Abstract
Plant extracts of Cissus hastata, indigenously known as Semperai, have been used as an effective traditional remedy against coughs. Recently, the leaf extract was potentially shown to have anti-hemorrhoid activity, although there is a lack of scientific data due to its folklore usage. Hence, the therapeutic properties of the phytochemicals and metabolites of Semperai remain elusive. Therefore, this study aims to determine the total phenolic content and phytochemical compounds of the plant leaf extract. Total phenolic content and antioxidant activity were determined by the Folin–Ciocalteau method and the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, respectively. Phytochemical compounds present in the leaf methanol extract were analyzed by a qualitative method. Results showed the extract comprised a total of 21.3 mg GAE/g of phenolic content with reference to gallic acid. The antioxidant activity was almost absence with an IC50 of 7.80 µg/mL when compared to trolox and gallic acid. Presence of the red to orange precipitate in reference to gallic acid indicate alkaloid content, while the appearance of black-blue/green color in reference to gallic acid are referred to as tannins. The steroids were represented by an upper red layer and a yellowish sulfuric acid with green fluorescence in comparison to cholesterol. Nonetheless, saponin was not detected in the extract, as indicated by the absence of the persisting foam in the test solution when compared with sodium dodecyl sulphate. In conclusion, despite not having an antioxidant property, the methanol extract of Semperai comprised a fair amount of phenolic compounds, including tannins, alkaloids, and steroids, which, potentially, are highly anti-inflammatory towards hemorrhoids.
1. Introduction
Since ancient times, herbal medicines have been touted as the most reasonable and easily accessible predominant source of traditional remedies against various illnesses [1]. A wide variety of phytochemical compounds have been derived from plants, which possess therapeutics properties, most commonly antioxidant and anti-inflammatory properties [2]. Natural antioxidants of plant bioactive flavonoids presented a better alternative to synthetic antioxidants due to their indigenous origin and stronger efficiency in reducing oxidative stress [3]. Hence, plants possessing such properties are of great importance and the most preferred traditional folk medicine with less harmful side effects [4].
Cissus hastata, commonly known as Semperai, is widely distributed in Southeast Asian countries, such as the Philippines, Myanmar, Vietnam, Brunei, Thailand, and Malaysia and is also found in the east coast of Australia [5]. The leaves are lanceolate shaped that narrow to a reddish pointy tip with the average width and length of 2–15.5 cm and 1.5–8 cm, respectively [6]. This indigenous vegetation is a prolific climbing plant with long flexible red tendrils, scrambling around other low vegetation and high branches of trees. Semperai bears flowers and fruits, which attract many bird species, such as Oriolus chinensis, Dicaeum cruentatum, and Pycnonotus goiavier [7]. The small flowers are produced in bunches along the stem and develop into round berries that turn black once ripen. The leaves have long been used as folklore medicine, pounded into poultices or as boiled extracts to treat illnesses, such as muscular pain, asthma, and ulcers [8]. In addition to the leaves, the stems and fruits of Cissus hastata are also believed to have expectorant and anti-emetic properties that are beneficial against coughs.
Despite the promising benefits, Cissus hastata remains understudied and less explored, unlike other Cissus species, such as Cissus arnottiana, which has been evidently shown to contain alkaloids, flavonoids, tannins, terpenoids, saponins, glycosides, and carbohydrates [9]. Another study reported the antimicrobial, anti-bacterial, and anti-inflammatory properties of Cissus aralioides, presented by the alkaloid and steroid components [10]. More recently, the flavonoids and polyphenols of another species, Cissus trifoliata, were shown to possess antitumor activity against hepatocarcinoma and breast cancer cells [11]. Wu and colleague reported Cissus vitiginea leaf extract as a strong antioxidant, proven as an excellent inhibitor towards urinary tract pathogens, including E. coli, Enterococcus sp., Proteus sp., and Klebsiella sp. [12]. Another finding concluded the antioxidant property of both the leaves and roots of Cissus cornifolia ethanol extract [13].
Solvent extraction techniques are highly effective and thereby necessary in handling complex sample matrices of different characters [14]. Therefore, this study aims to elucidate the phytochemical compounds and the total of phenolic content of the leaves methanolic extract of Cissus hastata using the solvent extraction approach. The findings of this study will be fundamental for the development of the indigenous plant as a therapeutic agent. Such a potent natural antioxidant would be beneficial against diseases, such as hemorrhoids, which have been shown to be reduced by the traditional use of the plant. More importantly this study presented scientific data on Cissus hastata medicinal properties toward evidence-based therapeutics.
