Antibacterial, Antidiabetic, and Toxicity Effects of Two Brown Algae: Sargassum buxifolium and Padina gymnospora
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification: Algae Material
2.2. Preparation of Extracts
2.3. NMR Analysis
2.4. Determination of the Fatty-Acid Profile by Fatty-Acid Methyl Esters (FAMEs) and Gas Chromatography (GC)
2.5. Biological Activity Assays
2.5.1. Evaluation of the α-Glucosidase Inhibitory Activity
2.5.2. Antibacterial Activity
Bacterial Strains
Preparation of Working Solution
Preparation of Inoculum
2.5.3. Antibacterial Assay
2.5.4. Statistical Analysis
2.6. Toxicity Assays
2.6.1. Animals
2.6.2. Acute One Dose Assay
2.6.3. Acute Three Doses Assay
2.6.4. Subchronic Assay
2.6.5. Acute Toxicity Testing (LD50) Using the Lorke Method
2.6.6. Statistical Analysis
3. Results
3.1. Biological Activity
3.1.1. Antibacterial
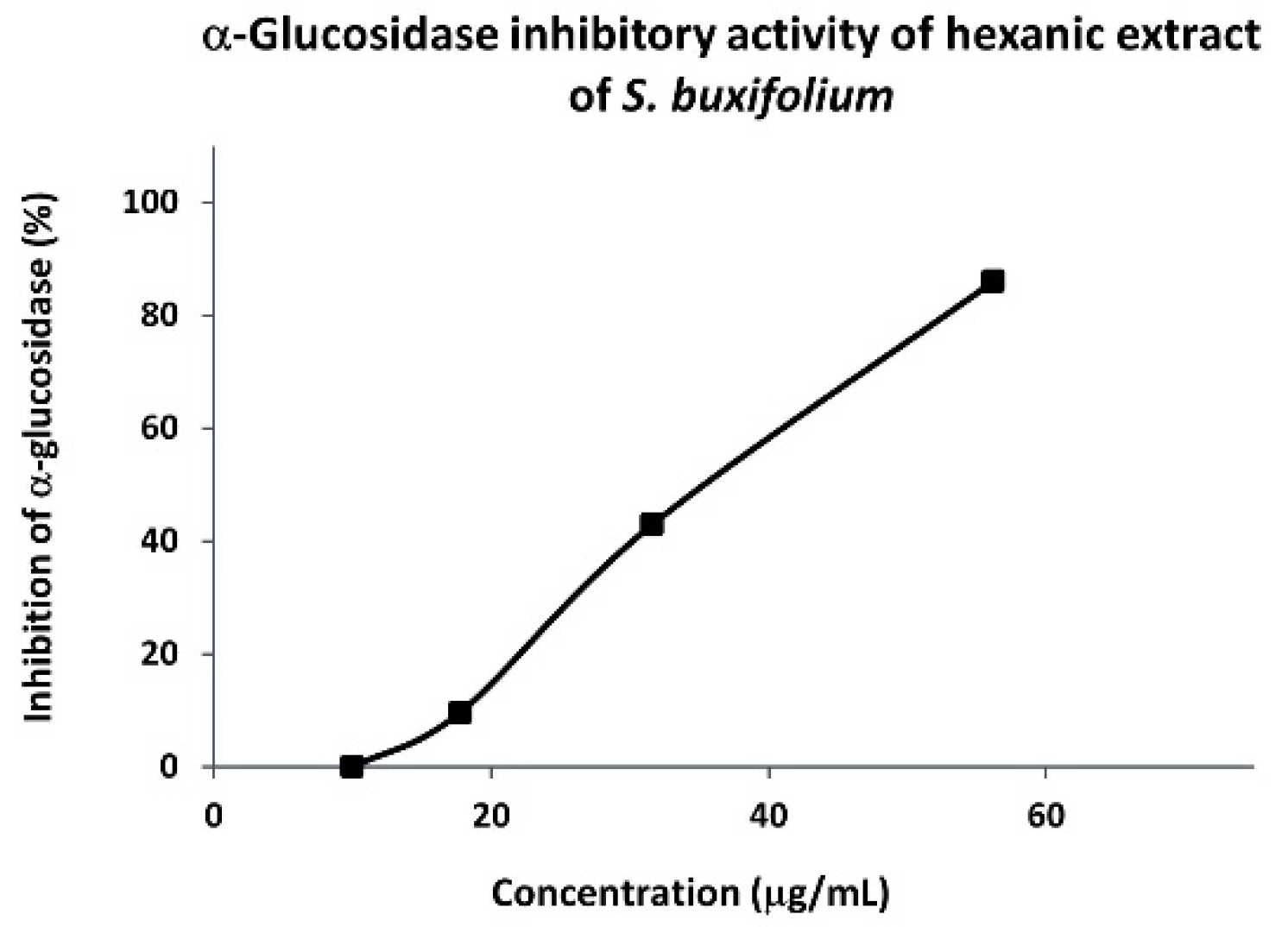
3.1.2. α-Glucosidase Inhibitory Activity of Hexanic Extracts
3.2. Toxicity of S. buxifolium and P. gymnospora Hexanic Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McArthur, K.A.; Mitchell, S.S.; Tsueng, G.; Rheingold, A.; White, D.J.; Grodberg, J.; Lam, K.S.; Potts, B.C.M. Lynamicins A−E, Chlorinated Bisindole Pyrrole Antibiotics from a Novel Marine Actinomycete. J. Nat. Prod. 2008, 71, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine Natural Products: A New Wave of Drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Andarwulan, N. Screening of In-Vitro Anti-Inflammatory and Antioxidant Activity of Sargassum ilicifolium Crude Lipid Extracts from Different Coastal Areas in Indonesia. Mar. Drugs 2021, 19, 252. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Elnahas, O.S.; Assar, N.H.; Gad, A.M.; El Hosary, R. Nanocrystals of Fusidic Acid for Dual Enhancement of Dermal Delivery and Antibacterial Activity: In Vitro, Ex Vivo and In Vivo Evaluation. Pharmaceutics 2020, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a Better Understanding of Medicinal Uses of the Brown Seaweed Sargassum in Traditional Chinese Medicine: A Phytochemical and Pharmacological Review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Pedroche, F.F.; Sentíes, A. Diversidad de Macroalgas Marinas En México. Una Actualización Florística y Nomenclatura. Cymbella 2020, 1, 4–55. [Google Scholar]
- Fredericq, S.; Cho, T.O.; Earle, S.A.; Gurgel, C.F.; Krayesky, D.M.; Mateo-Cid, L.E.; Catalina Mendoza-González, A.; Norris, J.N.; Suárez, A.M. Seaweed of the Gulf of Mexico. In Gulf of Mexico Origin, Waters, and Biota; Felder, D.L., Camp, D.K., Eds.; Texas A&M University Press: College Station, TX, USA, 2009; Volume 1, pp. 187–260. ISBN 1-60344-094-1. [Google Scholar]
- Nazarudin, M.F.; Alias, N.H.; Balakrishnan, S.; Wan Hasnan, W.N.I.; Noor Mazli, N.A.I.; Ahmad, M.I.; Md Yasin, I.-S.; Isha, A.; Aliyu-Paiko, M. Chemical, Nutrient and Physicochemical Properties of Brown Seaweed, Sargassum polycystum C. Agardh (Phaeophyceae) Collected from Port Dickson, Peninsular Malaysia. Molecules 2021, 26, 5216. [Google Scholar] [CrossRef]
