Abstract
Arbuscular mycorrhizal fungi, plant-growth-promoting bacteria (PGPB) and vermicompost constitute important environmental and economic resources for improving the production and quality of tomato fruits. The present research aims to determine the single and combined effect of Glomus fasciculatum (Gf) fungus, Azotobacter chroococcum (Azot), PGPB and vermicompost leachate (VL) organic fertilizer on the yield and quality of tomato fruit. Thus, an open-field experiment was established with seven treatments, a control and three replicates. Total soluble solids, vitamin C, acidity, fruit mass and fruit diameter were evaluated as fruit quality variables; the yield was recorded and estimated in tons per hectare−1. The results showed that Gf, Azot and VL were effective in promoting tomato yield and fruit quality. As a trend, the triple combination (Gf + Azot + VL) evidently obtained the highest values of total soluble solids, vitamin C and fruit acidity. The range of improvement concerning the fruit size was 66.6% (single treatment) compared to 78.5% (triple combination). The maximum yield of 54.5 t/ha−1 was recorded for the Gf + Azot + VL combination. Therefore, G. fasciculatum, A. chroococcum and VL are considered useful as organic alternatives for open-field tomato biofertilization programs in tropical countries.
1. Introduction
Tomato (Solanum lycopersicum L.) is one of the most important crops in the world in terms of planted area, production volume and profit generation for farmers [1,2]. Currently, the international tomato markets are considering organoleptic properties and nutritional qualities as food quality criteria, thereby increasing competitiveness at the time of export [3]. Recently, 37,257 tons (t) of industrial tomatoes have been produced worldwide. The five main producing countries are the United States of America, China, Italy, Spain and Turkey [4]. However, to achieve and maintain competitiveness in international markets, three actions should be considered: (I) developing new varieties adapted to the region [5]; (II) selecting microbiological species adequately [6]; and (III) considering quality in terms of higher vitamin content and other measurable parameters in addition to yield [7].
Some secondary metabolites of high commercial value, such as vitamins and antioxidants, are among the most valued properties, in addition to protein content [8]. In tomato production, vitamin C increases with organic compared to inorganic fertilization [9]. Thus, one of the most used strategies in plant nutrition systems with more sustainable agroecological approaches is the use of native microbiota.
The symbiotic effects eventually occur through a complex biological interaction and biochemical process, where the nutrients are the most important; the best plant response depends on the availability and mobility of nutrients in the soil [10]. This response is to the presence of the microorganisms Glomus fasciculatum (Gf) and Azotobacter chroococcum (Azot) in the soil and rhizosphere, whose interaction favors the physical and chemical soil properties, resulting in the activation of plant photosynthetic processes [11]. Plant-growth-promoting rhizobacteria (PGPR), humic acids and organic derivatives such as vermicompost leachate (VL) and arbuscular mycorrhizal fungus are enhancing the functions of soil texture, structure (physical, chemical and biological) and yield effects in tomato crops [12,13,14].
Some Azotobacter isolates influence secondary metabolite and phytohormone synthesis, which are involved in tomato yield and quality. The phytohormones influence the metabolic pathways (amino acids, vitamins, phospholipids and fatty acids) and induce ethylene synthesis, as well as, thereby, carbohydrate production and translocation in fruit. The auxins, cytokinins and gibberellins promote plant growth, biomass and agricultural yield [15,16].
The arbuscular mycorrhiza fungal species Glomus fasciculatum improves nutrient absorption, mainly of phosphorus and nitrogen. It increases water absorption due to the greater development of the root system and strengthens the immune systems of tomato plants, increasing yield and quality as well as resistance to biotic and abiotic stress conditions [17]. However, studies have assessed the effect of synergistic PGPR on the microorganisms contained in organic matter with the derivatives of vermicompost leachate [18]. Therefore, the organic fertilization strategy considers the adaptability of native PGPR strains and the ability to promote plant growth, which influences fruit quality and lowers environmental impact [19]. In this regard, vermicompost leachate contains macronutrients (N, K, Ca and Mg) and micronutrients (B, Fe and Zn), as well as abundant microbial activity (bacteria, fungi and yeasts). In addition, its composition of bioactive substances, such as humic and fulvic acids, and phytohormones is important for plant growth, development and crop yield [20]. The simple or combined effect of native microbiological strains with potential use in agriculture should especially be known in order to measure the effect of organic derivatives in terms of productivity and quality [21,22]. Therefore, the present research was carried out with the aim of determining the single and combined effects of G. fasciculatum, A. chroococcum and vermicompost leachate on the yield and quality of tomato fruit.
