Abstract
Developing innovative, eco-friendly fungicide alternatives is crucial to mitigate the substantial threat fungal pathogens pose to crop yields. In this study, we assessed the in vitro effectiveness of SiO2, CuO, and γFe2O3 nanoparticles against Rhizoctonia solani. Furthermore, greenhouse experiments were conducted in artificially infested soil to evaluate the in vivo impact of nanoparticles under study. Two application methods were employed: soil drenching with 10 mL per pot at concentrations of 50, 100, and 200 mg L−1, and seedling dipping in nanoparticle suspensions at each concentration combined with soil drench. The combined treatment of 200 mg L−1 γFe2O3 or CuO nanoparticles showed the highest in vitro antifungal activity. Conversely, SiO2 nanoparticles demonstrated the lowest in vitro activity. Notably, the application of 200 mg/L SiO2 via the dipping and soil drenching methods decreased counts of silicate-solubilizing bacteria and Azospirillum spp. Whereas, application of 100 mg L−1 γFe2O3 nanoparticles via soil drenching increased soil bacterial counts, and CuO nanoparticles at 50 mg L−1 through dipping and soil drenching had the highest dehydrogenase value. γFe2O3 nanoparticles improved plant photosynthetic pigments, reduced malondialdehyde levels, and minimized membrane leakage in lettuce plants. A root anatomical study showed that 200 mg L−1 CuO nanoparticles induced toxicity, whereas 200 mg L−1 γFe2O3 or SiO2 nanoparticles positively affected root diameter, tissue structure, and various anatomical measurements in lettuce roots. γFe2O3 nanoparticles hold promise as a sustainable alternative for managing crop diseases.
1. Introduction
Agriculture is the primary pillar of the developing economy, providing food for a better quality of life. The agricultural sector is currently facing a wide range of challenges, including unpredictable climate change, soil contamination with harmful environmental pollutants, such as fertilizers and pesticides, combined with the dramatic rise in food demand due to the growing global population [1]. Recent years have witnessed significant advances and innovations in agriculture to address the challenges of sustainable food security [2]. Nanomaterials have emerged as valuable tools in enhancing agricultural capabilities [3]. Globally, nanomaterial production reached 260,000–309,000 metric tons in 2010 and continued to grow, with consumption ranging from 225,060 to 585,000 metric tons between 2014 and 2019 [4]. These materials, known for their unique properties, have found increasing application in agriculture, including various types such as metal oxides, silicates, ceramics, and more [5]. Utilizing nanomaterials in agriculture aims to reduce the use of plant protection products, minimize nutrient losses during fertilization, and optimize revenue through improved nutrient management [6]. Notably, one of the promising applications in plant pathology involves using nanomaterials to control plant diseases and enhance plant growth [7].
The soil-borne phytopathogenic fungus Rhizoctonia solani Kuhn (teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk; basidiomycetes) is responsible for high yield losses in a number of economically important crops worldwide [8]. It can cause pre- and post-emergence damping off and bottom rots, commonly producing sclerotia on dead tissues [9]. R. solani causes bottom rot on lettuce, an economically important disease resulting in yield losses up to 70% [10]. The control of the pathogen is difficult because of its wide host range and its ability to survive as sclerotia under adverse environmental conditions. In practice, the control of diseases caused by R. solani relies mainly on fungicides [11]. However, increasing concern about the health and environmental hazards associated with the use of agrochemicals has resulted in the search for viable alternatives. Hence, it became very important and urgent to find effective strategies to control this disease [12].
Nanomaterials, including nano-metal, nano-metal oxides, and metal salts, possess unique properties that make them potential elicitors for enhancing antimicrobial activity against plant pathogens. Due to their large surface-to-volume ratio and small size compared to the same substances in their normal size, these nanomaterials could be effective in improving plant health and fighting against plant diseases [13,14,15,16,17]. Lettuce plants, susceptible to a range of soil-borne pathogenic fungi, face significant threats that can result in considerable yield and quality losses. Among these diseases, bottom rot stands out as one of the most destructive, particularly affecting lettuce plants during their head stage, causing decay, and often rendering the heads unsuitable for the market due to severe rotting. Even minor infections necessitate additional lower leaf trimming, leading to increased harvesting labor and reduced lettuce quality and head weight [18].
Copper nanoparticles (Cu NPs) are envisioned as pivotal in the next generation of nanomaterials due to their cost-effectiveness [19]. Copper oxide variants (CuO and Cu2O) are widely used antimicrobial agents, with CuO being cost-effective, easily combinable with polymers, and possessing stable chemical and physical properties [20]. High-ionic-scale metal oxides like CuO are of particular interest for their large surface areas and crystal shapes that enhance their antimicrobial potency [21]. Additionally, copper serves as a vital plant micronutrient, contributing to plant growth and disease resistance. Copper nanoparticles (CuO NPs) influence plant nutrition and disease defense, with Cu2ONPs acting as an effective nano-fertilizer to enhance disease resistance, as seen in systems like asparagus/fusarium crown and root rot [22]
Iron oxide nanoparticles demonstrated a substantial reduction in Fusarium oxysporum conidial germination rate and fungal growth, leading to a decrease in pathogen numbers on infected plants. Their pesticide effect proves effective against various pathogens, including fungi, bacteria, and viruses. Additionally, iron-oxide nanoparticles reduced fusaric acid production and increased mannitol content, thereby decreasing phytotoxin production in infected plants and resulting in a reduced disease index [23].
Besides its nutritional importance, Si stimulates plant resistance mechanisms against both biotic and abiotic stress [24]. It is known to suppress various plant diseases across crops, including multiple diseases in rice, as well as powdery mildew in wheat and cucumber [25]. Reducing Si particle size to the nano level through a safe synthesis method could enhance its efficacy in improving plant growth, resistance and suppressing plant pathogenic fungi [26].
The aim of this study was to (i): evaluate the efficacy of SiO2, CuO, and γFe2O3 nanoparticles as novel fungicides against Rhizoctonia solani, the pathogen responsible for bottom rot disease in lettuce; (ii): to assess the impact of these nanoparticles on soil health parameters and plant responses to the treatments. The study innovatively explores the use of nanoparticles as a potent and sustainable alternative for managing crop diseases, specifically Rhizoctonia solani, responsible for bottom rot in lettuce. It demonstrates their effectiveness through in vitro antifungal activity, enhanced plant health, and root anatomical improvements, offering a promising, environmentally friendly fungicide alternative.
2. Materials and Methods
2.1. Synthesis of Nanomaterials
A comprehensive explanation of the synthesis methods, along with their corresponding characterization, is available in the supporting information.
