The Role of Jasmonates in Modulating Growth, Trichome Density, and Cannabinoid Accumulation in Cannabis sativa L.
Abstract
1. Introduction
1.1. Origin and Classification of Cannabis sativa L.
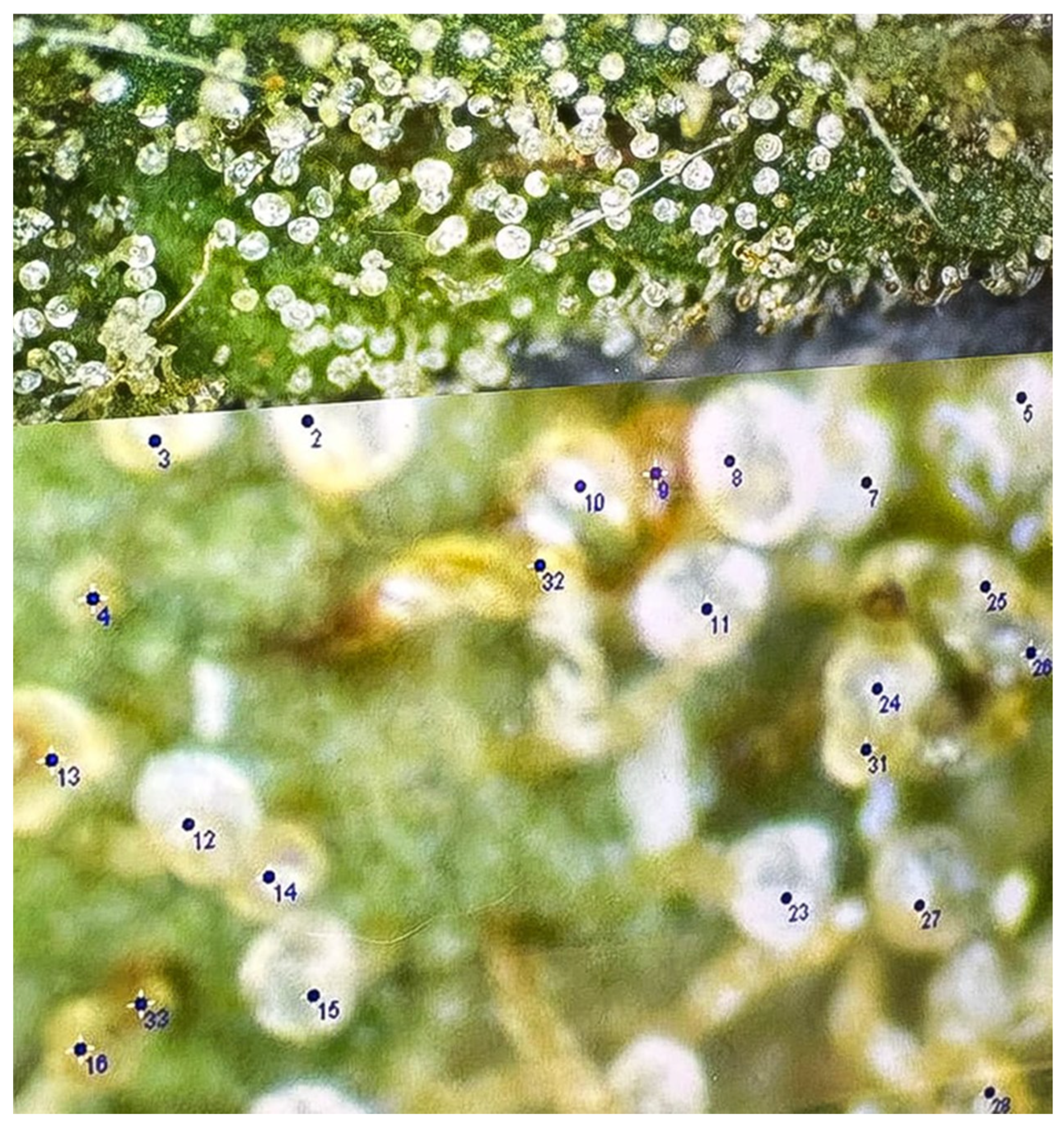
1.2. Role of Trichomes in Cannabinoid Synthesis and Economic Relevance
2. Methodology
3. Trichome Biology in Cannabis sativa L.
- A.
