Multiple Administration of Dexamethasone Possesses a Deferred Long-Term Effect to Glycosylated Components of Mouse Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Real-Time RT–PCR
2.3. Western-Blot
2.4. Alcian Blue Staining for a Total and Highly Sulfated GAG Content
2.5. Dot-Blot Analysis for HS and CS Content
2.6. Statistical Analysis
3. Results
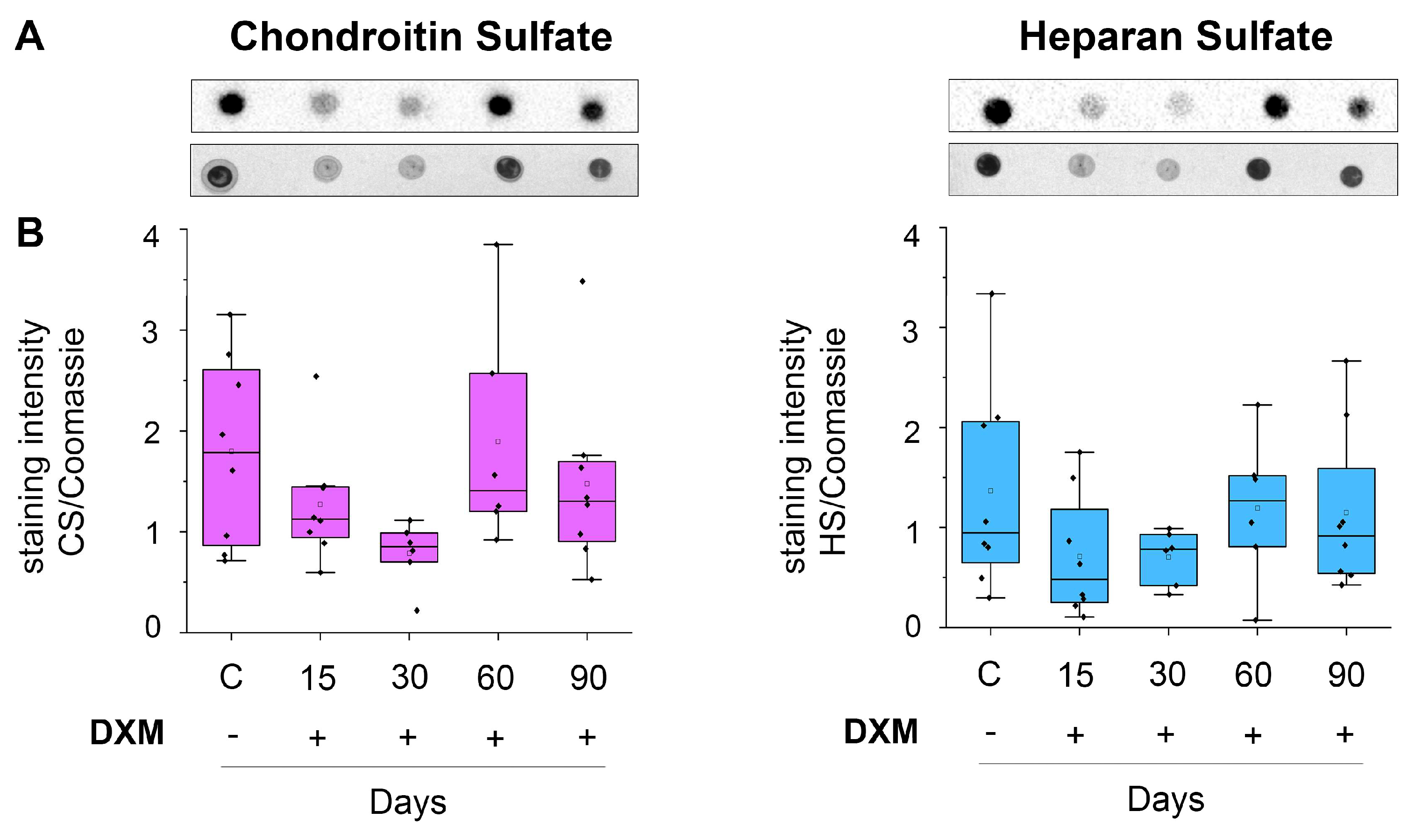
3.1. DXM Affects Content of Total and Sulfated GAGs in the Mouse Brain Tissue
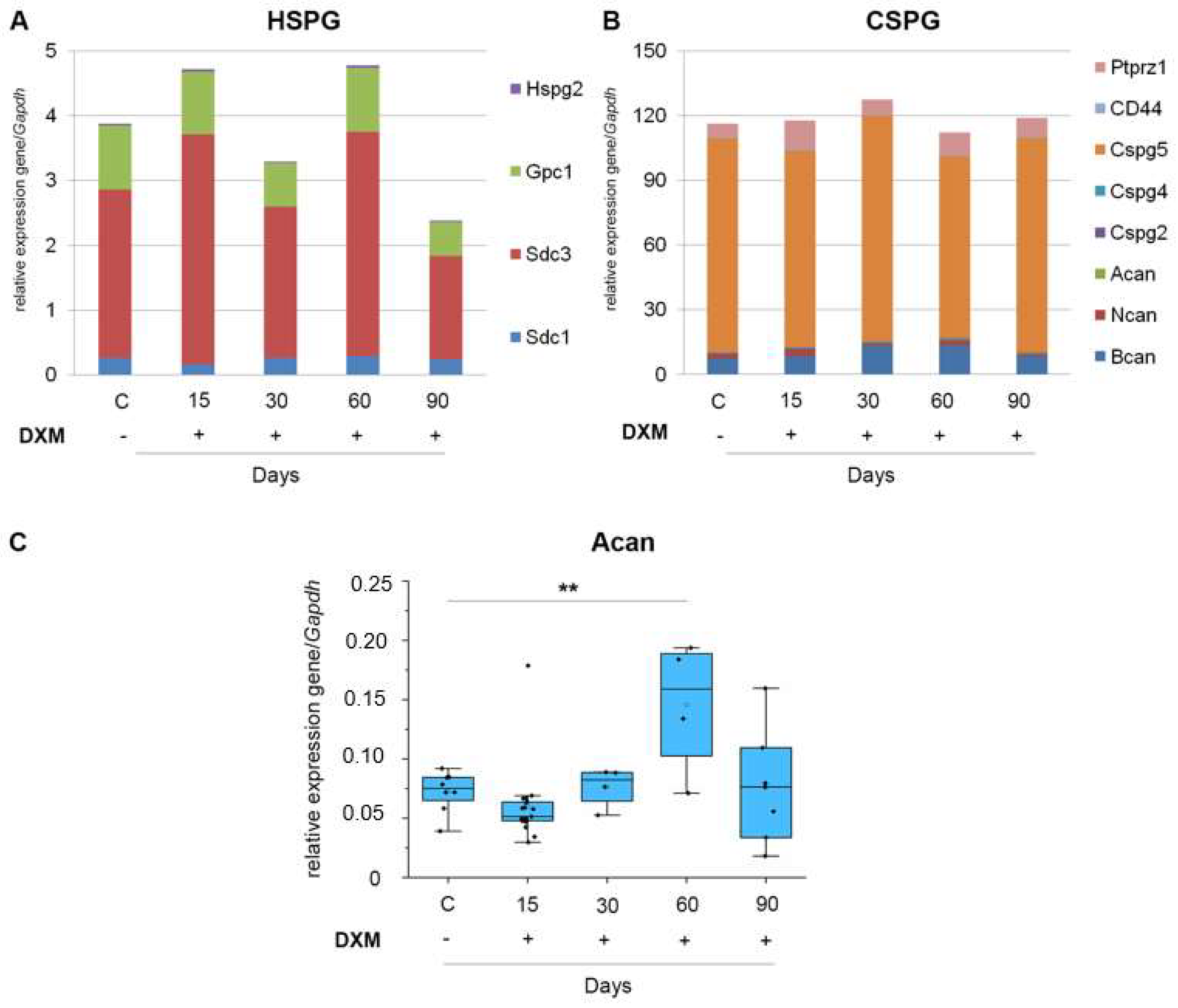
3.2. DXM Down-Regulates Transcriptional Activity of HS-Biosynthesis-Involved Genes
3.3. DXM Administration Does Not Affect the Expression of PG Core Protein-Coding Genes
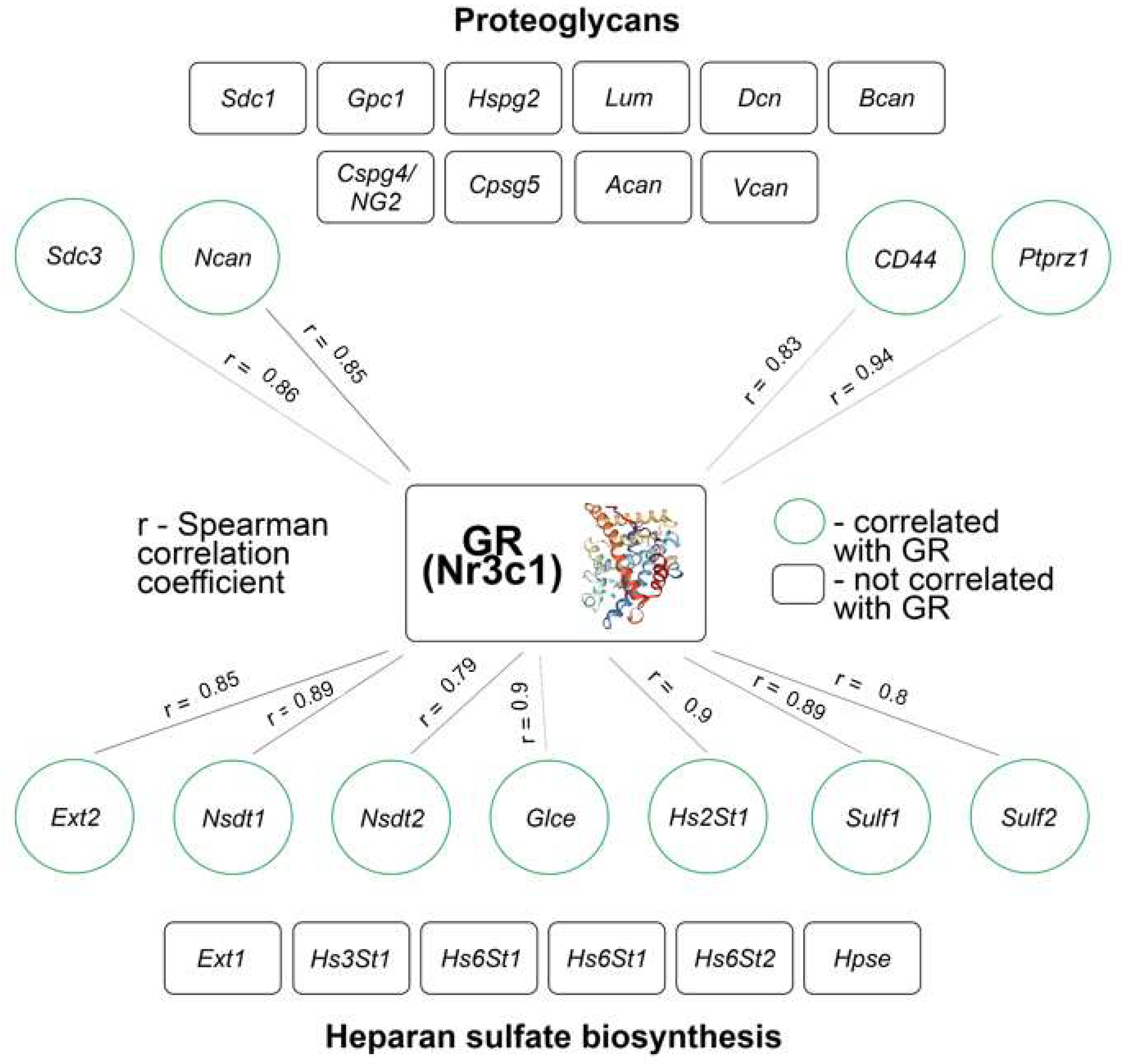
3.4. DXM Decreases GR mRNA Level of Expression in Mouse Brain Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, G.J.; Appleton, M.; Kipp, Z.A.; Loria, A.S.; Min, B.; Hinds, T.D.H., Jr. Glucocorticoids, their uses, sexual dimorphisms, and diseases: New concepts, mechanisms, and discoveries. Physiol. Rev. 2024, 104, 473–532. [Google Scholar] [CrossRef] [PubMed]
- Khadka, S.; Druffner, S.R.; Duncan, B.C.; Busada, J.T. Glucocorticoid regulation of cancer development and progression. Front. Endocrinol. 2023, 18, 1161768. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.T.; Wang, L.H. New dimensions of glucocorticoids in cancer treatment. Steroids 2016, 111, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, J.G.; Lombardi, M.; Seropian, I.M.; Raleigh, J.M.V.; Vergallo, R.; Larribau, M.; Agatiello, C.R.; Trani, C.; Burzotta, F. Chronic systemic glucocorticoid therapy is associated with increased risk of major vascular complications and cardiac tamponade after transcatheter aortic valve implantation: A systemic review and meta-analysis. Minerva Cardiol. Angiol. 2023, 72, 284–291. [Google Scholar] [CrossRef]