- González-Nieto, D.; Oliveira, M.C.; Núñez Resendiz, M.L.; Dreckmann, K.M.; Mateo-Cid, L.E.; Sentíes, A. Molecular Assessment of the Genus Sargassum (Fucales, Phaeophyceae) from the Mexican Coasts of the Gulf of Mexico and Caribbean, with the Description of S. xochitlae sp. nov. Phytotaxa 2020, 461, 254–274. [Google Scholar] [CrossRef]
- García-García, A.M.E.; Cabrera-Becerril, E.; Núñez-Reséndiz, M.L.; Dreckmann, K.M.; Sentíes, A. Actualización Taxonómica de Las Algas Pardas (Phaeophyceae, Ochrophyta) Marinas Bentónicas Del Atlántico Mexicano. Acta Bot. Mex. 2021, 128, 1–25. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomy, A.H.; Abdelmohsen, U.R. Pharmacological and Natural Products Diversity of the Brown Algae Genus Sargassum. RSC Adv. 2020, 10, 24951–24972. [Google Scholar] [CrossRef]
- Avila-Ortiz, A.G.; Pedroche, F.F. El Género Padina (Dictyotaceae, Phaeophyceae) En La Región Tropical Del Pacífico Mexicano. Monogr. Ficol. 2005, 2, 139–170. [Google Scholar]
- Díaz-Martínez, S.; Zuccarello, G.C.; Chávez, G.A.S.; Pedroche, F.F.; Avila-Ortiz, A.G. Species of Padina (Dictyotales, Phaeophyceae) in Tropical Mexican Waters Based on Molecular-Assisted Taxonomy. Phycologia 2016, 55, 673–687. [Google Scholar] [CrossRef]
- Baliano, A.P.; Pimentel, E.F.; Buzin, A.R.; Vieira, T.Z.; Romão, W.; Tose, L.V.; Lenz, D.; de Andrade, T.U.; Fronza, M.; Kondratyuk, T.P.; et al. Brown Seaweed Padina gymnospora Is a Prominent Natural Wound-Care Product. Rev. Bras. Farm. 2016, 26, 714–719. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic Potential of Marine Brown Algae—A Mini Review. J. Diabetes Res. 2020, 2020, 1230218. [Google Scholar] [CrossRef]
- Je, J.-G.; Lee, H.-G.; Fernando, K.H.N.; Jeon, Y.-J.; Ryu, B. Purification and Structural Characterization of Sulfated Polysaccharides Derived from Brown Algae, Sargassum binderi: Inhibitory Mechanism of INOS and COX-2 Pathway Interaction. Antioxidants 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 2 December 2022).
- Arciniegas, A.; Pérez-Castorena, A.L.; Nieto-Camacho, A.; Kita, Y.; Romo de Vivar, A. Anti-Hyperglycemic, Antioxidant, and Anti-Inflammatory Activities of Extracts and Metabolites from Sida Acuta and Sida rhombifolia. Quim. Nova 2016, 40, 176–181. [Google Scholar] [CrossRef]
- Ye, X.-P.; Song, C.-Q.; Yuan, P.; Mao, R.-G. α-Glucosidase and α-Amylase Inhibitory Activity of Common Constituents from Traditional Chinese Medicine Used for Diabetes Mellitus. Chin. J. Nat. Med. 2010, 8, 349–352. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Pérez-López, A.; Becerril-Montes, P.; Salazar-Aranda, R.; Said-Fernández, S.; Torres, N.W. de Evaluation of the Flora of Northern Mexico for in vitro Antimicrobial and Antituberculosis Activity. J. Ethnopharmacol. 2007, 109, 435–441. [Google Scholar] [CrossRef]
