Optimizing Ethephon Concentrations for Male Plant Feminization and Enhanced Seed Yield in Dioecious Thai Hemp (Cannabis sativa L. cv. RPF3)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Materials
2.2. Ethephon Preparation
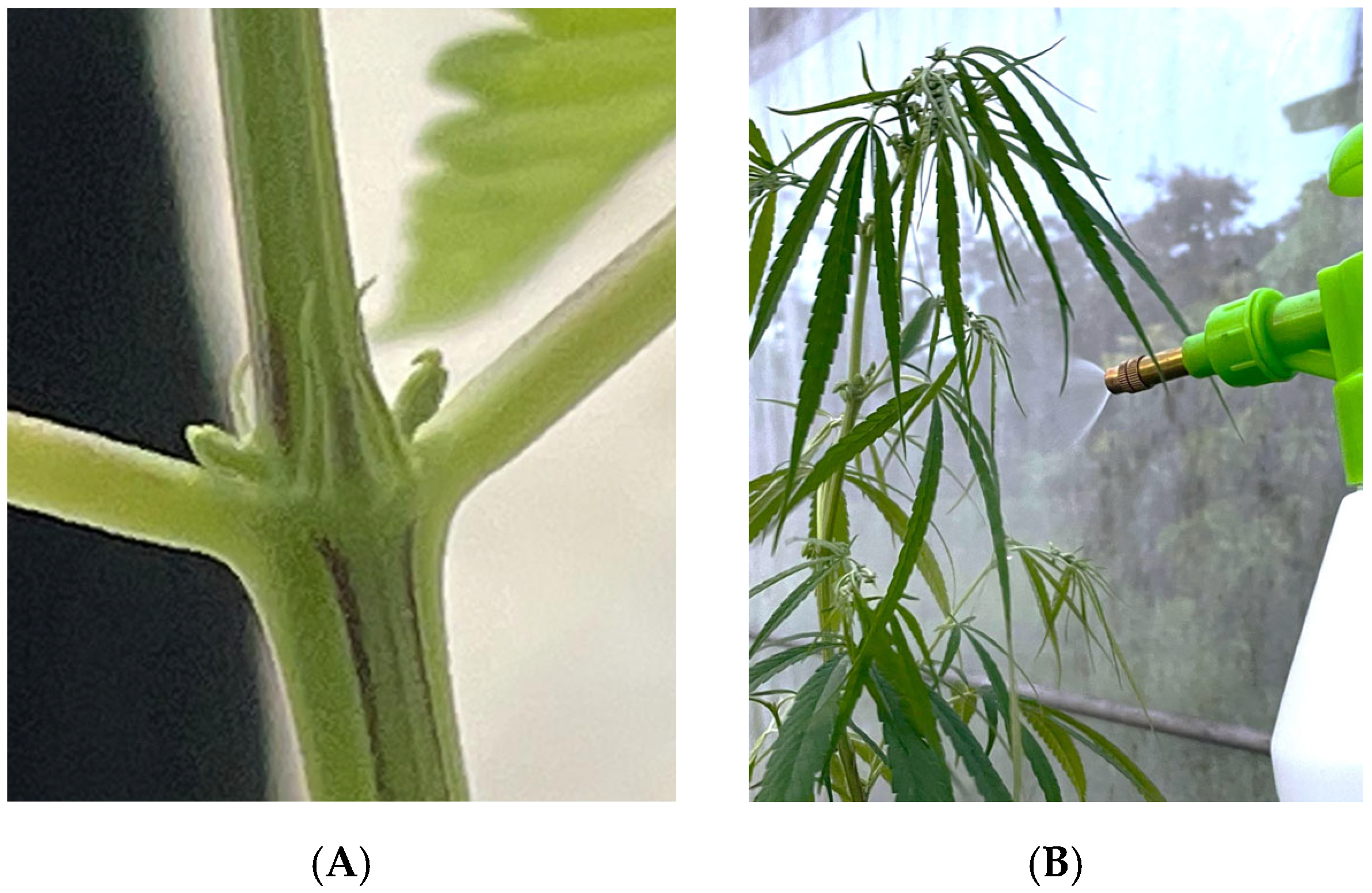
2.3. Ethephon Application
2.4. Data Collection
2.5. Statistical Analysis
3. Results
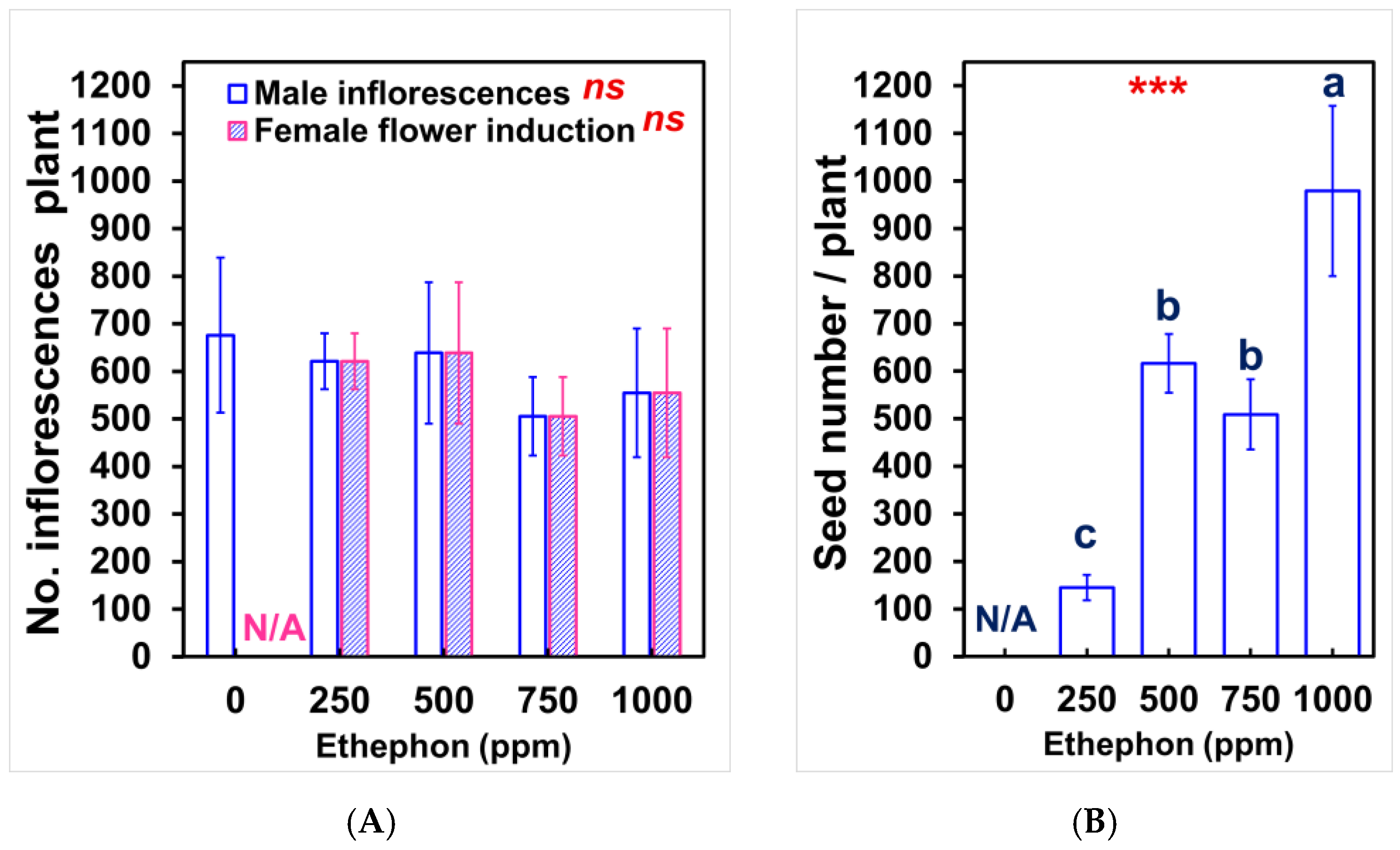
3.1. Sex Expression Timing and Ratios
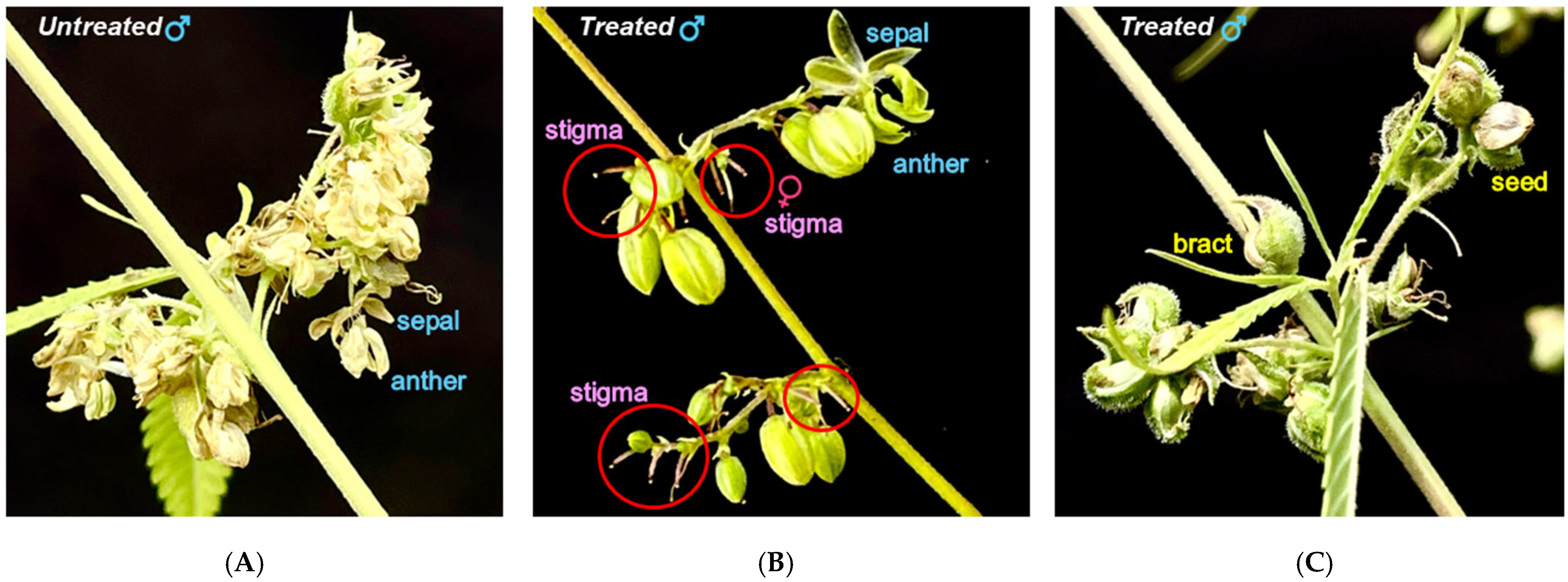
3.2. Ethephon-Induced Feminization and Reproductive Conversion in Males
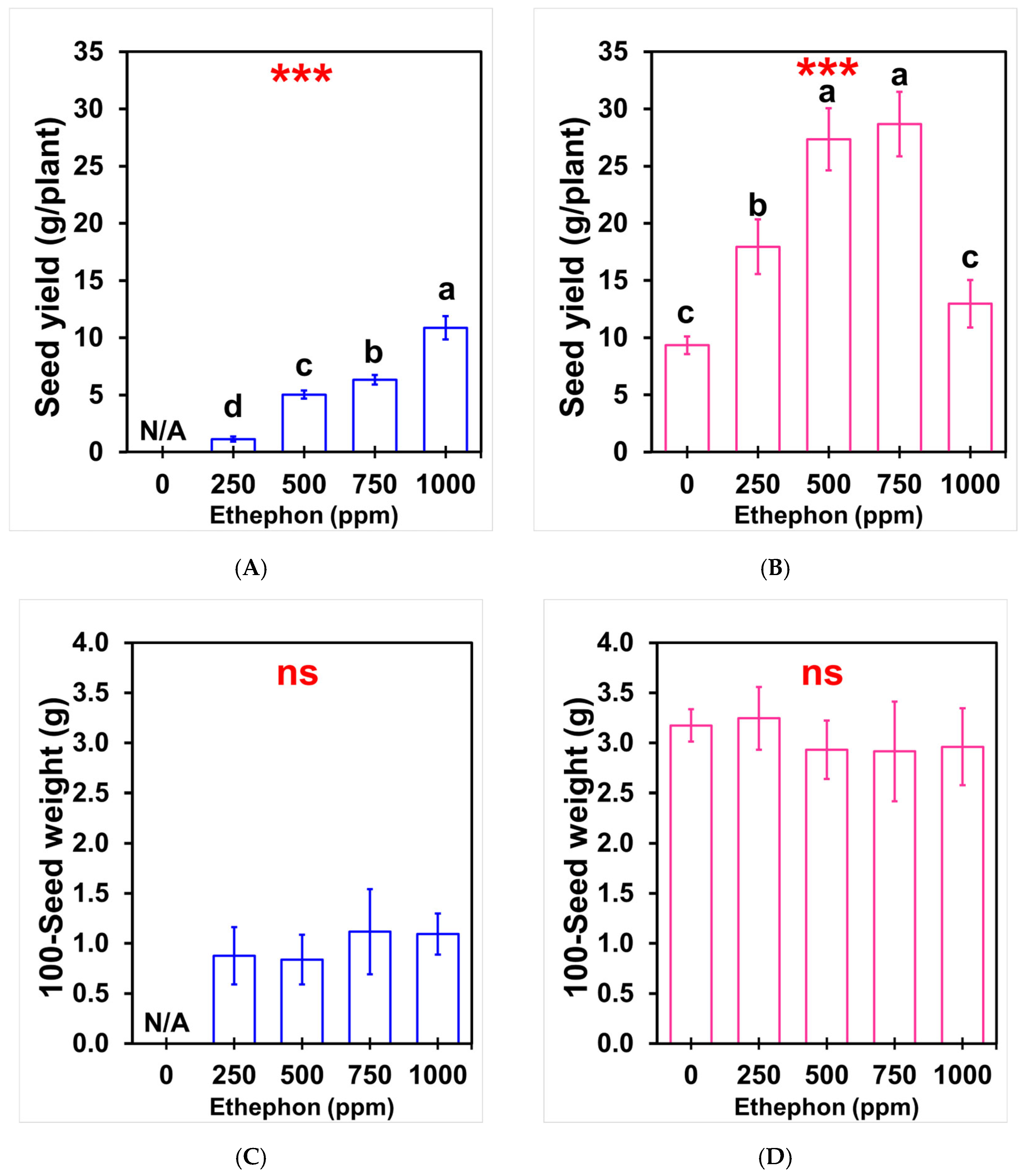
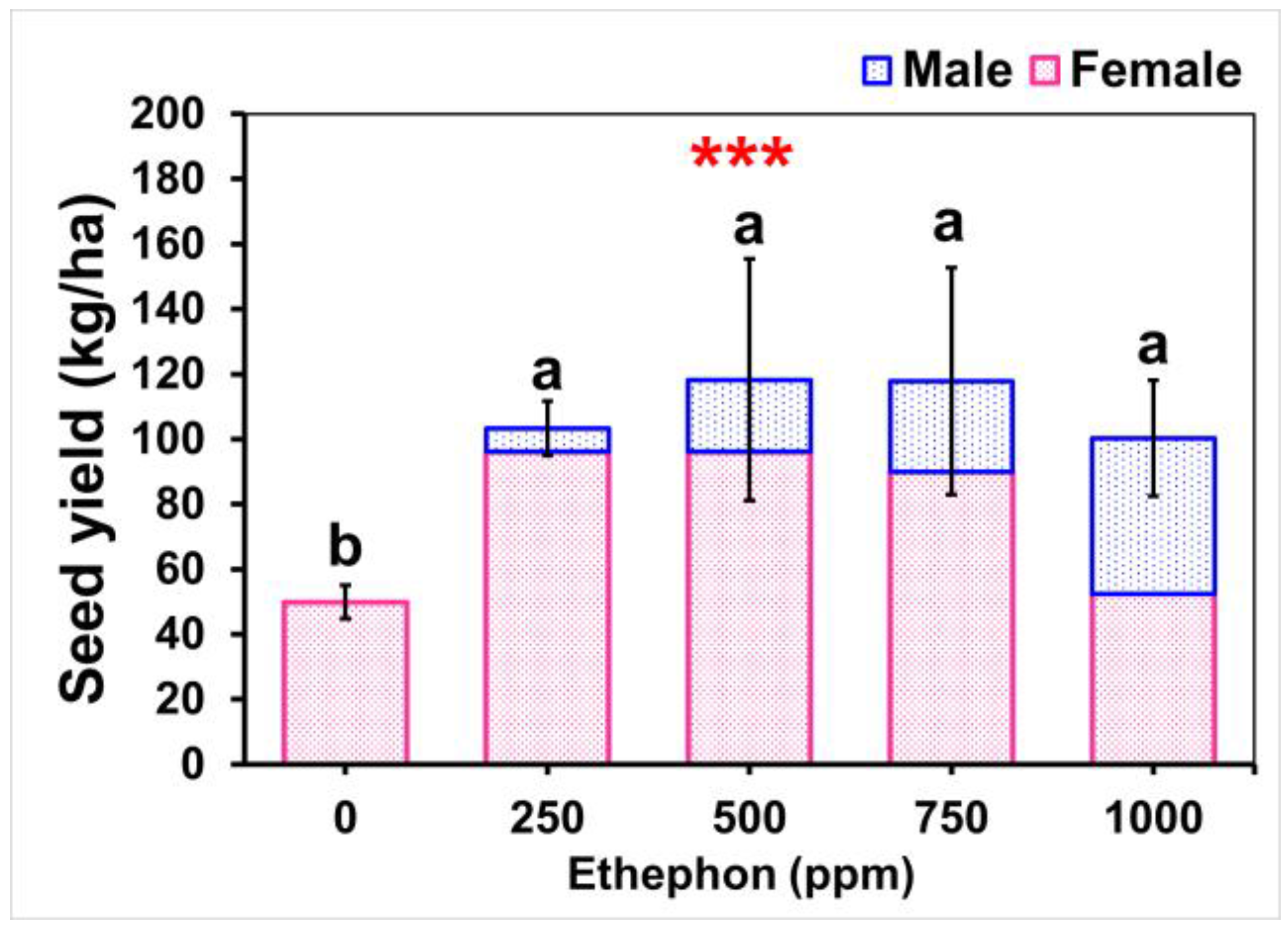
3.3. Effects of Ethephon on Seed Yield and Weight Parameters
3.4. Population Productivity and Concentration–Response Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schluttenhofer, C.; Yuan, L. Challenges towards Revitalizing Hemp: A Multifaceted Crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef] [PubMed]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the