- Chacón, A.G.M.; Wang, C.; Waqar, D.; Syeda, S.A.; Kumar, R.; Meghana, D.R. Long-term usage of oral glucocorticoids leading to adrenal insufficiency: A comprehensive review of the literature. Cureus 2017, 15, e38948. [Google Scholar] [CrossRef]
- Hashim, Z.; Nath, A.; Khan, A.; Gupta, M.; Kumar, A.; Chatterjee, R.; Dhiman, R.K.; Hoenigl, M.; Tripathy, N.K. Effect of glucocorticoids on the development of COVID-19-associated pulmonary aspergillosis: A meta-analysis of 21 studies and 5174 patients. Mycoses 2023, 66, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Pofi, R.; Caratti, G.; Ray, D.W.; Tomlinson, J.W. Treating the side effects of exogenous glucocorticoids; can we separate the good from the bad? Endocr. Rev. 2023, 44, 975–1011. [Google Scholar] [CrossRef] [PubMed]
- Alcubierre, D.D.; Ferrari, D.; Mauro, G.; Isidori, A.M.; Tomlinson, J.W.; Pofi, R. Glucocorticoids and cognitive function: A walkthrough in endogenous and exogenous alterations. J. Endocrinol. Investig. 2023, 46, 1961–1982. [Google Scholar] [CrossRef]
- Koning, A.C.A.M.; Habets, P.C.; Bogaards, M.; Kroon, J.; van Santen, H.M.; de Bont, J.M.; Meijer, O.C. Mineralcorticoid receptor status in the human brain after dexamethasone treatment: A single case study. Endocr. Connect. 2022, 11, e210425. [Google Scholar] [CrossRef]
- Srinivasan, M.; Lahiri, D.K. Glucocorticoid-Induced Leucine Zipper in Central Nervous System Health and Disease. Mol. Neurobiol. 2017, 54, 8063–8070. [Google Scholar] [CrossRef]
