Root ABA Accumulation Delays Lateral Root Emergence in Osmotically Stressed Barley Plants by Decreasing Root Primordial IAA Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Exogenous ABA Treatment
2.3. Immunolocalization of Hormones
2.4. Staining of Nuclei by Schiff Reagent
2.5. Statistics
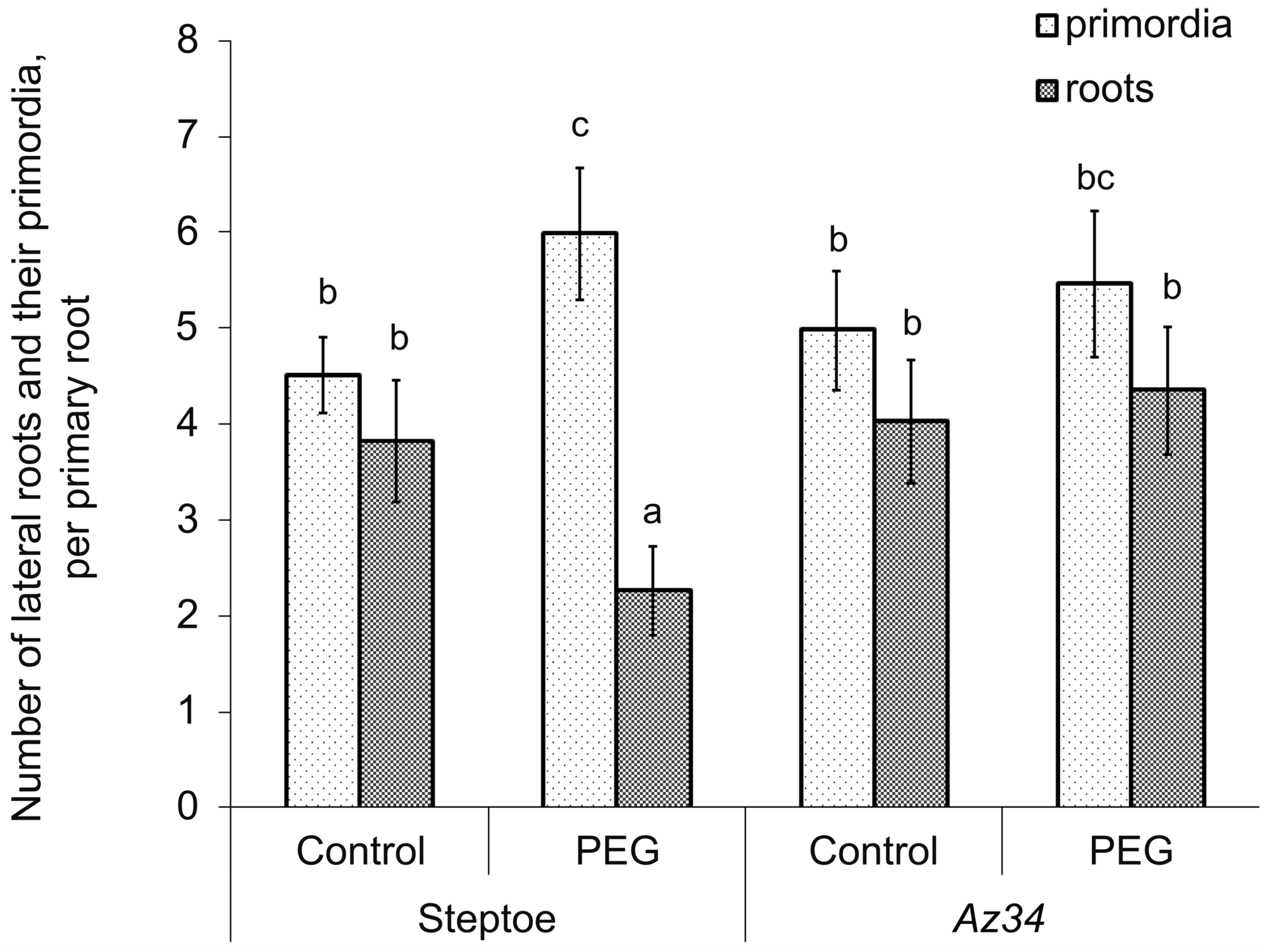
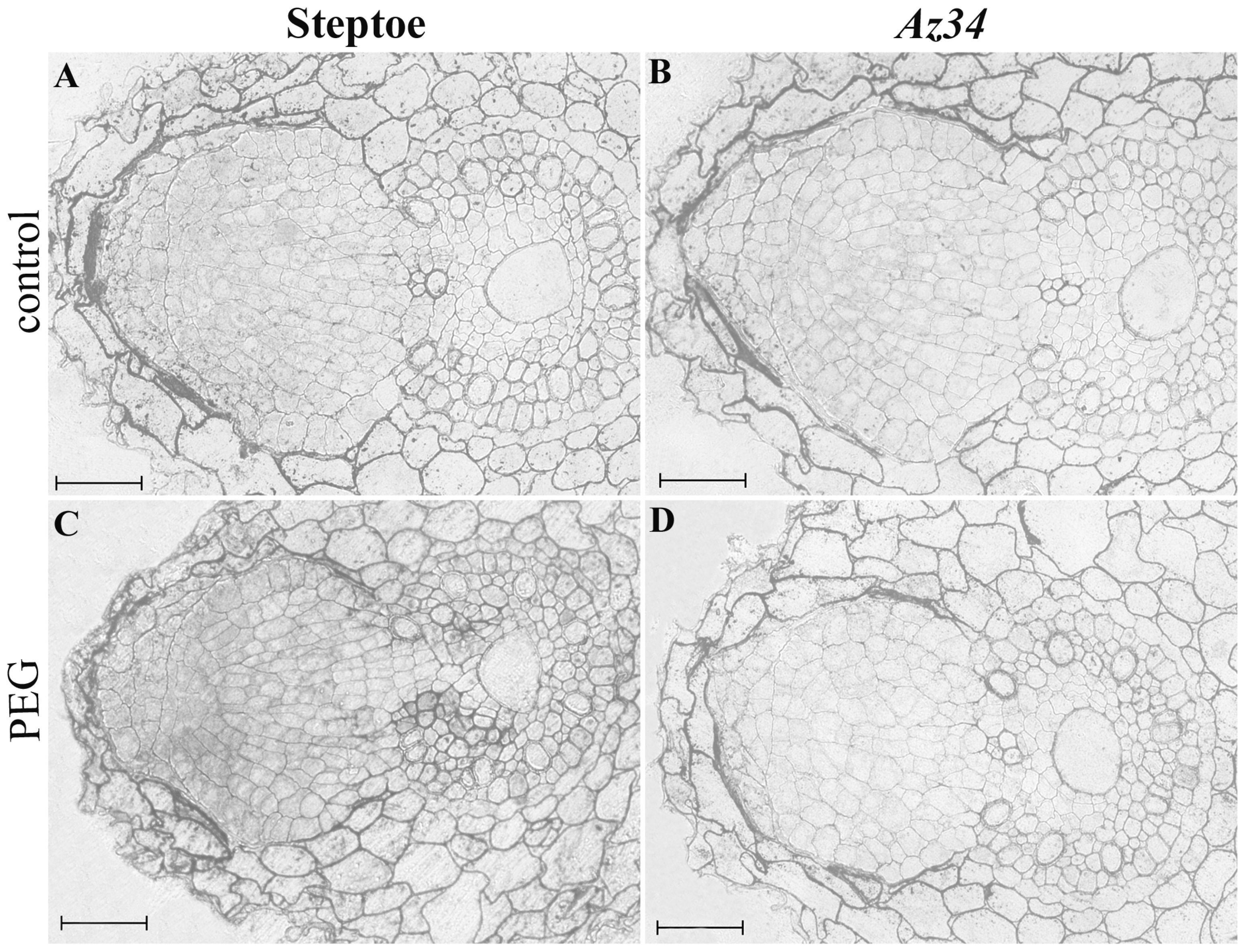
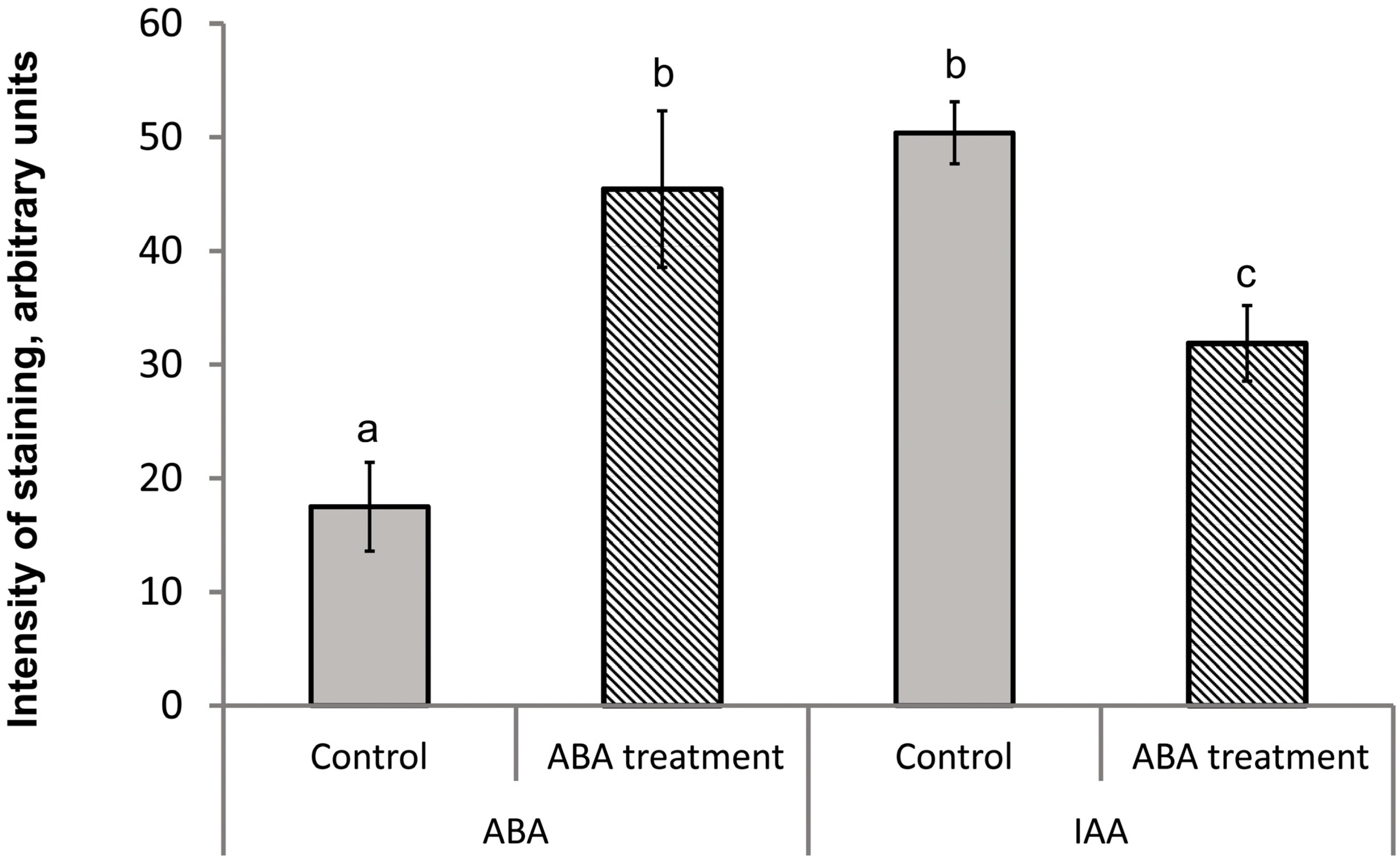
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, L.; Wang, R.G.; Mao, G.; Koczan, J.M. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 2006, 142, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Lindner, H.; Robbins II, N.E.; Dinneny, J.R. Growing out of stress: The role of cell- and organ-scale growth control in plant water-stress responses. Plant Cell 2016, 28, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A. Roots: The acquisition of water and nutrients from the heterogeneous soil environment. Prog. Bot. 2010, 71, 307–337. [Google Scholar] [CrossRef]
- Robbins II, N.E.; Dinneny, J.R. The divining root: Moisture-driven responses of roots at the micro- and macro-scale. J. Exp. Bot. 2015, 66, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Orman-Ligeza, B.; Morris, E.C.; Parizot, B.; Lavigne, T.; Babé, A.; Ligeza, A.; Klein, S.; Sturrock, C.; Xuan, W.; Novák, O.; et al. The xerobranching response represses lateral root formation when roots are not in contact with water. Curr Biol. 2018, 28, 3165–3173. [Google Scholar] [CrossRef]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.Y.; Lee, Y.N.; Kim, S.G.; Lee, Y.H.; Park, W.J.; Park, C.M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef]
- Emenecker, R.J.; Strader, L.C. Auxin-abscisic acid interactions in plant growth and development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef]
- De Smet, I.; Signora, L.; Beeckman, T.; Inzé, D.; Foyer, C.H.; Zhang, H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003, 33, 543–555. [Google Scholar] [CrossRef]
- Harris, J.M. Abscisic acid: Hidden architect of root system structure. Plants 2015, 4, 548–572. [Google Scholar] [CrossRef]