2. Materials and Methods
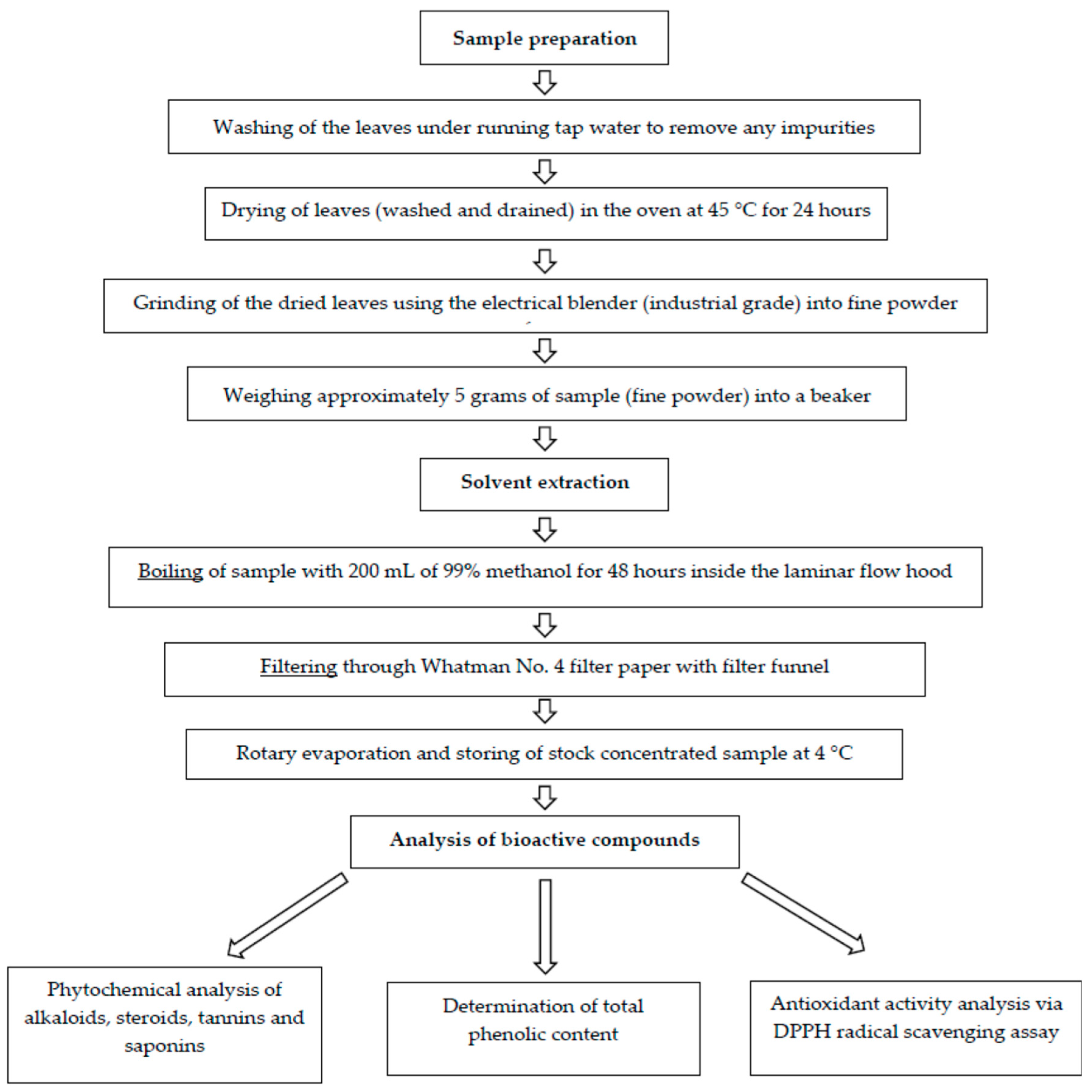
The experimental outline of the study starting from sample preparation until compound analysis is presented in Figure 1.
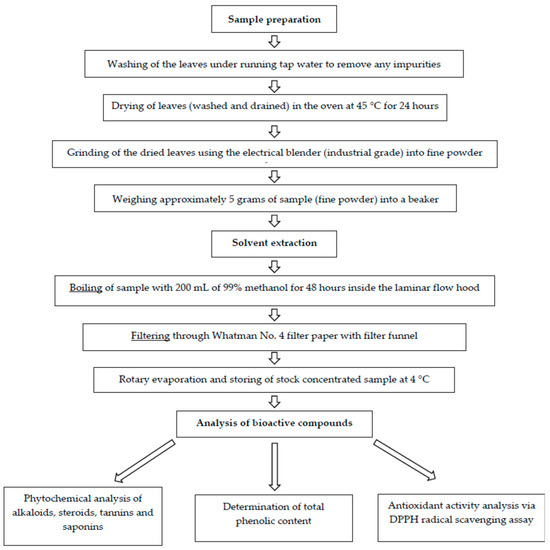
Figure 1.
Experimental outline of Cissus hastata leaf crude extract preparation toward analysis of the bioactive compounds.
2.1. Preparation of Plant Material
Cissus hastata plants were provided by Darussyifa’ Malaysia located in Bangi and were transported to the Institute for Molecular Medicine and Biotechnology (IMMB), Faculty of Medicine UiTM Sg. Buloh campus. The leaves were separated from the bark and collected before being washed under running tap water to remove any impurities. The clean leaves were dried in a 45 °C oven for 24 h. The dried leaves were then grounded into fine powder using an electric blender. Approximately 5 g of the leaf powder was soaked in 200 mL of methanol for 48 h. Subsequently, the mixture was filtered using filter paper (Whatman No. 4), and the supernatant was further dried with a rotary evaporator. The dried crude extract was stored at 4 °C until further analysis.