- Navarro-Navarro, M.; Ruiz-Bustos, P.; Valencia, D.; Robles-Zepeda, R.; Ruiz-Bustos, E.; Virués, C.; Hernandez, J.; Domínguez, Z.; Velazquez, C. Antibacterial Activity of Sonoran Propolis and Some of Its Constituents Against Clinically Significant Vibrio Species. Foodborne Pathog. Dis. 2013, 10, 150–158. [Google Scholar] [CrossRef]
- Baizman, E.R.; Branstrom, A.A.; Longley, C.B.; Allanson, N.; Sofia, M.J.; Gange, D.; Goldman, R.C. Antibacterial Activity of Synthetic Analogues Based on the Disaccharide Structure of Moenomycin, an Inhibitor of Bacterial Transglycosylase. Microbiology 2000, 146, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D. A New Approach to Practical Acute Toxicity Testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Zubia, M.; Payri, C.; Deslandes, E. Alginate, Mannitol, Phenolic Compounds and Biological Activities of Two Range-Extending Brown Algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J. Appl. Phycol. 2008, 20, 1033–1043. [Google Scholar] [CrossRef]
- Yende, S.; Chaugule, B.; Harle, U. Therapeutic Potential and Health Benefits of Sargassum Species. Pharmacogn. Rev. 2014, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.A.; Alves, L.G.; de Queiroz, K.C.S.; Santos, M.G.L.; Marques, C.T.; Chavante, S.F.; Rocha, H.A.O.; Leite, E.L. Partial Characterization and Anticoagulant Activity of a Heterofucan from the Brown Seaweed Padina gymnospora. Braz. J. Med. Biol. Res. 2005, 38, 523–533. [Google Scholar] [CrossRef]
- Dong, S.; Huang, Y.; Zhang, R.; Wang, S.; Liu, Y. Four Different Methods Comparison for Extraction of Astaxanthin from Green Alga Haematococcus pluvialis. Sci. World J. 2014, 2014, 694305. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Edible Oils: Discrimination by 1H Nuclear Magnetic Resonance: Edible Oils: Discrimination by 1 H Nuclear Magnetic Resonance. J. Sci. Food Agric. 2003, 83, 338–346. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Y.; Liu, T.; Zhang, L.; Liu, H.; Guan, H. Comparative Studies on the Characteristic Fatty Acid Profiles of Four Different Chinese Medicinal Sargassum Seaweeds by GC-MS and Chemometrics. Mar. Drugs 2016, 14, 68. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; H-kittikun, A. Lipids from Cephalothorax and Hepatopancreas of Pacific White Shrimp (Litopenaeus vannamei): Compositions and Deterioration as Affected by Iced Storage. Food Chem. 2012, 134, 2066–2074. [Google Scholar] [CrossRef]
- Vidal, N.P.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. Quality of Farmed and Wild Sea Bass Lipids Studied by 1H NMR: Usefulness of This Technique for Differentiation on a Qualitative and a Quantitative Basis. Food Chem. 2012, 135, 1583–1591. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Rapid Simultaneous Determination by Proton NMR of Unsaturation and Composition of Acyl Groups in Vegetable Oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