- Bulbous Trichomes:
- The smallest glandular form, often difficult to visualize without magnification.
- Less studied, but still capable of secreting small amounts of resinous metabolites [24].
- B.
- Capitate-Sessile Trichomes:
- Characterized by a sesquiterpene and cannabinoid-rich head directly attached to the epidermal surface.
- Typically contain eight secretory disk cells [25].
- Commonly found on bracts and sugar leaves, but less productive than capitate-stalked types.
- C.
- Capitate-Stalked Trichomes:
- Responsible for the vast majority of cannabinoid production.
- Highly visible due to their elongated stalk and large, bulbous gland head, making them a key target for horticulture/agronomic manipulation and breeding.
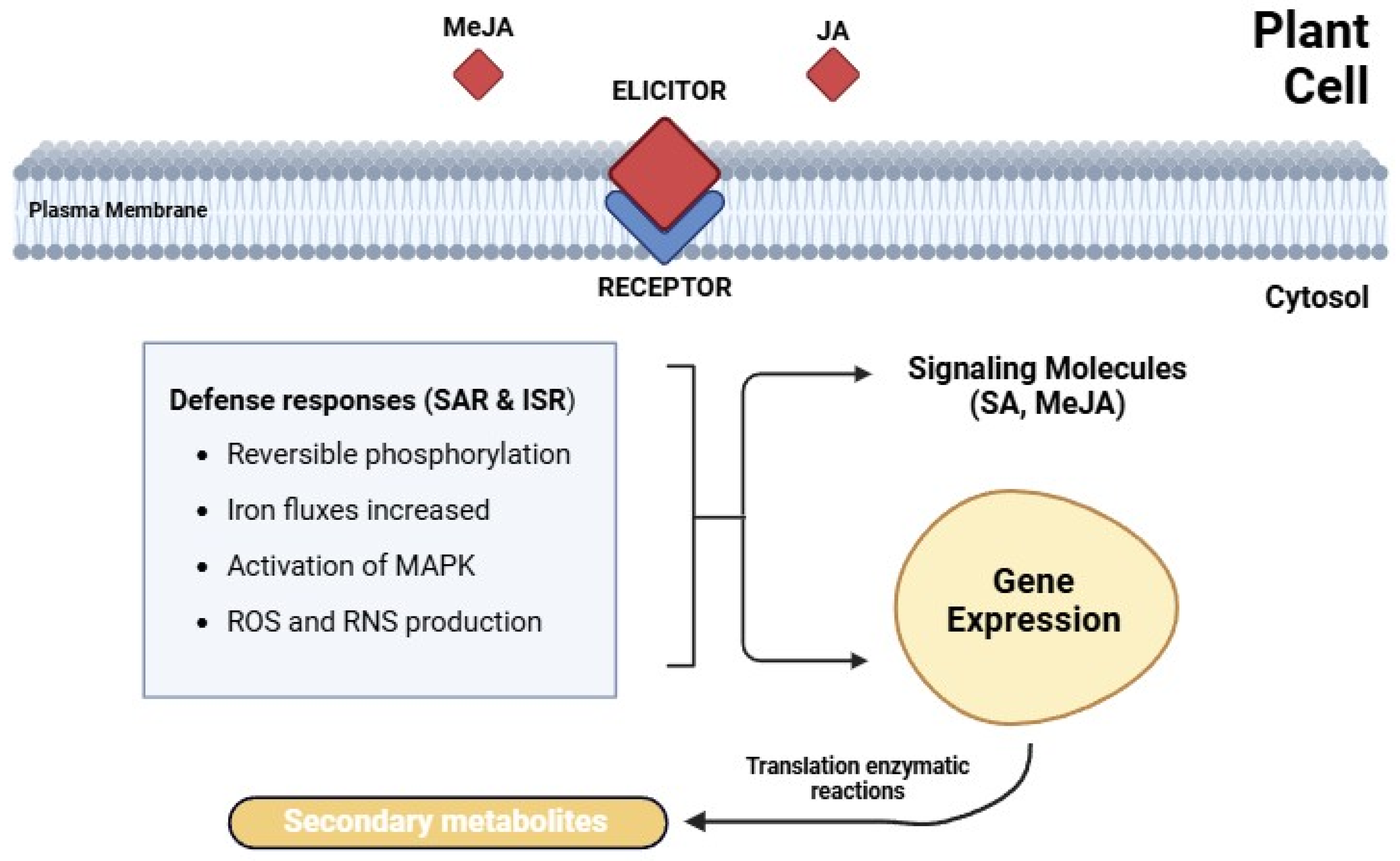
4. Jasmonates: Biosynthesis, Signaling, and Functions
4.1. MeJA as an Elicitor of Secondary Metabolism
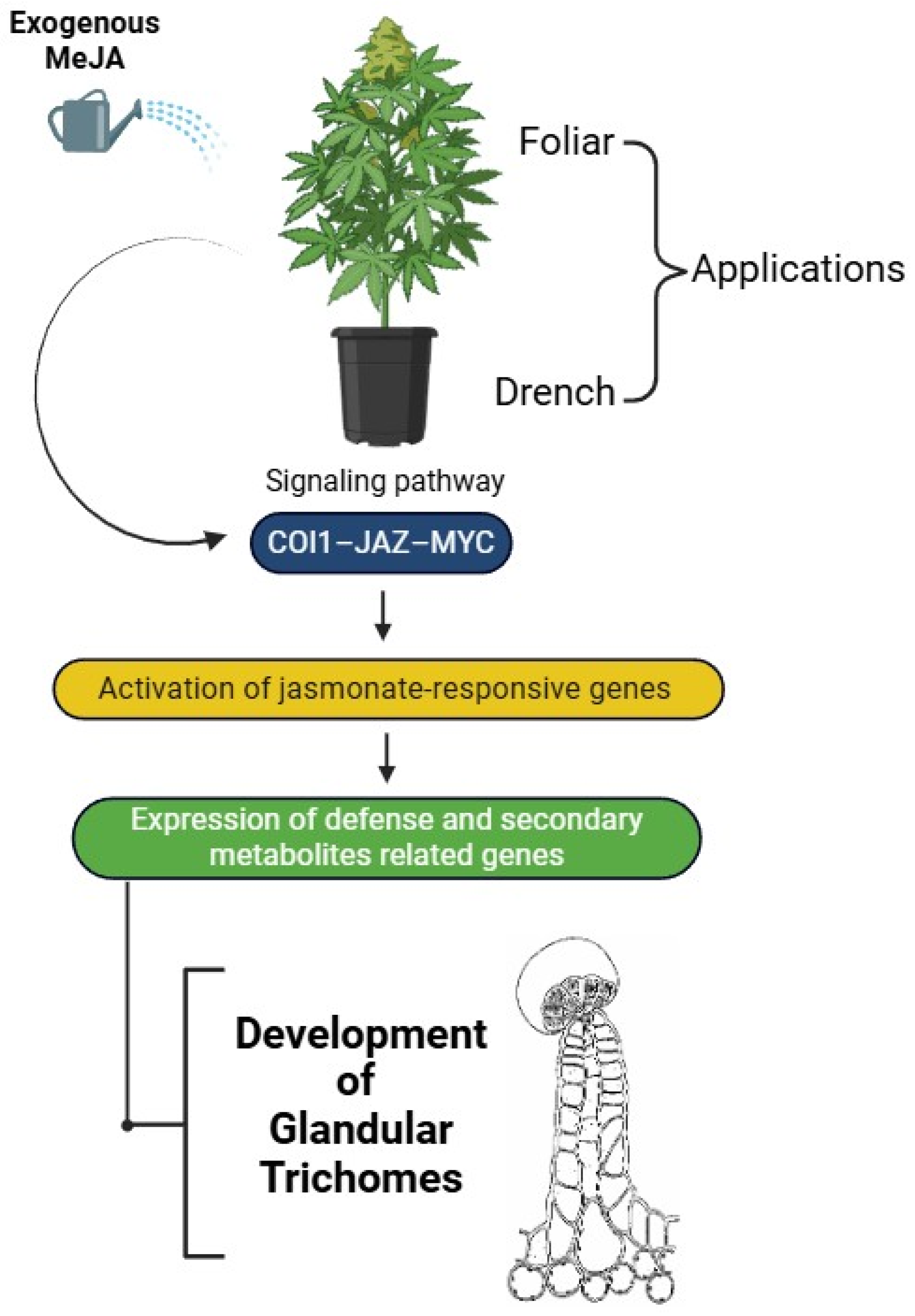
4.2. Deeper Mechanistic Insights: How MeJA Modulates Trichome Development and Cannabinoid Biosynthesis
4.2.1. MeJA Signaling Pathway and Gene Regulation in C. sativa
4.2.2. Specific Regulators in Cannabis
4.2.3. Role of Epigenetics and Post-Translational Modifications
4.2.4. Metabolite Fluxes and Enzymatic Activity
4.2.5. Crosstalk with Other Hormones at the Molecular Level
5. Practical Applications in Cannabis Cultivation
5.1. Controlled Environment Agriculture
5.2. Field Production and Stress Resistance
5.3. Integration with Other Elicitors or Stressors
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. Phytother. Res. 2018, 32, 2306–2318. [Google Scholar] [CrossRef] [PubMed]
- Small, E. Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. Botany 2015, 93, 729–753. [Google Scholar] [CrossRef]
- Li, H. An archaeological and historical account of cannabis in China. Econ. Bot. 1973, 28, 437–448. [Google Scholar] [CrossRef]
- Piluzza, G.; Delogu, G.; Cabras, A.; Marceddu, S.; Bullitta, S. Differentiation between fiber and drug types of hemp (Cannabis sativa L.) from a collection of wild and domesticated accessions. Genet. Resour. Crop Evol. 2013, 60, 2331–2342. [Google Scholar] [CrossRef]
- Preedy, V.R. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Siracusa, L.; Ruberto, G.; Cristino, L. Recent Research on Cannabis sativa L.: Phytochemistry, New Matrices, Cultivation Techniques, and Recent Updates on Its Brain-Related Effects (2018–2023). Molecules 2023, 28, 3387. [Google Scholar] [CrossRef]