2.2. Chemicals and Reagents
All chemicals and reagents used were of analytical grade. Trolox, DPPH was purchased from Sigma (Saint Louis, MO, USA). Methanol, sodium carbonate, and ferric chloride was purchased from HmbG Chemicals (Hamburg, Germany). Folin–Ciocalteau reagent, and chloroform was acquired from Thermo Fisher Scientific (Waltham, MA, USA). Bismuth nitrate, nitric acid, and potassium iodide were purchased from Merck Millipore (Burlington, MA, USA). Gallic acid and bismuth nitrate were procured from Bendosen (Johor Bharu, Malaysia) and HiMedia Laboratories (Kennett Square, PA, USA), respectively.
2.3. Screening of Phytochemical Constituents
The methanolic leaf extract of C. hastata was screened for the presence of alkaloids, tannins, steroids, and saponins. The qualitative results were interpretated according to the outcomes observed for each test.
2.3.1. Determination of Total Phenolic Content
Total phenolic content of the crude extract was measured by the Folin–Ciocalteau method adapted from Singleton and Rossi (1965) [15]. The crude extract stock was prepared by dissolving approximately 0.1 mg of the crude extract in 1 mL of methanol. Subsequently, 100 µL of sample stock was added to 500 µL of Folin–Ciocalteau reagent and was mixed for 1 min. Next, 1 mL of 7% sodium carbonate solution was added to the mixture followed by incubation at 30 °C in the dark for 1 h. The absorbance of the reaction mixture was measured at 760 nm. The standard calibration curve for gallic acid of five serially diluted concentrations was prepared in the same manner. Total phenolic content was expressed as mg gallic acid equivalent (GAE) per gram of extract.
2.3.2. Test for Alkaloids
Approximately 1 mL of crude extract (stock concentration 10 mg/mL) was mixed with 160 μL of Dragendorffs’ reagent (12 mL of 1.33 M bismuth nitrate in 30% nitric acid and 50 mL of 3.26 M potassium iodide adjusted to 100 mL with distilled water). The presence of alkaloids was compared to that of the standard, caffeine (10 mg/mL).
2.3.3. Test for Steroids
Screening for steroids was performed by the Salkowski test, whereby 1 mL of crude extract (10 mg/mL) was mixed thoroughly with 500 μL of chloroform. Subsequently, 1 mL of concentrated sulfuric acid was added to the mixture. The presence of steroids was compared to that of the standard, cholesterol.
2.3.4. Test for Tannins
Tannins were determined by adding 90 μL of 1% w/v ferric chloride solution into 10 mg crude extract dissolved in 1 mL methanol. The presence of tannins was compared against gallic acid as the standard.
2.3.5. Test for Saponins
Saponin content was determined by mixing 90 μL of dimethylsulfoxide (DMSO) and 5 mL of distilled water with 10 mg of the crude extract dissolved in 1 mL ethanol. The mixture was shaken thoroughly, and the presence of saponins was compared to the standard, sodium dodecyl sulphate (SDS).
2.3.6. Free Radical Scavenging Activity by the DPPH Assay
The DPPH test is based on the ability of the stable 2,2-diphenyl-1-picrylhydrazyl free radical to react with hydrogen donors. The DPPH radical displays an intense UV-VIS absorption spectrum at 517 nm. The free radical scavenging activity of the leaf extract using the DPPH assay was performed according to the method described by Noreen et al., (2017). Firstly, the crude extract stock of 10 mg/mL was diluted with methanol into different concentrations of 50, 100, 200, and 400 mg/mL. Gallic acid and trolox were used as the standards, which were similarly prepared into four different concentrations. The DPPH stock solution of 1 M was prepared in methanol and was diluted to a working concentration of 0.1 mM. For the assay, 3.6 mL of the 0.1 mM DPPH was mixed with 0.4 mL plant extract in the respective tubes of the four test concentrations. Both standards were also prepared for the assay by the same manner. The ‘blank’ (control) was comprised of 0.4 mL methanol and 3.6 mL DPPH solution only. All samples were prepared in triplicate and incubated in the dark at room temperature for 30 min. The absorbance was measured at 517 nm against blank. The scavenging activity was expressed as the percentage (%) of DPPH inhibition and calculated as the following:
The % inhibition of both standards and samples was calculated for each concentration, and graphs were plotted (% inhibition against concentration). The IC50 value was calculated from the graphs (effective concentration in µg/mL of samples that reduces the DPPH absorbance by 50%).
