot esculenta Crantz) under Multiple Abiotic Stresses. Open Life Sci. 2024, 19, 20220993. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Mansour, E.; Abdul-Hamid, M.I.; Yasin, M.T.; Qabil, N.; Attia, A. Identifying Drought-Tolerant Genotypes of Barley and Their Responses to Various Irrigation Levels in a Mediterranean Environment. Agric. Water Manag. 2017, 194, 58–67. [Google Scholar] [CrossRef]
- Wongnoi, S.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P. Physiology, Growth and Yield of Different Cassava Genotypes Planted in Upland with Dry Environment during High Storage Root Accumulation Stage. Agronomy 2020, 10, 576. [Google Scholar] [CrossRef]
- Olanrewaju, O.O.; Olufayo, A.A.; Oguntunde, P.G.; Ilemobade, A.A. Water Use Efficiency of Manihot esculenta Crantz under Drip Irrigation System in South Western Nigeria. Eur. J. Sci. Res. 2009, 27, 576–587. [Google Scholar]
- de Oliveira, E.C.; Miglioranza, É. Density and Stomata Distribution in Cassava Manihot esculenta Crantz IAC 576-70. Sci. Agropecu. 2014, 5, 135–140. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Physiological Characteristics of Cassava Tolerance to Prolonged Drought in the Tropics: Implications for Breeding Cultivars Adapted to Seasonally Dry and Semiarid Environments. Braz. J. Plant Physiol. 2007, 19, 257–286. [Google Scholar] [CrossRef]
- Pardales, J.R., Jr.; Esquisel, C.B. Effect of Drought During the Establishment Period on the Root System Development of Cassava. Jpn. J. Crop Sci. 1996, 65, 93–97. [Google Scholar] [CrossRef]
- Sangakkara, U.R.; Attanayake, K.B.; Gajanayake, J.N.; Bandaranayake, P. Impact of Mulches on Growth and Yields of Cassava and Sweet Potato in Tropical Asia. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Odubanjo, O.O.; Olufayo, A.A.; Oguntunde, P.G. Water Use, Growth, and Yield of Drip Irrigated Cassava in a Humid Tropical Environment. Soil Water Res. 2011, 6, 10–20. [Google Scholar] [CrossRef]
- Barakat, M.A.S.; Abd El-Mageed, T.A.; Elsayed, I.N.; Semida, W.M. Effect of Soil Mulching on Growth, Productivity, and Water Use Efficiency of Potato (Solanum tuberosum L.) under Deficit Irrigation. Arch. Agric. Environ. Sci. 2020, 5, 328–336. [Google Scholar] [CrossRef]
- Hall, R.D.; D’Auria, J.C.; Ferreira, A.C.S.; Gibon, Y.; Kruszka, D.; Mishra, P.; Van de Zedde, R. High-Throughput Plant Phenotyping: A Role for Metabolomics? Trends Plant Sci. 2022, 27, 549–563. [Google Scholar] [CrossRef]
- Kengkanna, J.; Jakaew, P.; Amawan, S.; Busener, N.; Bucksch, A.; Saengwilai, P. Phenotypic Variation of Cassava Root Traits and Their Responses to Drought. Appl. Plant Sci. 2019, 7, e01238. [Google Scholar] [CrossRef]
- Shoukat, S.; Tufail, A. Drought Stress Management in Potatoes: Physiological, Biochemical, and Agronomic Strategies. Appl. Agric. Sci. 2025, 3, 1–8. [Google Scholar] [CrossRef]
- Helal, N.A.S.; Eisa, S.S.; Attia, A. Morphological and Chemical Studies on Influence of Water Deficit on Cassava. World J. Agric. Sci. 2013, 9, 369–376. [Google Scholar]
- Da Silva, E.C.; Nogueira, R.; Da Silva, M.A.; De Albuquerque, M.B. Drought Stress and Plant Nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Ghadirnezhad Shiade, S.R.; Fathi, A.; Taghavi Ghasemkheili, F.; Amiri, E.; Pessarakli, M. Plants’ Responses under Drought Stress Conditions: Effects of Strategic Management Approaches—A Review. J. Plant Nutr. 2022, 46, 2198–2230. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, X.; Nawaz, G.; Yin, J.; Yang, J. Physiological and Biochemical Responses of Four Cassava Cultivars to Drought Stress. Sci. Rep. 2020, 10, 6968. [Google Scholar] [CrossRef] [PubMed]
