The Effect of Phytocannabinoids and Endocannabinoids on Nrf2 Activity in the Central Nervous System and Periphery
Abstract
1. Introduction
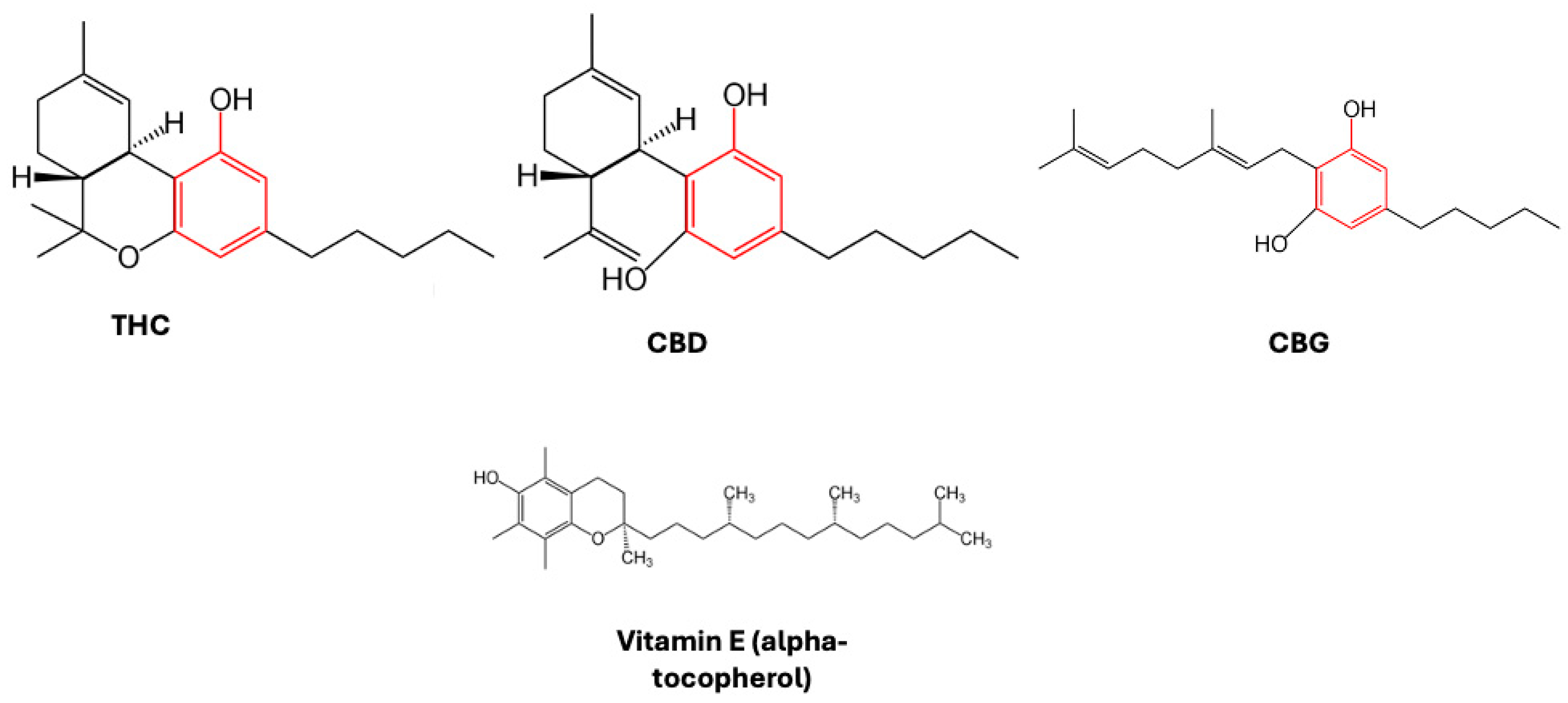
2. Cannabinoids and Endocannabinoids as Antioxidants
3. The Antioxidant Mechanisms
4. Nrf2 Pathway
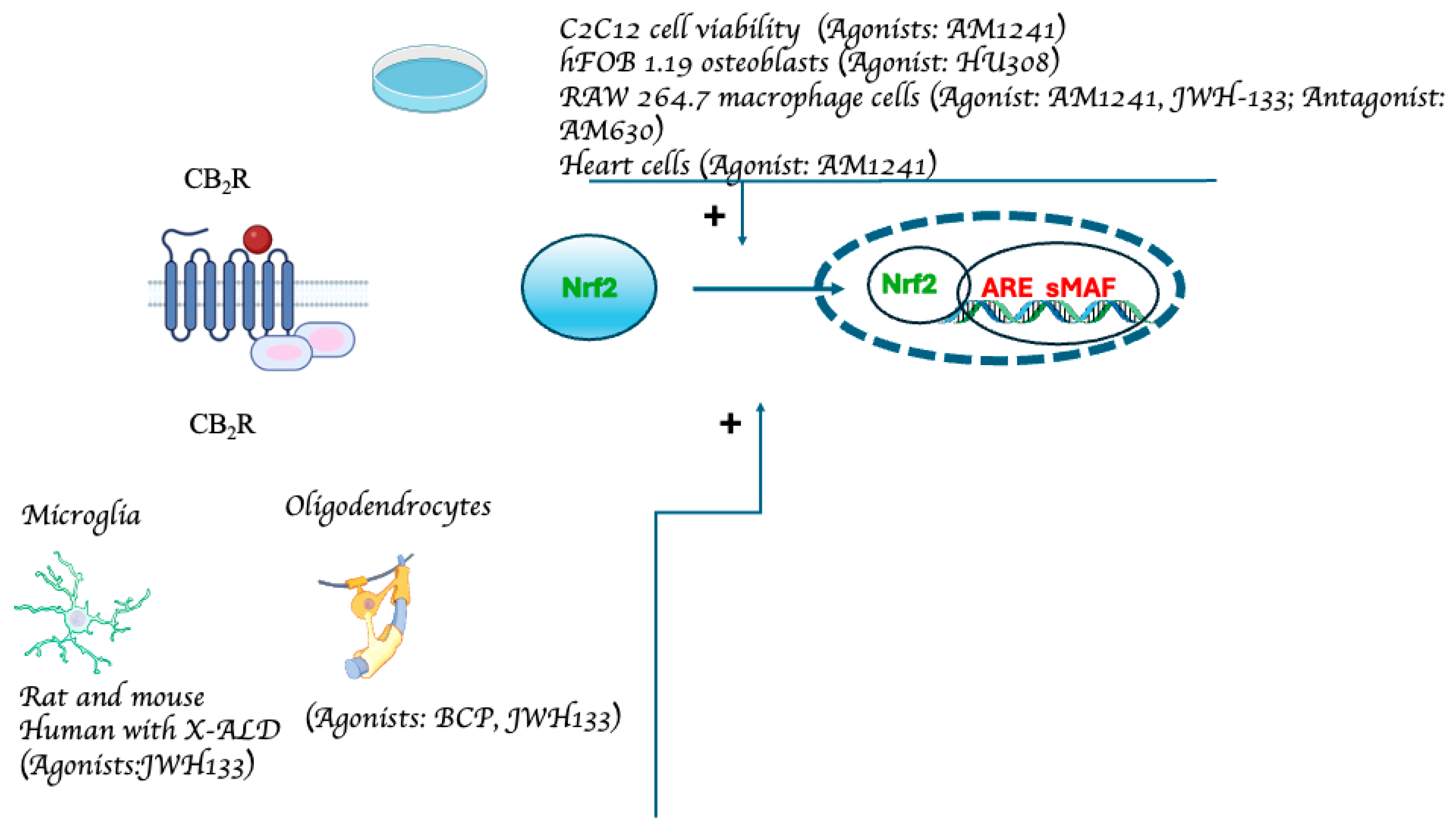
5. Effects of Phytocannabinoids and Endocannabinoids on Nrf2 Activity in the Central Nervous System (CNS)
6. The Effects of Phytocannabinoids and Endocannabinoids on Nrf2 Activity in the Periphery
7. Conclusions
8. Literature Search Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UN Commission Reclassifies Cannabis, yet Still Considered Harmful | UN News. Available online: https://news.un.org/en/story/2020/12/1079132 (accessed on 10 April 2024).