3. Results
3.1. Phytochemicals Screening
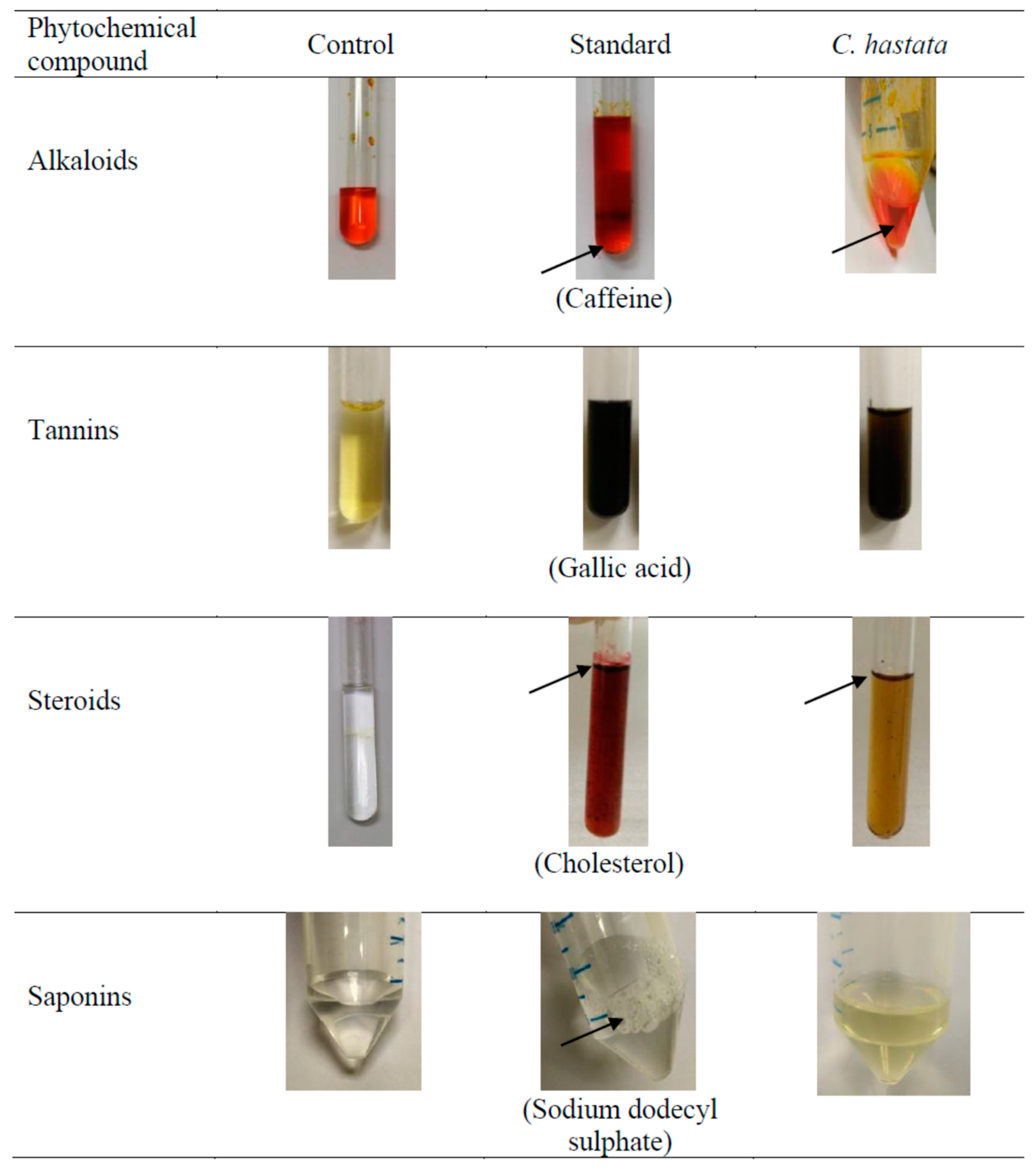
All changes observed in the appearance of the test mixture in the phytochemicals screening are presented in Figure 2. The leaf methanol extract of C. hastata was shown to contain alkaloids, indicated by the presence of the orange-red precipitate. Another phytochemical found in the leaves was steroids, presented as a reddish-brown coloration of the interface, which is referred to as terpenoid. Meanwhile, the presence of tannins was confirmed by the black or blue-green coloration of the sample. However, no saponins were detected in the leaves extract, as indicated by the absence of foam (froths). Qualitative results of the phytochemical compounds of C. hastata are expressed as (+) for the presence and (-) for the absence, presented in Table 1.
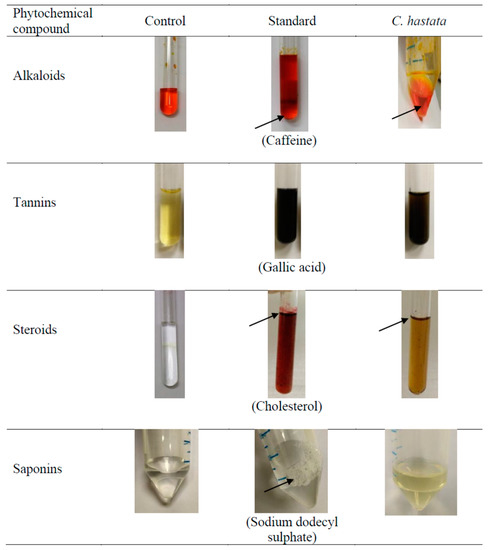
Figure 2.
Result of the phytochemical compounds screening of C. hastata leaf methanolic extract. Notes: The “black arrow” shows observed changes in the appearance of the test mixture.

Table 1.
Phytochemical compounds of C. hastata.
3.2. Total Phenolic Content
The leaf methanolic extract of C. hastata contained 21.30 mg GAE/g of extract of total phenolic content (Table 2).

Table 2.
Total phenolic content of C. hastata.