- Avivi, S.; Sanjaya, B.R.L.; Ogita, S.; Hartatik, S.; Soeparjono, S. Morphological, Physiological and Molecular Responses of Indonesian Cassava to Drought Stress. Aust. J. Crop Sci. 2020, 14, 1723–1727. [Google Scholar] [CrossRef]
- Orek, C. A Review of the Functions of Transcription Factors and Related Genes Involved in Cassava (Manihot esculenta Crantz) Response to Drought Stress. Trop. Plants 2023, 2, 14. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Pereira, L.F.M.; Santos, H.L.; Zanetti, S.; Brito, I.A.d.O.; Tozin, L.R.d.S.; Rodrigues, T.M.; Silva, M.d.A. Morphology, Biochemistry, and Yield of Cassava as Functions of Growth Stage and Water Regime. S. Afr. J. Bot. 2022, 149, 222–239. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and Salt Tolerance in Plants. CRC Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Hsu, P.-K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling Mechanisms in Abscisic Acid-Mediated Stomatal Closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef]
- Rai, G.K.; Khanday, D.M.; Choudhary, S.M.; Kumar, P.; Kumari, S.; Martínez-Andújar, C.; Martínez-Melgarejo, P.A.; Rai, P.K.; Pérez-Alfocea, F. Unlocking Nature’s Stress Buster: Abscisic Acid’s Crucial Role in Defending Plants against Abiotic Stress. Plant Stress 2024, 11, 100359. [Google Scholar] [CrossRef]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription Factors Interact with ABA through Gene Expression and Signaling Pathways to Mitigate Drought and Salinity Stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef]
- Vandegeer, R.; Miller, R.E.; Bain, M.; Gleadow, R.M.; Cavagnaro, T.R. Drought Adversely Affects Tuber Development and Nutritional Quality of the Staple Crop Cassava (Manihot esculenta Crantz). Funct. Plant Biol. 2013, 40, 195. [Google Scholar] [CrossRef]
- Alves, A.A.C. Cassava Botany and Physiology. In Cassava: Biology, Production and Utilization; CABI: Wallingford, UK, 2001; pp. 67–89. [Google Scholar]
- El-Sharkawy, M.A.; Cadavid, L.F. Response of Cassava to Prolonged Water Stress Imposed at Different Stages of Growth. Exp. Agric. 2002, 38, 333–350. [Google Scholar] [CrossRef]
- Nejad, T.S. Effect of Drought Stress on Shoot/Root Ratio. World Acad. Sci. Eng. Technol. 2011, 5, 539–541. [Google Scholar]
- Lahai, M.T.; Ekanayake, I.J.; George, J.B. Leaf Chlorophyll Content and Tuberous Root Yield of Cassava in Inland Valley. Afr. Crop Sci. J. 2003, 11, 107–117. [Google Scholar] [CrossRef]
- Santanoo, S.; Ittipong, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Vongcharoen, K.; Theerakulpisut, P. Photosynthetic Performance, Carbohydrate Partitioning, Growth, and Yield among Cassava Genotypes under Full Irrigation and Early Drought Treatment in a Tropical Savanna Climate. Plants 2024, 13, 2049. [Google Scholar] [CrossRef]
- de Oliveira, E.J.; Morgante, C.V.; de Tarso Aidar, S.; de Melo Chaves, A.R.; Antonio, R.P.; Cruz, J.L.; Filho, M.A.C. Evaluation of Cassava Germplasm for Drought Tolerance under Field Conditions. Euphytica 2017, 213, 188. [Google Scholar] [CrossRef]
- Aina, O.O.; Dixon, A.G.O.; Akinrinde, E.A. Effect of Soil Moisture Stress on Growth and Yield of Cassava in Nigeria. Pak. J. Biol. Sci. 2007, 10, 3085–3090. [Google Scholar] [CrossRef]
- Turyagyenda, F.L.; Kizito, E.B.; Baguma, Y.; Osiru, D. Evaluation of Ugandan cassava germplasm for drought tolerance. Int. J. Agric. Crop Sci. 2013, 5, 212–226. [Google Scholar]
