Astrocytoma Mimicking Herpetic Meningoencephalitis: The Role of Non-Invasive Multimodal Monitoring in Neurointensivism
Abstract
1. Introduction
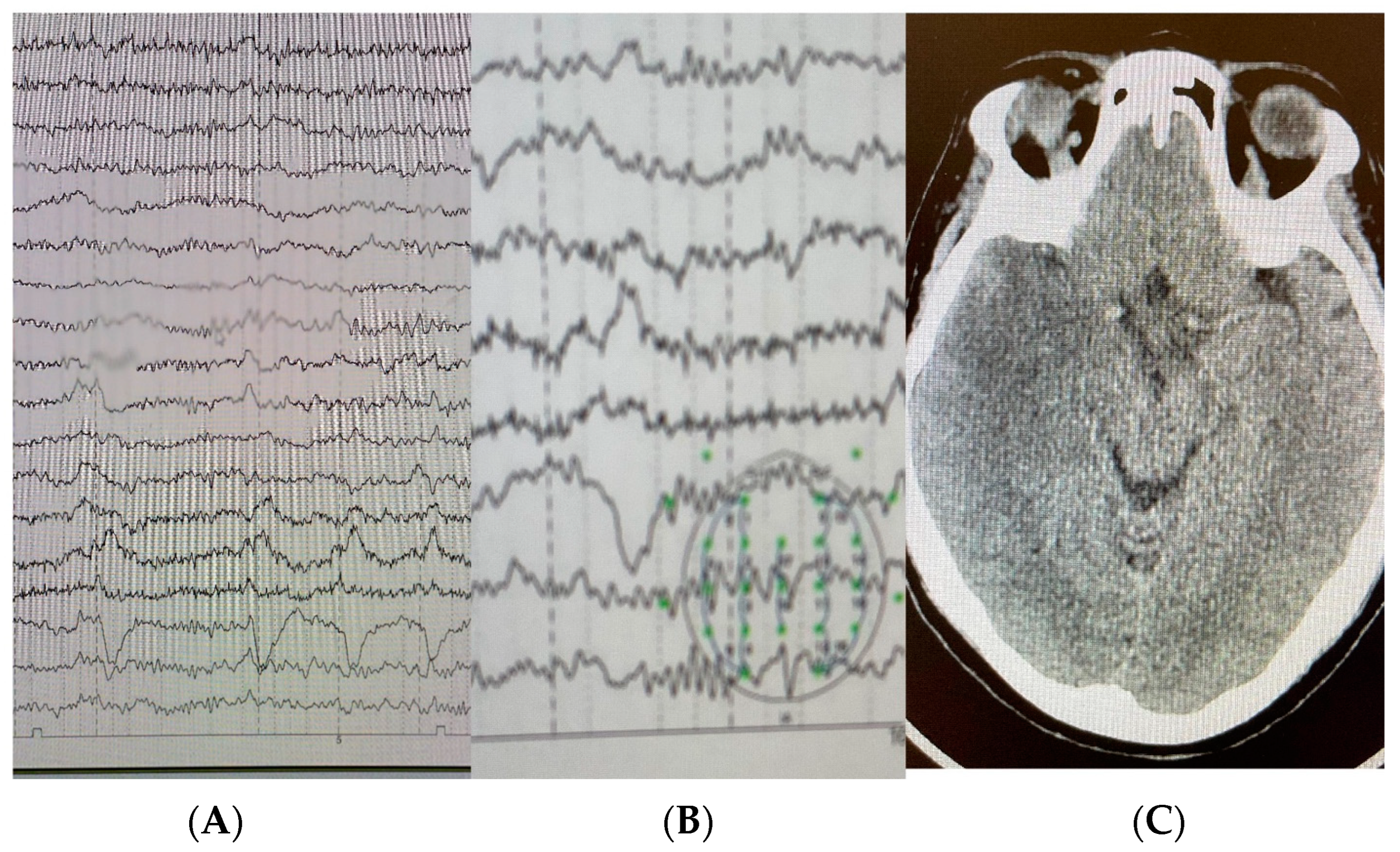
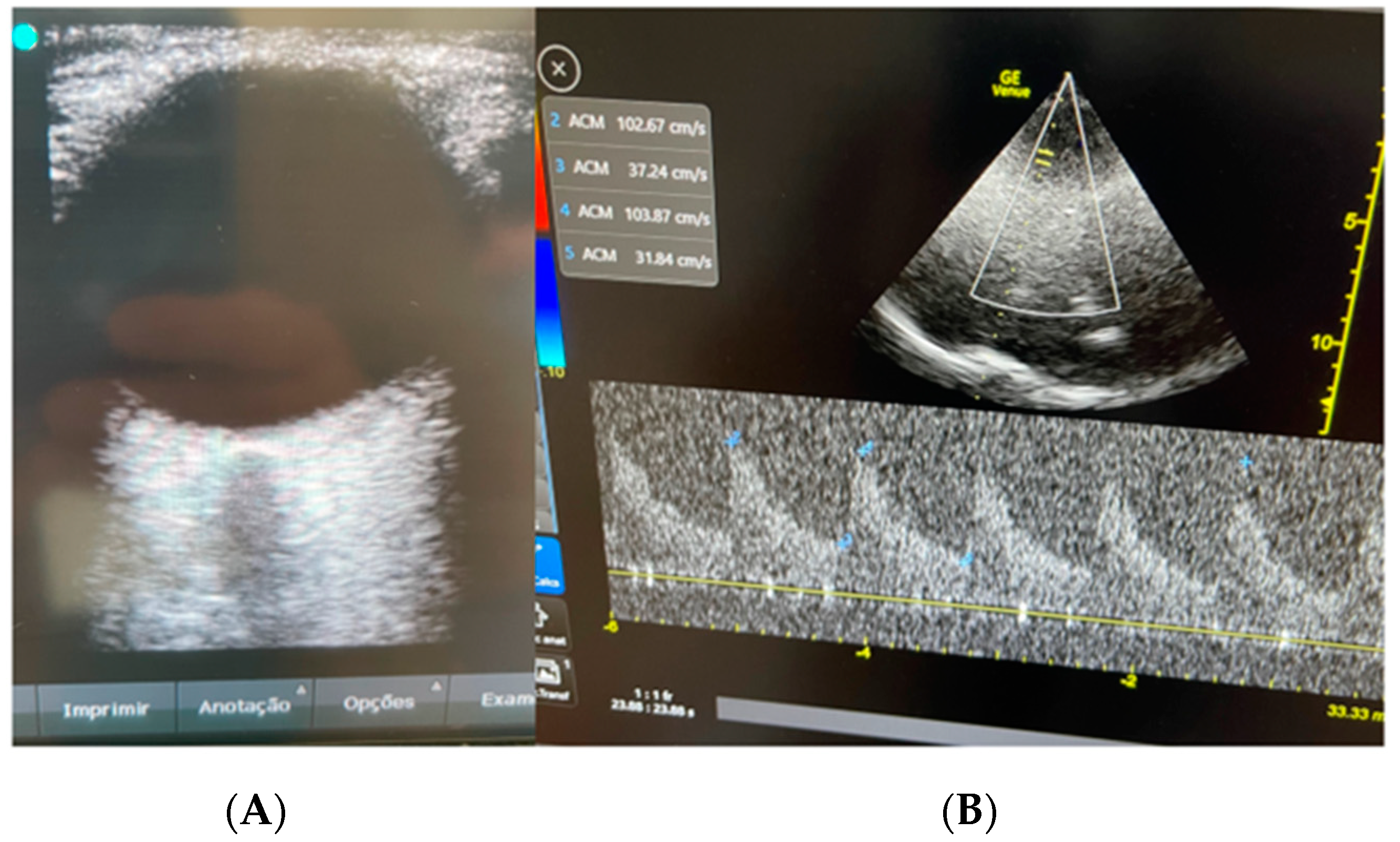
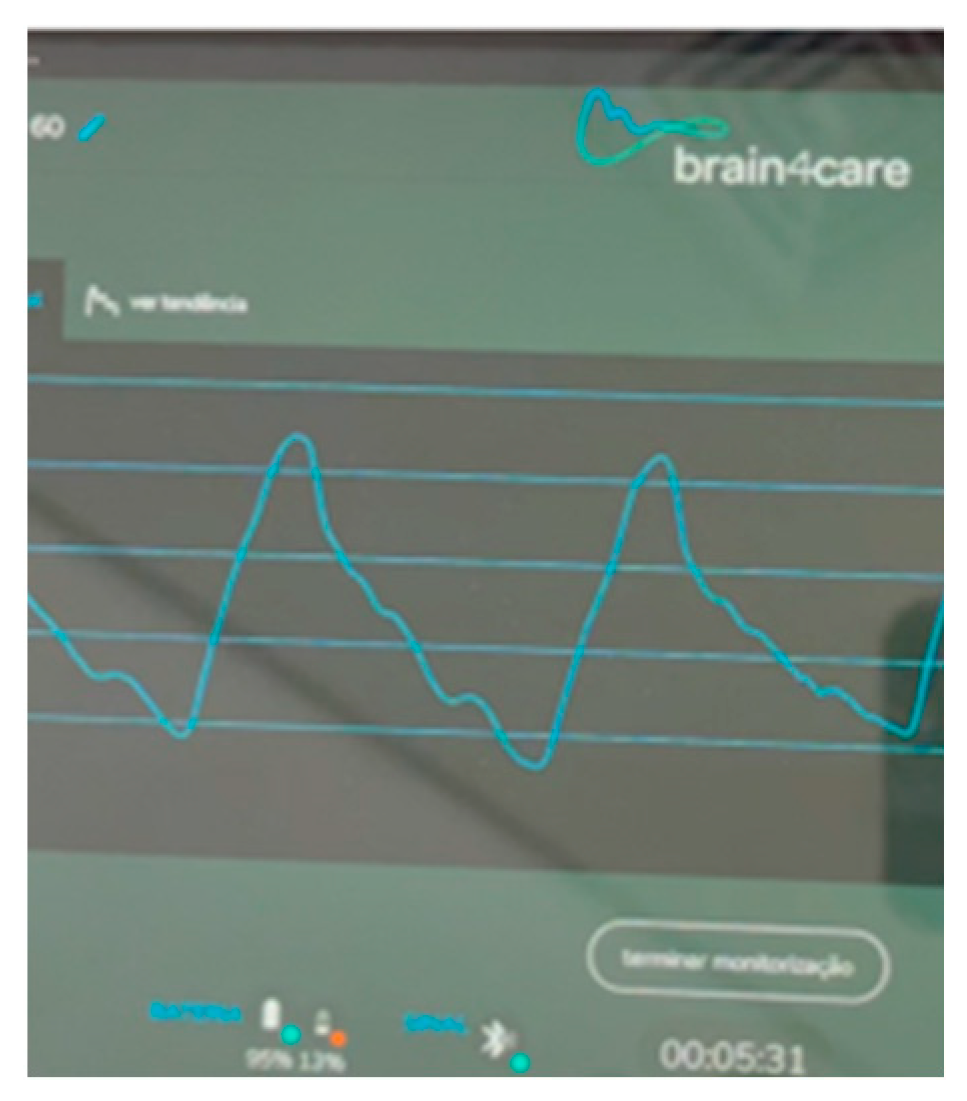
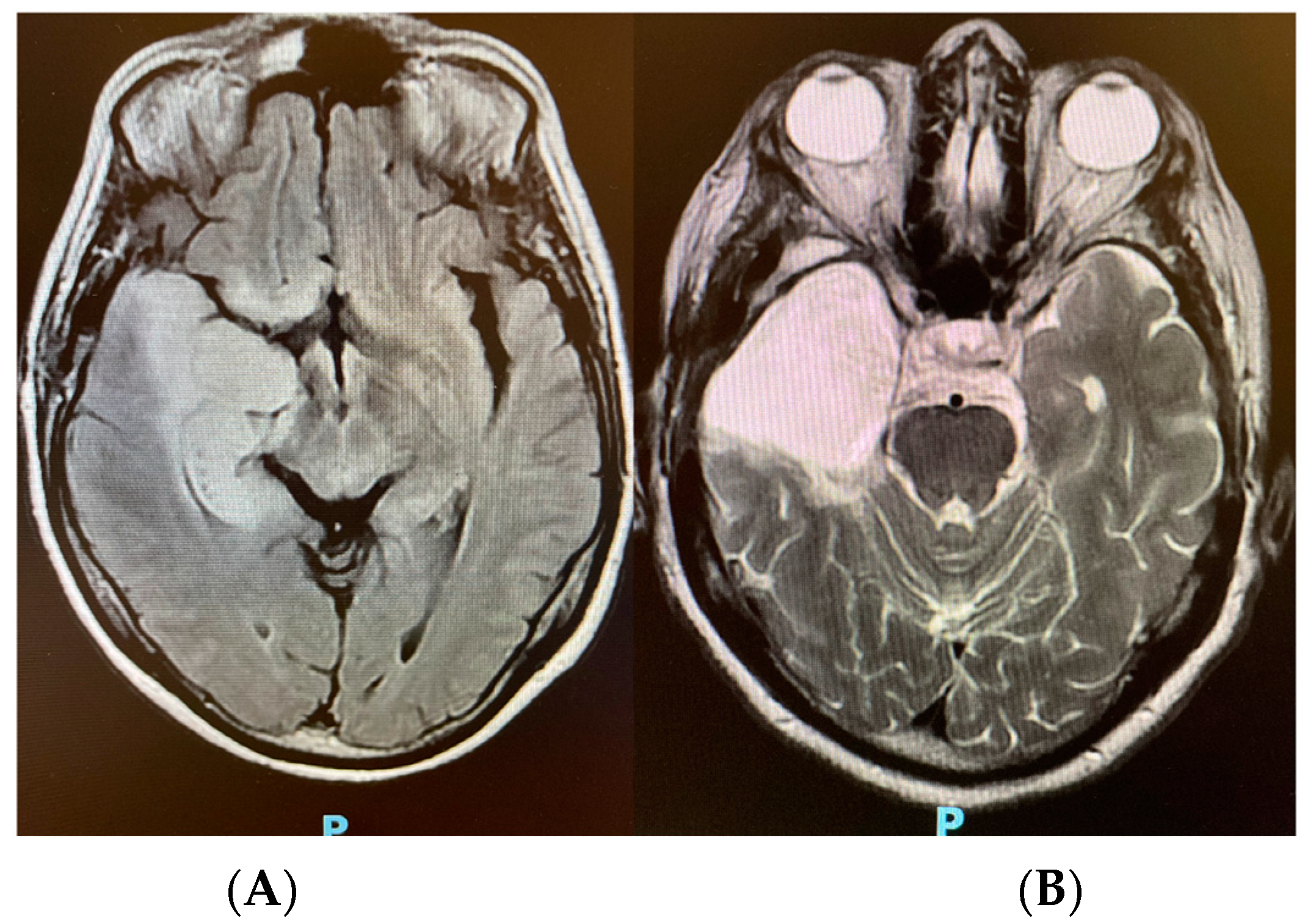
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramli, N.M.; Bae, Y.J. Structured Imaging Approach for Viral Encephalitis. Neuroimaging Clin. N. Am. 2023, 33, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, X.J.; Tu, D.Z.; Li, X.L.; Wei, B. Advances in viral encephalitis: Viral transmission, host immunity, and experimental animal models. Zool. Res. 2023, 44, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Rohani, H.; Arjmand, R.; Mozhgani, S.H.; Shafiee, A.; Amini, M.J.; Forghani-Ramandi, M.M. The Worldwide Prevalence of Herpes Simplex Virus Encephalitis and Meningitis: A Systematic Review and Meta-Analysis. Turk. Arch. Pediatr. 2023, 58, 580–587. [Google Scholar] [CrossRef]
- Thomas, D.L. 2021 updates to the World Health Organization classification of adult-type and pediatric-type diffuse gliomas: A clinical practice review. Chin. Clin. Oncol. 2023, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Chiang, G.C.; Kovanlikaya, I.; Choi, C.; Ramakrishna, R.; Magge, R.; Shungu, D.C. Magnetic Resonance Spectroscopy, Positron Emission Tomography and Radiogenomics—Relevance to Glioma. Front. Neurol. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Tadevosyan, A.; Kornbluth, J. Brain Herniation and Intracranial Hypertension. Neurol. Clin. 2021, 39, 293–318. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, S.; Li, B.; Zhang, J.; Ran, M.; Qi, B. A review of invasive intracranial pressure monitoring following surgery for hypertensive cerebral hemorrhage. Front. Neurol. 2023, 14, 1108722. [Google Scholar] [CrossRef]