Quality of Protein from Hemp Seed (Cannabis sativa L.) Products Through the Use of the Protein Digestibility-Corrected Amino Acid Score Method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Chen, H.; Xu, B.; Wang, Y.; Li, W.; He, D.; Zhang, Y.; Zhang, X.; Xing, X. Emerging Natural Hemp Seed Proteins and Their Functions for Nutraceutical Applications. Food Sci. Hum. Wellness 2023, 12, 929–941. [Google Scholar] [CrossRef]
- Divashuk, M.G.; Alexandrov, O.S.; Razumova, O.V.; Kirov, I.V.; Karlov, G.I. Molecular Cytogenetic Characterization of the Dioecious Cannabis sativa with an XY Chromosome Sex Determination System. PLoS ONE 2014, 9, e85118. [Google Scholar] [CrossRef] [PubMed]
- Sawler, J.; Stout, J.M.; Gardner, K.M.; Hudson, D.; Vidmar, J.; Butler, L.; Page, J.E.; Myles, S. The Genetic Structure of Marijuana and Hemp. PLoS ONE 2015, 10, e0133292. [Google Scholar] [CrossRef]
- Moliterni, V.M.C.; Cattivelli, L.; Ranalli, P.; Mandolino, G. The Sexual Differentiation of Cannabis sativa L.: A Morphological and Molecular Study. Euphytica 2004, 140, 95–106. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Too Dense or Not Too Dense: Higher Planting Density Reduces Cannabinoid Uniformity but Increases Yield/Area in Drug-Type Medical Cannabis. Front. Plant Sci. 2022, 13, 713481. [Google Scholar] [CrossRef]
- Faux, A.-M.; Draye, X.; Lambert, R.; d’Andrimont, R.; Raulier, P.; Bertin, P. The Relationship of Stem and Seed Yields to Flowering Phenology and Sex Expression in Monoecious Hemp (Cannabis sativa L.). Eur. J. Agron. 2013, 47, 11–22. [Google Scholar] [CrossRef]
- Piluzza, G.; Delogu, G.; Cabras, A.; Marceddu, S.; Bullitta, S. Differentiation between Fiber and Drug Types of Hemp (Cannabis sativa L.) from a Collection of Wild and Domesticated Accessions. Genet. Resour. Crop Evol. 2013, 60, 2331–2342. [Google Scholar] [CrossRef]
- Byers, R.E.; Baker, L.R.; Sell, H.M.; Herner, R.C.; Dilley, D.R. Ethylene: A Natural Regulator of Sex Expression of Cucumis melo L. Proc. Natl. Acad. Sci. USA 1972, 69, 717–720. [Google Scholar] [CrossRef]