2. Materials and Methods
2.1. Location and Soil Conditions
The experimental study was performed in open field conditions at the Universidad de Granma in Bayamo, Cuba, at 76°43′42′′ W, 20°16′48′′ N and 76 m above sea level. The type of soil was carbonated fluffy brown, characterized by having A, B and C horizons. It was formed under a salinization process with a clay-loam texture and a sialic B horizon, with a cation exchange capacity of ≥30 cmol/kg−1, clay and free iron contents of ≤3%, organic matter: 3.5%, pH: 6.8, P2O5: 4.2 mg/100 g−1 of soil and K2O: 18.87 mg/100 g−1 of soil [23].
2.2. Biological Material
Glomus fasciculatum (Gf) was donated from the Instituto de Ecología y Sistemática (IES), Habana, Cuba, and reproduced in greenhouse conditions (temperature 25 ± 3 °C, humidity 75%, field capacity for 75 days) on sorghum plants (Sorghum bicolor L. Moench) obtained from the Granma seed company. Plastic pots (40 × 17 × 17 cm) were used with 10 kg of substrate: sterile perlite, vermiculite and soil (vol/vol, 4:3:3), respectively. The Gf inoculum recorded a colonization factor of 65% (250 spores/g soil), and 0.50 g of Gf inoculum was applied to the roots of each plant. Azotobacter chroococcum (Azot) was donated from the Instituto Nacional de Investigaciones Fundamentales de la Agricultura Tropical (INIFAT), Habana, Cuba. A liquid formulation was used, inoculating 10 mL/plant with a concentration of 4 × 109 CFU/mL−1 adjusted using the spectrophotometric method [24]. The vermicompost leachate (VL) treatment was prepared by mixing one part of the solid vermicompost humus with five parts of water, and was allowed to settle for 48 h, then used at doses of 200 mL/plant. The chemical characterization corresponded to pH: 6.9, N 1.3%, P: 778 PPM, K: 4.8 PPM, and the base exchange capacity was 1870 milliequivalent (mEq) k−1; the evaluations were carried out at the Soil Laboratory at Granma from the Soil Institute of Cuba. All of the treatments were applied two times, 7 and 15 days after transplantation (DAT). The soil was prepared with animal traction, and irrigation was applied twice a week with a sprinkler irrigation system. Weed cleaning, removal of shoots and pest monitoring were carried out weekly according to technical instructions for tomato plants [25]. No pests or diseases were observed in the experiment. The weather variables were recorded: the temperature was 22 ± 4 °C, the relative humidity was 71 ± 2% and there was 45 mm of rain during the period.
2.3. Seed and Treatment Description
The tomato cv. Vyta is widely cultivated due to its climatic adaptability, pest tolerance and high agricultural yields. The seeds had previously been disinfected with 1% sodium hypochlorite (NaClO) solution for five minutes and germinated in an alveoli tray with a substrate consisting of sterile perlite, vermiculite and soil (vol/vol, 4:3:3) until their growth reached 12–14 cm. The field transplant was carried out at a planting distance of 0.90 m × 0.30 m. The plants were inoculated only with Gf, Azot and VL, then combined with Gf + Azot, Gf + VL, Azot + VL or Gf + Azot + VL. A group plant control was used without microorganisms or VL.
2.4. Yield and Quality of Tomato Fruit
With 20 fruits/treatment (n = 20) at 95 days after transplant (DAT), the equatorial and axial diameter (EDF, cm) were evaluated with a digital Vernier. Dry mass/fruit (DM, g) was assessed triplicate: the samples were dried in an electric oven at 70 °C for 72 h and the mass was recorded on an analytical balance (Sartorius entries 224I 1S). Total soluble carbohydrates (TSS, %): in Brix degrees (°Bx) were evaluated by the refractometric method, using a WYA-2S/ VWR refractometer obtained from the USA. The yield was evaluated at three moments, 85, 95 and 105 days after transplant, by counting the fruit mass per plant, which was estimated in tons per hectare−1. Vitamin C (mg kg−1) was determined by the volumetric oxidation–reduction method by extraction with a solution of acetic acid and meta-phosphoric acid until a pink color appeared, using the 2,6-dichlorophenolindophenol as a titrant [26].