- Cucciolini, G.; Motroni, V.; Czosnyka, M. Intracranial pressure for clinicians: It is not just a number. J. Anesth. Analg. Crit. Care 2023, 3, 31. [Google Scholar] [CrossRef]
- Vitiello, L.; Salerno, G.; De Bernardo, M.; D’Aniello, O.; Capasso, L.; Marotta, G.; Rosa, N. Ultrasound Detection of Intracranial Hypertension in Brain Injuries. Front. Med. 2022, 9, 870808. [Google Scholar] [CrossRef]
- Godoy, D.A.; Brasil, S.; Iaccarino, C.; Paiva, W.; Rubiano, A.M. The intracranial compartmental syndrome: A proposed model for acute brain injury monitoring and management. Crit. Care 2023, 27, 137. [Google Scholar] [CrossRef]
- Folchini, C.M.; Karuta, S.C.V.; Ricieri, M.C.; Motta, F.A.; Manços, G.R.; Frigieri, G.; Maeda, A.K. From disease to noninvasive intracranial monitoring. Arq. Neuropsiquiatr. 2022, 80, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Nag, D.S.; Sahu, S.; Swain, A.; Kant, S. Intracranial pressure monitoring: Gold standard and recent innovations. World J. Clin. Cases 2019, 7, 1535–1553. [Google Scholar] [CrossRef] [PubMed]
- Billington, M.; Kandalaft, O.R.; Aisiku, I.P. Adult Status Epilepticus: A Review of the Prehospital and Emergency Department Management. J. Clin. Med. 2016, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.I. Management of convulsive status epilepticus: Recent updates. Encephalitis 2023, 3, 39–43. [Google Scholar] [CrossRef]
- Peng, W.; Lu, L.; Wang, P.; Zhou, Y.; Xiong, W.; Li, J.; Tian, L.; Liu, J.; Tang, Y.; Wei, J.; et al. The initial treatment in convulsive status epilepticus in China: A multi-center observational study. Epilepsy Res. 2023, 197, 107245. [Google Scholar] [CrossRef]