- Lu, C.; Chen, M.-X.; Liu, R.; Zhang, L.; Hou, X.; Liu, S.; Ding, X.; Jiang, Y.; Xu, J.; Zhang, J.; et al. Abscisic acid regulates auxin distribution to mediate maize lateral root development under salt stress. Front. Plant Sci. 2019, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, C.A.; López-Bucio, J.; Cruz-Ramírez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yuan, Z.; Meng, Q.; Huang, G.; Périn, C.; Bureau, C.; Meunier, A.C.; Ingouff, M.; Bennett, M.J.; Liang, W.; et al. Dynamic regulation of auxin response during rice development revealed by newly established hormone biosensor markers. Front Plant Sci. 2017, 7, 256. [Google Scholar] [CrossRef] [PubMed]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Ondzighi-Assoume, C.A.; Chakraborty, S.; Harris, J.M. Environmental nitrate stimulates abscisic acid accumulation in Arabidopsis root tips by releasing it from inactive stores. Plant Cell 2016, 3, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Sharipova, G.; Veselov, D.; Kudsoyarova, G.; Fricke, W.; Dodd, I.C.; Katsuhara, M.; Furuichi, T.; Ivanov, I.; Veselov, S. Exogenous application of abscisic acid (ABA) increases root and cell hydraulic conductivity and abundance of some aquaporin isoforms in the ABA deficient barley mutant Az34. Ann. Bot. 2016, 118, 777–785. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef]
- Guo, D.; Liang, J.; Li, L. Abscisic acid (ABA) inhibition of lateral root formation involves endogenous ABA biosynthesis in Arachis hypogaea L. Plant Growth Regul. 2009, 58, 173–179. [Google Scholar] [CrossRef]
- Signora, L.; De Smet, I.; Foyer, C.H.; Zhang, H. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J. 2001, 28, 655–662. [Google Scholar] [CrossRef]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of endogenous auxin levels in plant root development. Int. J. Mol. Sci. 2017, 18, 2587. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Saab, I.N.; Sharp, R.E.; Pritchard, J. Effect of inhibition of ABA accumulation on the spatial distribution of elongation in the primary root and mesocotyl of maize at low water potentials. Plant Physiol. 1992, 99, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Friero, I.; Alarcón, M.V.; Gordillo, L.; Salguero, J. Abscisic acid is involved in several processes associated with root system architecture in maize. Acta Physiol. Plant. 2022, 44, 28. [Google Scholar] [CrossRef]