- Barison, A.; Pereira da Silva, C.W.; Campos, F.R.; Simonelli, F.; Lenz, C.A.; Ferreira, A.G. A Simple Methodology for the Determination of Fatty Acid Composition in Edible Oils through 1H NMR Spectroscopy. Magn. Reson. Chem. 2010, 48, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini-Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Lenihan-Geels, G.; Bishop, K.; Ferguson, L. Alternative Sources of Omega-3 Fats: Can We Find a Sustainable Substitute for Fish? Nutrients 2013, 5, 1301–1315. [Google Scholar] [CrossRef]
- Wächter, G.A.; Franzblau, S.G.; Montenegro, G.; Hoffmann, J.J.; Maiese, W.M.; Timmermann, B.N. Inhibition of Mycobacterium tuberculosis Growth by Saringosterol from Lessonia nigrescens. J. Nat. Prod. 2001, 64, 1463–1464. [Google Scholar] [CrossRef]
- Nagayama, K. Bactericidal Activity of Phlorotannins from the Brown Alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, E.; Quesada, A.; Rahman, M.M.; Gibbons, S.; Vagias, C.; Roussis, V. Dolabellanes with Antibacterial Activity from the Brown Alga Dilophus spiralis. J. Nat. Prod. 2011, 74, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, X.; Conte, M.M.; Khalil, Z.; Capon, R.J. Spiralisones A–D: Acylphloroglucinol Hemiketals from an Australian Marine Brown Alga, Zonaria spiralis. Org. Biomol. Chem. 2012, 10, 9671–9676. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.-H.; Kim, D.-H.; Lee, S.-H.; Yoon, N.-Y.; Kim, J.H.; Kim, T.H.; Chung, Y.-H.; Kim, S.-B.; Kim, Y.-M.; Kim, H.-W.; et al. In Vitro Antibacterial Activity and Synergistic Antibiotic Effects of Phlorotannins Isolated from Eisenia bicyclis Against Methicillin-Resistant Staphylococcus aureus: Anti-MRSA Activity and Synergistic Effect of Eisenia bicyclis. Phytother. Res. 2013, 27, 1260–1264. [Google Scholar] [CrossRef]
- Fang, H.-Y.; Chokkalingam, U.; Chiou, S.-F.; Hwang, T.-L.; Chen, S.-L.; Wang, W.-L.; Sheu, J.-H. Bioactive Chemical Constituents from the Brown Alga Homoeostrichus formosana. Int. J. Mol. Sci. 2014, 16, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Dasagrandhi, C.; Kim, S.-H.; Kim, B.-G.; Eom, S.-H.; Kim, Y.-M. In Vitro Antibacterial Activity of Phlorotannins from Edible Brown Algae, Eisenia Bicyclis Against Streptomycin-Resistant Listeria monocytogenes. Indian J. Microbiol. 2018, 58, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial Properties of Fucoidans from the Brown Algae Fucus vesiculosus L. of the Barents Sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A Brown Algal Phlorotannin with Biological Potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.-H.; Lee, D.-S.; Jung, Y.-J.; Park, J.-H.; Choi, J.-I.; Yim, M.-J.; Jeon, J.-M.; Kim, H.-W.; Son, K.-T.; Je, J.-Y.; et al. The Mechanism of Antibacterial Activity of Phlorofucofuroeckol-A against Methicillin-Resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9795–9804. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Cao, M.-J.; Liu, G.-M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial Activity and Mechanisms of Depolymerized Fucoidans Isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- De Figueiredo, C.S.; de Menezes Silva, S.M.P.; Abreu, L.S.; da Silva, E.F.; da Silva, M.S.; Cavalcanti de Miranda, G.E.; de O. Costa, V.C.; Le Hyaric, M.; de Siqueira Junior, J.P.; Barbosa Filho, J.M.; et al. Dolastane Diterpenes from Canistrocarpus cervicornis and Their Effects in Modulation of Drug Resistance in Staphylococcus aureus. Nat. Prod. Res. 2019, 33, 3231–3239. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial Free Fatty Acids: Activities, Mechanisms of Action and Biotechnological Potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Jackman, J.; Valle-González, E.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Rima, M.; Trognon, J.; Latapie, L.; Chbani, A.; Roques, C.; El Garah, F. Seaweed Extracts: A Promising Source of Antibiofilm Agents with Distinct Mechanisms of Action against Pseudomonas aeruginosa. Mar. Drugs 2022, 20, 92. [Google Scholar] [CrossRef]
- Moubayed, N.M.S.; Al Houri, H.J.; Al Khulaifi, M.M.; Al Farraj, D.A. Antimicrobial, Antioxidant Properties and Chemical Composition of Seaweeds Collected from Saudi Arabia (Red Sea and Arabian Gulf). Saudi J. Biol. Sci. 2017, 24, 162–169. [Google Scholar] [CrossRef]
- Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Najmi, A.; Alam, M.S.; Rehman, Z.; Moni, S.S. Spectral Analysis and Antibacterial Activity of the Bioactive Principles of Sargassum tenerrimum J. Agardh Collected from the Red Sea, Jazan, Kingdom of Saudi Arabia. Braz. J. Biol. 2023, 83, e249536. [Google Scholar] [CrossRef]
- Kamei, Y.; Sueyoshi, M.; Hayashi, K.; Terada, R.; Nozaki, H. The Novel Anti-Propionibacterium Acnes Compound, Sargafuran, Found in the Marine Brown Alga Sargassum macrocarpum. J. Antibiot. 2009, 62, 259–263. [Google Scholar] [CrossRef]
- Nogueira, L.; Morais, E.; Brito, M.; Santos, B.; Vale, D.; Lucena, B.; Figueredo, F.; Guedes, G.; Tintino, S.; Souza, C.; et al. Evaluation of Antibacterial, Antifungal and Modulatory Activity of Methanol and Ethanol Extracts of Padina sanctae-crucis. Afr. Health Sci. 2014, 14, 372–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dussault, D.; Vu, K.D.; Vansach, T.; Horgen, F.D.; Lacroix, M. Antimicrobial Effects of Marine Algal Extracts and Cyanobacterial Pure Compounds against Five Foodborne Pathogens. Food Chem. 2016, 199, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, K.; Karthikai Devi, G.; Anantharaman, P.; Balasubramanian, T. Antimicrobial Potential of Selected Brown Seaweeds from Vedalai Coastal Waters, Gulf of Mannar. Asian Pac. J. Trop. Biomed. 2011, 1, 114–120. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Pliego-Cortés, H.; Robledo, D. Seaweed Resources of Mexico: Current Knowledge and Future Perspectives. Bot. Mar. 2019, 62, 275–289. [Google Scholar] [CrossRef]