- Shi, J.; Ma, C.; Qi, D.; Lv, H.; Yang, T.; Peng, Q.; Chen, Z.; Lin, Z. Transcriptional responses and flavor volatiles biosynthesis in methyl jasmonate-treated tea leaves. BMC Plant Biol. 2015, 15, 233. [Google Scholar] [CrossRef]
- Falkner, A.; Kolodinsky, J.; Mark, T.; Snell, W.; Hill, R.; Luke, A.; Shepherd, J.; Lacasse, H. The reintroduction of hemp in the USA: A content analysis of state and tribal hemp production plans. J. Cannabis Res. 2023, 5, 17. [Google Scholar] [CrossRef]
- Campbell, L.G.; Peach, K.; Wizenberg, S.B. Dioecious hemp (Cannabis sativa L.) plants do not express significant sexually dimorphic morphology in the seedling stage. Sci. Rep. 2021, 11, 16825. [Google Scholar] [CrossRef]
- Xie, Z.; Mi, Y.; Kong, L.; Gao, M.; Chen, S.; Chen, W.; Meng, X.; Sun, W.; Chen, S.; Xu, Z. Cannabis sativa: Origin and history, glandular trichome development, and cannabinoid biosynthesis. Hortic. Res. 2023, 10, uhad150. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, G.I.; Gendall, A.R.; Johnson, K.L.; Welling, M.T. Understanding the role of oxylipins in Cannabis to enhance cannabinoid production. Front. Plant Sci. 2025, 16, 1568548. [Google Scholar] [CrossRef] [PubMed]
- Tanney, C.A.S.; Backer, R.; Geitmann, A.; Smith, D.L. Cannabis Glandular Trichomes: A Cellular Metabolite Factory. Front. Plant Sci. 2021, 12, 721986. [Google Scholar] [CrossRef]
- Glas, J.J.; Schimmel, B.C.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef]
- Punja, Z.K.; Sutton, D.B.; Kim, T. Glandular trichome development, morphology, and maturation are influenced by plant age and genotype in high THC-containing cannabis (Cannabis sativa L.) inflorescences. J. Cannabis Res. 2023, 5, 12. [Google Scholar] [CrossRef]
- Da Cunha Leme Filho, J.F.; Spencer, S.; Creager, K.E.; Saunders, G.G.; Diatta, A.; Didaran, F.; Boren, A.C.; Gage, K.L. Implementation of a Standardized Cloning and Propagation Protocol for Optimizing Cannabis sativa L. Cultivation. J. Agric. Sci. 2025, 17, p1. [Google Scholar] [CrossRef]
- Da Cunha Leme Filho, J.F.; Thomason, W.; Evanylo, G.; Zhang, X.; Strickland, M.; Chim, B.K.; Diatta, A. Biochemical and physiological responses of Cannabis sativa to an integrated plant nutrition system. Agron. J. 2020, 112, 5237–5248. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Methyl Jasmonate Application Induces Increased Densities of Glandular Trichomes on Tomato, Lycopersicon esculentum. J. Chem. Ecol. 2005, 31, 2211–2216. [Google Scholar] [CrossRef]
- Hahm MS, e.a. Foliar application of methyl jasmonate in vertical farming enhances trichome formation and phytochemical content of Cannabis sativa. Horticulturae 2024, 10, 98. [Google Scholar]