- Martin-Vertedor, A.I.; Dodd, I.C. Root-to-shoot signalling when soil moisture is heterogeneous: Increasing the proportion of root biomass in drying soil inhibits leaf growth and increases leaf abscisic acid concentration. Plant Cell Environ. 2011, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Veselov, D.S.; Sharipova, G.V.; Veselov, S.Y.; Dodd, I.C.; Ivanov, I.; Kudoyarova, G.R. Rapid changes in root HvPIP2;2 aquaporins abundance and ABA concentration are required to enhance root hydraulic conductivity and maintain leaf water potential in response to increased evaporative demand. Funct. Plant Biol. 2018, 45, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, L.B.; Korobova, A.V.; Kudoyarova, G.R. Abscisic acid accumulation in the roots of nutrient-limited plants: Its impact on the differential growth of roots and shoots. J. Plant Physiol. 2008, 165, 1274–1279. [Google Scholar] [CrossRef]
- Veselov, D.S.; Sharipova, G.V.; Veselov, S.U.; Kudoyarova, G.R. The effects of NaCl treatment on water relations, growth and ABA content in barley cultivars differing in drought tolerance. J. Plant Growth Regul. 2008, 27, 380–386. [Google Scholar] [CrossRef]
- Veselov, S.U.; Kudoyarova, G.R.; Egutkin, N.L.; Gyuli-Zade, V.G.; Mustafina, A.R.; Kof, E.K. Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole-3-acetic acid. Physiol. Plant 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Korobova, A.V.; Veselov, S.Y.; Dodd, I.C.; Kudoyarova, G.R. ABA mediation of shoot cytokinin oxidase activity: Assessing its impacts on cytokinin status and biomass allocation of nutrient deprived durum wheat. Funct. Plant Biol. 2009, 36, 66–72. [Google Scholar] [CrossRef]