- Landa-Cansigno, C.; Hernández-Domínguez, E.E.; Monribot-Villanueva, J.L.; Licea-Navarro, A.F.; Mateo-Cid, L.E.; Segura-Cabrera, A.; Guerrero-Analco, J.A. Screening of Mexican Tropical Seaweeds as Sources of α-Amylase and α-Glucosidase Inhibitors. Algal Res. 2020, 49, 101954. [Google Scholar] [CrossRef]
- Nagappan, H.; Pee, P.P.; Kee, S.H.Y.; Ow, J.T.; Yan, S.W.; Chew, L.Y.; Kong, K.W. Malaysian Brown Seaweeds Sargassum siliquosum and Sargassum polycystum: Low Density Lipoprotein (LDL) Oxidation, Angiotensin Converting Enzyme (ACE), α-Amylase, and α-Glucosidase Inhibition Activities. Food Res. Int. 2017, 99, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, C.; Fu, X. Screening α-Glucosidase Inhibitors from Four Edible Brown Seaweed Extracts by Ultra-Filtration and Molecular Docking. LWT 2021, 138, 110654. [Google Scholar] [CrossRef]
- Akhila, J.S.; Shyamjith, D.; Alwar, M.C. Acute Toxicity Studies and Determination of Median Lethal Dose. Curr. Sci. 2007, 93, 917–920. [Google Scholar]

 , 3.125 µg/mL;
, 3.125 µg/mL;  , 6.25 µg/mL;
, 6.25 µg/mL;  , 12.5 µg/mL;
, 12.5 µg/mL;  , 25 µg/mL;
, 25 µg/mL;  , 50 µg/mL;
, 50 µg/mL;  , 100 µg/mL;
, 100 µg/mL;  , 200 µg/mL;
, 200 µg/mL;  , 400 µg/mL;
, 400 µg/mL;  , gentamicin 12 µg/mL;
, gentamicin 12 µg/mL; , bacteria). All values represent mean of triplicate determinations ± SD. Significant differences (p < 0.05) from bacterial growth control are marked with asterisks.
, bacteria). All values represent mean of triplicate determinations ± SD. Significant differences (p < 0.05) from bacterial growth control are marked with asterisks.
 , 3.125 µg/mL;
, 3.125 µg/mL;  , 6.25 µg/mL;
, 6.25 µg/mL;  , 12.5 µg/mL;
, 12.5 µg/mL;  , 25 µg/mL;
, 25 µg/mL;  , 50 µg/mL;
, 50 µg/mL;  , 100 µg/mL;
, 100 µg/mL;  , 200 µg/mL;
, 200 µg/mL;  , 400 µg/mL;
, 400 µg/mL;  , gentamicin 12 µg/mL;
, gentamicin 12 µg/mL; , bacteria). All values represent mean of triplicate determinations ± SD. Significant differences (p < 0.05) from bacterial growth control are marked with asterisks.
, bacteria). All values represent mean of triplicate determinations ± SD. Significant differences (p < 0.05) from bacterial growth control are marked with asterisks.
 , 3.125 µg/mL;
, 3.125 µg/mL;  , 6.25 µg/mL;
, 6.25 µg/mL; 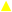 , 12.5 µg/mL;
, 12.5 µg/mL;  , 25 µg/mL;
, 25 µg/mL;  , 50 µg/mL;
, 50 µg/mL; , 100 µg/mL;
, 100 µg/mL; , 200 µg/mL;
, 200 µg/mL; , 400 µg/mL;
, 400 µg/mL;  , gentamicin or meropenem 12 µg/mL;
, gentamicin or meropenem 12 µg/mL; , bacteria). All values represent mean of triplicate determinations ± SD. Significant differences (p < 0.05) from bacterial growth control are marked with asterisks.
, bacteria). All values represent mean of triplicate determinations ± SD. Significant differences (p < 0.05) from bacterial growth control are marked with asterisks.