- Nafe, R.; Hattingen, E. The spectrum of molecular pathways in gliomas-an up-to-date review. Biomedicines 2023, 11, 2281. [Google Scholar] [CrossRef]
- Madalena, K.M.; Lerch, J.K. The Effect of Glucocorticoid and Glucocorticoid Receptor Interactions on Brain, Spinal Cord, and Glial Cell Plasticity. Neural Plast. 2017, 2017, 8640970. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, D.; Chen, Y.; Cao, Z.; Fan, Z. Dexamethasone protects against arsanilic acid-induced rat vestibular dysfunction through the BDNF and JNK ½ signaling pathways. Mol. Med. Rep. 2019, 19, 1781–1790. [Google Scholar] [CrossRef]
- Andersen, C. The effect of glucocorticoids in the normal cerebral hemisphere of brain tumour patients. Acta Neurol. Scand. 1998, 98, 433–438. [Google Scholar] [CrossRef]
- Poole, J.J.A.; Mostaço-Guidolin, L.B. Optical microscopy and the extracellular matrix structure: A review. Cells 2021, 10, 1760. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, C.; Hrabětová, S. Brain extracellular space: The final frontier of neuroscience. Biophys. J. 2017, 113, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K. Extracellular matrix: Key structural and functional meshwork in health and disease. FEBS J. 2019, 286, 2826–2829. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Pipergkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Da Costa, D.S.; Reis, R.L.; Pashkuleva, I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu. Rev. Biomed. Eng. 2017, 19, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Strokotova, A.V.; Grigorieva, E.V. Glucocorticoid effects on proteoglycans and glycosaminoglycans. Int. J. Mol. Sci. 2022, 23, 15678. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Lee, Y.H.; Tsai, S.Y.; Hsu, C.Y.; Sun, Y.Y.; Yang, L.Y.; Tsai, S.H.; Yang, W.C.V. Methylprednisolone inhibits the expression of glial fibrillary acidic protein and chondroitin sulfate proteoglycans in reactivated astrocytes. Glia 2008, 56, 1390–1400. [Google Scholar] [CrossRef]
- Zhong, Y.; Bellamkonda, R.V. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007, 1148, 15–27. [Google Scholar] [CrossRef]
- Wei, F.; Song, J.; Zhang, C.; Lin, J.; Xue, R.; Shan, L.-D.; Gong, S.; Zhang, G.X.; Qin, Z.H.; Xu, G.Y. Chronic stress impairs the aquaporin-4-mediated glymphatic transport through glucocorticoid signaling. Psychopharmacology 2019, 236, 1367–1384. [Google Scholar] [CrossRef]
- Tsidulko, A.Y.; Bezier, C.; de La Bourdonnaye, G.; Suhovskih, A.V.; Pankova, T.M.; Kazanksaya, G.M.; Aidagulova, S.V.; Grigorieva, E.V. Conventional Anti-glioblastoma Chemotherapy Affects Proteoglycan Composition of Brain Extracellular Matrix in Rat Experimental Model In Vivo. Front. Pharmacol. 2018, 2, 1104. [Google Scholar] [CrossRef] [PubMed]
- Tsidulko, A.Y.; Shevelev, O.B.; Khotskina, A.S.; Kolpakova, M.A.; Suhovskih, A.V.; Kazanskaya, G.M.; Volkov, A.M.; Aidagulova, S.V.; Zavyalov, E.L.; Grigorieva, E.V. Chemotherapy-Induced Degradation of Glycosylated Components of the Brain Extracellular Matrix Promotes Glioblastoma Relapse Development in an Animal Model. Front. Oncol. 2021, 11, 713139. [Google Scholar] [CrossRef]
- Miyake, N.; Miyake, K.; Sakai, A.; Yamamoto, M.; Suzuki, H.; Shimada, T. Treatment of adult metachromatic leukodystrophy model mice using intrathecal administration of type 9 AAV vector encoding arylsulfatase A. Sci. Rep. 2021, 11, 20513. [Google Scholar] [CrossRef]