- U.S. Moves to Reclassify Cannabis as a Lower Risk Drug 2024. ScienceInsider, 1 May 2024.
- Procaccia, S.; Lewitus, G.M.; Lipson Feder, C.; Shapira, A.; Berman, P.; Meiri, D. Cannabis for Medical Use: Versatile Plant Rather Than a Single Drug. Front. Pharmacol. 2022, 13, 894960. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Köfalvi, A. Cannabis: A Treasure Trove or Pandora’s Box? Mini Rev. Med. Chem. 2017, 17, 1223–1291. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Marzo, V.D.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Voicu, V.; Brehar, F.-M.; Toader, C.; Covache-Busuioc, R.-A.; Corlatescu, A.D.; Bordeianu, A.; Costin, H.P.; Bratu, B.-G.; Glavan, L.-A.; Ciurea, A.V. Cannabinoids in Medicine: A Multifaceted Exploration of Types, Therapeutic Applications, and Emerging Opportunities in Neurodegenerative Diseases and Cancer Therapy. Biomolecules 2023, 13, 1388. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, Efficacy, and Mechanisms of Action of Cannabinoids in Neurological Disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Sam, A.H.; Salem, V.; Ghatei, M.A. Rimonabant: From RIO to Ban. J. Obes. 2011, 2011, 432607. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds to Cannabinoid Receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kerbrat, A.; Ferré, J.-C.; Fillatre, P.; Ronzière, T.; Vannier, S.; Carsin-Nicol, B.; Lavoué, S.; Vérin, M.; Gauvrit, J.-Y.; Le Tulzo, Y. Acute Neurologic Disorder from an Inhibitor of Fatty Acid Amide Hydrolase. N. Engl. J. Med. 2016, 375, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- van Esbroeck, A.C.M.; Janssen, A.P.A.; Cognetta, A.B.; Ogasawara, D.; Shpak, G.; van der Kroeg, M.; Kantae, V.; Baggelaar, M.P.; de Vrij, F.M.S.; Deng, H.; et al. Activity-Based Protein Profiling Reveals off-Target Proteins of the FAAH Inhibitor BIA 10-2474. Science 2017, 356, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, D.; Mattoteia, D.; Pollastro, F.; Negri, R.; Lopatriello, A.; Chianese, G.; Minassi, A.; Collado, J.A.; Munoz, E.; Taglialatela-Scafati, O. The Oxidation of Phytocannabinoids to Cannabinoquinoids. J. Nat. Prod. 2020, 83, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- UNODC—Bulletin on Narcotics—1962 Issue 3—004. Available online: https://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1962-01-01_3_page005.html (accessed on 27 June 2024).