3.3. Free Radical Scavenging Activity
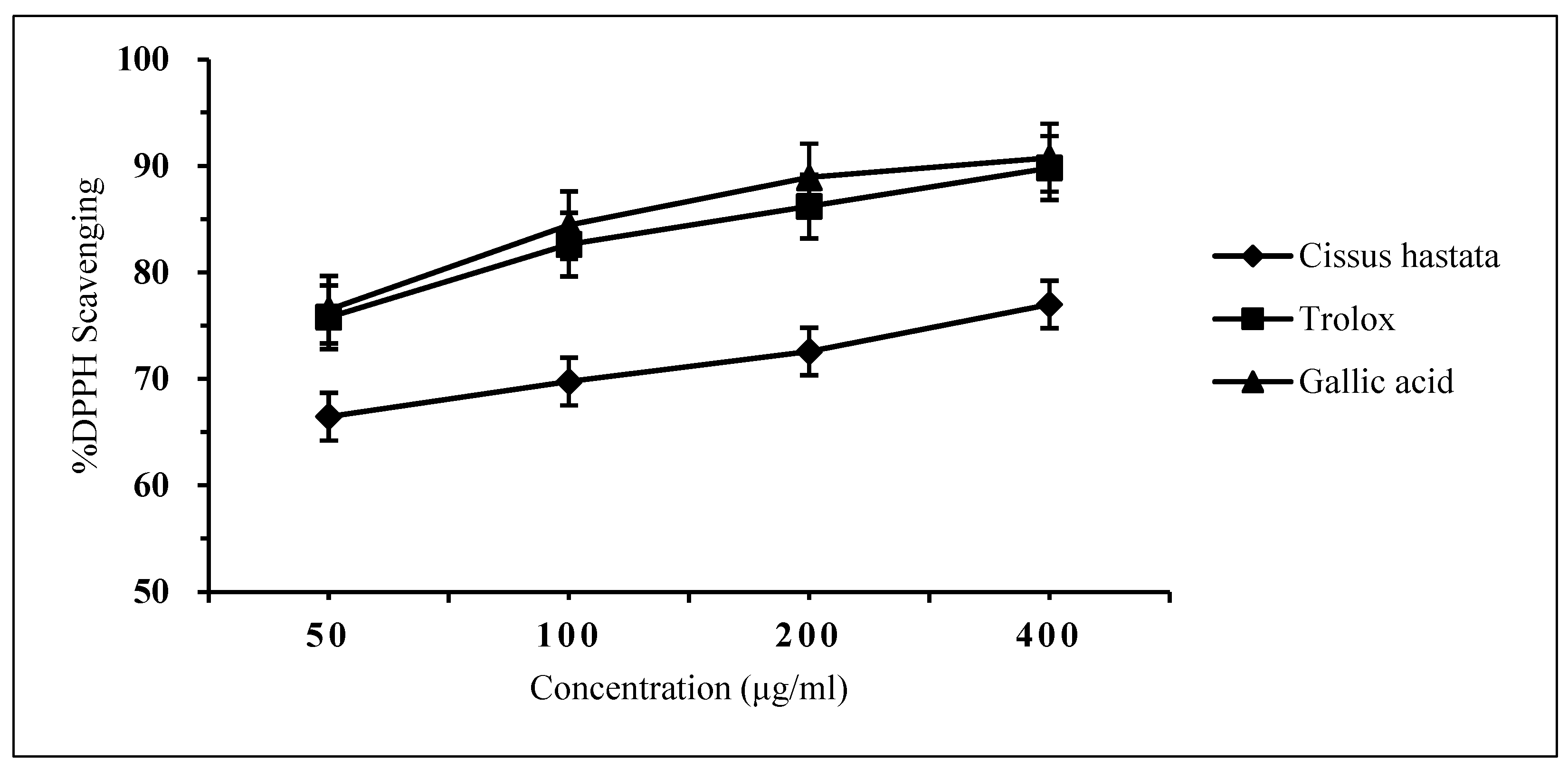
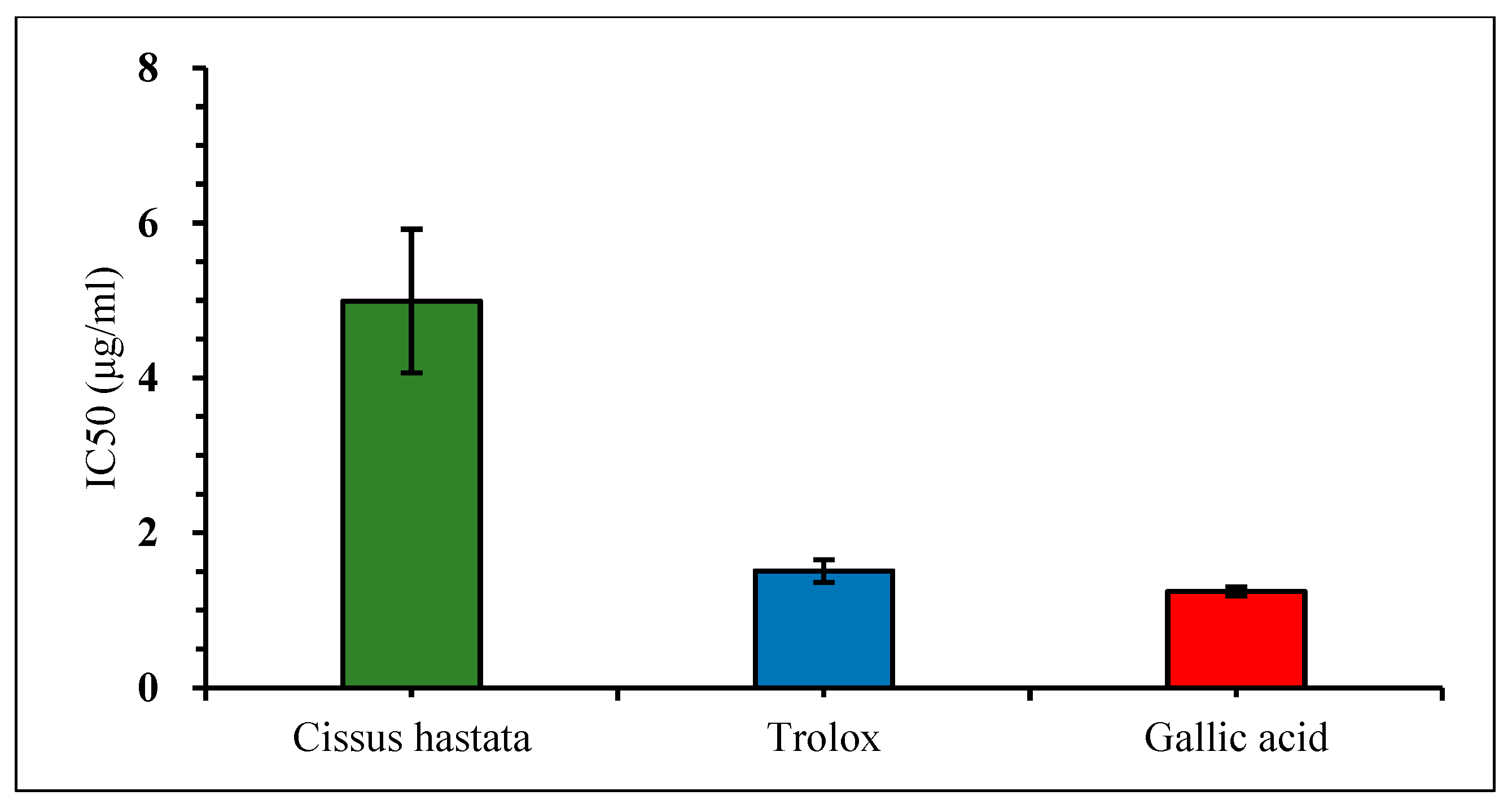
The free radical scavenging effect of the C. hastata methanolic leaf extract was measured by the DPPH assay. The result showed very weak inhibition against DPPH by C. hastata of only 6.45% even at a high concentration of 400 μg/mL. This non-remarkable scavenging activity of C. hastata was confirmed when compared to the standards, trolox and gallic acid, which showed strong inhibitory activity of 92.93% and 90.84%, respectively, at a similar concentration of 400 μg/mL (Figure 3). As expected, C. hastata presented the highest IC50 value of 7.27 μg/mL, which is non-comparable to both standards that showed much lower IC50 values of just under 0.3 μg/mL (Figure 4).
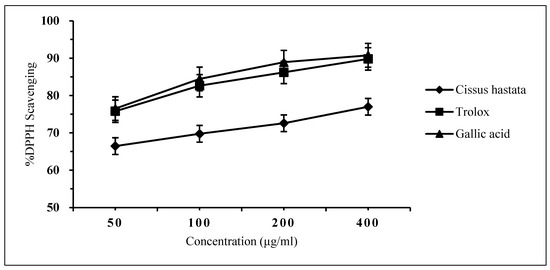
Figure 3.
Inhibitory activity against DPPH by C. hastata methanolic leaf extract and the standards (trolox and gallic acid).
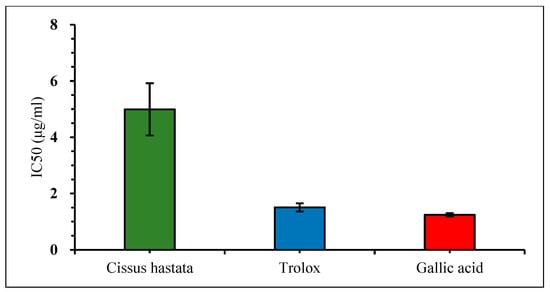
Figure 4.
Comparison of the IC50 value (DPPH radical scavenging activity) among C. hastata methanolic leaf extract, trolox, and gallic acid.
4. Discussion
Phytochemicals analysis of the C. hastata methanolic leaf extract revealed the presence of secondary metabolites, which have been previously reported in other plants to possess medicinal properties (Figure 2). To the best of our knowledge, this study is the first to report the presence of alkaloids, steroids, and tannins in C. hastata. Alkaloids are the end product of plant metabolism, which at the same time, serve as reservoirs for nitrogen, an essential plant nutrient. Primarily, these plant metabolites are protective agents against predators, which render their therapeutics importance as anesthetic, cardioprotective, and anti-inflammatory agents [16]. Another secondary metabolite detected in C. hastata was steroids, similar with finding of a previous study, albeit from other Cissus species, Cissus sicyoides. Regardless of the species, plant sterols are essential for plant growth and reproduction while exerting responses to various abiotic and biotic stresses [17]. These potent phytosterols have long been used as cholesterol lowering agents of a natural source, while they are also widely beneficial as immunosuppressive and anti-inflammatory agents [18]. The C. hastata tested in this current study also contains tannins, which are mainly found in leaf, bud, seed, root, and stem tissues of all plants, which regulate growth of these tissues. More importantly, this phytochemical is essential in the plant defense chemical mechanism against pathogens and herbivores, whereby the bitter taste of tannins is least preferred by herbivorous predators. However, if eaten, tannins will biochemically target the animal’s digestive enzymes and inhibit digestion, sometimes to the extent that the animal dies. Apart from that, tannins also protect plants from ultraviolet radiation [19].