2.5. Experimental Design and Statistical Analyses
A completely randomized block design was used, with three blocks for each treatment. The block contained 222 plants for treatment. All data were verified for normality using the Kolmogorov–Smirnov test, and variance homogeneity was assessed using Bartlett’s test. A univariate analysis of variance (ANOVA) was performed, and Tukey’s test was used [27].
3. Results
3.1. Fruit Size and Quality Standards
For the fruit size variable, significant differences were found between the treatments (p ≤ 0.05, Tukey’s test). The Gf + Azot + VL (7 cm) and Azot + VL (6.9 cm) treatments recorded the highest EDF values, without statistical differences between them, followed by the Gf + VL (6.3 cm), Gf + Azot (5.8 cm), VL (5.2 cm) and Azot (4.8 cm) treatments, while Gf (4.5 cm) and control (4.1 cm) obtained the lowest values. A 71% increase in the EDF variable was attributed to the effect of the Gf + Azot + VL and Azot + VL treatments compared to the control. For the ADF variable, the highest value was recorded for the combined treatment with Gf + Azot + VL (4.9 cm), which showed differences to the rest of the treatments, followed by the Azot + VL (4.5 cm), Gf + VL (4.5 cm), Gf + Azot (4.5), Gf (4.2), VL (4.1) and Azot (4.1) treatments. The control (3.1) obtained the lowest value. The triple combination Gf + Azot + VL showed a 58% increase in the ADF variable compared to the control (Table 1).

Table 1.
Effect of single and combined treatments of Glomus fasciculatum, Azotobacter chroococcum and vermicompost leachate on the equatorial and axial diameters of tomato fruits.
When the DM variable was analyzed, significant differences were found between the treatments (Tukey’s test, p < 0.05). The highest values were reached with the Gf + Azot + VL (12%) and Gf + VL (11.7) treatments, which differed from the rest of the treatments, and the control obtained the lowest dry mass (7.8%). The triple combination of Gf + Azot + VL and the treatment with Gf + VL showed 54% and 50% increases, respectively, in the DM variable compared to the control (Figure 1).
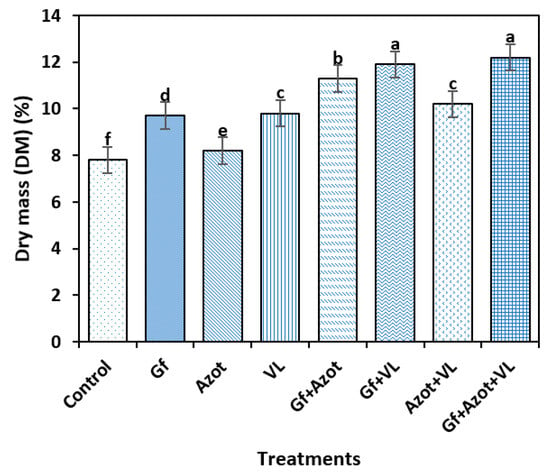
Figure 1.
Effect of Glomus fasciculatum (Gf), Azotobacer chroococcum (Azot) and vermicompost leachate (VL) on dry mass (DM) of tomato fruit. Vertical bars ± standard error (SE) are shown; the different letters differed significantly (Tukey’s test, p < 0.05).
For the TSS variable, significant differences were found between the treatments (Tukey’s test, p < 0.05). The highest value was reached with Gf + Azot + VL (6.5%), which differed from the rest of the treatments, and the control obtained the lowest mass (4.0%). The triple combination of Gf + Azot + VL showed a 62% increase in the TSS variable compared to the control (Figure 2).
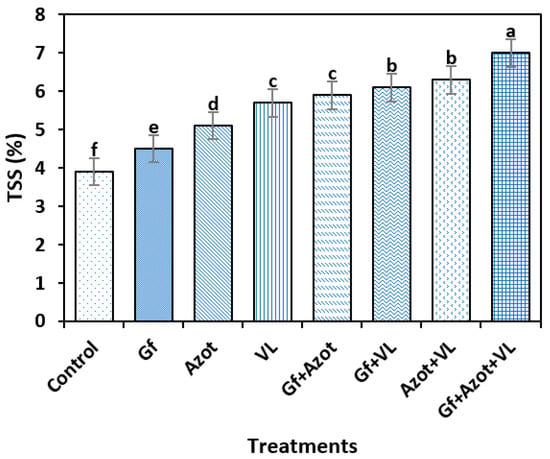
Figure 2.