- Wang, X.; Shen, C.; Meng, P.; Tan, G.; Lv, L. Analysis and review of trichomes in plants. BMC Plant Biol. 2021, 21, 70. [Google Scholar] [CrossRef]
- Kim, E.; Mahlberg, P. Immunochemical localization of tetrahydrocannabinol (THC) in cryofixed glandular trichomes of Cannabis (Cannabaceae). Am. J. Bot. 1997, 84, 336. [Google Scholar] [CrossRef]
- Mahlberg, P.G.; Kim, E.S. Accumulation of Cannabinoids in Glandular Trichomes of Cannabis (Cannabaceae). J. Ind. Hemp 2004, 9, 15–36. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Hammond, C.T.; Mahlberg, P.G. Morphogenisis of capitate glandular hairs of Cannabis sativa (Cannabaceae). Am. J. Bot. 1977, 64, 1023–1031. [Google Scholar] [CrossRef]
- Turner, J.C.; Hemphill, J.K.; Mahlberg, P.G. Cannabinoid composition and gland distribution in clones of Cannabis sativa L. (Cannabaceae). Bull. Narc. 1978, 30, 55–65. [Google Scholar] [PubMed]
- Oksanen, E. Trichomes form an important first line of defence against adverse environment-New evidence for ozone stress mitigation: Trichomes protect against hostile environment. Plant Cell Environ. 2018, 41, 1497–1499. [Google Scholar] [CrossRef]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, M.; Tooker, J.F.; Luthe, D.S.; Felton, G.W. Plants on early alert: Glandular trichomes as sensors for insect herbivores. New Phytol. 2009, 184, 644–656. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Grebenok, R.J.; Galbraith, D.W.; Bowers, W.S. Insect-Induced Synthesis of Phytoecdysteroids in Spinach, Spinacia oleracea. J. Chem. Ecol. 1999, 25, 1739–1757. [Google Scholar] [CrossRef]
- Cheong, J.J.; Choi, Y.D. Methyl jasmonate as a vital substance in plants. Trends Genet 2003, 19, 409–413. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Acosta, I.F.; Farmer, E.E. Jasmonates. Arab. Book 2010, 8, e0129. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Ryan, C.A. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA 1990, 87, 7713–7716. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Chen, H.; Jones, A.D.; Howe, G.A. Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 2006, 580, 2540–2546. [Google Scholar] [CrossRef]
- Shi, J.; Schilling, S.; Melzer, R. Morphological and genetic analysis of inflorescence and flower development in hemp (Cannabis sativa L.). BioRxiv 2024. [Google Scholar] [CrossRef]
- Shoji, T.; Ogawa, T.; Hashimoto, T. Jasmonate-induced nicotine formation in tobacco is mediated by tobacco COI1 and JAZ genes. Plant Cell Physiol. 2008, 49, 1003–1012. [Google Scholar] [CrossRef]