2.2. Enmiration, Isolation, Identification of Fungal Pathogens Responsible for Bottom Rot
Lettuce plant samples affected by bottom rot disease were collected from El-Mansoria and Nahia fields in Giza governorate. The lower stems and roots were rinsed, sterilized with 3% sodium hypochlorite for three minutes, and placed on potato dextrose agar (PDA) medium for fungal growth. The isolated fungi were identified based on morphological characteristics, following references [27,28]. The percentage of colony frequency was calculated using Equation (1):
where “n” represents the number of colonies for each pathogen, and “N” is the total number of colonies. For in vivo experiments, a rice hull medium was prepared by sterilizing a mixture of rice grains, sand, and water, which was then inoculated with the fungus mycelium and incubated at 28 °C for 7 days.
2.3. Pathogenicity Tests
The fungal isolates obtained from lettuce plant samples affected by bottom rot disease (Rhizoctonia solani F. oxysporum and F. solani) isolates were tested for pathogenicity on lettuce (Lactuca sativa Var. longifolia) in 25 cm diameter plastic pots filled with a sterile 1:2 sand and peat moss substrate mix. Soil inoculation with R. solani using hull rice culture (3% w/w) was carried out a week before transplantation. Each treatment involved three pots, and re-isolation from the bottom was performed to fulfill Koch’s postulates. Disease incidence and severity were calculated as per [29] using Equation (2).
Disease severity was evaluated based on the progression of yellowing, root rot, and overall plant decay at the end of the experiment, and recorded as the percentage of symptomatic plants. Disease severity assessment was determined using a modified 0–5 scale by [30] where 0 = 0%, 1 = 0–10%, 2 = 10–25%, 3 = 25–50%, 4 = 50–75%, and 5 = 75–100%. The disease severity percentage (DS%) was calculated based on the following Equation (3):
where n is the number of plants in each numerical rate, N is the total number of plants multiplied by the maximum numerical rate, and r is equal to 5.
2.4. In Vitro Examination of Antifungal Activity of the Prepared Nanomaterials
According to the pathogenicity test, R.solani was the most causative agent for bottom rot among other pathogens, so it was selected to complete the study. The antifungal activity of SiO2NPs, CuONPs, Fe2O3 NPs, and Rizolex® fungicide against R. solani was evaluated using a modified method outlined by [31]. SiO2NPs, CuONPs, and Fe2O3 NPs were added to autoclaved PDA media at concentrations of 50, 100, and 200 µg L−1, while Rizolex® fungicide was added at a concentration of 2.5 g L−1. PDA plates without any additives were set as control. Subsequently, 0.5 cm diameter disks of a 7-day-old fungal growth were placed on the center of each Petri dish. The radial growth was measured after 7 days of incubation or when the fungal growth was completely covered in the control treatment.
The inhibition percentage was estimated using the following equation:
where C represents the mycelium growth in the control (cm), and T represents the mycelium growth in the treatments (cm).
degree of inhibition in radial growth (%) = (C − T)/C × 100
2.5. In Vivo Examination of Antifungal Activity of the Prepared Nanomaterials
Sandy clay–loam soil was sterilized with 5% formalin and air dried for 7 days [32]. Each 25 cm diameter pot contained 4 kg of sterilized soil infected with R. solani. Lettuce seedlings were treated with SiO2NPs, CuONPs, and Fe2O3 NPs, suspensions (50, 100, and 200 mg L−1) for 2 h prior to planting. Soil drench treatments with the same solutions were administered by injecting 10 mL around the lettuce roots. Control groups received distilled water. The plants were grown to maturity using standard practices, and the incidence and severity of bottom rot disease were recorded 45 days after transplanting. Soil properties were assessed following the method by [33]. Treatments details are presented in Table 1.

Table 1.
Description of the greenhouse experiment.
2.6. Soil Biological Activities
To evaluate the ecological impact of nanomaterials under study, the following biological measurements were conducted.
2.6.1. Microbial Populations
To assess the total microbial count, rhizosphere soil samples were collected 45 days after transplanting and stored at 4 °C to maintain microbiological activity. The plate count technique was employed using potato dextrose agar medium (PDA) [34] and nutrient agar medium [35] for enumerating total fungi and bacteria, respectively. Serial dilution and standard count techniques were utilized to isolate and enumerate free-living nitrogen fixers, phosphate-solubilizing bacteria, and silicate-solubilizing bacteria. Free-living nitrogen fixers were cultured on N-free media, Azospirillum spp. on N-deficient medium [36], Azotobacter spp. on Modified Ashby’s broth medium [37], phosphate solubilizers on Pikovskaya’s agar medium [38], and silicate bacteria on Aleksandrov’s agar medium [39].
2.6.2. Soil Enzymes Activities
The dehydrogenase activity in the soil was determined using the method described by [40]. The urease activity was measured following the protocol by [41]. The alkaline phosphatase in the soil was assessed according to [42]. The nitrogenase activity in the plant rhizosphere was determined by the acetylene reduction method according to [36].
2.7. Plant Sampling and Analysis
2.7.1. Estimation of Photosynthetic Pigments
To determine the total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid contents, 0.5 g of leaf tissue was extracted using 10 mL of 80% acetone. The pigment estimation was carried out using a spectrophotometer (UV1901PC) at 645, 663, and 470 nm following the method of [43]. Acetone (80%) was used as the blank. The equations for the calculations are as follows:
2.7.2. Cell Membrane Stability Index
Membrane stability was assessed by measuring lipid peroxidation products, indicated by thiobarbituric acid (TBA) reactive substances equivalent to malondialdehyde (MDA), following [44]. Electrolyte leakage (EL) from leaf tissue was measured using a conductivity meter (Adwa-AD32). Three replicates were used, where samples were immersed in de-ionized water, and the conductivity was measured immediately and after 1 h. The electrolyte leakage rate was calculated as the net conductivity after 1 h divided by the total conductivity after boiling, as described by [45].
2.7.3. Anatomical Structure
Anatomical preparations of root cross-sections were conducted using the paraffin embedding method according to [46]. The root midribs of infected and healthy lettuce plants were killed and fixed for at least 48 h in formalin glacial acetic acid before being dehydrated. They were then serially sectioned by a rotary microtome at 20 μm thickness, and finally double stained with crystal violet and erythrosine, cleared in carbol xylene, and mounted in Canada balsam. Observations of the anatomy slides were performed using a light microscope (CX22LED, Olympus) and documented using a digital microscope camera (Optilab Advance V2, Miconos).