- Borges, R.S.; Batista, J.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; Da Silva, A.B.F. Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as Antioxidant Agents and Their Intervention Abilities in Antioxidant Action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef]
- Borges, R.S.; da Silva, A.B.F. Chapter E12—Cannabidiol as an Antioxidant. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. e122–e130. ISBN 978-0-12-800756-3. [Google Scholar]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−) Δ9-Tetrahydrocannabinol Are Neuroprotective Antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Int. J. Mol. Sci. 2024, 25, 3360. [Google Scholar] [CrossRef]
- Suzen, S.; Tucci, P.; Profumo, E.; Buttari, B.; Saso, L. A Pivotal Role of Nrf2 in Neurodegenerative Disorders: A New Way for Therapeutic Strategies. Pharmaceuticals 2022, 15, 692. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Cuadrado, A. Structural and Functional Characterization of Nrf2 Degradation by Glycogen Synthase Kinase 3/β-TrCP. Free. Radic. Biol. Med. 2015, 88, 147–157. [Google Scholar] [CrossRef]
- Ma, Q.; He, X. Molecular Basis of Electrophilic and Oxidative Defense: Promises and Perils of Nrf2. Pharmacol. Rev. 2012, 64, 1055–1081. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Gordan, J.D.; Jin, J.; Harper, J.W.; Diehl, J.A. The Keap1-BTB Protein Is an Adaptor That Bridges Nrf2 to a Cul3-Based E3 Ligase: Oxidative Stress Sensing by a Cul3-Keap1 Ligase. Mol. Cell. Biol. 2004, 24, 8477–8486. [Google Scholar] [CrossRef]
- Furukawa, M.; Xiong, Y. BTB Protein Keap1 Targets Antioxidant Transcription Factor Nrf2 for Ubiquitination by the Cullin 3-Roc1 Ligase. Mol. Cell. Biol. 2005, 25, 162–171. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative Stress Sensor Keap1 Functions as an Adaptor for Cul3-Based E3 Ligase to Regulate Proteasomal Degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Zhang, D.D.; Lo, S.-C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 Is a Redox-Regulated Substrate Adaptor Protein for a Cul3-Dependent Ubiquitin Ligase Complex. Mol. Cell. Biol. 2004, 24, 10941–10953. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 Regulation of Cellular Defense Mechanisms against Electrophiles and Reactive Oxygen Species. Adv. Enzyme Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct Evidence That Sulfhydryl Groups of Keap1 Are the Sensors Regulating Induction of Phase 2 Enzymes That Protect against Carcinogens and Oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef]
- Tong, K.I.; Katoh, Y.; Kusunoki, H.; Itoh, K.; Tanaka, T.; Yamamoto, M. Keap1 Recruits Neh2 through Binding to ETGE and DLG Motifs: Characterization of the Two-Site Molecular Recognition Model. Mol. Cell. Biol. 2006, 26, 2887–2900. [Google Scholar] [CrossRef]
- Katsuoka, F.; Yamamoto, M. Small Maf Proteins (MafF, MafG, MafK): History, Structure and Function. Gene 2016, 586, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Sherratt, P.J.; Nioi, P.; Yang, C.S.; Pickett, C.B. Nrf2 Controls Constitutive and Inducible Expression of ARE-Driven Genes through a Dynamic Pathway Involving Nucleocytoplasmic Shuttling by Keap1. J. Biol. Chem. 2005, 280, 32485–32492. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef] [PubMed]
- Tucci, P.; Lattanzi, R.; Severini, C.; Saso, L. Nrf2 Pathway in Huntington’s Disease (HD): What Is Its Role? Int. J. Mol. Sci. 2022, 23, 15272. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A. NRF2 in Neurodegenerative Diseases. Curr. Opin. Toxicol. 2016, 2, 46–53. [Google Scholar] [CrossRef]