- Yukimune, Y.; Tabata, H.; Higashi, Y.; Hara, Y. Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat. Biotechnol. 1996, 14, 1129–1132. [Google Scholar] [CrossRef]
- Welling, M.T.; Deseo, M.A.; O’Brien, M.; Clifton, J.; Bacic, A.; Doblin, M.S. Metabolomic analysis of methyl jasmonate treatment on phytocannabinoid production in Cannabis sativa. Front. Plant Sci. 2023, 14, 1110144. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. Cell Mol. Biol. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2019, 46, 197–212. [Google Scholar] [CrossRef]
- Wang, H.; Ma, C.; Li, Z.; Ma, L.; Wang, H.; Ye, H.; Xu, G.; Liu, B. Effects of exogenous methyl jasmonate on artemisinin biosynthesis and secondary metabolism in Artemisia annua L. Ind. Crops Prod. 2010, 31, 214–218. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, L.; Duan, J.; Miki, B.; Wu, K. HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell 2005, 17, 1196–1204. [Google Scholar] [CrossRef]
- Dicke, M.; Bruin, J. Chemical information transfer between plants: Back to the future. Biochem. Syst. Ecol. 2001, 29, 981–994. [Google Scholar] [CrossRef]
- Hou, X.; Lee, L.Y.; Xia, K.; Yan, Y.; Yu, H. DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 2010, 19, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Dimopoulos, N.; Guo, Q.; Liu, L.; Nolan, M.; Das, R.; Garcia-de Heer, L.; Mieog, J.C.; Barkla, B.J.; Kretzschmar, T. An In Vitro Phytohormone Survey Reveals Concerted Regulation of the Cannabis Glandular Trichome Disc Cell Proteome. Plants 2025, 14, 694. [Google Scholar] [CrossRef]
- Huang, X.; Chen, W.; Zhao, Y.; Chen, J.; Ouyang, Y.; Li, M.; Gu, Y.; Wu, Q.; Cai, S.; Guo, F.; et al. Deep learning-based quantification and transcriptomic profiling reveal a methyl jasmonate-mediated glandular trichome formation pathway in Cannabis sativa. Plant J. 2024, 118, 1155–1173. [Google Scholar] [CrossRef]
- Schuurink, R.; Tissier, A. Glandular trichomes: Micro-organs with model status? New Phytol. 2020, 225, 2251–2266. [Google Scholar] [CrossRef]
- Garrido, J.; Rico, S.; Corral, C.; Sánchez, C.; Vidal, N.; Martínez-Quesada, J.J.; Ferreiro-Vera, C. Exogenous application of stress-related signaling molecules affect growth and cannabinoid accumulation in medical cannabis (Cannabis sativa L.). Front. Plant Sci. 2022, 13, 1082554. [Google Scholar] [CrossRef]