- Petersen, P.T.; Bodilsen, J.; Jepsen, M.P.G.; Larsen, L.; Storgaard, M.; Hansen, B.R.; Helweg-Larsen, J.; Wiese, L.; Lüttichau, H.R.; Andersen, C.; et al. Clinical features and prognostic factors in adults with viral meningitis. Brain 2023, 146, 3816–3825. [Google Scholar] [CrossRef] [PubMed]
- Armangué, T.; Olivé-Cirera, G.; Martínez-Hernandez, E.; Rodes, M.; Peris-Sempere, V.; Guasp, M.; Ruiz, R.; Palou, E.; González, A.; Marcos, M.; et al. Neurologic complications in herpes simplex encephalitis: Clinical, immunological and genetic studies. Brain 2023, 146, 4306–4319. [Google Scholar] [CrossRef]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers 2015, 1, 15016. [Google Scholar] [CrossRef]
- Canac, N.; Jalaleddini, K.; Thorpe, S.G.; Thibeault, C.M.; Hamilton, R.B. Review: Pathophysiology of intracranial hypertension and noninvasive intracranial pressure monitoring. Fluids Barriers CNS 2020, 17, 40. [Google Scholar] [CrossRef]
- Ren, J.; Wu, X.; Huang, J.; Cao, X.; Yuan, Q.; Zhang, D.; Du, Z.; Zhong, P.; Hu, J. Intracranial Pressure Monitoring-Aided Management Associated with Favorable Outcomes in Patients with Hypertension-Related Spontaneous Intracerebral Hemorrhage. Transl. Stroke Res. 2020, 11, 1253–1263. [Google Scholar] [CrossRef]
- Choucha, A.; Boissonneau, S.; Beucler, N.; Graillon, T.; Ranque, S.; Bruder, N.; Fuentes, S.; Velly, L.; Dufour, H. Meningoencephalitis with refractory intracranial hypertension: Consider decompressive craniectomy. J. Neurosurg. Sci. 2023, 67, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choo, Y.H.; Jeong, H.; Kim, M.; Ha, E.J.; Oh, J.; Lee, S. Recent Updates on Controversies in Decompressive Craniectomy and Cranioplasty: Physiological Effect, Indication, Complication, and Management. Korean J. Neurotrauma. 2023, 19, 128–148. [Google Scholar] [CrossRef] [PubMed]