- Adjebeng-Danquah, J.; Gracen, V.E.; Offei, S.K.; Asante, I.K.; Manu-Aduening, J. Genetic Variability in Storage Root Bulking of Cassava Genotypes under Irrigation and No Irrigation. Agric. Food Secur. 2016, 5, 9. [Google Scholar] [CrossRef]
- Abiola, M.B.; Oyetunji, O.J. Determining the Tolerance of Selected Cassava (Manihot esculenta Crantz) Genotypes to Drought and Salinity Using Chlorophyll, Phenol and Yield as Screening Tools. Int. J. Plant Soil Sci. 2021, 33, 60–73. [Google Scholar] [CrossRef]
- Nadia, H.S.; Paul, K.; Cornelius, M.W.; Elijah, M.A. Phenotypic Evaluation of Cassava Genotypes (Manihot esculenta) under Moisture Stress. Afr. J. Agric. Res. 2021, 17, 836–843. [Google Scholar] [CrossRef]
- Turyagyenda, L.F.; Kizito, E.B.; Ferguson, M.; Baguma, Y.; Agaba, M.; Harvey, J.J.W.; Osiru, D.S.O. Physiological and Molecular Characterization of Drought Responses and Identification of Candidate Tolerance Genes in Cassava. AoB Plants 2013, 5, plt007. [Google Scholar] [CrossRef]
- Ravi, V.; Raju, S.; More, S.J. Evaluation of Potential Increase in Photosynthetic Efficiency of Cassava (Manihot esculenta Crantz) Plants Exposed to Elevated Carbon Dioxide. Funct. Plant Biol. 2024, 51, FP23254. [Google Scholar] [CrossRef]
- Alves, A.A.C.; Setter, T.L. Response of Cassava to Water Deficit: Leaf Area Growth and Abscisic Acid. Crop Sci. 2000, 40, 131–137. [Google Scholar] [CrossRef]
- Tang, B.; Man, J.; Romero, F.; Bergmann, J.; Lehmann, A.; Rillig, M.C. Mycorrhization Enhances Plant Growth and Stabilizes Biomass Allocation under Drought. Glob. Change Biol. 2024, 30, e17438. [Google Scholar] [CrossRef]
- Bergantin, V.R.; Yamauchi, A.; Pardales, J.R., Jr.; Bolatete , D.M., Jr. Screening Cassava Genotypes for Resistance to Water Deficit during Crop Establishment. Philipp. J. Crop Sci. 2004, 29, 29–39. [Google Scholar]
- Malele, K.P. Genotypic Variation in Water Use Efficiency, Gaseous Exchange and Yield of Four Cassava Landraces Grown under Rainfed Conditions in South Africa. Ph.D. Thesis, University of Venda, Thohoyandou, South Africa, 2020. [Google Scholar]
- Wasonga, D.O.; Kleemola, J.; Alakukku, L.; Mäkelä, P.S.A. Growth Response of Cassava to Deficit Irrigation and Potassium Fertigation during the Early Growth Phase. Agronomy 2020, 10, 321. [Google Scholar] [CrossRef]
- Cock, J.H.; Connor, D.J. Cassava. In Crop Physiology Case Histories for Major Crops; Elsevier: Amsterdam, The Netherlands, 2021; pp. 588–633. [Google Scholar]
- Sevanto, S. Drought Impacts on Phloem Transport. Curr. Opin. Plant Biol. 2018, 43, 76–81. [Google Scholar] [CrossRef]
- Zierer, W.; Rüscher, D.; Sonnewald, U.; Sonnewald, S. Tuber and Tuberous Root Development. Annu. Rev. Plant Biol. 2021, 72, 551–580. [Google Scholar] [CrossRef]
- Feller, U. Drought Stress and Carbon Assimilation in a Warming Climate: Reversible and Irreversible Impacts. J. Plant Physiol. 2016, 203, 84–94. [Google Scholar] [CrossRef]
- Adiele, J.G. Growing Out of Hunger: Towards an Improved Understanding of the Water and Nutrient Limited Yield of Cassava. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2020. [Google Scholar]
- Nyaika, J.; Abayomi, L.; Parmar, A.; Coast, O. Cyanide in Cassava: Understanding the Drivers, Impacts of Climate Variability, and Strategies for Food Security. Food Energy Secur. 2024, 13, e573. [Google Scholar] [CrossRef]
- Koundinya, A.V.V.; Ajeesh, B.R.; Lekshmi, N.S.; Hegde, V.; Sheela, M.N. Classification of Genotypes, Leaf Retention, Pith Density and Carbohydrate Dynamics in Cassava under Water Deficit Stress Conditions. Acta Physiol. Plant 2023, 45, 83. [Google Scholar] [CrossRef]
