Abstract
The current investigation delved into the utilization of cattle and municipal sanitary inocula for anaerobic digestion of poultry wastes, addressing a crucial and pragmatic challenge in waste management. The emphasis on poultry waste is pertinent due to its well-documented impediments in anaerobic digestion, attributed to heightened levels of ammonia and volatile fatty acids (VFAs). The strategic selection of cattle and municipal sanitary inocula suggests an approach aimed at bolstering the anaerobic digestion process. In this study, we evaluated the use of cattle and municipal sanitary inocula for the anaerobic digestion of various poultry wastes, which is often challenged by high levels of ammonia and volatile fatty acids (VFAs). The substrates tested included belt waste (Poultry A), poultry litter plus feed residues (Poultry B), tray hatchery ©, and stillage. These substrates were processed in two continuous stirred tank reactors (CSTRs), R-1 (with antibiotic monensin) and R-2 (without monensin). Initially, both reactors operated with the same hydraulic retention time (HRT), using a substrate ratio of stillage: belt: tray hatchery (S:B:T) of 70:15:15. On the 41st day, the HRT was adjusted to 20 days, and the substrate ratio was changed to S:A:T 70:40:40. The specific methane yield for R-1 started at 10.768 L g−1 COD, but decreased to 2.65 L g−1 COD by the end of the experiment. For R-2, the specific methane yield varied between 0.45 L g−1 COD and 0.243 L g−1 COD. Microbial composition in the reactors changed over time. In R-1, bacteroides were consistently dominant, while firmicutes were less abundant compared to R-2. Proteobacteria were initially low in abundance, but spirochetes were found in both reactors throughout the experiment. The study concluded that Poultry B substrates, due to their rich nutrient and trace element composition, are suitable for biogas plants. Municipal sanitary inocula also showed promise due to their resilience in high ammonia concentrations. Further research into biofilm interactions is recommended to better understand microbial responses to high ammonia concentrations, which can lead to propionate production in anaerobic digestion (AD).
1. Introduction
Anaerobic digestion (AD) is a crucial biotechnology for managing various types of organic waste, such as municipal waste and agricultural and animal manure, to mitigate pollution and generate energy, along with producing biofertilizers [1]. Poultry litter, particularly from poultry manure, is one of the substrates for AD. The AD process for poultry litter can annually mitigate about 5723 tonnes of CO2 equivalent, affecting soil nutrients, causing eutrophication in water bodies due to phosphorus, and contributing to air pollution through ammonia emission [2]. The high nitrogen and lignocellulose content of poultry litter makes it an unfamiliar substrate for AD [3].
The main challenge in nitrogen-rich waste is ammonia inhibition [4]. Inoculum selection is crucial for AD process stability and stress tolerance due to high levels of volatile fatty acids (VFAs) and ammonia [5]. In a study conducted with various inoculum sources (liger and Saint-Brieuc), methane production was affected by an increment in ammonia (NH3-N) concentration at 9.8 g L−1, causing a retardation in biochemical methane potential and an enhanced lag phase (>30 days), ultimately leading to complete inhibition at an ammonia concentration of 21.8 g·L−1. Liger was concluded to be the most suitable inoculum due to process stability at various organic loading rates [4]. Various microbial communities work together to accomplish AD, resulting in biomethane production. The coordinated activities of these microbial consortia make studying microbial dynamics challenging when investigating the AD of complex organics. The final stage of AD involves acetoclastic, hydrogenotrophic, and methylotrophic reactions, culminating in biogas formation. It has been demonstrated that hydrogenotrophic pathways are more feasible during methanogenesis, while acetoclastic archaea are prone to metabolic variations under high ammonia exposure. The syntrophic acetate oxidation (SAO) pathway involves the breakdown of produced acetate, and SAO is considered the predominant pathway coupled with hydrogenotrophic methanogenesis under high ammonia stress in AD systems [6,7]. Acclimatized Methanosarcinaceae could perform AD in high ammonium (5–7 NH4+-N g L−1) and acetate (9 g L−1) concentrations in batch tests [7].
However, overloading of substrates also leads to instability in AD [8]. In some studies, the accumulation of VFAs due to a high organic loading rate (OLR of 2.5 g TSL−1d−1) and hydraulic retention time (HRT of 40 d) induced inhibitory effects in AD [9]. Adding tannery fleshing wastes into the digester at a low organic loading rate of 5 gVSL-1 remains successful, whereas at high organic loading rates of 10 to 20 gVSL−1, the failure of the digester occurs due to inhibition of ammonia at a level of 1.50 ± 0.08 gNH4+ -N L−1, leading to steady-state inhibition, and at 2.42 ± 0.10 gNH4+-N L−1, system failure ensues. Pathways in AD are also influenced by high ammonia stress; hydrogen-dependent methyloptrophic is the major methanogenesis route at an OLR of 2.09 g VS L−1 d−1, whereas at the high OLR of 4 g VS L−1 d−1, the hydrogenotrophic route is the main pathway in the AD being run in the CSTRs [10].
Various techniques, such as co-digestion and bioaugmentation, have been tried previously to alleviate the effects of ammonium toxicity on the AD process. In the present study, the first goal was to monitor R-1, which operated for 190 days, to evaluate biogas production from poultry litter plus feed residues substrate against the cattle inoculum. Additionally, the similar reactor was tested for the effect of monensin on biogas production. The main reason for monensin assessment is that about 75% of antimicrobials are excreted by chickens due to their physiology [11]. The second goal of the study was to gain insight regarding the influence of various substrates against the municipal sanitary inoculum in R-2. The final goal of the study was to study the microbial community dynamics in both reactors.
2. Materials and Methods
Substrates Collection, Processing and Storage
Poultry substrates were collected from various locations within the poultry farm at the University of Illinois at Urbana–Champaign, USA. The collected substrate sites were labeled as A, B, and C. Site A was a dumping ground for poultry litter, B consisted of mixed poultry litter with feed residues, and C was the tray hatchery with pure litter. All three substrates were diluted with deionized water at a 1:1 ratio and placed in plastic containers, which were then stored in a cold room at −20 °C. The inoculum used was a combination of cattle and municipal sanitary waste from the Urbana Wastewater Municipal Sanitary Plant.
Two continuous stirred-tank reactors, each with a functional volume of 3 L (total capacity 4 L), were operated under mesophilic conditions at 37 °C and 200 rpm, as detailed in Table 1. The focus of the experiment was biogas generation using different poultry wastes in the two CSTRs, namely R-1 containing antibiotic monensis (based on literature survey) and R-2 (without any antibiotic). The organic loading rate (OLR), representing the amount of organic waste introduced into the digester per unit volume per day, and hydraulic retention time (HRT), indicating the average time substrates remain inside the reactor, was calculated. Chicken wastes were appropriately diluted with tap water, stirred thoroughly, and introduced into the continuous stirred-tank reactors.

Table 1.
Operating Conditions for CSTRs.
Total solids (TS) and volatile solids (VS) were analyzed using the dry and ash method. Dry porcelain crucibles were subjected to a 135 °C forced-air oven for 2 h, or aluminum crucibles were utilized for dry matter analysis. The crucibles were then transferred to desiccators and allowed to cool for 15–20 min. The crucible number and weight were recorded for each sample, with all samples run in duplicate.
For the determination of volatile solids, the crucibles were placed in a muffle furnace at 600 °C for 2 h and 45 min. After this time, the crucibles were again placed in desiccators for cooling.