2.8. Statistical Analysis
The collected data were analyzed using analysis of variance (ANOVA) based on a randomized complete block design (RCBD). Statistical analysis was performed using WASP software Version 12.4.12.79. To compare the mean differences between treatments, the Least Significant Difference (LSD) test was employed. The significance level was set at p < 0.05 [47].
3. Results and Discussion
3.1. Characterization of Synthesized Nanomaterials
Nano silica was prepared according to the method described by [48], Magnetic nanoparticles (MNPs) was prepared according to [49]. Nano-Cu was prepared by chemical precipitation according to the procedure described by [50,51]. Comprehensive Scanning Electron Microscope (SEM) for each type of the prepared nanomaterials can be found in the Supplementary Materials.
3.2. Isolation and Frequency Percentages of Causal Agents of Lettuce Bottom Rot
Table 2 presents data on the isolation and frequency percentages of causal agents of lettuce bottom rot. The results indicate that two fungal genera were identified from the discolored bottom rot of lettuce cv. Nader collected from four villages (El Mansoria and Nahia) in Giza governorate. The fungi were identified based on morphological criteria and microscopic features, and were identified as Fusarium oxysporum Schlecht, F. solani, and Rhizoctonia solani Kuhn. Rhizoctonia solani was the most prominent fungi, accounting for 63.64% of the total isolates. Fusarium solani was also frequently isolated, with a frequency of 21.21%, followed by F. oxysporum, which was isolated at a lower frequency of 15.15%.

Table 2.
Isolation and frequency percentages of causal agents of lettuce bottom rot.
3.3. Pathogenicity Test
The data presented in Table 3 indicate that the Nader cultivar is susceptible to Rhizoctonia solani, as evidenced by the similar symptoms observed in the field, including discoloration of the internal tissues at the bottom. R. solani was successfully isolated from the symptomatic tissues and recorded a high disease index and severity on Nader cultivar, at 83.33% and 64.77%, respectively. On the other hand, Fusarium solani showed a low disease index and severity, at 33.33% and 3.24%, respectively. The lowest disease incidence was observed when Nader cultivar was inoculated with F. oxysporum.

Table 3.
Pathogenicity test of causal agents of lettuce bottom rot.
3.4. Antifungal Performance of Nano-SiO2, Nano-γFe2O3 and Nano-Copper against R. solani In Vitro Experiment
Based on the in vitro experiment (Table 4), ferric and copper oxide nanoparticles exhibited moderate inhibitory effects on the mycelial growth of R. solani at all concentrations compared to commercial fungicide. The highest reduction in mycelium growth (23.33%) was observed with Fe2O3 at 200 mg L−1, followed by Nano-γFe2O3 at 100 mg L−1 (18.22% reduction) and 50 mg L−1 (17.11% reduction). Nano-copper oxide at 200 mg L−1 resulted in an 11.89% reduction Figure 1. However, SiO2 nanoparticles did not affect the mycelial growth. The detrimental effects of CuO and γ-Fe2O3 nanoparticles on R. solani mycelium may be attributed to interactions with P- and S-containing molecules inside or outside fungal cells, disrupting cell wall functions, protein synthesis, and ion exchanges, ultimately leading to cell death. Previous studies have highlighted similar mechanisms of action for CuO nanoparticles [52] and iron oxide nanoparticles [53]. On the other hand, the small size and larger surface area to volume ratio of nanoparticles in addition to their ability to reduce the oxygen supply for respiration enhance their antifungal potential. Thus, CuO and γ-Fe2O3 nanoparticles demonstrate potential as alternative control measures against fungal pathogens affecting stored vegetables.

Table 4.
Effect of the prepared nanomaterials on linear growth of Rhizoctonia solani on lettuce plants.
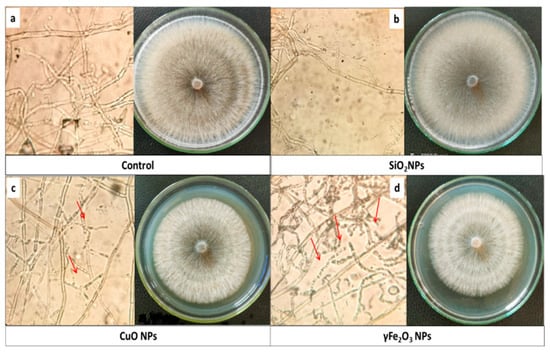
Figure 1.
(a) is the control R. solani mycelium with right-angled branching and constriction, (b) is effect of SiO2NPs, (c) is the effect of CuONPs, (d) is the effect of γFe2O3NPs (magnification 40× and red arrows pointed degradation ends).
3.5. Greenhouse Experiment
Under greenhouse conditions, SiO2, CuO, and γFe2O3 nanoparticles were evaluated for their efficacy in controlling lettuce bottom rot disease. Physical and chemical characteristics of the used soil, revealing sandy clay–loam texture with pH 7.7, EC 2. 16 ds/m, organic matter 1.58%, total nitrogen 0.13%, total phosphorus 0.025%, available phosphorus 0.005%, and total potassium 0.178%.
The results presented in Table 5 demonstrated that all tested γFe2O3 and CuO nanoparticles effectively reduced the severity of lettuce bottom rot compared to control and fungicide treatments. Significantly, γFe2O3 and CuO nanoparticles at a concentration of 200 mg L−1 exhibited higher effectiveness in reducing disease severity than SiO2 nanoparticles at a concentration of 100 mg L−1 and fungicide treatments. The reduction in disease severity was 24.84% and 34.11% for γFe2O3 and CuO nanoparticles, respectively, while SiO2 nanoparticles and fungicide treatments resulted in reductions of 36.18% and 18.32%, respectively. These findings highlight the considerable potential of γFe2O3 and CuO nanoparticles in controlling lettuce bottom rot disease.

Table 5.
Effect of the nanomaterials on head rot of lettuce plants under greenhouse conditions.
Additionally, the combination of SiO2, CuO, or Fe2O3 nanoparticles as a dipping and soil drench treatment proved to be the most effective strategy for controlling Rhizoctonia root rot caused by R. solani. Despite the low in vitro efficiency of SiO2 nanoparticles, they showed considerable in vivo potential which may be attributed to the formation of physical barriers through Si accumulation in plant cells [44]. In addition to the direct effect of Fe2O3 nanoparticles on pathogen growth, they also induce higher activity of antioxidant enzymes due to iron involvement in enzyme activity and RNA synthesis [44]. CuO nanoparticles interact with microorganisms by permeating cell membranes, oxidizing membrane lipids, altering proteins, and denaturing nucleic acids, ultimately resulting in cell death [54].