- Goncharov, I.; Weiner, L.; Vogel, Z. Delta9-Tetrahydrocannabinol Increases C6 Glioma Cell Death Produced by Oxidative Stress. Neuroscience 2005, 134, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Philippot, G.; Forsberg, E.; Tahan, C.; Viberg, H.; Fredriksson, R. A Single Δ9-Tetrahydrocannabinol (THC) Dose During Brain Development Affects Markers of Neurotrophy, Oxidative Stress, and Apoptosis. Front. Pharmacol. 2019, 10, 1156. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.; Leánez, S.; Pol, O. The Inhibitory Effects of Cobalt Protoporphyrin IX and Cannabinoid 2 Receptor Agonists in Type 2 Diabetic Mice. Int. J. Mol. Sci. 2017, 18, 2268. [Google Scholar] [CrossRef]
- Abioye, A.; Ayodele, O.; Marinkovic, A.; Patidar, R.; Akinwekomi, A.; Sanyaolu, A. Δ9-Tetrahydrocannabivarin (THCV): A Commentary on Potential Therapeutic Benefit for the Management of Obesity and Diabetes. J. Cannabis Res. 2020, 2, 6. [Google Scholar] [CrossRef]
- Kumar Kalvala, A.; Bagde, A.; Arthur, P.; Kumar Surapaneni, S.; Ramesh, N.; Nathani, A.; Singh, M. Role of Cannabidiol and Tetrahydrocannabivarin on Paclitaxel-Induced Neuropathic Pain in Rodents. Int. Immunopharmacol. 2022, 107, 108693. [Google Scholar] [CrossRef]
- Yi, M.; Cruz Cisneros, L.; Cho, E.J.; Alexander, M.; Kimelman, F.A.; Swentek, L.; Ferrey, A.; Tantisattamo, E.; Ichii, H. Nrf2 Pathway and Oxidative Stress as a Common Target for Treatment of Diabetes and Its Comorbidities. Int. J. Mol. Sci. 2024, 25, 821. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Celenza, G.; Petricca, S. Multi-Target Effects of ß-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants 2022, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.C.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; de Bem, A.F. β-Caryophyllene Protects the C6 Glioma Cells against Glutamate-Induced Excitotoxicity through the Nrf2 Pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chu, S.; Yang, Y.; Zhang, Z.; Pang, Z.; Chen, N. Neuroinflammatory In Vitro Cell Culture Models and the Potential Applications for Neurological Disorders. Front. Pharmacol. 2021, 12, 671734. [Google Scholar] [CrossRef] [PubMed]
- Horvath, R.J.; Nutile-McMenemy, N.; Alkaitis, M.S.; DeLeo, J.A. Differential Migration, LPS-Induced Cytokine, Chemokine, and NO Expression in Immortalized BV-2 and HAPI Cell Lines and Primary Microglial Cultures. J. Neurochem. 2008, 107, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; De Caris, M.G.; Maggi, E.; Armeli, F.; Coccurello, R.; Bisogno, T.; D’Erme, M.; Maccarrone, M.; Mancini, P.; Businaro, R. Fatty Acid Amide Hydrolase (FAAH) Inhibition Modulates Amyloid-Beta-Induced Microglia Polarization. Int. J. Mol. Sci. 2021, 22, 7711. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. Promising Neuroprotective Effects of β-Caryophyllene against LPS-Induced Oligodendrocyte Toxicity: A Mechanistic Study. Biochem. Pharmacol. 2019, 159, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. [Google Scholar] [CrossRef]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Juknat, A.; Gao, F.; Kaushansky, N.; Coppola, G.; Vogel, Z. Pathways and Gene Networks Mediating the Regulatory Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, in Autoimmune T Cells. J. Neuroinflammation 2016, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Coppola, G.; Geschwind, D.; Vogel, Z. Differential Transcriptional Profiles Mediated by Exposure to the Cannabinoids Cannabidiol and Δ9-Tetrahydrocannabinol in BV-2 Microglial Cells. Br. J. Pharmacol. 2012, 165, 2512–2528. [Google Scholar] [CrossRef]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Gao, F.; Coppola, G.; Geschwind, D.; Vogel, Z. Microarray and Pathway Analysis Reveal Distinct Mechanisms Underlying Cannabinoid-Mediated Modulation of LPS-Induced Activation of BV-2 Microglial Cells. PLoS ONE 2013, 8, e61462. [Google Scholar] [CrossRef] [PubMed]