- Boualem, A.; Troadec, C.; Camps, C.; Lemhemdi, A.; Morin, H.; Sari, M.-A.; Fraenkel-Zagouri, R.; Kovalski, I.; Dogimont, C.; Perl-Treves, R.; et al. A Cucurbit Androecy Gene Reveals How Unisexual Flowers Develop and Dioecy Emerges. Science 2015, 350, 688–691. [Google Scholar] [CrossRef]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The Yin-Yang of Hormones: Cytokinin and Auxin Interactions in Plant Development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- Yang, H.-W.; Akagi, T.; Kawakatsu, T.; Tao, R. Gene Networks Orchestrated by MeGI: A Single-Factor Mechanism Underlying Sex Determination in Persimmon. Plant J. 2019, 98, 97–111. [Google Scholar] [CrossRef]
- Galoch, E. The Hormonal Control of Sex Differentiation in Dioecious Plants of Hemp (Cannabis sativa). The Influence of Plant Growth Regulators on Sex Expression in Male and Female Plants. Acta Soc. Bot. Pol. 1978, 47, 153–162. [Google Scholar] [CrossRef]
- Ram, H.Y.M.; Jaiswal, V.S. Induction of Female Flowers on Male Plants of Cannabis sativa L. by 2-Chloroethanephos-Phonic Acid. Experientia 1970, 26, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Rudich, J.; Halevy, A.H.; Kedar, N. Ethylene Evolution from Cucumber Plants as Related to Sex Expression. Plant Physiol. 1972, 49, 998–999. [Google Scholar] [CrossRef]
- García, A.; Aguado, E.; Martínez, C.; Loska, D.; Beltrán, S.; Valenzuela, J.L.; Garrido, D.; Jamilena, M. The Ethylene Receptors CpETR1A and CpETR2B Cooperate in the Control of Sex Determination in Cucurbita Pepo. J. Exp. Bot. 2020, 71, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M. Ethylene in Plant Biology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 1–414. [Google Scholar]
- Vandenbussche, F.; Pierik, R.; Millenaar, F.F.; Voesenek, L.A.; Van Der Straeten, D. Reaching out of the Shade. Curr. Opin. Plant Biol. 2005, 8, 462–468. [Google Scholar] [CrossRef]