Effect of Glomus fasciculatum (Gf), Azotobacter chroococcum (Azot) and vermicompost leachate (VL) on total soluble solids (TSS) in tomato fruit. Vertical bars ± standard error (SE) are shown; the different letters differed significantly (Tukey’s test, p < 0.05).
3.2. Impact on Agricultural Yield
For the yield variable, significant differences were found between the treatments (Tukey’s test, p < 0.05). The highest value was reached with Gf + Azot + VL (55 t ha−1), which differed from the rest of the treatments, and the control obtained the lowest value (26 t ha−1). The triple combination Gf + Azot + VL potentiated the agricultural yield (211%) compared to the control (Figure 3).
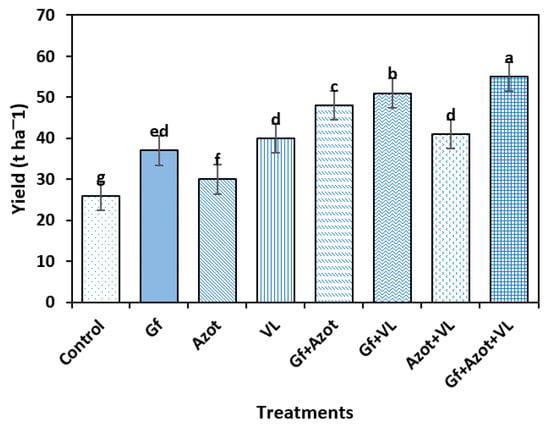
Figure 3.
Effect of Glomus fasciculatum (Gf), Azotobacer chroococcum (Azot) and vermicompost leachate (VL) on tomato yield. Vertical bars ± standard error (SE) are shown; the different letters differed significantly (Tukey’s test, p < 0.05).
3.3. Vitamin C
For the vitamin C content, significant differences were found between the treatments (Tukey’s test, p < 0.05). The highest value was reached with Gf + Azot + VL (229 mg kg−1), which differed from the rest of the treatments, and the control obtained the lowest content (149 mg kg−1). The triple combination Gf + Azot + VL showed a 54% increase in the vitamin C content compared to the control (Figure 4).
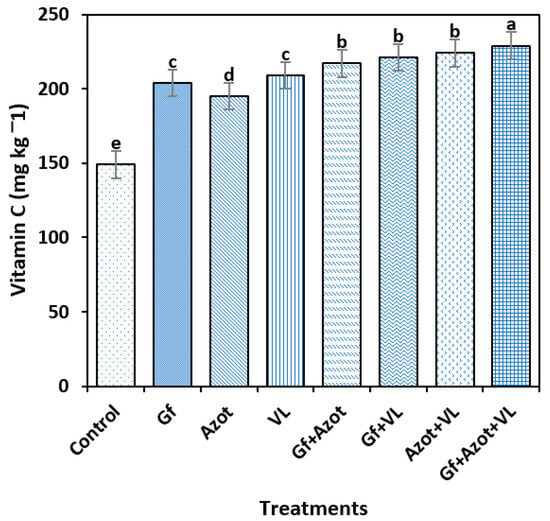
Figure 4.
The vitamin C content of tomato fruit was assessed by applying Glomus fasciculatum (Gf), Azotobacte chroococcum (Azot) and vermicompost leachate (VL), either alone or combined. Vertical bars ± standard error (SE) are shown; the different letters differed significantly (Tukey’s test, p < 0.05).
4. Discussion
Studies performed by Romero-Rodríguez et al. [28] demonstrated that arbuscular mycorrhiza inoculation exerts a positive effect on tomato fruit size and caliber by leading to a significant increase of 30% in the axial and equatorial diameters of the fruit. This result is attributed to the combined effect of Gf + Azot + VL, which activates the phytohormones (cytokinins) involved in the cellular division of fruit [29]. Veselova et al. [30] reported an increase of 24% in tomato fruit quality by inoculating the plant-growth-promoting bacterium Bacillus subtilis.
Alarcón-Zayas et al. [31] demonstrated that the increase in tomato fruit size was probably related to the stimulating and regulating action of the humic acids found in vermicompost leachate, as well as improved nutrient absorption. Certainly, two of the most documented effects of G. fasciculatum and Azotobacter species in commercial tomato production are vegetative promotion and root growth, which have effects on yield and quality, especially under stress conditions; this organic alternative is an important complement to tomato crop fertilization [32]. The Gf + Azot + VL combination enhanced the TSS levels in tomato fruits, which can be attributed to an improvement in the water adsorption, nutrients and physiological–metabolic activity of the plants due to combined biofertilizers for microorganisms (Gf, Azot) and vermicompost leachate [33]. Where the biofertilizers were applied, the synthesis of carbohydrates in the fruit was improved, which allowed for a higher concentration of TSS [34].