- Wilkinson, S.W.; Hannan Parker, A.; Muench, A.; Wilson, R.S.; Hooshmand, K.; Henderson, M.A.; Moffat, E.K.; Rocha, P.; Hipperson, H.; Stassen, J.H.M.; et al. Long-lasting memory of jasmonic acid-dependent immunity requires DNA demethylation and ARGONAUTE1. Nat. Plants 2023, 9, 81–95. [Google Scholar] [CrossRef]
- Adachi, H.; Nakano, T.; Miyagawa, N.; Ishihama, N.; Yoshioka, M.; Katou, Y.; Yaeno, T.; Shirasu, K.; Yoshioka, H. WRKY Transcription Factors Phosphorylated by MAPK Regulate a Plant Immune NADPH Oxidase in Nicotiana benthamiana. Plant Cell 2015, 27, 2645–2663. [Google Scholar] [CrossRef] [PubMed]
- Vincent, S.A.; Kim, J.-M.; Pérez-Salamó, I.; To, T.K.; Torii, C.; Ishida, J.; Tanaka, M.; Endo, T.A.; Bhat, P.; Devlin, P.F.; et al. Jasmonates and Histone deacetylase 6 activate Arabidopsis genome-wide histone acetylation and methylation during the early acute stress response. BMC Biol. 2022, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.Z.; Jiang, J.; Duan, C.G. The Crosstalk Between Epigenetic Mechanisms and Alternative RNA Processing Regulation. Front. Genet. 2020, 11, 998. [Google Scholar] [CrossRef]
- Stout, J.M.; Boubakir, Z.; Ambrose, S.J.; Purves, R.W.; Page, J.E. The hexanoyl-CoA precursor for cannabinoid biosynthesis is formed by an acyl-activating enzyme in Cannabis sativa trichomes. Plant J. 2012, 71, 353–365. [Google Scholar] [CrossRef]
- Fukazawa, J.; Mori, K.; Ando, H.; Mori, R.; Kanno, Y.; Seo, M.; Takahashi, Y. Jasmonate inhibits plant growth and reduces gibberellin levels via microRNA5998 and transcription factor MYC2. Plant Physiol. 2023, 193, 2197–2214. [Google Scholar] [CrossRef]
- Brian Traw, M.; Dawson, T.E. Reduced Performance of Two Specialist Herbivores (Lepidoptera: Pieridae, Coleoptera: Chrysomelidae) on New Leaves of Damaged Black Mustard Plants. Environ. Entomol. 2002, 31, 714–722. [Google Scholar] [CrossRef]
- Dalin, P.; Ågren, J.; Björkman, C.; Huttunen, P.; Kärkkäinen, K. Leaf Trichome Formation and Plant Resistance to Herbivory; Springer: Dordrecht, The Netherlands, 2008; pp. 89–105. [Google Scholar]
- Penninckx, I.A.; Thomma, B.P.; Buchala, A.; Métraux, J.P.; Broekaert, W.F. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 1998, 10, 2103–2113. [Google Scholar] [CrossRef]
- Da Cunha Leme Filho, J.F.; Chim, B.K.; Bermand, C.; Diatta, A.A.; Thomason, W.E. Effect of organic biostimulants on cannabis productivity and soil microbial activity under outdoor conditions. J. Cannabis Res. 2024, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Shiponi, S. The Highs and Lows of P Supply in Medical Cannabis: Effects on Cannabinoids, the Ionome, and Morpho-Physiology. Front. Plant Sci. 2021, 12, 657323. [Google Scholar] [CrossRef]