3.6. Effect of Metal Oxide NPs on Soil Biological Activities
3.6.1. Effect on Microbial Community
Biotic and abiotic factors influence microbial populations both in terms of diversity and numbers in soil. These factors may include soil plant litter, root exudates, pathogens, management factors like mineral fertilizers, soil moisture, and soil organic matter, which in turn, affect crop production and the sustainability of soil health [55]. Therefore, we tested the population density of total mesophilic microflora and the principal enzymatic activities.
Data exhibited in Table 6 show that the highest bacterial count was observed with the 200 mg L−1 SiO2 dipping and soil drench method. However, at the same concentration, there was a notable decrease in the counts of silicate-solubilizing bacteria and Azospirillum, whereas the population of Azotobacter was stimulated. Azotobacter as an obligate aerobe is the predominant free-living diazotroph in soils [56]. Ref. [57] demonstrated that nanosilica significantly enhanced microbial populations, total biomass content, and silica content in maize. In particular, the population of phosphate-solubilizing bacteria (PSB) increased in soil treated with nanosilica, likely due to the increased availability of phosphorus, which is influenced by both phosphorus and silicon. Furthermore, the highest populations of nitrogen-fixing bacteria were observed in the nanosilica-treated soil, suggesting the potential for increased nitrogen availability to plants through nitrogen fixation. However, the counts of sulfur-oxidizing bacteria (SSB) decreased.

Table 6.
Microbial populations in the rhizosphere of lettuce plants infected with Rhizoctonia solani as affected by different concentrations of SiO2, CuO, and γFe2O3 nanoparticles.
The different CuO NPs treatments caused significant changes in the microbial community structure. Treatments with 50 and 100 mg L−1 CuO NPs resulted in a significant increase in bacterial count, actinomycetes, and free-living diazotrophs. However, the highest concentration of CuO NP (200 mg L−1) led to a significant decrease in bacterial and actinomycetes counts. Surprisingly, there was no significant difference in phosphate-solubilizing bacteria (PSB) and sulfur-oxidizing bacteria (SSB) counts among CuO NP treatments up to 100 mg L−1. However, a significant decrease was observed with the 200 mg L−1 treatment. It is well known that elemental copper can be toxic to beneficial bacteria and fungi in the environment [58,59].
γFe2O3 NPs used for soil drenching generally increased total bacterial, actinomycetes, PSB, SSB, and free-living diazotroph counts. The maximum bacterial and free-living diazotroph counts were observed with 100 mgL−1 Fe2O3 soil drench (T8) at 24.67 × 106 and 0.91 × 104 CFU/g dry soil, respectively. However, combined application treatments (dipping + soil drench) led to a significant decrease in total bacterial, actinomycetes, and fungal counts. Treatment with 200 mg L−1 γFe2O3 NPs for soil drenching (T9) had a positive stimulation effect on beneficial soil microbes, with maximum bacterial numbers of 11.2, 0.87, 0.71, and 0.36 × 104 CFU/g dry soil for PSB, SSB, and free-living diazotroph counts, respectively.
In a study by [60], the effect of magnetic iron oxide nanoparticles (Fe3O4 and γ-Fe2O3) on the soil bacterial community was investigated using molecular approaches. Results showed that the addition of these nanoparticles could stimulate bacterial growth and alter the community structure. Iron is an essential nutrient for microorganisms, as it is involved in cell growth and regulation through iron–sulfur (Fe-S) clusters. These clusters sense environmental signals, such as oxygen and iron levels, and mediate adaptive responses [61]. The effects of magnetic iron oxide nanoparticles on bacterial populations can be related to both their properties and their impact on microbial metabolism [62,63]. Magnetic nanoparticles can easily penetrate soil due to their small size and stability, while their high surface-to-volume ratio makes them more prone to ion release compared to bulk materials. Additionally, nanoparticles have highly active surface sites, such as the Fe-OH site on iron oxide magnetic nanoparticles [29].
3.6.2. Effect on Soil Enzymatic Activities
Soil enzymatic activities reflect microbial performance and contribute to overall soil microbial activity [64,65]. Results illustrated in Figure 2 clearly indicated that, the combined treatment of dipping and soil drenching had the most significant effect, with a concentration of 200 mg L−1 showing the highest impact, followed by 100 mg L−1, while 50 mg L−1 had the least significant effect. The highest dehydrogenase activity was observed with 50 mg L−1 CuO NPs (dipping + soil drenching), followed by 100 mg L−1 γFe2O3 NPs (dipping + soil drenching) and 200 mg L−1 SiO2 NPs (dipping + soil drenching) [66]. Alkaline phosphatase activity was significantly influenced by combined treatments of metal oxide NPs (dipping + soil drenching), with 200 mg L−1 γFe2O3 NPs showing the highest activity. SiO2 and CuO NPs decreased alkaline phosphatase activity compared to the control treatment. Phosphatases are a group of enzymes that are of great agronomic value because they catalyze the hydrolysis of organic phosphorus compounds and transform them into an inorganic form which is assimilated by plants and microorganisms [66]. γFe2O3 and CuO NPs stimulated urease activity, especially when applied through combined treatments. The highest urease activity was observed with 100 mg L−1 γFe2O3 NPs (dipping + soil drenching) and 100 mg L−1 CuO NPs (dipping + soil drenching). SiO2 NPs had no significant effect on urease activity. All tested metal oxide NPs significantly stimulated soil nitrogenase activity, with SiO2 NPs showing the highest activity at a concentration of 200 mg L−1 (dipping + soil drenching). There was a direct correlation between SiO2 NPs concentration and nitrogenase activity [66].
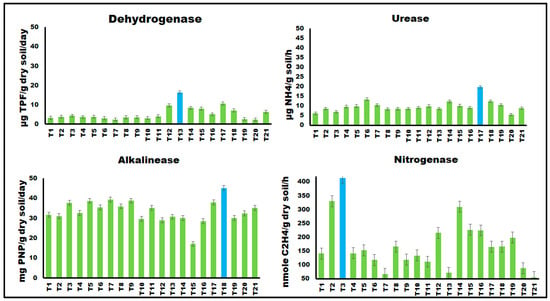
Figure 2.
Soil enzymatic activity of dehydrogenase, urease, alkaline phosphatase and nitrogenase in the rhizosphere of lettuce plants infected with Rhizoctonia solani and treated with different concentrations of SiO2, CuO, and γFe2O3 nanoparticles. Columns with blue indicate the highest activity.
These findings suggest that γFe2O3 NPs can enhance dehydrogenase, alkaline phosphatase, and urease activities through changes in the bacterial community. Iron is important for microorganisms as it acts as a cofactor for many enzymes [60,67]. CuO NPs have been shown to inhibit soil enzyme activities, including dehydrogenase, acid and alkaline phosphatase, and urease [68,69].