- Chang, L.; Wang, L.; Peng, C.; Tong, Z.; Wang, D.; Ding, G.; Xiao, J.; Guo, A.; Wang, X. The Chloroplast Proteome Response to Drought Stress in Cassava Leaves. Plant Physiol. Biochem. 2019, 142, 351–362. [Google Scholar] [CrossRef]
- Meyer, A.J.; Dreyer, A.; Ugalde, J.M.; Feitosa-Araujo, E.; Dietz, K.-J.; Schwarzländer, M. Shifting Paradigms and Novel Players in Cys-Based Redox Regulation and ROS Signaling in Plants-and Where to Go Next. Biol. Chem. 2021, 402, 399–423. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, P.; Lu, Y.; Bai, Y.; Wei, Y.; Liu, G.; Shi, H. MeRAV5 Promotes Drought Stress Resistance in Cassava by Modulating Hydrogen Peroxide and Lignin Accumulation. Plant J. 2021, 107, 847–860. [Google Scholar] [CrossRef]
- Chemonges, M.; Balyejusa, E.K.; Bisikwa, J.; Osiru, D.S.O. Phenotypic and Physiological Traits Associated with Drought Tolerant Cassava Cultivars in Uganda. Afr. Crop Sci. Conf. Proc. 2013, 11, 463–469. [Google Scholar]
- Du, C.; Li, L.; Effah, Z. Effects of Straw Mulching and Reduced Tillage on Crop Production and Environment: A Review. Water 2022, 14, 2471. [Google Scholar] [CrossRef]
- Mhazo, N.; Chivenge, P.; Chaplot, V. Tillage Impact on Soil Erosion by Water: Discrepancies Due to Climate and Soil Characteristics. Agric. Ecosyst. Environ. 2016, 230, 231–241. [Google Scholar] [CrossRef]
- Bwalya, B.; Mutandwa, E.; Chiluba, B.C. Awareness and Use of Sustainable Land Management Practices in Smallholder Farming Systems. Sustainability 2023, 15, 14660. [Google Scholar] [CrossRef]
- Ito, M.; Matsumoto, T.; Quinones, M.A. Conservation Tillage Practice in Sub-Saharan Africa: The Experience of Sasakawa Global 2000. Crop Prot. 2007, 26, 417–423. [Google Scholar] [CrossRef]
- Mavesere, F.; Dzawanda, B. Effectiveness of Pfumvudza as a Resilient Strategy against Drought Impacts in Rural Communities of Zimbabwe. GeoJournal 2023, 88, 3455–3470. [Google Scholar] [CrossRef]
- Friedlander, L.; Tal, A.; Lazarovitch, N. Technical Considerations Affecting Adoption of Drip Irrigation in Sub-Saharan Africa. Agric. Water Manag. 2013, 126, 125–132. [Google Scholar] [CrossRef]
- Kalra, A.; Goel, S.; Elias, A.A. Understanding Role of Roots in Plant Response to Drought: Way Forward to Climate-resilient Crops. Plant Genome 2023, 17, e20395. [Google Scholar] [CrossRef]
- Mupakati, T.; Tanyanyiwa, V.I. Cassava Production as a Climate Change Adaptation Strategy in Chilonga Ward, Chiredzi District, Zimbabwe. Jamba 2017, 9, 348. [Google Scholar] [CrossRef]
- Haider, S.; Bibi, K.; Munyaneza, V.; Zhang, H.; Zhang, W.; Ali, A.; Ahmad, I.A.; Mehran, M.; Xu, F.; Yang, C.; et al. Drought-Induced Adaptive and Ameliorative Strategies in Plants. Chemosphere 2024, 364, 143134. [Google Scholar] [CrossRef] [PubMed]
- Negin, B.; Moshelion, M. The Evolution of the Role of ABA in the Regulation of Water-Use Efficiency: From Biochemical Mechanisms to Stomatal Conductance. Plant Sci. 2016, 251, 82–89. [Google Scholar] [CrossRef]
- Burns, A.; Gleadow, R.; Cliff, J.; Zacarias, A.; Cavagnaro, T. Cassava: The Drought, War and Famine Crop in a Changing World. Sustainability 2010, 2, 3572–3607. [Google Scholar] [CrossRef]
- Ewa, F. Genetic Mapping and Evaluation of Cassava (Manihot esculenta Crantz) for Drought Tolerance and Early Bulking in Marginal Savannah Ecology of Nigeria. Ph.D. Thesis, University of Limpopo, Polokwane, South Africa, 2021. [Google Scholar]