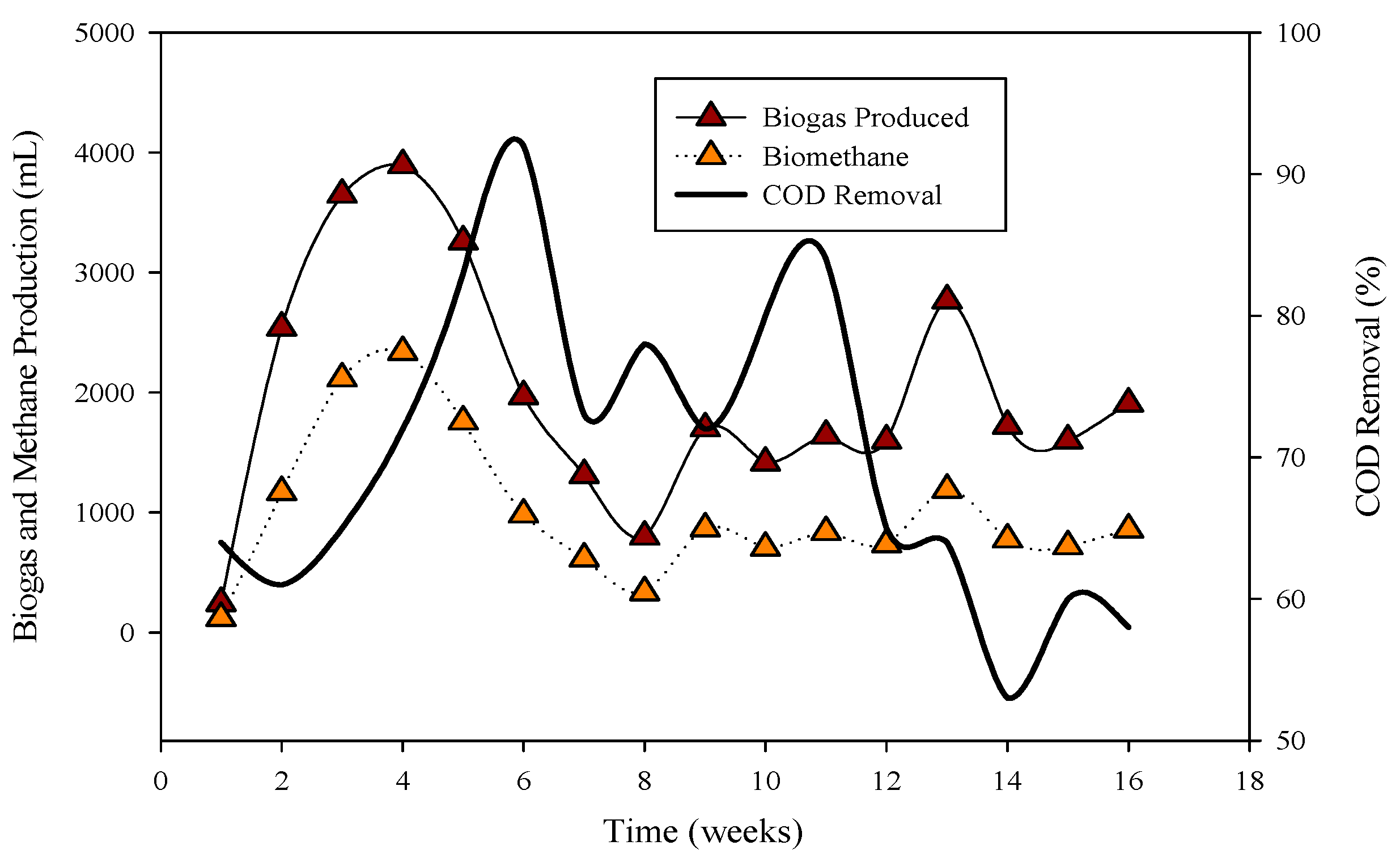
The pH of the effluent from the continuous stirred tank reactors (CSTRs), as shown in Figure 1, was measured daily using an Accumet AB15 pH meter (Fisher Scientific, Pittsburgh, PA, USA). Anaerobic bacteria/archaea, particularly methanogens, were found to be sensitive to pH variations, and the optimal pH range for efficient methanogenesis was identified as 6.8–7.2 [12].
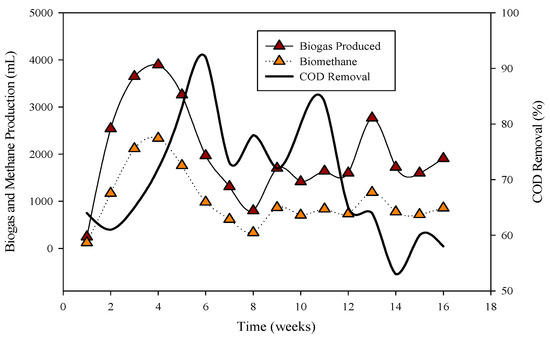
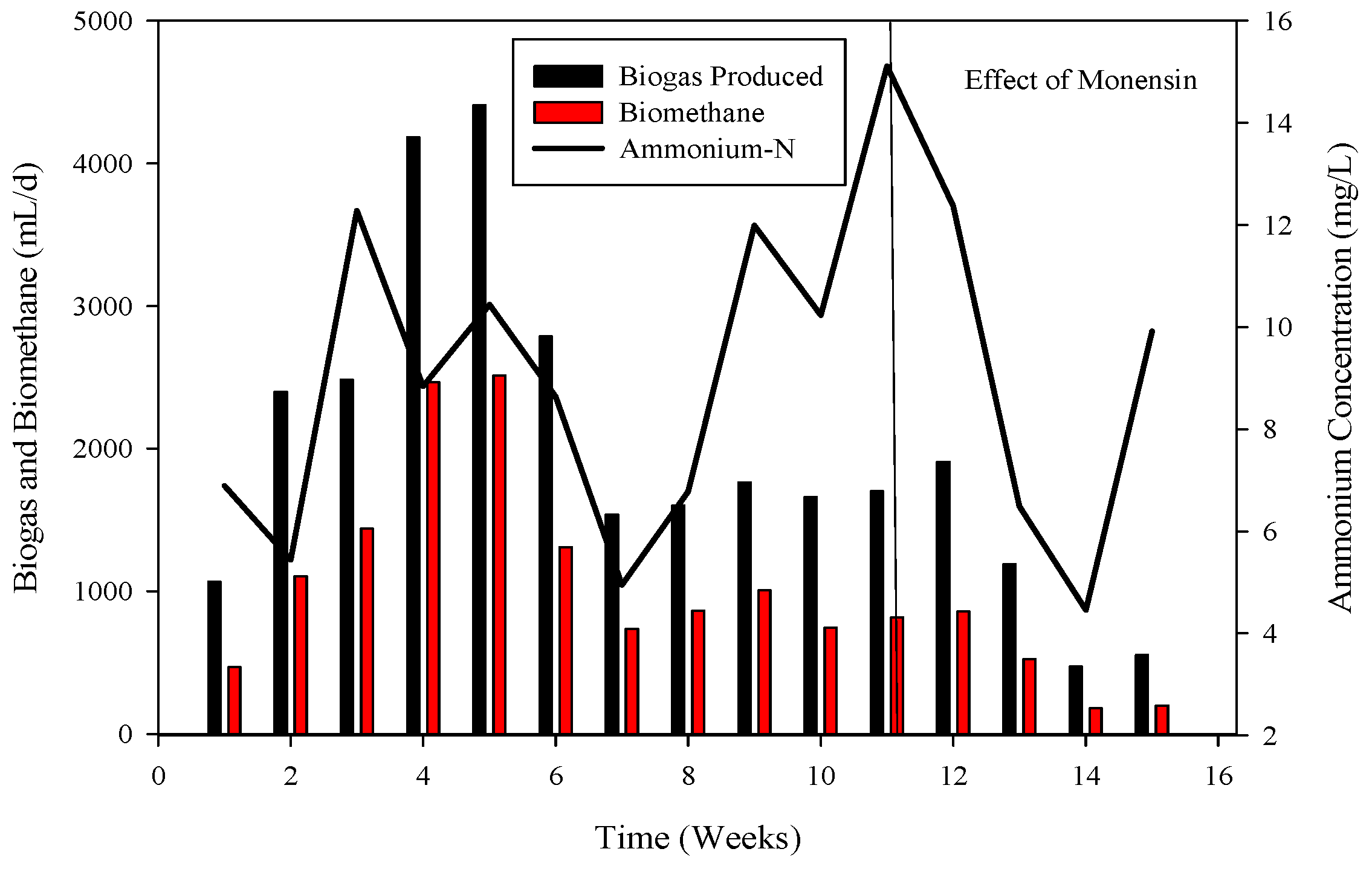
Figure 1.
Relation of biogas and methane quantity with COD removal percentage in CSTR 1 under the influence of monensin.
Ammonium nitrogen levels were measured at the end of the experiment. Biogas quantity was measured using a calibrated manometer. Volatile fatty acids (VFAs) were separated and quantified via high-performance liquid chromatography (HPLC) with 5 mM H2SO4 as the mobile phase and a BioRad Aminex HPX 87H column (Bio-Rad, Hercules, CA, USA) as the stationary phase.
The C:N ratio was determined via elemental CHNS analysis, and ammonia nitrogen was measured using the Chaney and Marbach colorimetric method. Methane analysis was conducted using a Gow-Mac Gas Chromatograph (GC) with a thermal conductivity detector. Biomethane analysis was performed to measure methane gas in the total gas production.
Every third day, 0.5 mL of biogas samples collected from bioreactors was injected, along with different standards, through a gas chromatograph (series 580 thermal conductivity GC, Gow-Mac Instrument Co., Bethlehem, PA, USA). The GC column, measuring 183 cm × 6.4 mm, was packed with Porapak Q, and the temperatures for the injection port, detector, and column were set at 80, 80, and 75 °C, respectively.