The use of VL and microbes (Gf, Azot) is widely documented in agriculture, in the open field under protected agriculture systems (greenhouse) and especially in drought stress conditions [35]. This result could be significant, since some organic molecules in VL can act as biochemical signals or messengers that modulate or influence ion movement through cell membranes, especially for K+ influence and Ca2+ flow. These mechanisms allow for stomatal opening, with a consequent higher uptake of CO2 and its higher concentration in the chloroplasts. Furthermore, these microorganisms and VL have bioactive components, such as auxin-like molecules, that interact with some plant hormone receptors, resulting in a more favorable effect on the concentration of sugars and soluble solids in fruit [36].
In the present research, the combination of microorganisms (Gf, Azot) and LV contributed to obtaining the highest agricultural yield under field conditions, which was attributed to better plant nutrition; G. fasciculatum was shown to be involved in nutrient absorption, mainly of phosphorous and nitrogen, as well as improved water absorption and increased resistance to biotic and abiotic stress [37].
Specialized strains of A. chroococcum can fix up to 300 kg ha−1 of nitrogen, thus guaranteeing the necessary nitrogen for morphological development and crop yield [38]. VL contains nitrogen, phosphorus and potassium, as well as the micronutrients zinc, iron, copper, manganese, molybdenum, boron, calcium, magnesium, sulfur and sodium, elements which are closely related to growth processes, flower formation and fruiting in tomato plants [39]. Due to the above, the triple combination of biofertilizers (Gf + Azot + VL) enhanced the formation of yield in cv. Vyta tomatoes in open field conditions.
A. chroococcum isolates influence the synthesis of secondary metabolites and phytohormones, which are involved in tomato yield and quality. The phytohormones influence the metabolic pathways (amino acids, vitamins, phospholipids and fatty acids) and induce ethylene synthesis, thus affecting carbohydrate production and translocation in fruit. The auxins, cytokinins and gibberellins promote plant growth, biomass and agricultural yield [15,16].
The highest vitamin C (ascorbic acid, AsA) contents observed in tomato fruit corresponded to the triple combination of biofertilization (Gf + Azot + VL). Vitamin C has been documented to be produced in plant tissues from glucose; its concentration increases during fruit ripening and the respiratory process, which is associated with an increase in ethylene levels, stimulating the enzyme L-gulono-1,4-lactone oxidase activity [40].
Vitamin C is an essential multifaceted phytonutrient for both the human diet and plant growth. The optimum levels of AsA accumulation combined with balanced redox homeostasis are required for normal plant development and the defense responses of plants to adverse environmental stimuli; microorganism-based biofertilizers are an organic alternative which can be used to enhance the content of vitamin C in tomato fruit [41]. Understanding quality in terms of antioxidant activity, AsA and mineral and protein contents, in addition to the most well-known indicators, can positively influence external indicators such as the size, color and organoleptic properties of fruits [33,42].
Moreover, the effects of the humic acids in VL impart trace enzymes that can adhere to ATP molecules and influence principal axis length growth and lateral root induction. The biological processes exerted by VL have also been observed to accelerate the oxidative effects and stability of organic matter and associated microorganisms, including GF and Azot [14]. In addition, it is possible that the most studied aspect of VL is its effect on the properties of soil porosity, where aeration, moisture-holding capacity and microbial exchange are favored [43,44,45,46].
5. Conclusions
This study applied Glomus fasciculatum (Gf), Azotobacter chroococcum (Azot) and vermicompost leachate (VL) in combination, determining the most effective treatment with which to increase tomato yield and quality. The results demonstrated the importance of using combined biofertilization practices as an alternative to the organic production of tomato crops in the tropical Caribbean.
Author Contributions
Conceptualization, A.A.-Z. and W.G.C.-C.; methodology, A.A.-Z. and W.G.C.-C.; software, D.M.-H. and E.O.R.-P.; validation, A.A.-Z., R.J.H.-P., L.G.H.-M., D.M.-H. and E.O.R.-P.; formal analysis, D.M.-H. and E.O.R.-P.; investigation, A.A.-Z.; resources, W.G.C.-C.; data curation, L.G.H.-M.; writing—original draft preparation, A.A.-Z., D.M.-H. and E.O.R.-P.; writing—review and editing, R.J.H.-P., L.G.H.-M. and W.G.C.-C.; visualization, L.G.H.-M.; supervision, R.J.H.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Universidad de Granma and Higher Education of the Cuba Republic, agreement No. 01042020-1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data will be made available if requested to corresponding author.