- Muiruri, K.S.; Fathima, A.A. Advances in Cassava Trait Improvement and Processing Technologies for Food and Feed; IntechOpen: London, UK, 2023. [Google Scholar]
- Yahaya, M.A.; Shimelis, H. Drought Stress in Sorghum: Mitigation Strategies, Breeding Methods and Technologies—A Review. J. Agron. Crop Sci. 2022, 208, 127–142. [Google Scholar] [CrossRef]
- Adjebeng-Danquah, J.; Acheremu, K.; Asante, S.K.; Kusi, F.; Osei, C. Evaluation of Early Bulking Cassava Accessions for High Yield Potential for the Guinea Savannah Zone of Ghana. Ghana J. Agric. Sci. 2012, 45, 61–70. [Google Scholar]
- Abah, S.P.; Mbe, J.O.; Dzidzienyo, D.K.; Njoku, D.; Onyeka, J.; Danquah, E.Y.; Offei, S.K.; Kulakow, P.; Egesi, C.N. Determination of Genomic Regions Associated with Early Storage Root Formation and Bulking in Cassava. Front. Plant Sci. 2024, 15, 1391452. [Google Scholar] [CrossRef]
- Karim, K.Y.; Ifie, B.; Dzidzienyo, D.; Danquah, E.Y.; Blay, E.T.; Whyte, J.B.A.; Kulakow, P.; Rabbi, I.; Parkes, E.; Omoigui, L.; et al. Genetic Characterization of Cassava (Manihot esculenta Crantz) Genotypes Using Agro-Morphological and Single Nucleotide Polymorphism Markers. Physiol. Mol. Biol. Plants 2020, 26, 317–330. [Google Scholar] [CrossRef]
- Diaguna, R.; Suwarto; Santosa, E.; Hartono, A.; Pramuhadi, G.; Nuryartono, N.; Yusfiandayani, R.; Prartono, T. Morphological and Physiological Characterization of Cassava Genotypes on Dry Land of Ultisol Soil in Indonesia. Int. J. Agron. 2022, 2022, 3599272. [Google Scholar] [CrossRef]
- Ochieng’Orek, C. Morphological, Physiological and Molecular Characterization of Drought Tolerance in Cassava (Manihot esculenta Crantz). Ph.D. Thesis, ETH, Zurich, Switzerland, 2014. [Google Scholar]
- Leon Pacheco, R.I.; Pérez Macias, M.; Fuenmayor Campos, F.C.; Rodríguez Izquierdo, A.J.; Rodríguez Izquierdo, G.A. Agronomic and Physiological Evaluation of Eight Cassava Clones under Water Deficit Conditions. Rev. Fac. Nac. Agron. Medellin 2020, 73, 9109–9119. [Google Scholar] [CrossRef]
- Wahyuni, T.S.; Noerwijati, K. Cassava Genotypes Selection for High Yield and High Starch Content in Advanced Yield Trials. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012127. [Google Scholar] [CrossRef]
- Pratiwi, H.; Wahyuni, T.S.; Nugrahaeni, N. Multiple Tolerances of Cassava Germplasm to Drought Stress and Red Spider Mite Attacks. Biosaintifika J. Biol. Biol. Educ. 2022, 14, 293–300. [Google Scholar] [CrossRef]
- Koundinya, A.V.V.; Nisha, A.; Ajeesh, B.R. Early Vigour: A Key to Drought Tolerance in Cassava Based on Physiological and Biochemical Traits Including Inherent Non-Enzymatic Antioxidant Activity. Sci. Hortic. 2024, 331, 113110. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, L.; Xu, X.; Li, D.; Qiu, H.; Cao, X. Evaluation of Long-Term Carbon Sequestration of Biochar in Soil with Biogeochemical Field Model. Sci. Total Environ. 2022, 822, 153576. [Google Scholar] [CrossRef]
- Jozay, M.; Zarei, H.; Khorasaninejad, S.; Miri, T. Maximising CO2 Sequestration in the City: The Role of Green Walls in Sustainable Urban Development. Pollutants 2024, 4, 91–116. [Google Scholar] [CrossRef]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-Mediated Carbon Capture and Utilization by Microalgae towards Sustainable CO2 Biofixation and Biomass Valorization—A Review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Rajalekshmi, K.; Bastin, B. Potential of Wastelands for Carbon Sequestration—A Review. Int. J. Chem. Stud. 2020, 8, 2873–2881. [Google Scholar] [CrossRef]