- Seldimirova, O.A.; Kudoyarova, G.R.; Kruglova, N.N.; Zaytsev, D.Y.; Veselov, S.Y. Changes in distribution of zeatin and indole-3-acetic acid in cells during callus induction and organogenesis in vitro in immature embryo culture of wheat. In Vitro Cell Dev. Biol.-Plant 2016, 52, 251. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Veselov, S.Y.; Kudoyarova, G.R. Effect on shoot water relations, and cytokinin and abscisic acid levels of inducing expression of a gene coding for isopentenyltransferase in roots of transgenic tobacco plants. J. Exp. Bot. 2010, 61, 3709–3717. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Veselova, S.; Hartung, W.; Farhutdinov, R.; Veselov, D.; Sharipova, G. Involvement of root ABA and hydraulic conductivity in the control of water relations in wheat plants exposed to increased evaporation demand. Planta 2011, 233, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, L.B.; Veselov, S.Y.; Veselov, D.S.; Filippenko, V.N.; Ivanov, E.A.; Ivanov, I.I.; Kudoyarova, G.R. Immunohistological localization and quantification of IAA in studies of root growth regulation. Russ. J. Plant Physiol. 2007, 54, 827–832. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Timergalina, L.N.; Akhiyarova, G.R.; Kudoyarova, G.R.; Korobova, A.V.; Ivanov, I.I.; Arkhipova, T.N.; Prinsen, E. Study of cytokinin transport from shoots to roots of wheat plants is informed by a novel method of differential localization of free cytokinin bases or their ribosylated forms by means of their specific fixation. Protoplasma 2018, 255, 1581–1594. [Google Scholar] [CrossRef]
- Ozerov, I.A.; Zhinkina, N.A.; Efimov, A.M.; Machs, E.M.; Rodionov, A.V. Feulgen-positive staining of the cell nuclei in fossilized leaf and fruit tissues of the Lower Eocene Myrtaceae. Bot. J. Linn 2006, 150, 315–321. [Google Scholar] [CrossRef]
- McSteen, P.; Zhao, Y. Plant hormones and signaling: Common themes and new developments. Dev. Cell 2008, 14, 467–473. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, D.P. Abscisic acid receptors: Multiple signal perception sites. Ann. Bot. 2008, 101, 311–317. [Google Scholar] [CrossRef]
- Shkolnik-Inbar, D.; Bar-Zvi, D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 2010, 22, 3560–3573. [Google Scholar] [CrossRef]