- Rasulo, F.A.; Bertuetti, R.; Robba, C.; Lusenti, F.; Cantoni, A.; Bernini, M.; Girardini, A.; Calza, S.; Piva, S.; Fagoni, N.; et al. The accuracy of transcranial Doppler in excluding intracranial hypertension following acute brain injury: A multicenter prospective pilot study. Crit. Care 2017, 21, 44. [Google Scholar] [CrossRef]
- Sriganesh, K.; Rao, G.S.; Smita, V. Early detection of severe intracranial hypertension by transcranial Doppler before the occurrence of clinical and radiologic manifestations. J. Neurosurg. Anesthesiol. 2011, 23, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Hayashi, T.; Anegawa, S.; Torigoe, R.; Nishio, N.; Moriyama, T.; Toda, K.; Udono, H. Transcranial Doppler sonography in acute intracranial hypertension model—Usefulness of pulsatility index. No Shinkei 1993, 45, 851–856. [Google Scholar]
- Pradeep, R.; Gupta, D.; Shetty, N.; Bhushan, A.K.; Haskar, K.; Gogineni, S.; Mehta, A.; Javali, M.; Acharya, P.T.; Srinivasa, R. Transcranial Doppler for Monitoring and Evaluation of Idiopathic Intracranial Hypertension. J. Neurosci. Rural Pract. 2020, 11, 309–314. [Google Scholar] [CrossRef]
- AIUM. Practice Parameter for the Performance of Transcranial Doppler Ultrasound. J. Ultrasound Med. 2023, 42, E36–E44. [Google Scholar] [CrossRef]
- Shahripour, R.B.; Azarpazhooh, M.R.; Akhuanzada, H.; Labin, E.; Borhani-Haghighi, A.; Agrawal, K.; Meyer, D.; Meyer, B.; Hemmen, T. Transcranial Doppler to evaluate postreperfusion therapy following acute ischemic stroke: A literature review. J. Neuroimaging 2021, 31, 849–857. [Google Scholar] [CrossRef]