The positive impact of nanosilicon dioxide (SiO2) and plant growth-promoting rhizobacteria (PGPR) on soil and plant health through increased microbial population and enzyme activity is consistent with previous research [70,71]. However, the effects of nanomaterials depend on various factors (NPs type, concentration, size, shape, exposure duration, and plant/animal species [71].
3.7. Effect of Metal Oxide NPs on Endogenous Factors
The impact of metal oxide NPs (SiO2, CuO, and γFe2O3) on endogenous factors in plant growth and development was assessed by measuring various physiological parameters, including total chlorophyll, chlorophyll a, chlorophyll b, carotenoid content, cell membrane stability index (MDA), and rate of membrane leakage.
3.7.1. NPs Effect on Photosynthetic Pigments
Metal oxide NP treatments were assessed for their impact on photosynthetic pigments, which serve as indicators of plant stress [72]. As shown in Figure 3,without nanomaterials, R. solani bottom rot disease caused a significant reduction in total chlorophyll (35.66%) and carotenoid content (50.41%) (T19). γFe2O3 NPs at 50 mg L−1 (soil drench) significantly increased the total chlorophylls (82.2%) and carotenoids (67.22%) (T7). Significant increases were also observed with 200 mg L−1 CuO-NPs (dipping) in total chlorophylls (70.24%) and carotenoids (78.47%) (T6). SiO2-NPs had no adverse effects on photosynthetic pigments at lower concentrations (50 and 100 mg L−1), but at 200 mg L−1, they reduced chlorophyll content (Figure 4). Iron and copper are essential elements for photosynthetic efficiency and growth, while silica stimulates chlorophyll biosynthesis and activity [73,74,75]. Metal NPs enhance chlorophyll structure and metabolic activities [75]. Copper promotes plant growth but inhibits it at higher doses [59]. In a recent study by [76], foliar or soaking treatment of wheat plants with CuO-NPs at 50 ppm increased total chlorophylls (23% and 10.5%) and carotenoids (20.2% and 12.3%). This increase in photosynthesis rate may be due to the increased biological and chemical activities of metals at the nanoscale and the correlative impact of nutrients such as magnesium, iron, zinc, sulfur, etc., on plants. Silica stimulates chlorophyll biosynthesis and improves photosystem II activity [75].
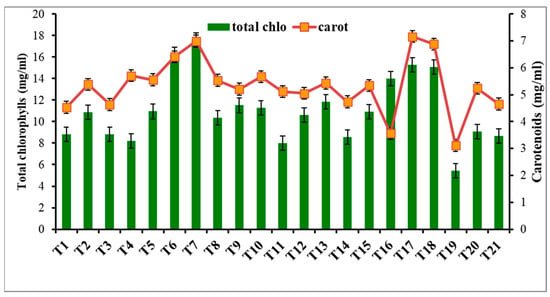
Figure 3.
Effect of metal oxide NPs on total chlorophyll and carotenoid contents.
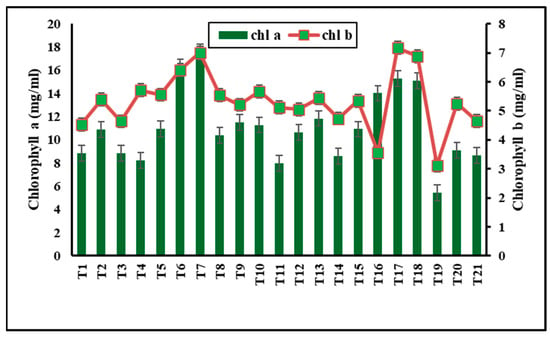
Figure 4.
Effect of metal oxide NPs on chlorophyll a, chlorophyll b contents.
3.7.2. Effect of Metal oxide NPs on Malondialdehyde (MDA) and Electrolyte Leakage (EC)
Root rot infection often leads to increased lipid peroxidation, as indicated by elevated levels of malondialdehyde (MDA) and other aldehydes [72].
However, in the case of lettuce plants, the application of 50 mg L−1 γFe2O3 nanoparticles (NPs) as a soil drench treatment (T7) resulted in the most significant reduction in MDA levels (Figure 5). The combined treatment of dipping and soil drenching with γFe2O3 NPs showed a greater decrease in MDA levels compared to other treatments. Similarly, a soil drench treatment with 200 mg L−1 CuO NPs (T6) significantly decreased MDA levels. Conversely, SiO2-NPs treatments had a negative impact on MDA levels compared to the control (T1), indicating their potential to exacerbate lipid peroxidation under stress conditions.
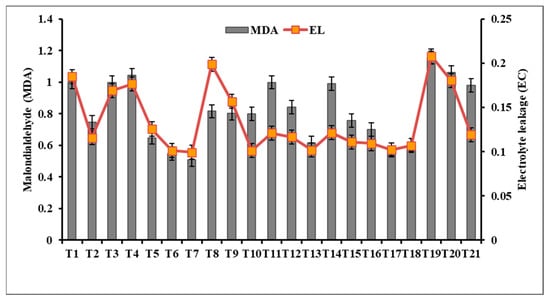
Figure 5.
Effect of metal oxide NPs on malondialdehyde (MDA) and electrolyte leakage (EC).
To assess membrane permeability and electrolyte leakage caused by Rhizoctonia solani bottom rot infection and the impact of metal oxide NPs treatments, electrolyte leakage was measured after 60 days. The dipping and soil drench treatments with 50 mg L−1 γFe2O3 NPs (T10) exhibited the highest reduction in electrolyte leakage, which was consistent with the results of total chlorophylls, carotenoids, and MDA analysis. The most effective concentration of CuO NPs was found in T6, where a soil drench treatment of 200 mg L−1 CuO NPs was applied [70]. In contrast, SiO2-NPs treatments, especially the 200 mg L−1 SiO2-NPs soil drench treatment (T3), resulted in a significant increase in electrolyte leakage, indicating changes in membrane permeability [45]. Nanoparticles have shown potential in mitigating various stresses in plants, as demonstrated by the positive effects of iron oxide nanoparticles (IONPs) and silicon nanoparticles (SiNPs) in alleviating the effects of cadmium stress in Phaseolus vulgaris plants, including reducing MDA content and electrolyte leakage [70].