Biogas was quantified daily by monitoring and measuring with a Milli Gas Counter (MGC-10, Ritter, Bochum, Germany). The detailed design and operating conditions are presented in Table 1.
The compositions of volatile fatty acids (VFA) were analyzed using HPLC. The samples were quantified within the linear range of the calibration curve (0.1–5 g L−1). The mobile phase consisted of 5 mM H2SO4, and the samples were run at 25 °C for one hour at a flow rate of 0.4 mL min−1, with an injection volume of 10 µL. The BioRad Aminex HPX 87H column was utilized. Negative controls, consisting of HPLC vials with 200 µL of water and media, were also run for VFAs.
Microanalysis for poultry characterization was conducted via ICP, while the C:N ratio for belt, tray, and hatchery waste was determined using CHN. Elemental analysis employed a Varian Vista Pro CCD simultaneous inductively coupled plasma optical emission spectrometer with a radial torch configuration and an SPS 3 auto sampler (Varian, Palo Alto, CA, USA). The samples were nebulized for transport into the radio frequency ICP, where each element emitted a specific spectrum. Wavelength intensities were measured with the photosensitive CCD microchip, and data were computed and stored using ICP Expert software (Varian720 ES). A combustion process with chromium oxide as a catalyst and mass separation via an internal GC column was employed.
For molecular studies, DNA was isolated from mesophilic CSTR AD effluent from poultry wastes following the manufacturer’s instructions (Fast DNA Spin Kit for soil, MP Biomedicals, Irvine, CA, USA). DNA concentration was determined using NanoDrop (ND 2000, Thermo Fisher Scientific, Waltham, MA, USA), and integrity was confirmed with a 1% agarose gel. DNA quantification was performed using the Qubit dsDNA BR assay kit (Life Technologies, Carlsbad, CA, USA). Equimolar ratios of quantified samples were combined into sample pools, which underwent further processing at the Keck Center (University of Illinois at Urbana-Champaign, Urbana, IL, USA). Sample pools were subjected to quality control, including qPCR and quality check on a High Sensitivity DNA chip (Agilent, Santa Clara, CA, USA).
Amplification of 16S rRNA genes was conducted at the University of Illinois at Urbana–Champaign Biotech Center using the Fluidigm system, allowing for parallel amplification of a specific region from a target gene prior to high-throughput sequencing. Amplicon libraries for the V4 region on the 16S rRNA were generated using the primer pair 515F (5′ GTGYCAGCMGCCGCGGTAA 3′) and 806R (5′ GGACTACNVGGGTWTCTAAT 3′). Sequencing of amplicons was performed using the Illumina MiSeq V3 platform (sequencing option: paired reads 2 × 300).
Bioinformatics analysis of sequencing results involved merging raw paired reads using PEAR [13] and quality checking with FastQC [14]. Resulting amplicons were processed using the QIIME pipeline [15]. Reads with quality scores below 25 were excluded from further analysis. Quality-controlled sequences were denoised using default settings and binned into operational taxonomic units (OTUs) at a 97% similarity cutoff [16] using Uclust 1.2.22 as the OTU picking method [17]. The cluster seed was used as the representative sequence. Chimeric sequences were detected based on previous research [17] and excluded from subsequent analysis. Non-chimeric sequences were aligned with the PyNAST tool [15] using the Green genes core set alignment as a reference [18]. Taxonomy assignments were inferred through comparisons with both the RDP [19] and BLASTn [20] databases. Rarefaction analysis was performed to eliminate sequence number heterogeneity per sample before calculating alpha and beta diversity statistics.
3. Results and Discussion
The study explores the impact of monensin, an antibiotic added to poultry feed and released in chicken excreta, on reactor 1 poultry substrates. The section also delves into the discussion of COD, ammonia-nitrogen, volatile fatty acid profile, and microbial guilds for both reactors 1 and 2.
3.1. Impact of Substrates Properties on Anaerobic Digestion
The physicochemical characteristics and elemental analysis of various poultry substrates are presented in Table 1, while sugar cane stillage characterization is detailed in Table 2. Poultry B substrate exhibited lower ammonia nitrogen compared to Poultry A and Poultry C. Moreover, Poultry B contained a higher calcium concentration than the other two substrates. Poultry C, on the other hand, had a higher phosphorus concentration compared to Poultry A and Poultry B substrates. Poultry B demonstrated a suitable C:N ratio and a Ni concentration of 1212 ppm for anaerobic co-digestion compared to the other two substrates in Table 2.

Table 2.
Physicochemical characterizations of different poultry substrates.
3.2. Process Stability and Biogas Generation
R-1 was operated for 120 days, inoculated with cattle inoculum and B poultry substrate (Figure 1). The experiment comprised three stages based on substrate addition. For 100 days, 1 mL of monensin was added daily, resulting in a significant improvement in daily biogas production, reaching a peak of 4407 mL d−1. Subsequently, the production gradually decreased and stabilized on day 53 at 1558 mL d−1. The reactor remained stable for 47 days until impacted by the antibiotic monensin. Biogas and methane production, sampled every 3rd day, are also illustrated in Figure 1.
The utilization of chemical oxygen demand (COD) by microbes is also depicted in Figure 1, showcasing a decrease in sCOD from 43 mg L−1 to 167 mg L−1 when monensin was applied.
3.3. Perturbed System with Monensin
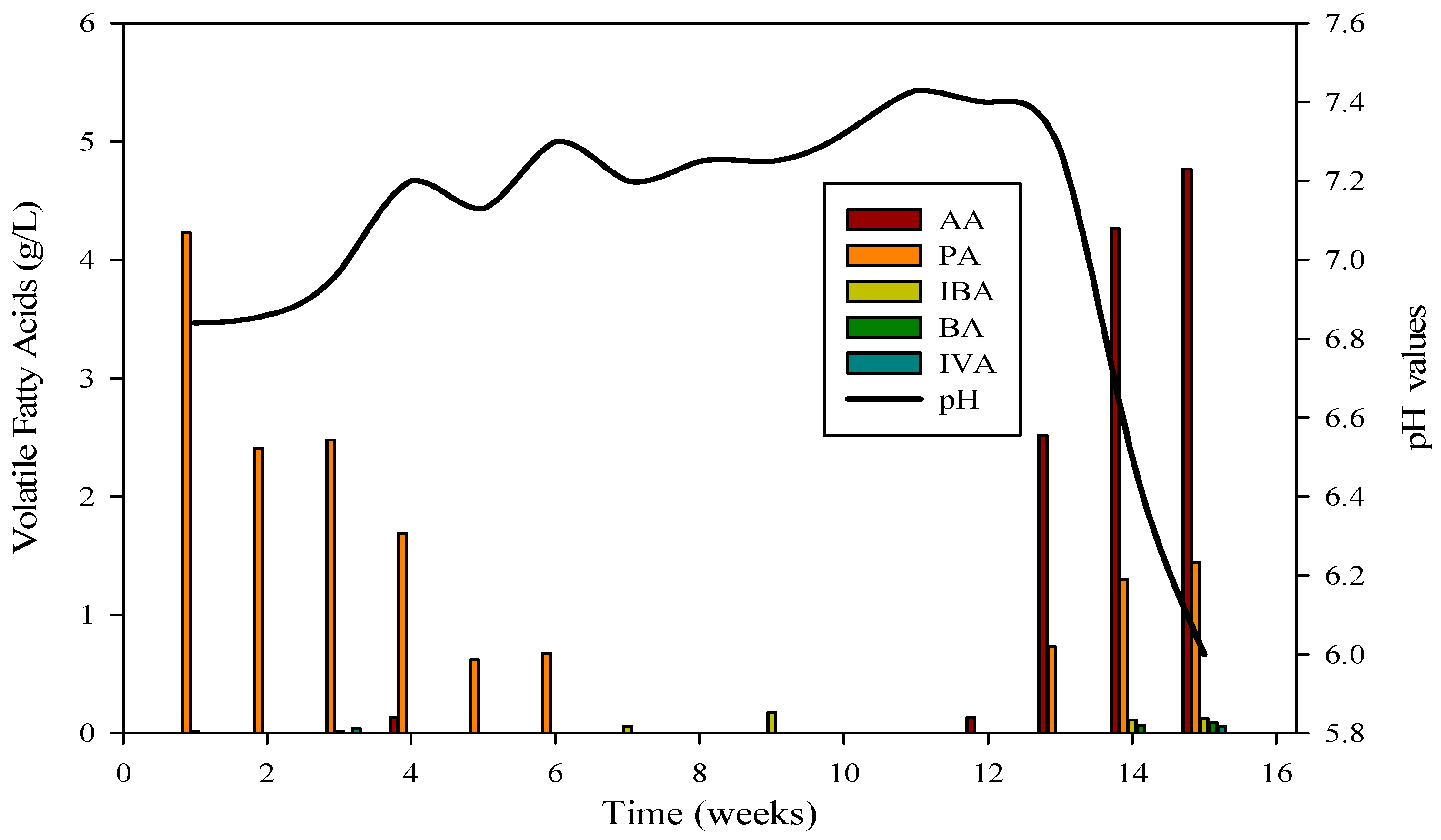
Monensin is typically introduced into poultry feed as a preventive measure against chicken diseases. Beginning on day 1, 1 mL of monensin was incorporated daily for eight consecutive days. Figure 2 illustrates the microbial utilization of various volatile fatty acids (VFAs) in R1. The application of monensin resulted in a decline in COD utilization, leading to the release of COD in the effluent (Figure 1). The impact of monensin application concerning VFAs is depicted in Figure 2. Initially, propionic acids dominated the VFA samples, ranging from 4332 mg L−1 to 674. However, in the middle samples, the absence of propionic acids was noted until the introduction of monensin. Subsequent to monensin addition, the VFA profile underwent a transformation, with acetic acid (2521 to 4768 mg L−1) and propionic acids (729 to 1447 mg L−1) becoming predominant. Conversely, isobutyric acids exhibited the lowest concentration (113 to 123 mg L−1), while butyric acids ranged from 67 to 86 mg L−1, and isovaleric acids were present at 58 mg L−1 in the final samples of R-1.