- Heinemann, H.; Hirte, J.; Seidel, F.; Don, A. Increasing Root Biomass Derived Carbon Input to Agricultural Soils by Genotype Selection—A Review. Plant Soil 2023, 490, 19–30. [Google Scholar] [CrossRef]
- Punyasu, N.; Thaiprasit, J.; Kalapanulak, S.; Saithong, T.; Postma, J.A. Modeling Cassava Root System Architecture and the Underlying Dynamics in Shoot–Root Carbon Allocation during the Early Storage Root Bulking Stage. Plant Soil 2024, 507, 863–880. [Google Scholar] [CrossRef]
- Rüscher, D.; Vasina, V.V.; Knoblauch, J.; Bellin, L.; Pommerrenig, B.; Alseekh, S.; Fernie, A.R.; Neuhaus, H.E.; Knoblauch, M.; Sonnewald, U.; et al. Symplasmic Phloem Loading and Subcellular Transport in Storage Roots Are Key Factors for Carbon Allocation in Cassava. Plant Physiol. 2024, 196, 1322–1339. [Google Scholar] [CrossRef]
- Feng, R.-J.; Ren, M.-Y.; Lu, L.-F.; Peng, M.; Guan, X.; Zhou, D.-B.; Zhang, M.-Y.; Qi, D.-F.; Li, K.; Tang, W.; et al. Involvement of Abscisic Acid-Responsive Element-Binding Factors in Cassava (Manihot esculenta) Dehydration Stress Response. Sci. Rep. 2019, 9, 12661. [Google Scholar] [CrossRef]
- Gudi, S.; Kumar, P.; Singh, S.; Tanin, M.J.; Sharma, A. Strategies for Accelerating Genetic Gains in Crop Plants: Special Focus on Speed Breeding. Physiol. Mol. Biol. Plants 2022, 28, 1921–1938. [Google Scholar] [CrossRef] [PubMed]
- Shikha, M.; Kanika, A.; Rao, A.R.; Mallikarjuna, M.G.; Gupta, H.S.; Nepolean, T. Genomic Selection for Drought Tolerance Using Genome-Wide SNPs in Maize. Front. Plant Sci. 2017, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed Leaf Senescence Induces Extreme Drought Tolerance in a Flowering Plant. Proc. Natl. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar] [CrossRef]
- Fan, W.; Hai, M.; Guo, Y.; Ding, Z.; Tie, W.; Ding, X.; Yan, Y.; Wei, Y.; Liu, Y.; Wu, C.; et al. The ERF Transcription Factor Family in Cassava: Genome-Wide Characterization and Expression Analyses against Drought Stress. Sci. Rep. 2016, 6, 37379. [Google Scholar] [CrossRef]
- Ou, W.; Mao, X.; Huang, C.; Tie, W.; Yan, Y.; Ding, Z.; Wu, C.; Xia, Z.; Wang, W.; Zhou, S.; et al. Genome-Wide Identification and Expression Analysis of the KUP Family under Abiotic Stress in Cassava (Manihot esculenta Crantz). Front. Physiol. 2018, 9, 17. [Google Scholar] [CrossRef]
- Roca Paixão, J.F.; Gillet, F.-X.; Ribeiro, T.P.; Bournaud, C.; Lourenço-Tessutti, I.T.; Noriega, D.D.; Melo, B.P.d.; de Almeida-Engler, J.; Grossi-de-Sa, M.F. Improved Drought Stress Tolerance in Arabidopsis by CRISPR/DCas9 Fusion with a Histone AcetylTransferase. Sci. Rep. 2019, 9, 8080. [Google Scholar] [CrossRef]
- Fayiah, J.S.; Ndunguru, J.C.; Gwakisa, P.S.; Nyuma, H.T. Survey for the Expression Levels of Drought Tolerant Genes in Cassava Varieties in Tanzania. Am. J. Multidiscip. Res. Dev. 2021, 3, 29–43. [Google Scholar]
- Chen, Y.; Weng, X.; Zhou, X.; Gu, J.; Hu, Q.; Luo, Q.; Wen, M.; Li, C.; Wang, Z.-Y. Overexpression of Cassava RSZ21b Enhances Drought Tolerance in Arabidopsis. J. Plant Physiol. 2022, 268, 153574. [Google Scholar] [CrossRef]
- Yang, T.; Li, H.; Li, L.; Wei, W.; Huang, Y.; Xiong, F.; Wei, M. Genome-Wide Characterization and Expression Analysis of α-Amylase and β-Amylase Genes Underlying Drought Tolerance in Cassava. BMC Genom. 2023, 24, 190. [Google Scholar] [CrossRef]