3.8. Anatomical Structure of Lettuce Root Infected with R. solani and Treated with Nano Metal Oxide
The control root (healthy plant) displayed a diameter of 680.957 µm at 500 µm depth (Figure 6a). The epidermis and cortical cells were intact, while the xylem vessels and phloem tissue were well developed. In contrast, the R. solani-infected control root (Figure 6b) exhibited hyphal growth along the epidermal walls, tissue disintegration, and disrupted xylem vessels. Crystal violet staining revealed substance accumulation on the infected control root walls. SiO2 NPs treatment showed a slight decrease in root diameter (464.466 µm) but improved phloem development when used as a dipping treatment. Combined dipping and soil drenching of SiO2 NPs resulted in a larger root diameter (657.726 µm) and well-defined tissue structure (Figure 6e). CuO NPs treatment increased the root diameter (792.395 µm) but caused deformation and crushed the phloem tissue. The anatomical study demonstrated the toxic effect of a 200 mg L−1 CuO NPs dipping and soil drenching treatment (T15). γ-Fe2O3 NPs treatment for soil drenching or combined dipping and soil drenching improved the root diameter (752.00 µm and 603.782 µm, respectively) and exhibited well-developed phloem tissue and visible primary xylem (Figure 6e,f).
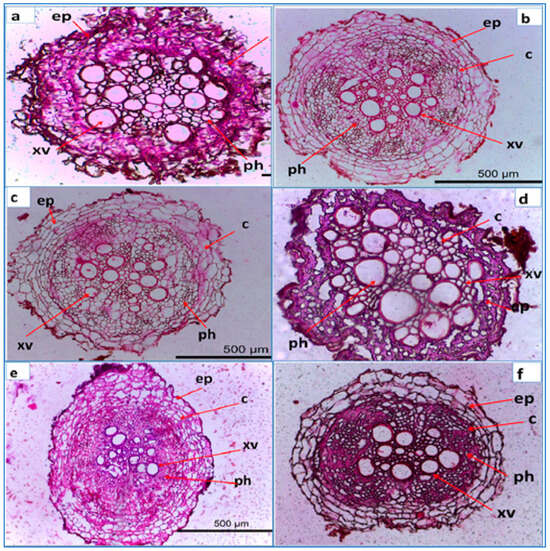
Figure 6.
Light micrographs of cross-section of the root of lettuce inoculated with R. solani and treated with different nano metal oxides, (a) treated with SiO2 for soil drenching; (b) SiO2 for soil drenching + dipping; (c) CuO for soil drenching; (d) CuO for soil drenching + dipping; (e) γFe2O3 for soil drenching ; (f) γFe2O3 for soil drenching + dipping. The abbreviations shown in the images refer to epidermis (ep), cortex (c), xylem vessel (xv), phloem tissue (ph). Transverse section of the root of lettuce under 20× magnification. Bar = 500 µm.
Overall, SiO2 NPs treatment enhanced phloem development, CuO NPs treatment increased root diameter but had adverse effects on tissue structure, and γ-Fe2O3 NPs treatment improved root diameter and mineral translocation. These findings highlight the potential of nano metal oxide treatments in promoting plant growth and resistance to R. solani infection.
4. Conclusions
This study underscores the urgent issue of fungal pathogens on crop yields, emphasizing the critical need for innovative and eco-friendly fungicide alternatives. The investigation into the in vitro effectiveness of SiO2, CuO, and γFe2O3 nanoparticles against Rhizoctonia solani showcases promising results. Particularly, the combined treatment of 200 mg L−1 γFe2O3 or CuO nanoparticles demonstrated exceptional in vitro antifungal activity. Furthermore, the in vivo impact of these nanoparticles, with two application methods, revealed additional insights into their potential benefits. γFe2O3 nanoparticles, in particular, exhibited a range of positive effects, including enhanced plant photosynthetic pigments, reduced oxidative stress, and improved root anatomical features. These findings underscore the potential of γFe2O3 nanoparticles as a sustainable alternative for managing crop diseases, opening doors to more effective and environmentally responsible agricultural practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres15010014/s1, Figure S1. SEM images of Nano-SiO2 (a), Nano- γFe2O3 (b) and Nano- Copper (c).
Author Contributions
The collaborative efforts of the authors were integral to this research. N.A.H.F., T.A.E. and T.M.S. contributed significantly to the conceptualization and methodology. Alongside A.A.T., S.F.E. and N.A.T., they played crucial roles in developing and validating the methodology. T.A.E. and T.E. handled software implementation and validation. N.A.H.F., T.A.E. and T.E. conducted data analysis, while investigation efforts were shared by N.A.H.F., T.A.E. and N.A.T., N.A.H.F. and T.A.E. managed project resources, and data curation involved N.A.H.F., T.A.E. and T.E. The manuscript’s initial draft was collaboratively written by N.A.H.F., T.M.S., T.A.E. and T.E., with T.E. taking the lead in review and editing. Visualization tasks were shared by N.A.H.F., T.A.E. and T.E., and project supervision was led by N.A.H.F. and T.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This research study does not involve the use of human subjects. As such, it falls outside the purview of the Institutional Review Board (IRB) requirements.