- Dorn, A.Y.; Thorpe, S.G.; Canac, N.; Jalaleddini, K.; Hamilton, R.B. A Review of the use of Transcranial Doppler Waveform Morphology for Acute Stroke Assessment. J. Clin. Neurosci. 2020, 81, 346–352. [Google Scholar] [CrossRef]
- Steinmeier, R.; Laumer, R.; Bondar, I.; Priem, R.; Fahlbusch, R. Cerebral hemodynamics in subarachnoid hemorrhage evaluated by transcranial Doppler sonography. Part 2. Pulsatility indices: Normal reference values and characteristics in subarachnoid hemorrhage. Neurosurgery 1993, 33, 10–18, discussion 18–19. [Google Scholar] [CrossRef]
- Hylkema, C. Optic Nerve Sheath Diameter Ultrasound and the Diagnosis of Increased Intracranial Pressure. Crit. Care Nurs. Clin. N. Am. 2016, 28, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, L.; De Bernardo, M.; Capasso, L.; Cornetta, P.; Rosa, N. Optic Nerve Ultrasound Evaluation in Animals and Normal Subjects. Front. Med. 2021, 8, 797018. [Google Scholar] [CrossRef] [PubMed]
- Major, R.; Girling, S.; Boyle, A. Ultrasound measurement of optic nerve sheath diameter in patients with a clinical suspicion of raised intracranial pressure. Emerg. Med. J. 2011, 28, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.; Raghavendran, K.; Zhao, L.; Rajajee, V. A Prospective Study of Optic Nerve Ultrasound for the Detection of Elevated Intracranial Pressure in Severe Traumatic Brain Injury. Crit. Care Med. 2020, 48, e1278–e1285. [Google Scholar] [CrossRef]
- Aletreby, W.; Alharthy, A.; Brindley, P.G.; Kutsogiannis, D.J.; Faqihi, F.; Alzayer, W.; Balhahmar, A.; Soliman, I.; Hamido, H.; Alqahtani, S.A.; et al. Optic Nerve Sheath Diameter Ultrasound for Raised Intracranial Pressure: A Literature Review and Meta-analysis of its Diagnostic Accuracy. J. Ultrasound Med. 2022, 41, 585–595. [Google Scholar] [CrossRef]