- Sharp, R.E.; Wu, Y.; Voetberg, G.S.; Saab, I.N.; LeNoble, M.E. Confirmation that abscisic acid accumulation is required for maize primary root elongation at low water potentials. J. Exp. Bot. 1994, 45, 1743–1751. [Google Scholar] [CrossRef]
- Duan, L.; Dietrich, D.; Ng, C.H.; Chan, P.M.Y.; Bhalerao, R.; Bennett, M.L.; Dinneny, J.R. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 2013, 25, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Dodd, I.C.; Veselov, D.S.; Rothwell, S.A.; Veselov, S.U. Common and specific responses to availability of mineral nutrients and water. J. Exp. Bot. 2015, 66, 2133–2144. [Google Scholar] [CrossRef] [PubMed]






| Genotypes | Normal Conditions | PEG Treatment |
|---|---|---|
| Steptoe | 199 ± 6 | 206 ± 11 |
| Az34 | 184 ± 9 | 201 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhiyarova, G.; Veselov, D.; Ivanov, R.; Sharipova, G.; Ivanov, I.; Dodd, I.C.; Kudoyarova, G. Root ABA Accumulation Delays Lateral Root Emergence in Osmotically Stressed Barley Plants by Decreasing Root Primordial IAA Accumulation. Int. J. Plant Biol. 2023, 14, 77-90. https://doi.org/10.3390/ijpb14010007
Akhiyarova G, Veselov D, Ivanov R, Sharipova G, Ivanov I, Dodd IC, Kudoyarova G. Root ABA Accumulation Delays Lateral Root Emergence in Osmotically Stressed Barley Plants by Decreasing Root Primordial IAA Accumulation. International Journal of Plant Biology. 2023; 14(1):77-90. https://doi.org/10.3390/ijpb14010007
Chicago/Turabian StyleAkhiyarova, Guzel, Dmitriy Veselov, Ruslan Ivanov, Guzel Sharipova, Igor Ivanov, Ian C. Dodd, and Guzel Kudoyarova. 2023. "Root ABA Accumulation Delays Lateral Root Emergence in Osmotically Stressed Barley Plants by Decreasing Root Primordial IAA Accumulation" International Journal of Plant Biology 14, no. 1: 77-90. https://doi.org/10.3390/ijpb14010007
APA StyleAkhiyarova, G., Veselov, D., Ivanov, R., Sharipova, G., Ivanov, I., Dodd, I. C., & Kudoyarova, G. (2023). Root ABA Accumulation Delays Lateral Root Emergence in Osmotically Stressed Barley Plants by Decreasing Root Primordial IAA Accumulation. International Journal of Plant Biology, 14(1), 77-90. https://doi.org/10.3390/ijpb14010007