Informed Consent Statement
This research study does not involve the use of human or animal samples. As such, the requirement for informed consent is not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.; Bongiorno, G.; Cucino, V.; Cariola, A. Adopting new technologies during the crisis: An empirical analysis of agricultural sector. Technol. Forecast. Soc. Chang. 2023, 186, 122106. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, A.U.; Hasan, M.A.; Yadav, K.K.; Pinto, M.M.C.; Malik, N.; Yadav, V.K.; Khan, A.H.; Islam, S.; Sharma, G.K. Agro-nanotechnology as an emerging field: A novel sustainable approach for improving plant growth by reducing biotic stress. Appl. Sci. 2021, 11, 2282. [Google Scholar] [CrossRef]
- Grillo, R.; Abhilash, P.C.; Fraceto, L.F. Nanotechnology applied to bio-encapsulation of pesticides. J. Nanosci. Nanotechnol. 2016, 16, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Massalimov, I.; Ram, P.; Amr, I.M.I.; Ahmed, I.S.A. Modern Prospects of Nanotechnology in Plant Pathology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–372. [Google Scholar] [CrossRef]
- Wolf, P.F.J.; Lenz, R.; Baron, K.; Verreet, J.A. Quaternary integrated pest management concept for powdery mildew in sugar beet. I. Analysis of epidemic determinants to predict disease onset. J. Plant Dis. Prot. 2006, 113, 61–67. [Google Scholar] [CrossRef]
- Elshahawy, I.E.; El-Mohamedy, R.S. Biological control of Pythium damping-off and root-rot diseases of tomato using Trichoderma isolates employed alone or in combination. J. Plant Pathol. 2019, 101, 597–608. [Google Scholar] [CrossRef]
- Wallon, T.; Sauvageau, A.; Van der Heyden, H. Detection and quantification of rhizoctonia solani and rhizoctonia solani ag1-ib causing the bottom rot of lettuce in tissues and soils by multiplex qpcr. Plants 2021, 10, 57. [Google Scholar] [CrossRef]
- Martins, S.A.; Schurt, D.A.; Seabra, S.S.; Martins, S.J.; Ramalho, M.A.P.; Moreira, F.M.d.S.; da Silva, J.C.P.; da Silva, J.A.G.; de Medeiros, F.H.V. Common bean (Phaseolus vulgaris L.) growth promotion and biocontrol by rhizobacteria under Rhizoctonia solani suppressive and conducive soils. Appl. Soil Ecol. 2018, 127, 129–135. [Google Scholar] [CrossRef]
- Mondal, P.; Kumar, R.; Gogoi, R. Bioorganic Chemistry fungicidal evaluation against Sclerotium rolfsii, Rhizoctonia bataticola and Rhizoctonia solani. Bioorg. Chem. 2017, 70, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Raghupati, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticles suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.; Rubilar, O.; Pieretti, J.C.; Fincheira, P.; de Melo Santana, B.; Fernández-Baldo, M.A.; Benavides-Mendoza, A.; Seabra, A.B. Nanoparticles as a Promising Strategy to Mitigate Biotic Stress in Agriculture. Antibiotics 2023, 12, 338. [Google Scholar] [CrossRef]
- Day, J.M.; Döbereiner, J. Physiological aspects of N2-fixation by a Spirillum from Digitaria roots. Soil Biol. Biochem. 1976, 8, 45–50. [Google Scholar] [CrossRef]
- Xiong, L.; Tong, Z.H.; Chen, J.J.; Li, L.L.; Yu, H.Q. Morphology-dependent antimicrobial activity of Cu/Cu𝑥O nanoparticles. Ecotoxicology 2015, 24, 2067–2072. [Google Scholar] [CrossRef]
- Khatami, M.; Varma, R.; Heydari, M.; Peydayesh, M.; Sedighi, A.; Askari, H.A.; Rohani, M.; Baniasadi, M.; Arkia, S.; Seyedi, F.; et al. Copper oxide nanoparticles greener aynthesis using tea and its antifungal efficiency on Fusarium solani. Geomicrobiol. J. 2019, 36, 777–781. [Google Scholar] [CrossRef]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal Activity of Copper Oxide Nanoparticles against Root Rot Disease in Cucumber. J. Fungi 2022, 8, 911. [Google Scholar] [CrossRef]
- Elmer, W.H.; De La Torre-Roche, R.; Pagano, L.; Majumdar, S.; Zuverza-Mena, N.; Dimpka, C.; Gardea-Torresdey, J.; White, W. Effect of metalloid and metallic oxide nanoparticles on Fusarium wilt of watermelon. Plant. Dis. 2018, 102, 1394–1401. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; Al-Shammari, B.M.; Abdelaziz, A.M.; Nofel, M.M.; Gobara, M.; Elkhatib, W.F.; Eid, N.A.; Salem, M.S.; Attia, M.S. Protective role of iron oxide nanocomposites on disease index, and biochemical resistance indicators against Fusarium oxysporum induced-cucumber wilt disease: In vitro, and in vivo studies. Microb. Pathog. 2023, 180, 106131. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.A.W.; Duarte, H.S.S.; Rodrigues, F.A. Effect of foliar application of potassium silicate and fungicide on the severity of leaf rust and yellow leaf spot in wheat. Rev. Ceres 2013, 60, 726–730. [Google Scholar]
- Rodrigues, F.A.; Dallagnol, L.J.; Duarte, H.S.S.; Datnoff, L.E. Silicon control of foliar diseases in monocots and dicots. In Silicon and Plant Diseases; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Abdelrhim, A.S.; Mazrou, Y.S.A.; Nehela, Y.; Atallah, O.O.; El-Ashmony, R.M.; Dawood, M.F.A. Silicon Dioxide Nanoparticles Induce Innate Immune Responses and Activate Antioxidant Machinery in Wheat against Rhizoctonia solani. Plants 2021, 10, 2758. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, G.C.; James, P.W. Ainsworth and Bisby’s Dictionary of Fungi, 6th ed.; Commonwealth Mycological Institute: Kew, UK, 1971. [Google Scholar]
- Alexopoulos, C.J.; Mims, C.W.; Blackwell, M. Introductory Mycology, 4th ed.; John Wiley and Sons: New York, NY, USA, 1996. [Google Scholar]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and faster “Nano darts” kill more bacteria: A study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Abd-El-Khair, H.; El-Nagdi, W.M.A. Field application of bio-control agents for controlling fungal root rot and root-knot nematode in potato. Arch. Phytopathol. Plant Prot. 2014, 47, 1218–1230. [Google Scholar] [CrossRef]
- Fratemale, D.; Giamperi, L.; Ricci, D. Chemical Composition and antifungal activity of essential oil obtained from in vitro plants of Thymus mastichina L. J. Essent. Oil Res. 2003, 15, 278–281. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, D.; Zhao, P.; Yu, X.; Tu, B.; Wang, G. Effect of applying an arsenic-resistant and plant growth-promoting rhizobacterium to enhance soil arsenic phytoremediation by Populus deltoides LH05-17. J. Appl. Microbiol. 2011, 111, 1065–1074. [Google Scholar] [CrossRef]
- Black, C.A. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media for the Examination of Food; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Difco. Difco & BBL Manual: Manual of Microbiological Culture Media, 2nd ed.; Citeseer: College Park, MD, USA, 2009. [Google Scholar] [CrossRef]