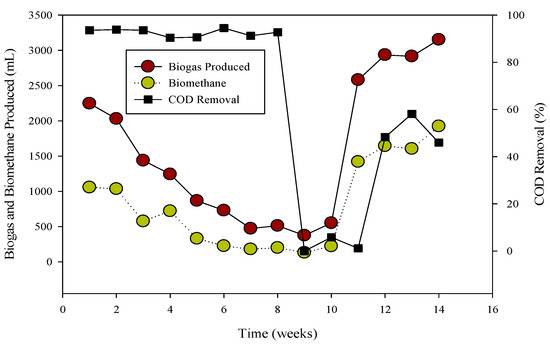
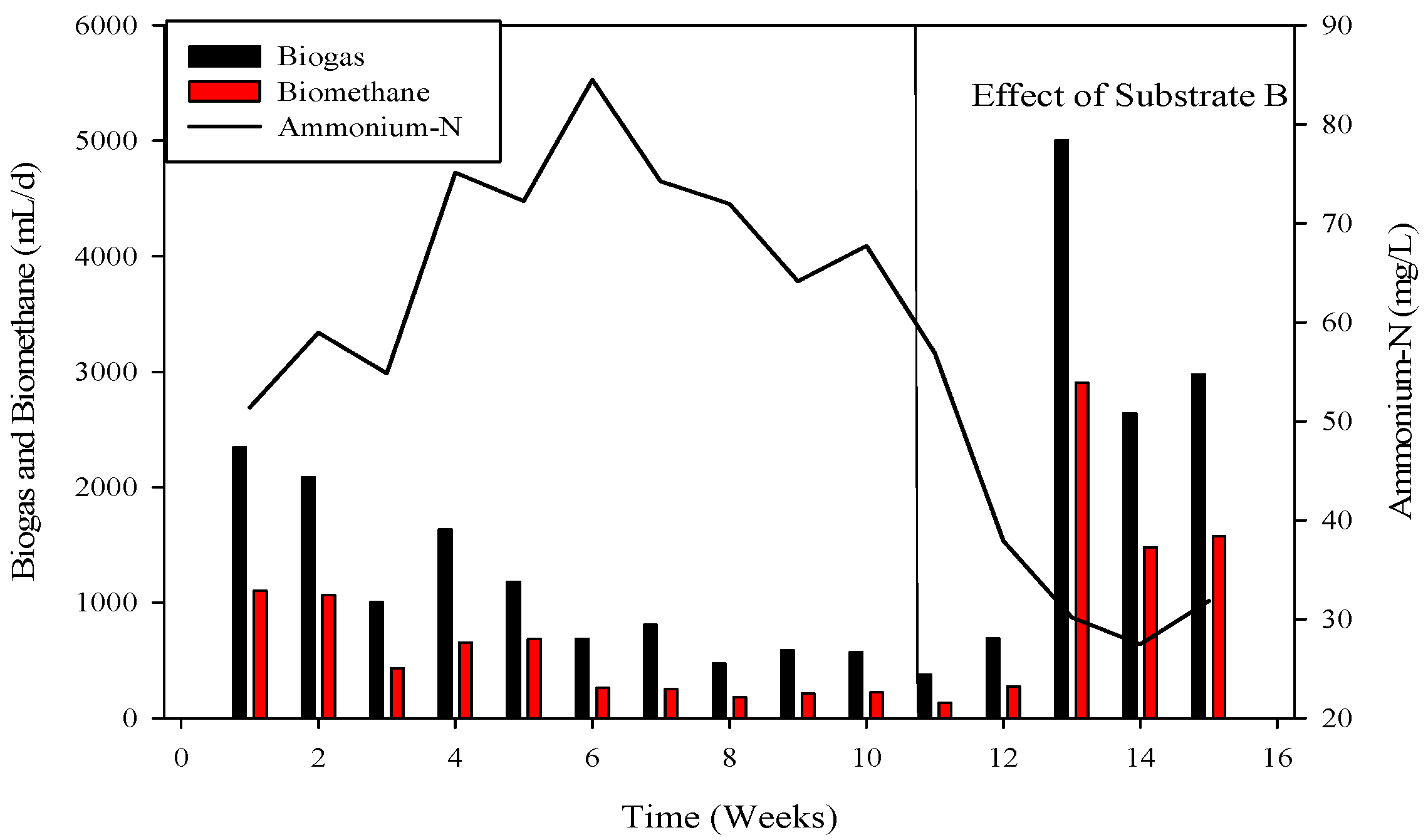
Figure 2.
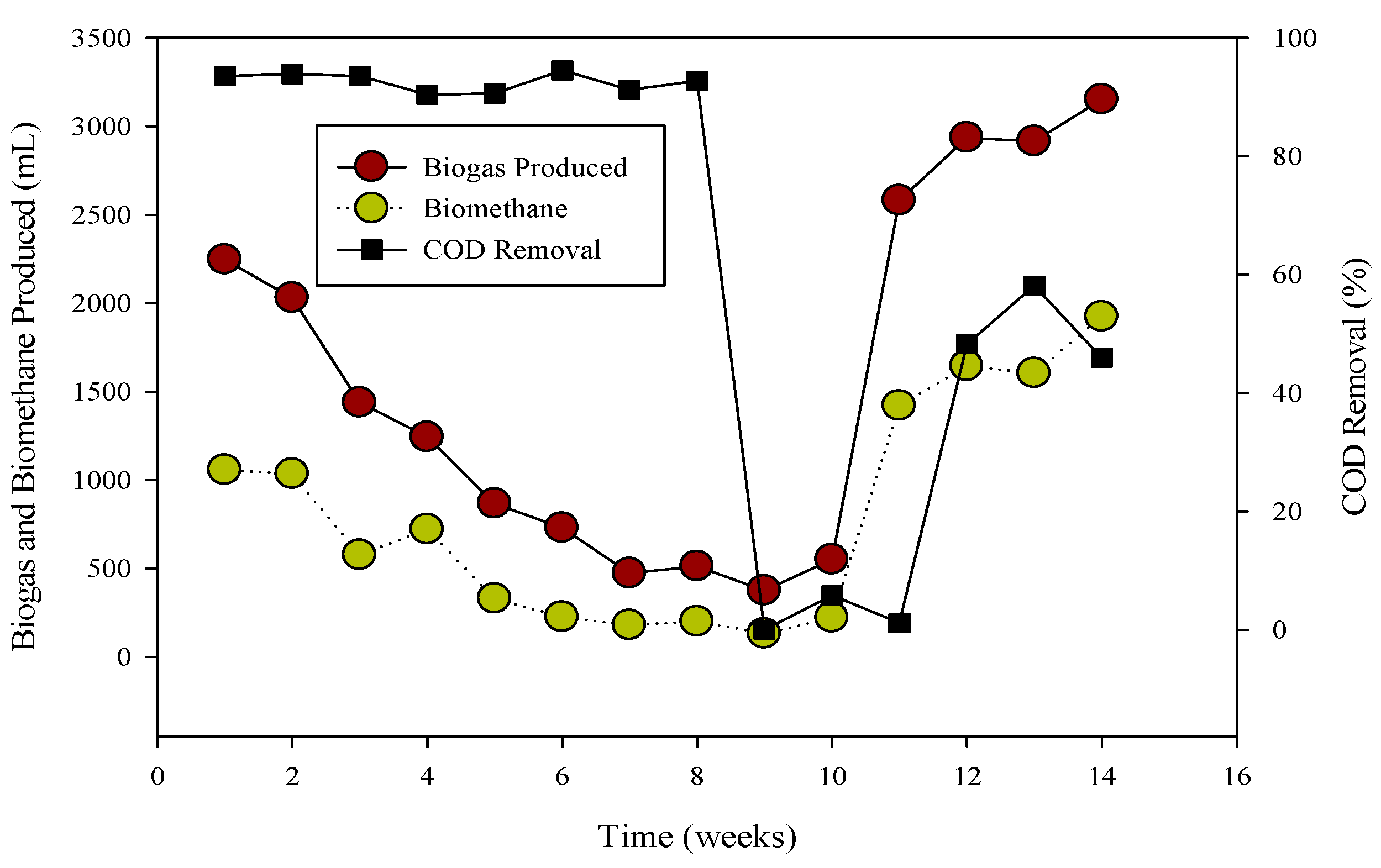
Relation of biogas and methane quantity with COD removal percentage in CSTR 2.