- Li, Y.; Shteyman, D.B.; Hachem, Z.; Ulay, A.A.; Fan, J.; Fu, B.M. Heparan Sulfate Modulation Affects Breast Cancer Cell Adhesion and Transmigration across In Vitro Blood-Brain Barrier. Cells 2024, 13, 190. [Google Scholar] [CrossRef]
- de Palma, L.; Marinelli, M.; Memè, L.; Pavan, M. Immunohistochemistry of the enthesis organ of the human Achilles tendon. Foot Ankle Int. 2004, 25, 414–418. [Google Scholar] [CrossRef]
- Aladev, S.D.; Sokolov, D.K.; Strokotova, A.V.; Kazanskaya, G.M.; Volkov, A.M.; Politko, M.O.; Shahmuradova, A.I.; Kliver, E.E.; Tsidulko, A.Y.; Aidagulova, S.V.; et al. Dexamethasone effects on the expression and content of glycosylated components of mouse brain tissue. Adv. Mol. Oncol. 2023, 10, 25–39. (In Russia) [Google Scholar] [CrossRef]
- Sokolov, D.K.; Shevelev, O.B.; Khotskina, A.S.; Tsidulko, A.Y.; Strokotova, A.V.; Kazanskaya, G.M.; Volkov, A.M.; Kliver, E.E.; Aidagulova, S.V.; Zavyalov, E.L.; et al. Dexamethasone inhibits heparan sulfate biosynthetic system and decreases heparan sulfate content in orthotopic glioblastoma tumors in mice. Int. J. Mol. Sci. 2023, 24, 10243. [Google Scholar] [CrossRef] [PubMed]
- Tanic, N.; Perovic, M.; Mladenovic, A.; Ruzdijic, S.; Kanazir, S. Effects of aging, dietary restriction and glucocorticoid treatment on housekeeping gene expression in rat cortex and hippocampus-evaluation by real time RT-PCR. J. Mol. Neurosci. 2007, 32, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Dai, S.; Yi, Y.; Liu, Y.; Zhang, S.; Luo, M.; Wang, H.; Xu, D. The combination of hprt and gapdh is the best compound reference genes in the fetal rat hippocampus. Dev. Neurobiol. 2020, 80, 229–238. [Google Scholar] [CrossRef]