Saponin is a phytochemical compound that possesses soap-like qualities and produces foam when mixed with water. When mixed with water, saponin will reduce the surface tension of water, allowing the formation of small stable bubbles [20]. Because of their surface-active properties, saponins are excellent foaming agents (very stable). In nature, plants rely on saponins as a mechanism to fight parasites. Similarly, when consumed by humans, saponins provide a similar defense against harmful organisms [21]. However, during the experiment, no foam persisted for 15 min in the sample of C. hastata, indicating there is no saponin present in the C. hastata. Hence, C. hastata cannot provide the mechanism to fight parasites.
The total phenolic content was measured by the Folin–Ciocalteau assay and was expressed as μg gallic acid equivalents per microgram of extract. The amount of total phenolic compound for C. hastata was 21.3 mg GAE/g. Phenolic compounds are commonly present in many plants, such as Cajanus Cajan and Teucrium Montanum, and the amount of phenolic content in each type of plant is different in relation to its antioxidant capacity. The higher the amount of the compound, the stronger the antioxidant capacity of the plants [22]. However, there was previously no study about the phenolic content of Cissus hastata or other species of Cissus. Phenol is needed in plants for plant development, particularly in lignin and pigment biosynthesis [23]. They also provide structural integrity and scaffolding support to plants. More importantly, phenolic phytoalexins secreted by wounded or otherwise perturbed plants, repel or kill many microorganisms, and some pathogens can counteract or nullify these defenses or even subvert them to their own advantage. Phenolic compounds are ubiquitous in plants, and when plant foods are consumed, these phytochemicals contribute to the intake of natural antioxidants in the human diets [24].
The antioxidant capacity of C. hastata plant extract was measured by DPPH assay. The reaction depends on the ability of the samples to scavenge free radicals, which can be visually observed by its color change from purple to yellow due to its hydrogen donating ability [25]. The shorter the time taken for the absorbance to reduce, the stronger the antioxidant activity of the plant extract. Antioxidants are the secondary metabolites of a plant, which in a small quantity can scavenges the free radicals and prevent several chronic diseases by donating their own electrons to reactive oxygen species and reactive nitrogen species (ROS/RNS) [26]. Overall, C. hastata shows very poor antioxidant activity, as shown by the weak inhibition against DPPH. The unremarkable scavenging activity was verified when compared to the standards, trolox and gallic acid, even at an equivalent concentration of 400 µg/mL. It can be said that C. hastata plant will not be suitable as therapeutics for inflammatory diseases. However, as this study focused on the leaves, future work is required for the other plant components, such as the stems and the roots, which may possibly demonstrate a considerable amount of antioxidant activity. Table 3 summarizes the comparison of the effectivity of the solvent extraction method used in this study against other extraction methods of previous studies, thus indicating extraction with 99% ethanol of the Cissus hastata leaves produced a comparable result.

Table 3.
List of the different solvent extraction methods on different Cissus sp. for the comparison of effectiveness in the determination of bioactive compounds.
5. Conclusions
The presence of alkaloids, steroids, and tannins in the C. hastata leaves was evident in the result of the phytochemical screening. On the other hand, the DPPH radical scavenging capacity was not detected in the leaf extract, which suggested low suitability of the C. hastata as a therapeutic candidate for inflammatory diseases. Nonetheless, more analysis is necessary to further validate the therapeutic potential of the other bioactive compounds found in this study. Hence, future work is aimed at approaching other extraction methods, both solvent and aqueous based on other parts of the plant (stem and root) toward a comprehensive phytochemical analysis.