The R-2 experiment spanned 90 days, during which various substrates were introduced into the continuous stirred tank reactors (CSTR) using municipal sanitary inoculum (Figure 2). Poultry substrate (A) and tray hatchery (T) were added for the initial 37 days, leading to an initial daily biogas production of 690 mL d−1, which increased to around 2000 mL d−1 within 8 days. R-2, with municipal sanitary inoculum, exhibited superior performance during the initial 12 days compared to R-1. Subsequently, daily biogas production declined, severely impacted by high ammonium-nitrogen concentrations (84.47 to 89.71 mM) from day 30 to 36, resulting in daily biogas production of 1083 mL d−1 and 683 mL d−1, respectively.
3.4. Volatile Fatty Acid (VFA) Profile
The evaluation of AD performance involved measuring concentrations of organic acids, with particular focus on propionic, butyric, acetic, and isobutyric acids, during reactor startup and operation. These acids, especially propionic, butyric, and acetic, were consistently detected at elevated levels, signaling the collaborative activity of diverse microbial communities. The VFA profile in Figure 3 confirmed the daily production of biogas and biomethane in R-1.
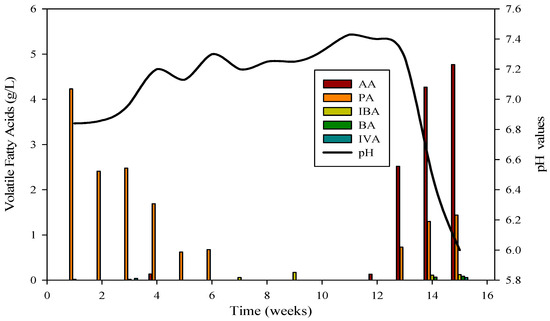
Figure 3.
VFA profile and its relation with pH in R1; AA = Acetic acid, PA = Propanoic acid, IBA = Isobutyric acid, BA = Butyric acid, IVA = Isovaleric acid.
3.5. Effect of Ammonia
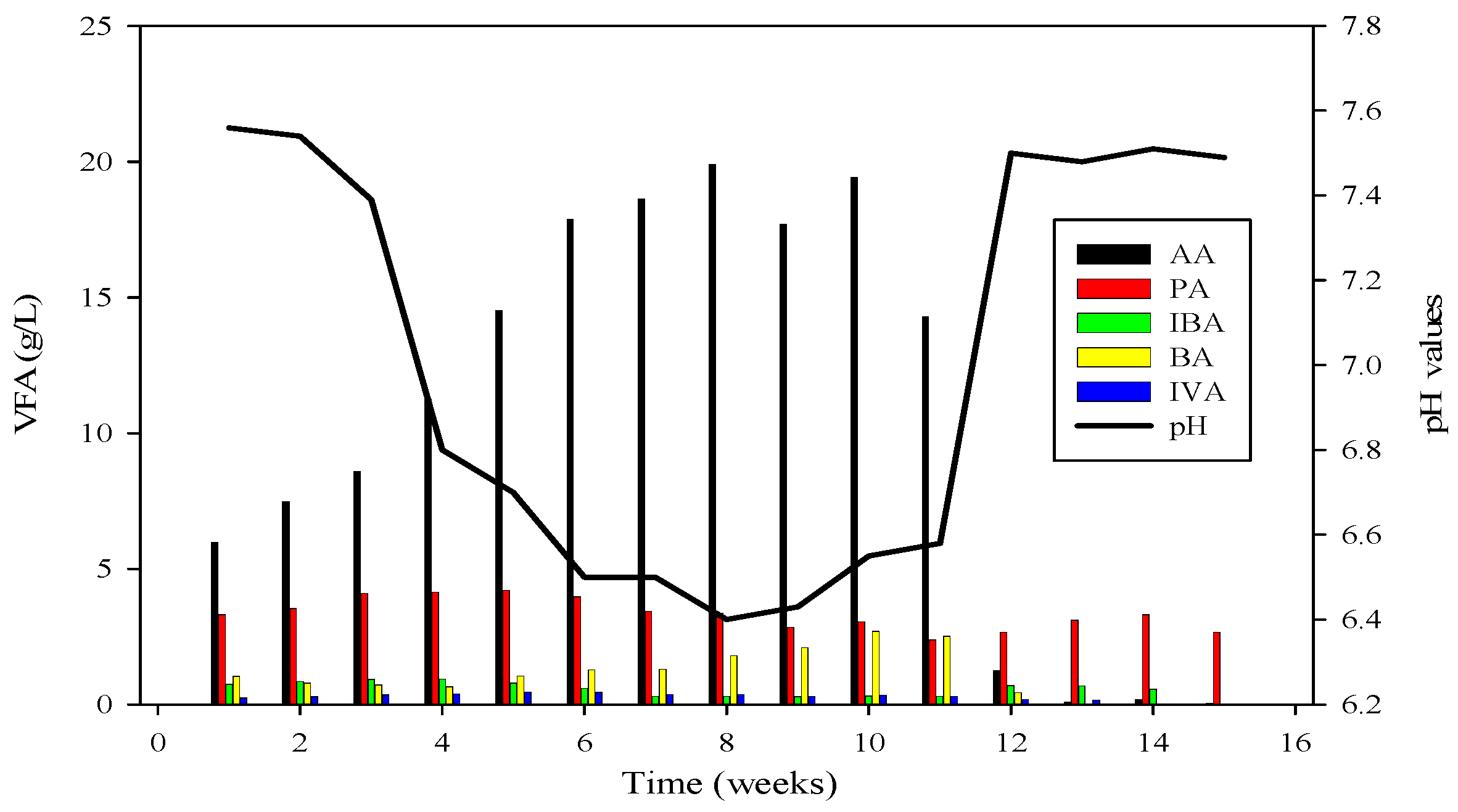
Ammonium nitrogen levels were assessed every third day in the CSTRs. During the initiation of reactor 2, the initial concentration of ammonium-nitrogen was 41.89 mM. Over time, it progressively increased, reaching its peak at 89.71 mM. Subsequently, through the alteration of substrate B, concentrations gradually decreased, with the last recorded levels at 14.85 mM, as depicted in Figure 4, Figure 5 and Figure 6.
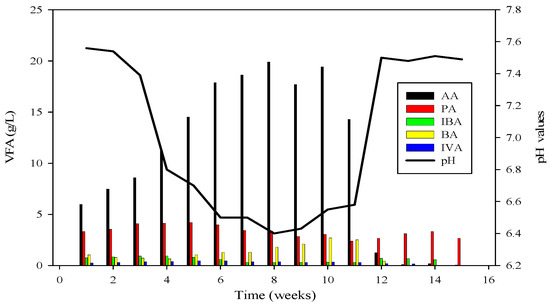
Figure 4.
VFA profile of R2 and its relation with pH; AA = Acetic acid, PA = Propanoic acid, IBA = Isobutyric acid, BA = Butyric acid, IVA = Isovaleric acid.
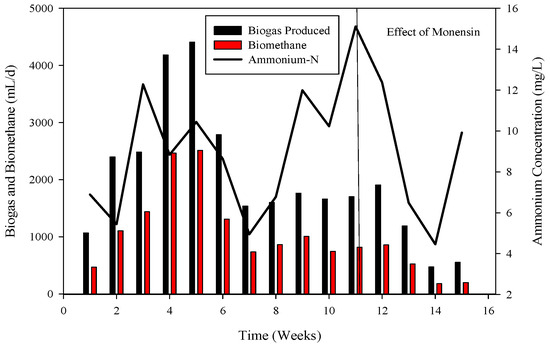
Figure 5.