- CTC. The Nature and Identification of Quantitative Trait Loci: A Community’s View. Nat. Rev. Genet. 2003, 4, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, S.; Mao, K.; Dong, Q.; Liang, B.; Li, C.; Wei, Z.; Li, M.; Ma, F. Mapping QTLs for Water-Use Efficiency Reveals the Potential Candidate Genes Involved in Regulating the Trait in Apple under Drought Stress. BMC Plant Biol. 2018, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, X.; Dai, S.; Cui, X.; Cao, X.; Liu, Z.; Shen, J. The Multilocular Trait of Rapeseed Is Ideal for High-yield Breeding. Plant Breed. 2020, 140, 65–73. [Google Scholar] [CrossRef]
- Utsumi, Y.; Tanaka, M.; Morosawa, T.; Kurotani, A.; Yoshida, T.; Mochida, K.; Matsui, A.; Umemura, Y.; Ishitani, M.; Shinozaki, K.; et al. Transcriptome Analysis Using a High-Density Oligomicroarray under Drought Stress in Various Genotypes of Cassava: An Important Tropical Crop. DNA Res. 2012, 19, 335–345. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Cereal Crop Proteomics: Systemic Analysis of Crop Drought Stress Responses Towards Marker-Assisted Selection Breeding. Front. Plant Sci. 2017, 8, 757. [Google Scholar] [CrossRef]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.F.; et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Klingler, J.P.; Batelli, G.; Zhu, J.-K. ABA Receptors: The START of a New Paradigm in Phytohormone Signalling. J. Exp. Bot. 2010, 61, 3199–3210. [Google Scholar] [CrossRef]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The Central Role of Transcription Factors in Bridging Biotic and Abiotic Stress Responses for Plants’ Resilience. New Crops 2024, 1, 100005. [Google Scholar] [CrossRef]
- Xie, H.; Yang, D.-H.; Yao, H.; Bai, G.; Zhang, Y.-H.; Xiao, B.-G. ITRAQ-Based Quantitative Proteomic Analysis Reveals Proteomic Changes in Leaves of Cultivated Tobacco (Nicotiana tabacum) in Response to Drought Stress. Biochem. Biophys. Res. Commun. 2016, 469, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Waheeda, K.; Kitchel, H.; Wang, Q.; Chiu, P.-L. Molecular Mechanism of Rubisco Activase: Dynamic Assembly and Rubisco Remodeling. Front. Mol. Biosci. 2023, 10, 1125922. [Google Scholar] [CrossRef]
- Zeng, C.; Ding, Z.; Zhou, F.; Zhou, Y.; Yang, R.; Yang, Z.; Wang, W.; Peng, M. The Discrepant and Similar Responses of Genome-Wide Transcriptional Profiles between Drought and Cold Stresses in Cassava. Int. J. Mol. Sci. 2017, 18, 2668. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and Its Actions during the Drought Stress in Plants. Physiol. Plant 2020, 172, 1321–1335. [Google Scholar] [CrossRef]
- Misra, B.B.; Acharya, B.R.; Granot, D.; Assmann, S.M.; Chen, S. The Guard Cell Metabolome: Functions in Stomatal Movement and Global Food Security. Front. Plant Sci. 2015, 6, 334. [Google Scholar] [CrossRef]
- Wang, L.; Ma, K.-B.; Lu, Z.-G.; Ren, S.-X.; Jiang, H.-R.; Cui, J.-W.; Chen, G.; Teng, N.-J.; Lam, H.-M.; Jin, B. Differential Physiological, Transcriptomic and Metabolomic Responses of Arabidopsis Leaves under Prolonged Warming and Heat Shock. BMC Plant Biol. 2020, 20, 86. [Google Scholar] [CrossRef]
- Ephraim, N.; Patrick, R.R.; Ssetumba, M.; Samuel, K.; Joseph, F.H.; Yona, B. Biochemical and Secondary Metabolites Changes under Moisture and Temperature Stress in Cassava (Manihot esculenta Crantz). Afr. J. Biotechnol 2014, 13, 3173–3186. [Google Scholar] [CrossRef]
- Mutanda, M.; Shimelis, H.; Chaplot, V.; Figlan, S. Managing Drought Stress in Wheat (Triticum aestivum L.) Production: Strategies and Impacts. S. Afr. J. Plant Soil 2025, 42, 1–12. [Google Scholar] [CrossRef]