- Mudge, K.W.; Swanson, B.T. Effect of Ethephon, Indole Butyric Acid, and Treatment Solution pH on Rooting and on Ethylene Levels within Mung Bean Cuttings. Plant Physiol. 1978, 61, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wen, C.-K. Preparation of Ethylene Gas and Comparison of Ethylene Responses Induced by Ethylene, ACC, and Ethephon. Plant Physiol. Biochem. 2010, 48, 45–53. [Google Scholar] [CrossRef]
- Cathey, H.M.; Stuart, N.W. Comparative Plant Growth-Retarding Activity of Amo-1618, Phosfon, and CCC. Bot. Gaz. 1961, 123, 51–57. [Google Scholar] [CrossRef]
- Moon, Y.-H.; Lee, Y.J.; Koo, S.C.; Hur, M.; Huh, Y.C.; Chang, J.-K.; Park, W.T. Effect of Timing of Ethephon Treatment on the Formation of Female Flowers and Seeds from Male Plant of Hemp (Cannabis sativa L.). Korean J. Plant Res. 2020, 33, 682–688. [Google Scholar] [CrossRef]
- Garcia-de Heer, L.; Guo, Q.; Mieog, J.; Nolan, M.; Liu, L.; Dimopoulos, N.; Melzer, R.; Kretzschmar, T. Transcriptomic Analysis of Ethephon-Induced Sex