- Rathman, D.; Vanden Berghe, W.; Dejager, L.; Libert, C.; Tavernier, J.; Beck, I.M.; De Bosscher, K. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell. Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aladev, S.D.; Sokolov, D.K.; Strokotova, A.V.; Kazanskaya, G.M.; Volkov, A.M.; Aidagulova, S.V.; Grigorieva, E.V. Multiple Administration of Dexamethasone Possesses a Deferred Long-Term Effect to Glycosylated Components of Mouse Brain. Neurol. Int. 2024, 16, 790-803. https://doi.org/10.3390/neurolint16040058
Aladev SD, Sokolov DK, Strokotova AV, Kazanskaya GM, Volkov AM, Aidagulova SV, Grigorieva EV. Multiple Administration of Dexamethasone Possesses a Deferred Long-Term Effect to Glycosylated Components of Mouse Brain. Neurology International. 2024; 16(4):790-803. https://doi.org/10.3390/neurolint16040058
Chicago/Turabian StyleAladev, Stanislav D., Dmitry K. Sokolov, Anastasia V. Strokotova, Galina M. Kazanskaya, Alexander M. Volkov, Svetlana V. Aidagulova, and Elvira V. Grigorieva. 2024. "Multiple Administration of Dexamethasone Possesses a Deferred Long-Term Effect to Glycosylated Components of Mouse Brain" Neurology International 16, no. 4: 790-803. https://doi.org/10.3390/neurolint16040058
APA StyleAladev, S. D., Sokolov, D. K., Strokotova, A. V., Kazanskaya, G. M., Volkov, A. M., Aidagulova, S. V., & Grigorieva, E. V. (2024). Multiple Administration of Dexamethasone Possesses a Deferred Long-Term Effect to Glycosylated Components of Mouse Brain. Neurology International, 16(4), 790-803. https://doi.org/10.3390/neurolint16040058