Dynamics of biogas and biomethane production in R1.
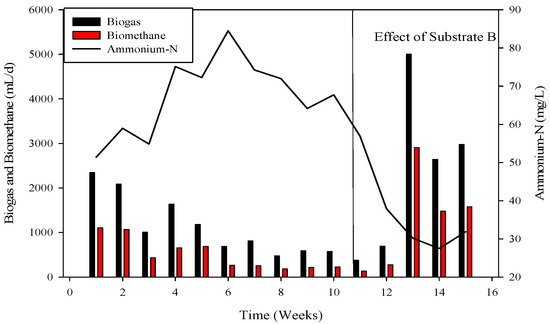
Figure 6.
Dynamics of biogas and biomethane production in R2.
To recover the reactor, poultry substrates (A and T) were reintroduced along with sugar cane thin stillage on day 37. This addition led to a decrease in ammonium-nitrogen concentration from 89.71 mM to 56.87 mM, as illustrated in Figure 5 and Figure 6. Although biogas production slightly increased, it subsequently decreased, reaching 532 mL d−1 at a 30-day hydraulic retention time (HRT). HRT was then reduced from day 47, changing from 30 days to 20 days. On day 57, co-digested poultry B with feed residues was added, initiating a gradual recovery. By day 70, the reactor had fully recovered, achieving a biogas production of 2581 mL d−1. From day 71 to 75, biogas production peaked, indicating a stabilized stage for R-2. Graphs depicting daily biogas and biomethane, as well as ammonia-nitrogen trends, are presented in Figure 7. Throughout the 90-day experiment, all graph trends showed a decreasing pattern in accordance with substrate dynamics. The recovery of the R-2 reactor was attributed to the superior C:N ratio of substrate B (litter with feed residues) compared to other substrates such as stillage (13.8:1), A (dumped poultry waste) (13.3), and T (tray hatchery) (8.1).
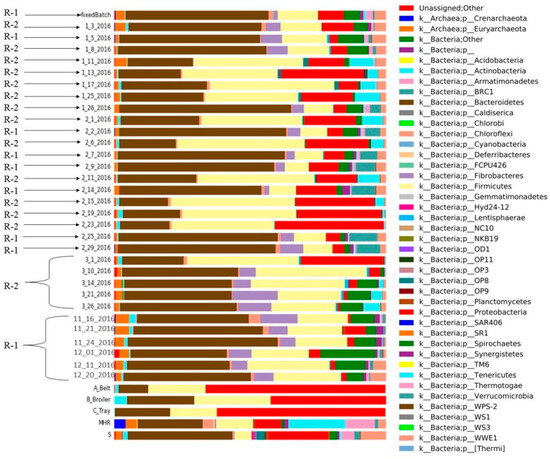
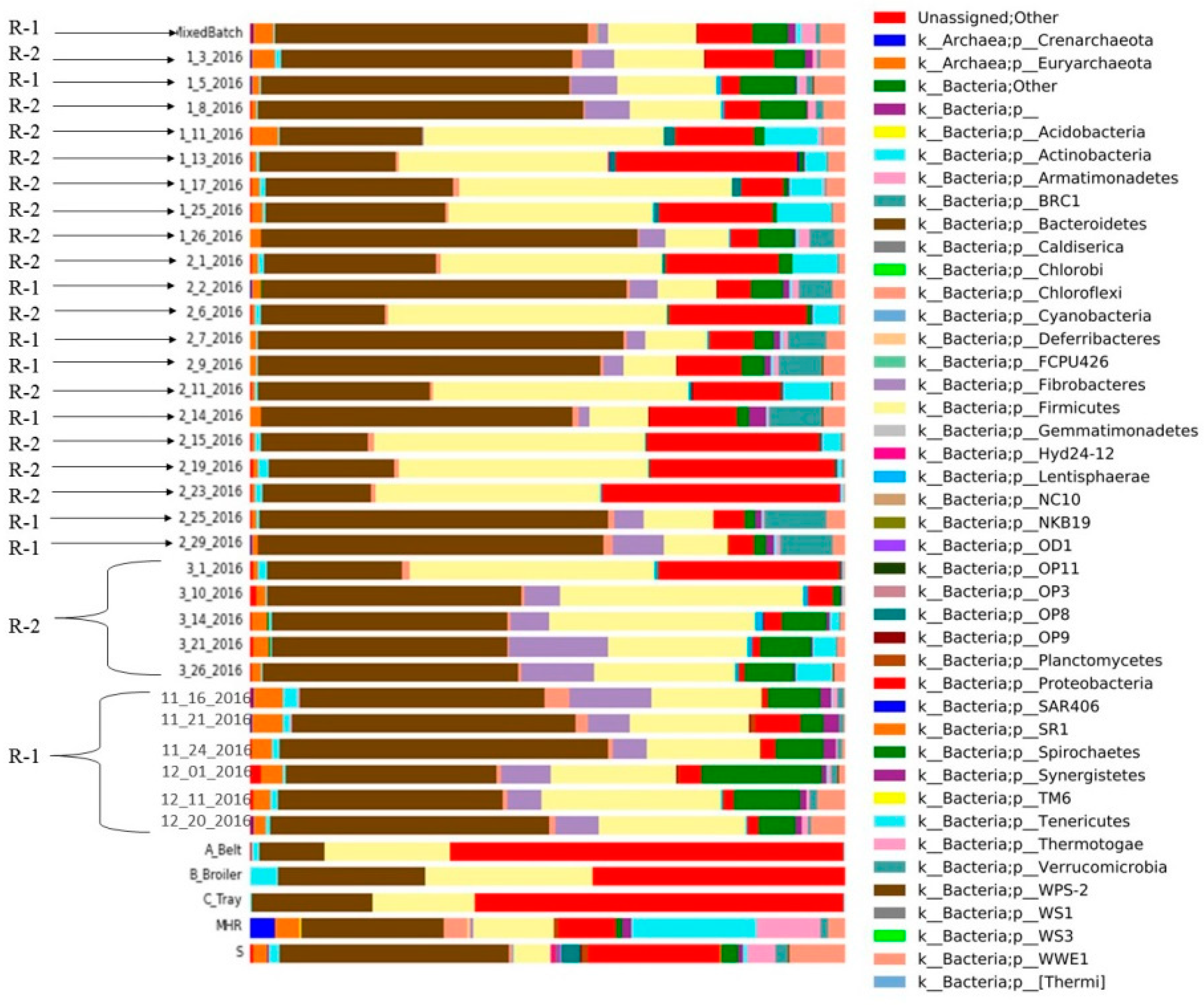
Figure 7.
Different microbial compositions of CSTRs (R-1; R-2) and mixed batch, substrates and inoculum sources (mesophilic and sanitary) with relative abundance (%) of the major phylum.
3.6. Microbial Composition
In R-1, bacteroides emerged as the dominant bacteria across all collected samples, while firmicutes exhibited lower abundance compared to R-2 (Figure 7). Proteobacteria were less prevalent in the initial samples of R-1 compared to the substrate and inoculum, and spirochetes were present in both R-1 and R-2. Notably, R-1 exhibited a higher abundance of spirochetes at the beginning than in the middle and end of the experiment. Fibrobacterial abundance was initially high in R-1 but decreased in later samples. Chloroflexi bacteria were consistently present in both reactors over the sampling period. Tenericutes were identified in R-2 but were absent in R-1.
Various inocula, including those from cattle and sanitary sources, were employed in the study, and these were applied to diverse substrates with distinct biochemical compositions obtained from poultry farms at the University of Illinois at Urbana–Champaign, USA. To enhance biogas production, thin stillage was introduced into CSTR-2 along with poultry substrates; however, the outcome proved unsatisfactory. The thin stillage exhibited limited compatibility with poultry waste for biogas generation. In CSTR-1, the addition of Monessen had a noteworthy impact, resulting in a reduction of both biogas and biomethane yield.
The cattle inocula experiment with substrate B spanned 120 days. Reactor 1 achieved stabilization after the 53rd day, maintaining stability for 47 days before the introduction of monensin disrupted it. Subsequently, the VFA profile underwent changes, with elevated concentrations of acetic acid and propionic acids compared to isobutyric and butyric acids, leading to a decline in pH from 7.4 to 6 (as depicted in Figure 3). VFAs play a crucial role in bioreactor stability by regulating pH, a critical parameter for the survival of methanogens. The optimum range for VFAs is 59.78–5786.96 mg·L−1, and inhibitory effects are minimal when concentrations are below 6000 mg·L−1.