 , 3.125 µg/mL;
, 3.125 µg/mL;  , 6.25 µg/mL;
, 6.25 µg/mL;  , 12.5 µg/mL;
, 12.5 µg/mL;  , 25 µg/mL;
, 25 µg/mL;  , 50 µg/mL;
, 50 µg/mL; , 100 µg/mL;
, 100 µg/mL; , 200 µg/mL;
, 200 µg/mL; , 400 µg/mL;
, 400 µg/mL;  , gentamicin or meropenem 12 µg/mL;
, gentamicin or meropenem 12 µg/mL; , bacteria). All values represent mean of triplicate determinations ± SD. Significant differences (p < 0.05) from bacterial growth control are marked with asterisks.
, bacteria). All values represent mean of triplicate determinations ± SD. Significant differences (p < 0.05) from bacterial growth control are marked with asterisks.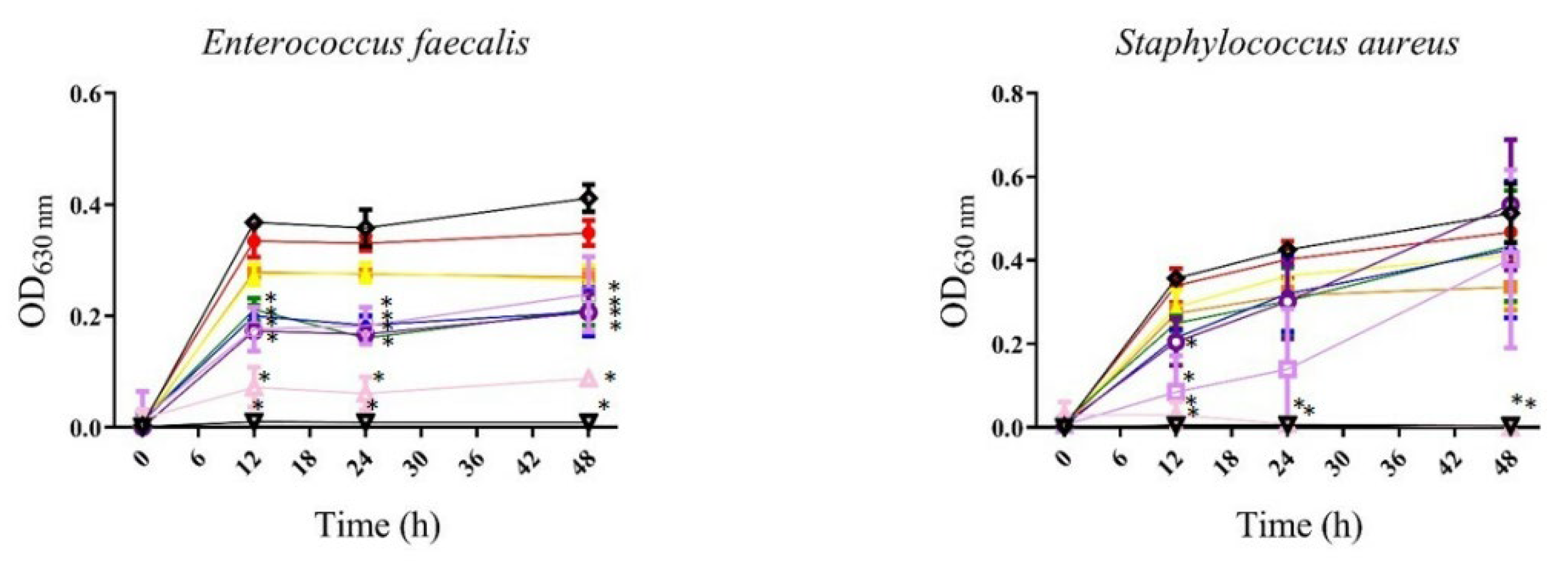
| Signal | Chemical Shift (ppm) | Proton Type |
|---|---|---|
| A | 0.83–0.92 (m) | Terminal-CH3 group of all FA (exception ω-3 FA) |
| B | 0.97 (t) | Terminal-CH3 group of unsaturated ω-3 FA |
| C | 1.17–1.40 (m) | -(CH2)n- group protons of FA chains |
| D | 1.56–1.66 (m) | Acyl-OCO-CH2-CH2- group protons of the beta position to carbonyl group |
| D′ | 1.70 (m) | Acyl-OCO-CH2-CH2 group protons of the beta position to carbonyl group of EPA |
| E | 1.94–2.12 (m) | -CH2-CH=CH-CH2- group protons in alpha position to double bond |
| F | 2.31 (t) | -OCO-CH2- group protons in alpha position to carbonyl group |
| G | 2.72–2.90 (m) | -CH=CH-CH2-CH=CH- group protons of polyunsaturated ω-6 and ω-3 acyl groups and FA |
| H | 5.30–5.42 (m) | -CH=CH- vinylic protons of FA chains |
| Name | S. buxifolium | P. gymnospora | ||
|---|---|---|---|---|
| Retention Time (min) | % Area | Retention Time (min) | % Area | |
| Methyl palmitate | 4.67 | 35.5 | 4.67 | 18.5 |
| Methyl palmitoleate | 5.07 | 16.4 | 5.07 | 8.1 |
| Methyl stearate | 8.74 | 3.3 | 8.74 | 1.1 |
| Methyl oleate | 9.31 | 7.5 | 9.32 | 40.2 |
| Methyl linoleate | 10.81 | 1.6 | 10.81 | 5.6 |
| Methyl linolenate | 13.35 | 1.1 | 13.36 | 1.7 |
| Bacteria | Strain | P. gymnospora | S. buxifolium | ||
|---|---|---|---|---|---|
| MIC50* | MIC90* | MIC50* | MIC90* | ||
| Gram-positive bacteria | Enterococcus faecalis ATCC 51299 | 200 | — | 25 | — |
| Staphylococcus aureus ATCC 25293 | — | — | 200 | 400 | |