- Brasil, S.; Frigieri, G.; Taccone, F.S.; Robba, C.; Solla, D.J.F.; de Carvalho Nogueira, R.; Yoshikawa, M.H.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.S. Noninvasive intracranial pressure waveforms for estimation of intracranial hypertension and outcome prediction in acute brain-injured patients. J. Clin. Monit. Comput. 2022, 37, 753–760. [Google Scholar] [CrossRef]
- Rabelo, N.N.; Silveira Chaves, P.H.; Gonçalves de Sena Barbosa, M.; Frigieri, G. Is It Possible to Monitor the Wave Form with Noninvasive Methods? World Neurosurg. 2021, 152, 231–232. [Google Scholar] [CrossRef]
- Brasil, S.; Solla, D.J.F.; Nogueira, R.C.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.D.S. A Novel Noninvasive Technique for Intracranial Pressure Waveform Monitoring in Critical Care. J. Pers. Med. 2021, 11, 1302. [Google Scholar] [CrossRef]
- Lin, E.S.; Poon, W.; Hutchinson, R.C.; Oh, T.E. Systems analysis applied to intracranial pressure waveforms and correlation with clinical status in head injured patients. Br. J. Anaesth. 1991, 66, 476–482. [Google Scholar] [CrossRef][Green Version]
- Frigieri, G.; Robba, C.; Machado, F.S.; Gomes, J.A.; Brasil, S. Application of non-invasive ICP waveform analysis in acute brain injury: Intracranial Compliance Scale. Intensive Care Med. Exp. 2023, 11, 5. [Google Scholar] [CrossRef]
- Fan, T.H.; Rosenthal, E.S. Physiological Monitoring in Patients with Acute Brain Injury: A Multimodal Approach. Crit. Care Clin. 2023, 39, 221–233. [Google Scholar] [CrossRef]
- Casault, C.; Couillard, P.; Kromm, J.; Rosenthal, E.; Kramer, A.; Brindley, P. Multimodal brain monitoring following traumatic brain injury: A primer for intensive care practitioners. J. Intensive Care Soc. 2022, 23, 191–202. [Google Scholar] [CrossRef]