Author Contributions
N.S.Z. performed a major part of the experiments, analyzed and interpreted the data, drafted the manuscript, and prepared figures and tables. W.A.S. performed part of the experiments and contributed to approval of a part of the total project funding. S.A.-R. conceptualized the study, designed the experiments, and interpreted the data. M.M. conceived, analyzed, and interpreted the data, reviewed drafts, and critically revised the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the research grant from the Universiti Teknologi MARA Office of the Deputy Vice-Chancellor (Research and Innovation) [100-TNCPI/PRI 16/6/2 (033/2021)].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledged SEGi University grant fund (SEGilRF/2018-4/FoM-3/78) and Darussyifa’ Malaysia foundation grant (100-TNCPI/PRI16/6/2(033/2021) for the provision of funds and materials for this study. The authors also acknowledged IMMB, Universiti Teknologi MARA for the research facility where the work for this study was performed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manikandan Ganapathymuru, S.A.S.; Lakshmanan, G. Review on Phytochemical and Pharmacological activities of the genus Cissus Linn. Int. J. Pharm. Res. 2016, 8, 1. [Google Scholar]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; De Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef]
- Kadir, S.L.A.; Yaakob, H.; Zulkifli, R. Potential anti-dengue medicinal plants: A review. J. Nat. Med. 2013, 67, 677–689. [Google Scholar] [CrossRef]
- Rodrigues, J.G.; Lombardi, J.A.; Lovato, M.B. Phylogeny of Cissus (Vitaceae) focusing on South American species. Taxon 2014, 63, 287–298. [Google Scholar] [CrossRef]
- Najmaddin, C.; Hussin, K.; Maideen, H. Comparative Leaf Anatomy of Selected Species in Vitaceae and Leeaceae. Am. J. Appl. Sci. 2013, 10, 414–417. [Google Scholar] [CrossRef][Green Version]
- Hwang, Y.H.; Yue, Z.E.J. Intended wildness: Utilizing spontaneous growth for biodiverse green spaces in a tropical city. J. Landsc. Arch. 2019, 14, 54–63. [Google Scholar] [CrossRef]
- Kavitha, S.; Manimekalai, G. A study on properties of Cissus quadrangularis plant—A review. Int. J. Res. Appl. Nat. Soc. Sci. 2015, 3, 15–18. [Google Scholar] [CrossRef]
- Sama, K.; Sivaraj, R. Pharmacognostical and phytochemical screening of fruit and leaves of Cissus arnottiana. Asian J. Pharm. Clin. Res. 2012, 5, 64–66. [Google Scholar]
- Oduje, A.A.; Awode, A.; Edah, A.; Sagay, I. Characterization and Phytochemical Screening of nHexane Oil Extract from Cissus aralioides Seeds. Int. J. Sci. Eng. Res. 2015, 6, 113. [Google Scholar]
- Méndez López, L.F. Metabolomic profile and Bioassay-guided Phytochemical analysis of the Stems from Cissus trifoliata, evaluation of their Antibacterial and Cytotoxic activity, and determination of the Mechanism of Action of one active compound. Int. J. Mol. Sci. 2020, 21, 930. [Google Scholar] [PubMed]
- Wu, S.; Rajeshkumar, S.; Madasamy, M.; Mahendran, V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Chipiti, T.; Ibrahim, M.A.; Koorbanally, N.A.; Islam, S. In vitro antioxidant activity and GC-MS analysis of the ethanol and aqueous extracts of Cissus cornifolia (Baker) Splanch (Vitaceae) parts. Acta Pol. Pharm. Drug Res. 2015, 72, 119–127. [Google Scholar]
- Alexovič, M.; Dotsikas, Y.; Bober, P.; Sabo, J. Achievements in robotic automation of solvent extraction and related approaches for bioanalysis of pharmaceuticals. J. Chromatogr. B 2018, 1092, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Belkhadir, Y.; Jaillais, Y.; Epple, P.; Balsemão-Pires, E.; Dangl, J.L.; Chory, J. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2011, 109, 297–302. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Alam Tufani, N.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent Patents Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, J.; Wang, H.; Song, T.; Hu, W.; Li, S. Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes. Molecules 2018, 23, 2073. [Google Scholar] [CrossRef]
- Santini, E.; Jarek, E.; Ravera, F.; Liggieri, L.; Warszynski, P.; Krzan, M. Surface properties and foamability of saponin and saponin-chitosan systems. Colloids Surfaces B Biointerfaces 2019, 181, 198–206. [Google Scholar] [CrossRef]
- Senathilake, K.; Karunanayake, E.; Samarakoon, S.; Tennekoon, K.; de Silva, E.; Adhikari, A. Oleanolic acid from antifilarial triterpene saponins of Dipterocarpus zeylanicus induces oxidative stress and apoptosis in filarial parasite Setaria digitata in vitro. Exp. Parasitol. 2017, 177, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, M.S. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac J. Sci. 2011, 33, 63–72. [Google Scholar]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Bhandari, R.; Sharma, G.; Jamarkatel-Pandit, N. Anti-oxidant activity of selected medicinal plants of the Himalayan Region. Int. J. Pharm. Sci. Res. 2015, 6, 473. [Google Scholar] [CrossRef]
- Aletan, U.; Adetola, E.; Abudullahi, A.; Onifade, O. Phytochemical analysis, invitro antioxidant activity and GC-MS studies of crude extracts of Cissus populnea stem. Commun. Phys. Sci. 2022, 8, 507–519. [Google Scholar]
- Soladoye, M.O.; Chukwuma, E.C. Phytochemical analysis of the stem and root of Cissus populnea (Vitaceae)—An important medicinal plant in Central Nigeria. Phytol. Balc. 2012, 18, 149–153. [Google Scholar]
- Shoibe, M.; Chy, N.U.; Alam, M.; Adnan, M.; Islam, Z.; Nihar, S.W.; Rahman, N.; Suez, E. In Vitro and In Vivo Biological Activities of Cissus adnata (Roxb.). Biomedicines 2017, 5, 63. [Google Scholar] [CrossRef]
- Hasan, M.S.; Uddin, G.; Shoibe, M.; Al Mahmud, A.; Banik, S. Evaluation of anxiolytic and hypoglycemic potential of Cissus adnata Roxb. in animal model. J. Complement. Integr. Med. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Kuppuramalingam, A.P.; Ramesh, B. Antioxidant activity of Cissus quadrangularis L. Stem in-vitro. World J. Pharm. Res. 2018, 7, 759–765. [Google Scholar] [CrossRef]
- Dhanasekaran, S. Phytochemical characteristics of aerial part of Cissus quadrangularis (L.) and its in-vitro inhibitory activity against leukemic cells and antioxidant properties. Saudi J. Biol. Sci. 2020, 27, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).