Reversion of Male Cannabis sativa Reveals Changes in Expression of Floral Homeotic Genes and a Distinct Trichome Morphology. J. Exp. Bot. 2025, eraf291. [Google Scholar] [CrossRef]
- Ruzgas, R.; Tilvikienė, V.; Barčauskaitė, K.; Viršilė, A.; Žydelis, R. Ethephon’s Effects on Sexual Expression and Cannabinoid Production in Monoecious and Dioecious Varieties of Hemp (Cannabis sativa L.): A Field Trial. Ind. Crops Prod. 2024, 210, 118064. [Google Scholar] [CrossRef]
- Thongplew, P.; Kangsopa, J.; Hermhuk, S.; Tongkoom, K.; Bhuyar, P.; Insalud, N. Sex Expression and Seed Yield Stability in Thai Hemp (Cannabis sativa L.): Seasonal Effects on Dioecious Cultivars for Optimized Seed Production. Int. J. Plant Biol. 2025, 16, 67. [Google Scholar] [CrossRef]
- Johnson, R. Hemp as an Agricultural Commodity. Congressional Research Service Report RL32725. Available online: https://www.everycrsreport.com/files/20121218_RL32725_ca894ef3e91fd4d909ccb71569b76d93d850ace6.pdf (accessed on 21 May 2025).