- Araneda-Cabrera, R.J.; Bermúdez, M.; Puertas, J. Assessment of the Performance of Drought Indices for Explaining Crop Yield Variability at the National Scale: Methodological Framework and Application to Mozambique. Agric. Water Manag. 2021, 246, 106692. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought Resistance in Spring Wheat Cultivars. I. Grain Yield Responses. Aust. J. Agric. Res. 1978, 29, 897. [Google Scholar] [CrossRef]
- Rosielle, A.A.; Hamblin, J. Theoretical Aspects of Selection for Yield in Stress and Non-Stress Environment. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T. Stress Tolerance in Soybeans. I. Evaluation of Three Screening Techniques for Heat and Drought Tolerance. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective Selection Criteria for Assessing Plant Stress Tolerance; AVRDC: Taipei, Taiwan, 1993. [Google Scholar]
- Gavuzzi, P.; Rizza, F.; Palumbo, M.; Campanile, R.G.; Ricciardi, G.L.; Borghi, B. Evaluation of Field and Laboratory Predictors of Drought and Heat Tolerance in Winter Cereals. Can. J. Plant Sci. 1997, 77, 523–531. [Google Scholar] [CrossRef]
- Ramirez-Vallejo, P.; Kelly, J.D. Traits Related to Drought Resistance in Common Bean. Euphytica 1998, 99, 127–136. [Google Scholar] [CrossRef]
- Jafari, A.; Paknejad, F.; JAMI, A.M. Evaluation of Selection Indices for Drought Tolerance of Corn (Zea mays L.) Hybrids. Int. J. Biol. Sci. 2009, 3, 33–38. [Google Scholar]
- Ewa, F.; Asiwe, J.N.A.; Okogbenin, E.; Ogbonna, A.C.; Egesi, C. KASPar SNP Genetic Map of Cassava for QTL Discovery of Productivity Traits in Moderate Drought Stress Environment in Africa. Sci. Rep. 2021, 11, 11268. [Google Scholar] [CrossRef]
- Amelework, A.B.; Bairu, M.W.; Marx, R.; Owoeye, L.; Laing, M.; Venter, S.L. On-Farm Multi-Environment Evaluation of Selected Cassava (Manihot esculenta Crantz) Cultivars in South Africa. Plants 2022, 11, 3339. [Google Scholar] [CrossRef]
- de Oliveira, C.R.S.d.; Borel, J.C.; Pereira, D.A.; Carvalho, B.P.d.; Medrado, E.d.S.; Ishikawa, F.H.; Oliveira, E.J.d. Genetic Parameters and Path Analysis for Root Yield of Cassava under Drought and Early Harvest. Crop Breed. Appl. Biotechnol. 2021, 21, e36162137. [Google Scholar] [CrossRef]
- Vieira, S.L.; Oliveira, C.R.S.d.; Pereira, D.A.; Borel, J.C.; Oliveira, E.J.d. Early Evaluation of Genotype x Harvest Interactions in Cassava Crops under Water Stress. Rev. Caatinga 2024, 37, e11458. [Google Scholar] [CrossRef]
- Setiawan, K.; Paresta, R.; Hadi, M.S.; Utomo, S.D.; Karyanto, A. Ardian Correlation and Path Analysis of Three Elite Clones of Cassava (Manihot esculenta Crantz). IOP Conf. Ser. Earth Environ. Sci. 2023, 1208, 012030. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutanda, M.; Amelework, A.B.; Ndou, N.; Figlan, S. Drought Stress in Cassava (Manihot esculenta): Management Strategies and Breeding Technologies. Int. J. Plant Biol. 2025, 16, 112. https://doi.org/10.3390/ijpb16040112
Mutanda M, Amelework AB, Ndou N, Figlan S. Drought Stress in Cassava (Manihot esculenta): Management Strategies and Breeding Technologies. International Journal of Plant Biology. 2025; 16(4):112. https://doi.org/10.3390/ijpb16040112
Chicago/Turabian StyleMutanda, Maltase, Assefa B. Amelework, Nzumbululo Ndou, and Sandiswa Figlan. 2025. "Drought Stress in Cassava (Manihot esculenta): Management Strategies and Breeding Technologies" International Journal of Plant Biology 16, no. 4: 112. https://doi.org/10.3390/ijpb16040112
APA StyleMutanda, M., Amelework, A. B., Ndou, N., & Figlan, S. (2025). Drought Stress in Cassava (Manihot esculenta): Management Strategies and Breeding Technologies. International Journal of Plant Biology, 16(4), 112. https://doi.org/10.3390/ijpb16040112