- Topjian, A.A.; Zhang, B.; Xiao, R.; Fung, F.W.; Berg, R.A.; Graham, K.; Abend, N.S. Multimodal monitoring including early EEG improves stratification of brain injury severity after pediatric cardiac arrest. Resuscitation 2021, 167, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Rajajee, V.; Soroushmehr, R.; Williamson, C.A.; Najarian, K.; Ward, K.; Tiba, H. Transcranial Color-Coded Sonography with Angle Correction As a Screening Tool for Raised Intracranial Pressure. Crit. Care Explor. 2023, 5, e0953. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.I.; Arntfield, R.T. Point-of-care transcranial Doppler by intensivists. Crit. Ultrasound J. 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Montrief, T.; Alerhand, S.; Jewell, C.; Scott, J. Incorporation of Transcranial Doppler into the ED for the neurocritical care patient. Am. J. Emerg. Med. 2019, 37, 1144–1152. [Google Scholar] [CrossRef]
- Chan, S.T.; Evans, K.C.; Song, T.Y.; Selb, J.; van der Kouwe, A.; Rosen, B.R.; Zheng, Y.P.; Ahn, A.; Kwong, K.K. Cerebrovascular reactivity assessment with O2-CO2 exchange ratio under brief breath hold challenge. PLoS ONE 2020, 15, e0225915. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Santori, G.; Czosnyka, M.; Corradi, F.; Bragazzi, N.; Padayachy, L.; Taccone, F.S.; Citerio, G. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2018, 44, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Diedler, J.; Czosnyka, M. Merits and pitfalls of multimodality brain monitoring. Neurocrit. Care 2010, 12, 313–316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarwal, A.; Robba, C.; Venegas, C.; Ziai, W.; Czosnyka, M.; Sharma, D. Are We Ready for Clinical Therapy based on Cerebral Autoregulation? A Pro-con Debate. Neurocrit. Care 2023, 39, 269–283. [Google Scholar] [CrossRef]