- Döbereiner, J.; Day, J.M.; Dart, P.J. Nitrogenase activity in the rhizosphere of sugar cane and some other tropical grasses. Plant Soil 1972, 37, 191–196. [Google Scholar] [CrossRef]
- Abd-el-Malek, Y.; Ishac, Y.Z. Evaluation of Methods Used in Counting Azotobacters. J. Appl. Bacteriol. 1968, 31, 267–275. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiologiya 1984, 17, 362–370. [Google Scholar]
- Zahra, M.K. Studies of Silicate Bacteria. Master’s Thesis, Faculty of Agriculture Cairo University, Giza, Egypt, 1969; p. 111. [Google Scholar]
- Casida, L.E., Jr. Microbial Metabolic Activity in Soil as Measured by Dehydrogenase Determinationst. Appl. Environ. Microbiol. 1977, 34, 630–636. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Senwo, Z.N.; Ranatunga, T.D.; Tazisong, I.A.; Taylor, R.W.; He, Z. Phosphatase activity of Ultisols and relationship to soil fertility indices. J. Food Agric. Environ. 2007, 5, 262–266. [Google Scholar]
- Young, A.J. The photoprotective role of carotenoids in higher plants. Physiol. Plant. 1991, 83, 702–708. [Google Scholar] [CrossRef]
- Zedan, A.; Omar, S. Nano selenium: Reduction of severe hazards of atrazine and promotion of changes in growth and geneexpression patterns on Vicia faba seedlings. Afr. J. Biotechnol. 2019, 18, 502–510. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Saeed, M.; Dodd, P.B.; Sohail, L. Anatomical studies of stems, roots and leaves of.pdf. J. Hortic. For. 2010, 2, 87–94. [Google Scholar]
- Hoshmand, A. Design of Experiments for Agriculture and the Natural Sciences, 2nd ed.; Chapman and Hall: New York, NY, USA, 2006. [Google Scholar]
- Premaratne, W.A.P.J.; Priyadarshana, W.M.G.I.; Gunawardena, S.H.P.; De Alwis, A.A.P. Synthesis of nanosilica from paddy husk ash and their surface functionalization. J. Sci. Univ. Kelaniya Sri Lanka 2013, 8, 33–48. [Google Scholar] [CrossRef]
- Jeon, C.S.; Baek, K.; Park, J.K.; Oh, Y.K.; Lee, S.D. Adsorption characteristics of As (V) on iron-coated zeolite. J. Hazard. Mater. 2009, 163, 804–808. [Google Scholar] [CrossRef]
- Chen, J.P.; Lim, L.L. Key Factors in Chemical Reduction by Hydrazine for Recovery of Precious Metals. Chemosphere 2002, 49, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Oussou-Azo, A.F.; Nakama, T.; Nakamura, M.; Futagami, T.; Vestergaard, M.C.M. Antifungal potential of nanostructured crystalline copper and its oxide forms. Nanomaterials 2020, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Nashwa, A.H.F.; Mervat, A.H. Reduction of Urea Transformation in Soil Using Aqueous Extracted Leaves of Neem (Azadirachta indica) and Olive (Olea europaea L). Indian J. Environ. Prot. IJEP 2020, 40, 12–22. Available online: https://www.e-ijep.co.in/january-2020/ (accessed on 15 January 2023).
- Nashwa, A.H.F.; Massoud, O.N.; Ebtsam, M.M.; Khalil, H.M. Biological Evaluation of Soil Cultivated with Egyptian Clover (Trifolium alexndrinum L.) through Long Term Trial at Bahtim Region, Egypt. Sciences 2015, 5, 515–525. Available online: https://www.curresweb.com/mejas/mejas/2015/515-525.pdf (accessed on 12 May 2023).
- Rangaraj, S.; Gopalu, K.; Rathinam, Y.; Periasamy, P.; Venkatachalam, R.; Narayanasamy, K. Effect of silica nanoparticles on microbial biomass and silica availability in maize rhizosphere. Biotechnol. Appl. Biochem. 2014, 61, 668–675. [Google Scholar] [CrossRef]
- Díaz-Raviña, M.; de Anta, R.C.; Bååth, E. Tolerance (PICT) of the Bacterial Communities to Copper in Vineyards Soils from Spain. J. Environ. Qual. 2007, 36, 1760–1764. [Google Scholar] [CrossRef]
- Bakshi, M.; Kumar, A. Copper-based nanoparticles in the soil-plant environment: Assessing their applications, interactions, fate and toxicity. Chemosphere 2021, 281, 130940. [Google Scholar] [CrossRef]
- He, S.; Feng, Y.; Ren, H.; Zhang, Y.; Gu, N.; Lin, X. The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J. Soils Sediments 2011, 11, 1408–1417. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Luo, J.; Xu, J.; Zhou, G.; Yu, Y.; Xue, L.; Yang, L.; He, S. Fe3O4 nanoparticles affect paddy soil microbial-driven carbon and nitrogen processes: Roles of surface coating and soil types. Environ. Sci. Nano 2022, 9, 2440–2452. [Google Scholar] [CrossRef]
- Ju-Nam, Y.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B. The behavior and effects of nanoparticles in the environment. Environ. Pollut. 2009, 157, 1063–1064. [Google Scholar] [CrossRef]
- Khalifa, M.; Fetyan, N.A.H.; Magid, M.S.A.; El-Sherry, N.I. Effectiveness of potassium silicate in suppression white rot disease and enhancement physiological resistance of onion plants, and its role on the soil microbial community. Middle East J. Agric. Res. 2017, 6, 376–394. [Google Scholar]
- Stone, M.M.; DeForest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Outten, F.W.; Theil, E.C. Iron-based redox switches in biology. Antioxid. Redox Signal. 2009, 11, 1029–1046. [Google Scholar] [CrossRef]
- Asadishad, B.; Chahal, S.; Akbari, A.; Cianciarelli, V.; Azodi, M.; Ghoshal, S.; Tufenkji, N. Amendment of Agricultural Soil with Metal Nanoparticles: Effects on Soil Enzyme Activity and Microbial Community Composition. Environ. Sci. Technol. 2018, 52, 1908–1918. [Google Scholar] [CrossRef]
- Jośko, I.; Oleszczuk, P.; Futa, B. The effect of inorganic nanoparticles (ZnO, Cr2O3, CuO and Ni) and their bulk counterparts on enzyme activities in different soils. Geoderma 2014, 232–234, 528–537. [Google Scholar] [CrossRef]
- Kukreti, B.; Sharma, A.; Chaudhary, P.; Agri, U.; Maithani, D. Influence of nanosilicon dioxide along with bioinoculants on Zea mays and its rhizospheric soil. 3 Biotech 2020, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M. Surfactant assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem. Eng. J. 2014, 235, 100–108. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Briat, J.F.; Duc, C.; Ravet, K.; Gaymard, F. Ferritins and iron storage in plants. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 806–814. [Google Scholar] [CrossRef]
- Aguirre, G.; Pilon, M. Copper delivery to chloroplast proteins and its regulation. Front. Plant Sci. 2016, 6, 1250. [Google Scholar] [CrossRef]
- Jurkow, R.; Sękara, A.; Pokluda, R.; Smoleń, S.; Kalisz, A. Biochemical response of oakleaf lettuce seedlings to different concentrations of some metal(oid) oxide nanoparticles. Agronomy 2020, 10, 997. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.H.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy assessment of biosynthesized copper oxide nanoparticles (Cuo-nps) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.) plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).