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key Cultivation Techniques for Hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New Developments in Fiber Hemp (Cannabis sativa L.) Breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Amaducci, S. Comparing Hemp (Cannabis sativa L.) Cultivars for Dual-Purpose Production under Contrasting Environments. Ind. Crops Prod. 2016, 87, 33–44. [Google Scholar] [CrossRef]
- Mansouri, H.; Salari, F.; Asrar, Z. Ethephon Application Stimulats Cannabinoids and Plastidic Terpenoids Production in Cannabis sativa at Flowering Stage. Ind. Crops Prod. 2013, 46, 269–273. [Google Scholar] [CrossRef]
- Stead, A.D. Pollination-Induced Flower Senescence: A Review. Plant Growth Regul. 1992, 11, 13–20. [Google Scholar] [CrossRef]
- Small, E.; Naraine, S.G.U. Expansion of Female Sex Organs in Response to Prolonged Virginity in Cannabis sativa (Marijuana). Genet. Resour. Crop Evol. 2016, 63, 339–348. [Google Scholar] [CrossRef]
- Roach, D.A.; Wulff, R.D. Maternal Effects in Plants. Annu. Rev. Ecol. Syst. 1987, 18, 209–235. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The Role of Gibberellin Signalling in Plant Responses to Abiotic Stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Törjék, O.; Bucherna, N.; Kiss, E.; Homoki, H.; Finta-Korpelová, Z.; Bócsa, I.; Nagy, I.; Heszky, L.E. Novel Male-Specific Molecular Markers (MADC5, MADC6) in Hemp. Euphytica 2002, 127, 209–218. [Google Scholar] [CrossRef]
- Flajšman, M.; Slapnik, M.; Murovec, J. Production of Feminized Seeds of High CBD Cannabis sativa L. by Manipulation of Sex Expression and Its Application to Breeding. Front. Plant Sci. 2021, 12, 718092. [Google Scholar] [CrossRef] [PubMed]
- Vonapartis, E.; Aubin, M.-P.; Seguin, P.; Mustafa, A.F.; Charron, J.-B. Seed Composition of Ten Industrial Hemp Cultivars Approved for Production in Canada. J. Food Compost. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Thawonkit, T.; Insalud, N.; Dangtungee, R.; Bhuyar, P. Integrating Sustainable Cultivation Practices and Advanced Extraction Methods for Improved Cannabis Yield and Cannabinoid Production. Int. J. Plant Biol. 2025, 16, 38. [Google Scholar] [CrossRef]


| Ethephon Concentration (ppm) | Days to First Sex Expression (DAG) | Sex Ratio (Male–Female, %) | |
|---|---|---|---|
| Male | Female | ||
| 0 | 45.3 ± 0.6 | 53.7 ± 3.7 | 44:56 |
| 250 | 46.7 ± 0.6 | 54.6 ± 3.3 | 44:56 |
| 500 | 45.4 ± 0.5 | 54.3 ± 3.1 | 44:56 |
| 750 | 45.2 ± 0.4 | 54.3 ± 2.9 | 44:56 |
| 1000 | 45.7 ± 1.2 | 54.3 ± 2.0 | 44:56 |
| F-test | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongplew, P.; Kangsopa, J.; Hermhuk, S.; Tongkoom, K.; Bhuyar, P.; Insalud, N. Optimizing Ethephon Concentrations for Male Plant Feminization and Enhanced Seed Yield in Dioecious Thai Hemp (Cannabis sativa L. cv. RPF3). Int. J. Plant Biol. 2025, 16, 111. https://doi.org/10.3390/ijpb16030111
Thongplew P, Kangsopa J, Hermhuk S, Tongkoom K, Bhuyar P, Insalud N. Optimizing Ethephon Concentrations for Male Plant Feminization and Enhanced Seed Yield in Dioecious Thai Hemp (Cannabis sativa L. cv. RPF3). International Journal of Plant Biology. 2025; 16(3):111. https://doi.org/10.3390/ijpb16030111
Chicago/Turabian StyleThongplew, Pheeraphan, Jakkrapong Kangsopa, Sutheera Hermhuk, Krittiya Tongkoom, Prakash Bhuyar, and Nednapa Insalud. 2025. "Optimizing Ethephon Concentrations for Male Plant Feminization and Enhanced Seed Yield in Dioecious Thai Hemp (Cannabis sativa L. cv. RPF3)" International Journal of Plant Biology 16, no. 3: 111. https://doi.org/10.3390/ijpb16030111
APA StyleThongplew, P., Kangsopa, J., Hermhuk, S., Tongkoom, K., Bhuyar, P., & Insalud, N. (2025). Optimizing Ethephon Concentrations for Male Plant Feminization and Enhanced Seed Yield in Dioecious Thai Hemp (Cannabis sativa L. cv. RPF3). International Journal of Plant Biology, 16(3), 111. https://doi.org/10.3390/ijpb16030111