| Staphylococcus epidermidis | 200 | — | 200 | 400 | |
| Gram-negative bacteria | Escherichia coli ATCC 25292 | — | — | — | — |
| Klebsiella pneumoniae | — | — | 400 | — | |
| Pseudomonas aeruginosa | — | — | — | — | |
| Salmonella typhimurium | — | — | — | — | |
| (a)Escherichia coli ESBL+ | — | — | — | — | |
| (a)Klebsiella pneumoniae ESBL+ ATCC 700603 | — | — | 400 | — | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Sansininea, J.J.; Tavera-Hernández, R.; Jiménez-Estrada, M.; Coronado-Aceves, E.W.; Espitia-Pinzón, C.I.; Díaz-Martínez, S.; Hernández-Anaya, L.; Rangel-Corona, R.; Avila-Ortiz, A.G. Antibacterial, Antidiabetic, and Toxicity Effects of Two Brown Algae: Sargassum buxifolium and Padina gymnospora. Int. J. Plant Biol. 2023, 14, 63-76. https://doi.org/10.3390/ijpb14010006
Alvarado-Sansininea JJ, Tavera-Hernández R, Jiménez-Estrada M, Coronado-Aceves EW, Espitia-Pinzón CI, Díaz-Martínez S, Hernández-Anaya L, Rangel-Corona R, Avila-Ortiz AG. Antibacterial, Antidiabetic, and Toxicity Effects of Two Brown Algae: Sargassum buxifolium and Padina gymnospora. International Journal of Plant Biology. 2023; 14(1):63-76. https://doi.org/10.3390/ijpb14010006
Chicago/Turabian StyleAlvarado-Sansininea, Jesús Javier, Rosario Tavera-Hernández, Manuel Jiménez-Estrada, Enrique Wenceslao Coronado-Aceves, Clara Inés Espitia-Pinzón, Sergio Díaz-Martínez, Lisandro Hernández-Anaya, Rosalva Rangel-Corona, and Alejandrina Graciela Avila-Ortiz. 2023. "Antibacterial, Antidiabetic, and Toxicity Effects of Two Brown Algae: Sargassum buxifolium and Padina gymnospora" International Journal of Plant Biology 14, no. 1: 63-76. https://doi.org/10.3390/ijpb14010006
APA StyleAlvarado-Sansininea, J. J., Tavera-Hernández, R., Jiménez-Estrada, M., Coronado-Aceves, E. W., Espitia-Pinzón, C. I., Díaz-Martínez, S., Hernández-Anaya, L., Rangel-Corona, R., & Avila-Ortiz, A. G. (2023). Antibacterial, Antidiabetic, and Toxicity Effects of Two Brown Algae: Sargassum buxifolium and Padina gymnospora. International Journal of Plant Biology, 14(1), 63-76. https://doi.org/10.3390/ijpb14010006