Acknowledgments
The authors thank to Commercial English editing organizations or MDPI for English edition.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Casanovas Cosío, E.; Suárez del Villar Labastida, A.; Álvarez Sánchez, A.; Avilleira Cruz, I. Valoración de la seguridad alimentaria cubana a partir de la superficie agrícola explotada y los rendimientos agrícolas. Rev. Univ. Soc. 2022, 14, 304–314. [Google Scholar]
- SIAP. Servicio de Información Agroalimentaria y Pesquera. Panorama Agroalimentario. Secretaria de Agricultura y Desarrollo Rural, México. 2020, p. 200. Available online: https://www.inforural.com.mx/wp-content/uploads/2020/11/Atlas-Agroalimentario-2020.pdf (accessed on 11 December 2023).
- Krivdáné Dorogi, D.A. Evaluation of the competitiveness of fresh tomato. Int. J. Hort. Sci. 2022, 28, 73–77. [Google Scholar] [CrossRef]
- González, I.A.; Cidoncha, C.M.; Ruiz, S.C. Tomate de Industria: Campaña 2021. Nav. Agrar. 2022, 250, 25–32. [Google Scholar]
- Rivero, A.G.; Keutgen, A.J.; Pawelzik, E. Antioxidant Properties of Tomato Fruit (Lycopersicon esculentum Mill.) as Affected by Cultivar and Processing Method. Horticulturae 2022, 8, 547. [Google Scholar] [CrossRef]
- Carrillo-Sosa, Y.; Terry-Alfonso, E.; Ruiz-Padrón, J.; Delgado-Arrieta, G. Efecto de la coinoculación de microorganismos eficientes-HMA en el rendimiento del cultivo del tomate (Solanum lycopersicum L.). Cult. Trop. 2023, 43, 3. [Google Scholar]
- Fan, H.; Zhang, Y.; Li, J.; Jiang, J.; Waheed, A.; Wang, S.; Rasheed, S.M.; Zhang, L.; Zhang, R. Effects of Organic Fertilizer Supply on Soil Properties, Tomato Yield, and Fruit Quality: A Global Meta-Analysis. Sustainability 2023, 15, 2556. [Google Scholar] [CrossRef]
- Colak, N.G.; Eken, N.T.; Ülger, M.; Frary, A.; Doğanlar, S. Mapping of quantitative trait loci for antioxidant molecules in tomato fruit: Carotenoids, vitamins C and E, glutathione and phenolic acids. Plant Sci. 2020, 292, 110393. [Google Scholar] [CrossRef]
- Sabin, F.; Tehmina, A.; Riaz, H.; Basharat, A. PGPR Mediated Bio-Fortification of Tomato Fruit Metabolites with Nutritional and Pharmacological Importance. Pak. J. Biotechnol. 2017, 14, 17–21. [Google Scholar]
- Liu, L.; Wang, S.; Guo, X.; Wang, H. Comparison of the effects of different maturity composts on soil nutrient, plant growth and heavy metal mobility in the contaminated soil. J. Environ. Manag. 2019, 250, 109525. [Google Scholar] [CrossRef]
- Chehade, L.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from food processing by-products: Agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J. Sci. Food. Agric. 2018, 98, 1426–1436. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Babalola, O.O.; Prigent-Combaret, C.; Cruz, C.; Stefan, M.; Kutu, F.; Glick, B.R. The application of plant growth-promoting rhizobacteria in Solanum lycopersicum production in the agricultural system: A review. PeerJ 2022, 10, e13405. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gu, M.; Yu, P.; Zhou, C.; Liu, X. Biochar and vermicompost amendments affect substrate properties and plant growth of basil and tomato. Agronomy 2020, 10, 224. [Google Scholar] [CrossRef]