| Date | CSF | ONSD | TCD | B4C |
|---|---|---|---|---|
| D 0 | Pan-herpes negative | |||
| Day 2 | 5.6 mm | MCA 59 cm/s; PI 1.2; RI 0.7 | P2 > P1 | |
| Day 2 | MCA 65 cm/s; PI 1.0; RI 0.5 | P1 > P2 | ||
| Day 5 | Pan-herpes negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flato, U.A.P.; Pereira, B.C.d.A.; Costa, F.A.; Vilela, M.C.; Frigieri, G.; Cavalcante, N.J.F.; Almeida, S.L.S.d. Astrocytoma Mimicking Herpetic Meningoencephalitis: The Role of Non-Invasive Multimodal Monitoring in Neurointensivism. Neurol. Int. 2023, 15, 1403-1410. https://doi.org/10.3390/neurolint15040090
Flato UAP, Pereira BCdA, Costa FA, Vilela MC, Frigieri G, Cavalcante NJF, Almeida SLSd. Astrocytoma Mimicking Herpetic Meningoencephalitis: The Role of Non-Invasive Multimodal Monitoring in Neurointensivism. Neurology International. 2023; 15(4):1403-1410. https://doi.org/10.3390/neurolint15040090
Chicago/Turabian StyleFlato, Uri Adrian Prync, Barbara Cristina de Abreu Pereira, Fernando Alvares Costa, Marcos Cairo Vilela, Gustavo Frigieri, Nilton José Fernandes Cavalcante, and Samantha Longhi Simões de Almeida. 2023. "Astrocytoma Mimicking Herpetic Meningoencephalitis: The Role of Non-Invasive Multimodal Monitoring in Neurointensivism" Neurology International 15, no. 4: 1403-1410. https://doi.org/10.3390/neurolint15040090
APA StyleFlato, U. A. P., Pereira, B. C. d. A., Costa, F. A., Vilela, M. C., Frigieri, G., Cavalcante, N. J. F., & Almeida, S. L. S. d. (2023). Astrocytoma Mimicking Herpetic Meningoencephalitis: The Role of Non-Invasive Multimodal Monitoring in Neurointensivism. Neurology International, 15(4), 1403-1410. https://doi.org/10.3390/neurolint15040090