The reduction in biogas and biomethane yield (Figure 6) resulted from pH reduction and disturbance of the methanogen niche, subsequently affecting COD utilization for optimal biogas and biomethane generation. Ammonium-nitrogen had a limited impact in this study due to the substrate’s low initial ammonia-nitrogen content (19.86 mM). Unlike anaerobic digestion of municipal solid waste, which tolerated 8.5 NH4-N L−1, the current study did not experience inhibition. In high-ammonia anaerobic digesters, the prevalent methanogenic pathway becomes hydrogenotrophic instead of acetoclastic, as the latter is more sensitive to ammonia.
Substrate B, added to the reactor, holds promise for community-scale digesters in poultry farms, addressing energy needs for heat and electricity. CSTR-2, utilizing municipal sanitary inocula from Urbana, USA, faced efficiency challenges with increasing COD, resulting in a near-halt in biogas production at 199 mL. However, the reactor recovered when the substrate was switched to poultry B, which included feed residues, potentially balancing the C:N ratio for anaerobic digestion.
4. Discussion
The current investigation delved into the utilization of cattle and municipal sanitary inocula for anaerobic digestion of poultry wastes, addressing a crucial and pragmatic challenge in waste management. The emphasis on poultry waste is pertinent due to its well-documented impediments in anaerobic digestion, attributed to heightened levels of ammonia and volatile fatty acids (VFAs). The strategic selection of cattle and municipal sanitary inocula suggests an approach aimed at bolstering the anaerobic digestion process. Cattle inocula, presumably teeming with beneficial microorganisms, contribute to the indispensable microbial community responsible for breaking down intricate organic compounds in poultry wastes. Conversely, municipal sanitary inocula bring a diverse array of microbial populations, stabilizing the digestion process and enhancing overall efficiency.
The novelty of this work lies in its innovative approach to addressing a significant challenge in waste management—the anaerobic digestion of poultry wastes. The focus on utilizing both cattle and municipal sanitary inocula represents a novel strategy to enhance the anaerobic digestion process, particularly in the face of well-documented challenges associated with poultry waste. The study is not only concerned with anaerobic digestion but specifically targets the challenges posed by poultry wastes. By addressing the documented impediments related to ammonia and volatile fatty acids (VFAs), the research demonstrates a nuanced understanding of the specific issues within poultry waste management. The incorporation of both cattle and municipal sanitary inocula represents a dual-inoculum strategy, introducing a unique and comprehensive approach to tackle the complexities of poultry waste. The decision to use cattle inocula, rich in beneficial microorganisms, and municipal sanitary inocula with a diverse microbial population showcases a deliberate effort to synergize different microbial communities for a more effective waste digestion process. The work emphasizes the importance of microbial communities in anaerobic digestion. By leveraging cattle inocula to contribute essential microorganisms for breaking down organic compounds in poultry wastes and municipal sanitary inocula to stabilize the digestion process, the study delves into the intricate dynamics of microbial communities, providing a deeper insight into their role in waste management. In summary, this work goes beyond traditional approaches by introducing a dual-inoculum strategy and strategically selecting inocula to address specific challenges associated with poultry waste. The emphasis on microbial community dynamics and efficiency enhancement adds a layer of sophistication to waste management practices, making this investigation a noteworthy contribution to the field.
The study addresses anticipated elevations in ammonia and VFAs. Ammonia, a prevalent byproduct in poultry waste, can impede microbial activity, compromising the efficacy of anaerobic digestion. Similarly, elevated VFAs can disrupt process stability. The research offers insights into how the chosen inocula may mitigate these challenges, potentially advancing more effective and sustainable waste treatment. Nickel emerges as a pivotal trace element in the anaerobic digestion process [21]. The study underscores nickel’s enduring impact on biogas and biomethane content, playing a vital role in the recovery of the R-2 CSTR reactor by modifying the addition of poultry B substrate.
The optimization of anaerobic digestion is achieved through shrimp shell biochar and nickel at varying concentrations: 81.8, 116.1, 134.7, and 99.2 mL g−1 VS [22]. Stillage with a high sulfate content of 1743 mg L−1, as examined by Oosterkamp et al. [23], is deemed unsuitable for co-digestion in the CSTR. Cost-effective accelerants like fly ash, bag filter gas dust, and laterite, housing trace elements necessary for the anaerobic digestion process, are recommended for optimal biogas and biomethane production in large-scale digesters. These accelerants not only provide trace elements but also foster new microbial syntrophies. Additionally, the study discusses the inclusion of ammonia-reducing additives such as zeolites and trace elements in anaerobic digestion as a means to mitigate ammonia levels [24].
Initially, propionic acids dominated the VFA samples, but over time, all VFAs were utilized until the introduction of monensin. Post-monensin, the VFA profile changed, with acetic and propionic acids becoming dominant, and isobutyric and butyric acids present in the least concentrations in the final R-1 samples. This aligns with similar outcomes observed in a study assessing the reduction of methane production with long-term monensin supplementation in dairy cattle [25].
However, increasing the organic loading rate (OLR) and reducing hydraulic retention time (HRT) had no optimal effect on biogas production due to the consistently high sulfate concentration of 1743.7 ppm. Despite the reduction in ammonium-nitrogen concentration in sugar cane stillage [23], biogas production continued to decrease.
Monensin addition influenced the VFA profile of R-1, resulting in a pH increase. Acetic acid production in R-1 was initially low but gradually rose over time until the substrate change to B (litter with feed residues). Reactor 2’s VFA data consistently exhibited propionic acid presence. Propionate, a key intermediate, accumulated notably in the presence of high ammonia concentrations in AD [26]. Volatile fatty acids could be one of the rate-limiting steps and can be overcome with the syntrophic association of bacteria and methanogen to understand the microorganisms and biochemical reactions for the optimization of biomethane [27]. Hook et al. [25] observed a similar inhibition of biomethane production with long-term supplementation of MON in dairy cattle. Conversely, a study by Beneragama et al. [28] found that the addition of cefazolin to dairy manure did not inhibit methane production at concentrations of 30, 60, and 90 mg L−1 in a thermophilic digester.
Notably, the literature reports toxic ammonia concentrations ranging from 1.7 to 14 g NH4+-NL−1 [12]. Furthermore, the presence of free ammonia, identified as the predominant toxic form in the anaerobic digestion process, exhibits an increase in concentration correlating with pH and temperature. This complexity contributes to the overall toxicity of ammonia [29]. Presently, it is widely acknowledged that total ammonia and free ammonia concentrations exceeding 3 g NH4+-N L−1 or 0.15 g NH3-N L−1, respectively, are considered toxic for methane production. This holds true regardless of variations in temperature and pH levels [12]. Ammonia tolerance by microbial species depends on the high energy availability of the functional microbial species and provides assistance in the optimization and operation of anaerobic digestion systems for optimum methane production [30].
The microbial composition in anaerobic digestion (AD) is significantly influenced by the makeup of raw materials. For instance, in the process of AD, substrates rich in ammonium may lead to the predominant metabolic pathway of hydrogen trophic methanogenesis due to the heightened sensitivity of acetoclastic methanogens to inhibitors [31]. Acetoclastic methanogens play a pivotal role in methane production in typical AD processes involving various organic substrates [32]. Figure 7 illustrates the diversity of microbes and their relative abundances at the phylum level derived from different substrates, inocula, and samples obtained from two distinct continuous stirred-tank reactors (CSTRs). In this study, Firmicutes phylotypes replaced Bacteroidetes phylotypes, and Methanosarcina sp. dominated all examined samples with elevated total ammonia nitrogen (TAN) levels [33]. Significant alterations in microbial community structure occurred with shorter hydraulic residences, while stability was observed with longer hydraulic residences [34]. Although the microbial composition of the three studied reactors was initially similar, it diverged considerably after a substrate change [35].
Some of the limitations of this work were as follows. The investigation may not provide insights into the long-term stability and sustainability of the anaerobic digestion process. It is crucial to consider the potential shifts in microbial communities and process dynamics over extended periods. While the focus is on waste management, the environmental impact of the anaerobic digestion process itself should have been considered. The study may not have extensively explored the potential for nutrient recovery from the anaerobic digestion process. Assessing the feasibility of extracting valuable by-products for agricultural use could enhance the overall sustainability of the waste management approach. We will consider their limitations in future investigations.
Poultry substrate B is recommended for small-scale biogas plants at poultry farms to maintain indoor worker exposure to ammonia below the permissible limit of 25 ppm. This substrate not only aids in energy generation but also contributes to combine health and power benefits. Future research should focus on studying ammonia-tolerant microbes and their interactions in biofilms under elevated ammonia levels, particularly in propionate degradation.
5. Conclusions
This study underscores the significance of appropriate inoculation in anaerobic digestion (AD) processes. By employing continuous stirred-tank reactors (CSTRs) and utilizing specific inocula, the investigation successfully optimized biogas production from various poultry substrates and stillage. Following are the key conclusions.