- Kiyasudeen, K.; Ibrahim, M.H.; Quaik, S.; Ismail, S.A. Prospects of organic waste management and the significance of earthworms. In Applied Environmental Science and Engineering for A Sustainable Future; Springer International Publishing: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Fusco, G.M.; Nicastro, R.; Rouphael, Y.; Carillo, P. The Effects of the Microbial Biostimulants Approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops. Foods 2022, 11, 2656. [Google Scholar] [CrossRef] [PubMed]
- Hindersah, R.; Kamaluddin, N.N.; Samanta, S.; Banerjee, S.; Sarkar, S. Role and perspective of Azotobacter in crops production. Sains Tanah J. Soil Sci. Agroclimatol. 2020, 17, 170–179. [Google Scholar] [CrossRef]
- Devi, N.O.; Tombisana Devi, R.K.; Debbarma, M. Effect of endophytic Bacillus and arbuscular mycorrhiza fungi (AMF) against Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici. Egypt. J. Biol. Pest. Control 2022, 32, 1. [Google Scholar] [CrossRef]
- Banerjee, A.; Bareh, D.A.; Joshi, S.R. Native microorganisms as potent bioinoculants for plant growth promotion in shifting agriculture (Jhum) systems. J. Soil Sci. Plant Nutr. 2017, 17, 127–140. [Google Scholar] [CrossRef]
- Le, T.A.; Pék, Z.; Takács, S.; Neményi, A.; Daood, H.G.; Helyes, L. The effect of plant growth promoting rhizobacteria on the water-yield relationship and carotenoid production of processing tomatoes. HortScience 2018, 53, 816–822. [Google Scholar] [CrossRef]
- Na, L.; Abail, Z.; Whalen, J.K.; Liang, B.; Hu, C.; Hu, R.; Wu, Y. Earthworms increase nitrogen uptake by lettuce and change short-term soil nitrogen dynamics. Appl. Soil Ecol. 2022, 176, 104488. [Google Scholar] [CrossRef]
- Aishwarya, M.; Dhanya, M.K.; Johnson, J.M.; Murugan, M.; Beena, R.; Paul, A. Individual and combined effects of beneficial fungal root endophytes Piriformospora indica and Glomus fasciculatum on growth, nutrient uptake and IAA production in small cardamom. J. Plant. Crops 2022, 50, 35–41. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Massa, D.; Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulant applications in low input horticultural cultivation systems. Italus Hortus 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Hernández Jiménez, A.; Pérez Jiménez, J.M.; Bosch Infante, D.; Castro Speck, N. Clasificación de los Suelos de Cuba; Ediciones INCA: La Habana, Cuba, 2015. [Google Scholar]
- Kipper, T.G.J.; da Silva, L.P.; Bender, S.; Vinderola, C.G.; de Fariña, L.O. Cell counting and bacterial inoculum standardization by spectrophotometric method for Bifidobacterium animalis ssp. Lactis INL1. Braz. J. Dev. 2020, 6, 77634–77643. [Google Scholar] [CrossRef]
- López-Marín, G.L. Manual Técnico del Cultivo del Tomate. Innovación Para la Seguridad Alimentaria y Nutricional de Centroamérica y Panamá; Instituto Nacional de Innovación y Transferencia en Tecnología Agropecuaria; IICA: Kingston, Jamaica, 2017; 121p, ISBN 978-9968-586-27-6. [Google Scholar]
- Veillet, S.; Busch, J.; Savage, G. Acceptability and antioxidant properties of a semi-dried and smoked tomato product. J. Food Agricult. Env. 2009, 7, 70–75. [Google Scholar]
- StatSoft, Inc. Statistica (Data Analysis Software System), version 6; Scientific Research: Tulsa, AZ, USA, 2001; pp. 91–94.