- Stack, G.M.; Snyder, S.I.; Toth, J.A.; Quade, M.A.; Crawford, J.L.; McKay, J.K.; Jackowetz, J.N.; Wang, P.; Philippe, G.; Hansen, J.L.; et al. Cannabinoids function in defense against chewing herbivores in Cannabis sativa L. Hortic. Res. 2023, 10, uhad207. [Google Scholar] [CrossRef]
| MeJA Concentration | Timing of Application | Observed Effects on Trichomes | Impact on Cannabinoid Yield | Associated Risks |
|---|---|---|---|---|
| 0.05–0.1 mM | Early-to-mid-flowering; 1–2 foliar sprays | Moderate increase in trichome density | 15–30% increase in THC/CBD content | Minimal vegetative impact; safe for most cultivars |
| 0.5–1 mM | Early flowering; may require repeated applications | Substantial increase in trichome initiation and stalk elongation | 30–60% increase, depending on cultivar | Mild stunting, potential for leaf chlorosis |
| 5–15 mM | Early flowering or late vegetative stage; single dose | Maximal trichome formation; high resin output | Up to 100% increase, but variable | Severe dwarfing, biomass reduction, and potential stress symptoms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Cunha Leme Filho, J.F.; Schuchman, S.; De Sarandy Raposo, R.; Diatta, A.A.; Didaran, F.; Sharma, S.; Walters, A.; Gage, K.L. The Role of Jasmonates in Modulating Growth, Trichome Density, and Cannabinoid Accumulation in Cannabis sativa L. Int. J. Plant Biol. 2025, 16, 68. https://doi.org/10.3390/ijpb16020068
Da Cunha Leme Filho JF, Schuchman S, De Sarandy Raposo R, Diatta AA, Didaran F, Sharma S, Walters A, Gage KL. The Role of Jasmonates in Modulating Growth, Trichome Density, and Cannabinoid Accumulation in Cannabis sativa L. International Journal of Plant Biology. 2025; 16(2):68. https://doi.org/10.3390/ijpb16020068
Chicago/Turabian StyleDa Cunha Leme Filho, Jose F., Spencer Schuchman, Rodrigo De Sarandy Raposo, Andre A. Diatta, Fardad Didaran, Shiksha Sharma, Alan Walters, and Karla L. Gage. 2025. "The Role of Jasmonates in Modulating Growth, Trichome Density, and Cannabinoid Accumulation in Cannabis sativa L." International Journal of Plant Biology 16, no. 2: 68. https://doi.org/10.3390/ijpb16020068
APA StyleDa Cunha Leme Filho, J. F., Schuchman, S., De Sarandy Raposo, R., Diatta, A. A., Didaran, F., Sharma, S., Walters, A., & Gage, K. L. (2025). The Role of Jasmonates in Modulating Growth, Trichome Density, and Cannabinoid Accumulation in Cannabis sativa L. International Journal of Plant Biology, 16(2), 68. https://doi.org/10.3390/ijpb16020068