- Poultry substrate B, processed in the R-1 CSTR with cow rumen inoculum, demonstrated superior and consistent biogas generation compared to cattle inoculum in R-1 and municipal sanitary inoculum in R-2. The study highlights the pivotal role of the optimal C: N ratio (17:1) in substrate B, contributing to successful AD.
- Co-digestion of belt waste (A) and tray hatchery resulted in substantial biogas and biomethane yield, particularly adaptable to cattle inoculum. Inhibitory effects observed in stillage, attributed to elevated sulfur content, led to a substrate switch to poultry B, ensuring cumulative biogas production and reactor stability on the 80th day.
- Bacteriodes predominated in R-1, while fermicutes exhibited lower abundance compared to R-2. Proteobacteria, initially less prevalent in R-1, showed consistent presence in the substrate and inoculum. Spirochetes were present in both reactors, with higher abundance in R-1 initially. Fibrobacteria showed high initial abundance but decreased by the experiment’s end. Chloroflexi bacteria were consistently present in both reactors, while Tenericutes were exclusive to R-2.
- The study proposes the use of natural accelerants like laterite and industrial accelerants such as fly ash, bag filter gas dust, and phosphorus dust from the fertilizer industry. These additions aim to enhance microbial syntrophy, providing essential trace elements to mitigate ammonia toxicity in the biogas digester, thereby improving overall processing within the circular economy.
Author Contributions
Conceptualization, Q.M. and R.I.M.; methodology, Q.M.; formal analysis, F.A.S.; investigation, F.A.S.; resources, R.I.M.; data curation, F.A.S.; writing—original draft preparation, F.A.S.; writing—review and editing, Q.M.; supervision, R.I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available from first author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Archana, K.; Visckram, A.S.; Senthil Kumar, P.; Manikandan, S.; Saravanan, A.; Natrayan, L. A review on recent technological breakthroughs in anaerobic digestion of organic biowaste for biogas generation: Challenges towards sustainable development goals. Fuel 2024, 358, 130298. [Google Scholar] [CrossRef]
- Beausang, C.; McDonnell, K.; Murphy, F. Anaerobic digestion of poultry litter—A consequential life cycle assessment. Sci. Total Environ. 2020, 735, 139494. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A. A comprehensive review of green policy, anaerobic digestion of animal manure and chicken litter feedstock potential–Global and Irish perspective. Renew. Sustain. Energy Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Mansour, M.-N.; Lendormi, T.; Drévillon, L.; Naji, A.; Louka, N.; Maroun, R.G.; Hobaika, Z.; Lanoisellé, J.-L. Influence of substrate/inoculum ratio, inoculum source and ammonia inhibition on anaerobic digestion of poultry waste. Environ. Technol. 2022, 1–14. [Google Scholar] [CrossRef]
- De Vrieze, J.; Gildemyn, S.; Vilchez-Vargas, R.; Jáuregui, R.; Pieper, D.H.; Verstraete, W.; Boon, N. Inoculum selection is crucial to ensure operational stability in anaerobic digestion. Appl. Microbiol. Biotechnol. 2015, 99, 189–199. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Iqbal, A.; Mackie, R.I. Inhibition of biomethane production under antibiotics in poultry litter. Arab. J. Geosci. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Fotidis, I.A.; Karakashev, D.; Kotsopoulos, T.A.; Martzopoulos, G.G.; Irini Angelidaki, I. Effect of ammonium and acetate on methanogenic pathway and methanogenic community composition. FEMS Microbiol. Ecol. 2013, 83, 38–48. [Google Scholar] [CrossRef]
- Rhee, C.; Park, S.-G.; Kim, D.W.; Yu, S.I.; Shin, J.; Hwang, S.; Shin, S.G. Tracking microbial community shifts during recovery process in overloaded anaerobic digesters under biological and non-biological supplementation strategies. Bioresour. Technol. 2021, 340, 125614. [Google Scholar] [CrossRef]
- Mahdy, A.; Bi, S.; Song, Y.; Qiao, W.; Dong, R. Overcome inhibition of anaerobic digestion of chicken manure under ammonia-stressed condition by lowering the organic loading rate. Bioresour. Technol. Rep. 2020, 9, 100359. [Google Scholar] [CrossRef]
- Christou, M.; Vasileiadis, S.; Kalamaras, S.; Karpouzas, D.; Angelidaki, I.; Kotsopoulos, T. Ammonia-induced inhibition of manure-based continuous biomethanation process under different organic loading rates and associated microbial community dynamics. Bioresour. Technol. 2021, 320, 124323. [Google Scholar] [CrossRef]
- Laloučková, K.; Skřivanová, E. Antibiotic resistance in livestock breeding: A review. Sci. Agric. Bohem. 2019, 50, 15–22. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 10 July 2023).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Kunin, V.; Engelbrektson, A.; Ochman, H.; Hugenholtz, P. Wrinkles in the rare biosphere: Pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ. Microbiol. 2010, 12, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.; McGarrell, D.M.; Marsh, T.; Garrity, G.M. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37 (Suppl. 1), D141–D145. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, Z.; Hu, Z.; Luo, X. Enhanced anaerobic digestion with the addition of chelator-nickel complexes to improve nickel bioavailability. Sci. Total Environ. 2021, 759, 143458. [Google Scholar] [CrossRef]
- Li, X.; Wu, M.; Xue, Y. Nickel-loaded shrimp shell biochar enhances batch anaerobic digestion of food waste. Bioresour. Technol. 2022, 352, 127092. [Google Scholar] [CrossRef] [PubMed]
- Oosterkamp, M.J.; Méndez-García, C.; Kim, C.-H.; Bauer, S.; Ibáñez, A.B.; Zimmerman, S.; Hong, P.-Y.; Cann, I.K.; Mackie, R.I. Lignocellulose-derived thin stillage composition and efficient biological treatment with a high-rate hybrid anaerobic bioreactor system. Biotechnol. Biofuels 2016, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, W.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China. Renew. Sustain. Energy Rev. 2018, 97, 186–199. [Google Scholar] [CrossRef]
- Hook, S.E.; Northwood, K.S.; Wright, A.D.; McBride, B.W. Long-term monensin supplementation does not significantly affect the quantity or diversity of methanogens in the rumen of the lactating dairy cow. Appllied Environ. Microbiol. 2009, 75, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Schnürer, A.; Dolfing, J.; Westerholm, M. Syntrophic entanglements for propionate and acetate oxidation under thermophilic and high-ammonia conditions. ISME J. 2023, 17, 1966–1978. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Yuan, Z.; Wang, R.; Angelidaki, I.; Zhu, G. Syntrophy mechanism, microbial population, and process optimization for volatile fatty acids metabolism in anaerobic digestion. Chem. Eng. J. 2023, 452, 139137. [Google Scholar] [CrossRef]
- Beneragama, N.; Moriya, Y.; Yamashiro, T.; Iwasaki, M.; Lateef, S.A.; Ying, C.; Umetsu, K. The survival of cefazolin-resistant bacteria in mesophilic co-digestion of dairy manure and waste milk. Waste Manag. Res. 2013, 31, 843–848. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Zhang, Y.; Yang, J.; Shen, W.; Yang, S.; Liu, B.; Yuan, Z.; Zhang, Y. Thermodynamic restrictions determine ammonia tolerance of functional floras during anaerobic digestion. Bioresour. Technol. 2023, 391, 129919. [Google Scholar] [CrossRef]
- Song, M.; Shin, S.G.; Hwang, S. Methanogenic population dynamics assessed by real-time quantitative PCR in sludge granule in upflow anaerobic sludge blanket treating swine wastewater. Bioresour. Technol. 2010, 101, S23–S28. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef] [PubMed]
- Belostotskiy, D.E.; Ziganshina, E.E.; Siniagina, M.; Boulygina, E.A.; Miluykov, V.A.; Ziganshin, A.M. Impact of the substrate loading regime and phosphoric acid supplementation on performance of biogas reactors and microbial community dynamics during anaerobic digestion of chicken wastes. Bioresour. Technol. 2015, 193, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Hinks, J.; Edwards, S.; Sallis, P.J.; Caldwell, G.S. The steady state anaerobic digestion of Laminaria hyperborea–Effect of hydraulic residence on biogas production and bacterial community composition. Bioresour. Technol. 2013, 143, 221–230. [Google Scholar] [CrossRef]
- De Francisci, D.; Kougias, P.G.; Treu, L.; Campanaro, S.; Angelidaki, I. Microbial diversity and dynamicity of biogas reactors due to radical changes of feedstock composition. Bioresour. Technol. 2015, 176, 56–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).