- Romero-Rodríguez, A.; Luna-Zendejas, H.S.; Solis-Oba, A.; Castro-Rivera, R.; Armenta-Bojórquez, A.D.; Solís-Oba, M.M. Evaluación de la calidad de tomate fertilizado con extracto de sargazo del Caribe mexicano y micorrizas. Mex. J. Biotechnol. 2022, 7, 15–31. [Google Scholar] [CrossRef]
- Chai, L.; Wang, H.; Yu, H.; Pang, E.; Lu, T.; Li, Y.; Li, Q. Girdling promotes tomato fruit enlargement by enhancing fruit sink strength and triggering cytokinin accumulation. Front. Plant Sci. 2023, 14, 1174403. [Google Scholar] [CrossRef] [PubMed]
- Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Cherepanova, E.A.; Alekseev, V.Y.; Maksimov, I.V. By modulating the hormonal balance and ribonuclease activity of tomato plants Bacillus subtilis induces defense response against potato virus X and potato virus Y. Biomolecules 2022, 12, 288. [Google Scholar] [CrossRef]
- Alarcón-Zayas, A.; Barreiro-Elorza, P.; Boicet-Fabré, T.; Ramos-Escalona, M.; Morales-León, J.Á. Influencia de ácidos húmicos en indicadores bioquímicos y físico-químicos de la calidad del tomate. Rev. Cubana Quím. 2018, 30, 243–255. [Google Scholar]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Abd El-Lateef, H.M.; Yasir, M.; Tanveer Shah, S.; Zohaib Ikram, M. Effect of Azospirillum and Azotobacter species on the performance of cherry tomato under different salinity levels. Gesunde Pflanz 2022, 74, 487–499. [Google Scholar] [CrossRef]
- Zazueta-Avitia, A.; Burboa-Meza, C.Y.; Ramírez-Alvarado, D.; Flores-Martínez, H.; Segura-Castruita, M.Á.; Gómez-Leyva, J.F. Caracterización de frutos tomate (Solanum lycopersicum) en plantas colonizadas por el hongo micorrízico arbuscular Rhizopagus irregularis en condiciones de estrés salino. Acta Univ. 2021, 31, e3120. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol. Res. 2020, 239, 126569. [Google Scholar] [CrossRef]
- Scaglia, B.; Nunes, R.R.; Rezende, M.O.O.; Tambone, F.; Adani, F. Investigating organic molecules responsible of auxin-like activity of humic acid fraction extracted from vermicompost. Sci. Total Environ. 2016, 562, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Math, S.; Arya, S.; Sonawane, H.; Patil, V.; Chaskar, M. Arbuscular mycorrhizal (Glomus fasciculatum) fungi as a plant immunity booster against fungal pathogen. Curr. Agricult. Res. J. 2019, 7, 99–107. [Google Scholar] [CrossRef]
- Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglou, M.; Chanioti, S.; Efthimiadou, A. Evaluation of plant growth promoting bacteria strains on growth, yield and quality of industrial tomato. Microorganisms 2021, 9, 2099. [Google Scholar] [CrossRef] [PubMed]
- Becagli, M.; Guglielminetti, L.; Cardelli, R. Effects of combined biochar and vermicompost solution on leachate characterization and nitrogen balance from a greenhouse tomato (Solanum lycopersicum) cultivation soil. Comm. Soil Sci. Plant Anal. 2021, 52, 1879–1893. [Google Scholar] [CrossRef]
- Yagmur, B.; Gunes, A. Evaluation of the Effects of Plant Growth Promoting Rhizobacteria (PGPR) on Yield and Quality Parameters of Tomato Plants in Organic Agriculture by Principal Component Analysis (PCA). Gesunde Pflanzen 2021, 73, 219–228. [Google Scholar] [CrossRef]
- Mellidou, I.; Koukounaras, A.; Kostas, S.; Patelou, E.; Kanellis, A.K. Regulation of vitamin C accumulation for improved tomato fruit quality and alleviation of abiotic stress. Genes 2021, 12, 694. [Google Scholar] [CrossRef]
- Andrade-Sifuentes, A.; Fortis-Hernández, M.; Preciado-Rangel, P.; Orozco-Vidal, J.A.; Yescas-Coronado, P.; Rueda-Puente, E.O. Azospirillum brasilense and solarized manure on the production and phytochemical quality of tomato fruits (Solanum lycopersicum L.). Agronomy 2020, 10, 1956. [Google Scholar] [CrossRef]
- Zhou, Z.; Tran, P.Q.; Breister, A.M.; Liu, Y.; Kieft, K.; Cowley, E.S.; Anantharaman, K. Metabolic: High-throughput profiling of microbial genomes for functional traits, metabolism, biogeochemistry, and community-scale functional networks. Microbiome 2022, 10, 33. [Google Scholar] [CrossRef]
- Demir, Z. Effects of microbial bio-fertilizers on soil physicochemical properties under different soil water regimes in greenhouse grown eggplant (Solanum melongena L.). Commun. Soil Sci. Plant Anal. 2020, 51, 1888–1903. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Ndukwe, J.K.; Aliyu, G.O.; Chukwu, K.O.; Ezugworie, F.N.; Igbokwe, V.C. Composting: An eco-friendly technology for sustainable agriculture. In Ecological and Practical Applications for Sustainable Agriculture; Springer: Singapore, 2020; pp. 179–206. [Google Scholar] [CrossRef]
- Chatterjee, R.; Debnath, A.; Mishra, S. Vermicompost and soil health. Soil Health 2020, 59, 69–88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).