- Juknat, A.; Gao, F.; Coppola, G.; Vogel, Z.; Kozela, E. miRNA Expression Profiles and Molecular Networks in Resting and LPS-Activated BV-2 Microglia-Effect of Cannabinoids. PLoS ONE 2019, 14, e0212039. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, M.; Ma, Z. Cannabinoid Type 2 Receptor Activation Inhibits MPP+-Induced M1 Differentiation of Microglia through Activating PI3K/Akt/Nrf2 Signal Pathway. Mol. Biol. Rep. 2023, 50, 4423–4433. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, J.; Goicoechea, L.; Planas-Serra, L.; Pastor, A.; Ruiz, M.; Calingasan, N.Y.; Guilera, C.; Aso, E.; Boada, J.; Pamplona, R.; et al. Activating Cannabinoid Receptor 2 Preserves Axonal Health through GSK-3β/NRF2 Axis in Adrenoleukodystrophy. Acta Neuropathol. 2022, 144, 241–258. [Google Scholar] [CrossRef]
- Tadijan, A.; Vlašić, I.; Vlainić, J.; Đikić, D.; Oršolić, N.; Jazvinšćak Jembrek, M. Intracellular Molecular Targets and Signaling Pathways Involved in Antioxidative and Neuroprotective Effects of Cannabinoids in Neurodegenerative Conditions. Antioxidants 2022, 11, 2049. [Google Scholar] [CrossRef]
- Galán-Ganga, M.; Del Río, R.; Jiménez-Moreno, N.; Díaz-Guerra, M.; Lastres-Becker, I. Cannabinoid CB2 Receptor Modulation by the Transcription Factor NRF2 Is Specific in Microglial Cells. Cell. Mol. Neurobiol. 2020, 40, 167–177. [Google Scholar] [CrossRef]
- Jin, M.C.; Yoo, J.-M.; Sok, D.-E.; Kim, M.R. Neuroprotective Effect of N-Acyl 5-Hydroxytryptamines on Glutamate-Induced Cytotoxicity in HT-22 Cells. Neurochem. Res. 2014, 39, 2440–2451. [Google Scholar] [CrossRef]
- Elmazoglu, Z.; Rangel-López, E.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Túnez, I.; Aschner, M.; Santamaría, A.; Karasu, Ç. Cannabinoid-Profiled Agents Improve Cell Survival via Reduction of Oxidative Stress and Inflammation, and Nrf2 Activation in a Toxic Model Combining hyperglycemia+Aβ1-42 Peptide in Rat Hippocampal Neurons. Neurochem. Int. 2020, 140, 104817. [Google Scholar] [CrossRef]
- Kuret, T.; Kreft, M.E.; Romih, R.; Veranič, P. Cannabidiol as a Promising Therapeutic Option in IC/BPS: In Vitro Evaluation of Its Protective Effects against Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 5055. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.; Zhang, X.; Zhang, H.; Liu, Y.; Wang, C.; Miao, L.; Li, Y.; Huang, Y.; Teng, H.; et al. Cannabidiol Protects the Liver from α-Amanitin-Induced Apoptosis and Oxidative Stress through the Regulation of Nrf2. Food Chem. Toxicol. 2023, 182, 114196. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xuan, Y.; Zhu, B.; Wang, X.; Tian, X.; Zhao, L.; Wang, Y.; Jiang, X.; Wen, N. Protective Effects of Cannabidiol on Chemotherapy-Induced Oral Mucositis via the Nrf2/Keap1/ARE Signaling Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 4619760. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol Induces Antioxidant Pathways in Keratinocytes by Targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef]
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A Review of Its Therapeutic Potentials, Advances in Its Nanodelivery, Recent Patents, and Clinical Trials. Phytother. Res. 2021, 35, 5440–5458. [Google Scholar] [CrossRef]
- Jang, Y.S.; Jeong, S.; Kim, A.-R.; Mok, B.R.; Son, S.J.; Ryu, J.-S.; Son, W.S.; Yun, S.K.; Kang, S.; Kim, H.J.; et al. Cannabidiol Mediates Epidermal Terminal Differentiation and Redox Homeostasis through Aryl Hydrocarbon Receptor (AhR)-Dependent Signaling. J. Dermatol. Sci. 2023, 109, 61–70. [Google Scholar] [CrossRef]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic Application of Cannabidiol on UVA and UVB Irradiated Rat Skin. A Proteomic Study. J. Pharm. Biomed. Anal. 2021, 192, 113656. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jia, H.; Yang, Q.; Shan, W.; Chen, X.; Huang, X.; Liu, T.; Sun, R. Specific Activation of CB2R Ameliorates Psoriasis-Like Skin Lesions by Inhibiting Inflammation and Oxidative Stress. Inflammation 2023, 46, 1255–1271. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, M.; Wang, L.; Yu, T.; Jiang, S.; Jiang, P.; Sun, Y.; Pi, J.; Zhao, R.; Guan, D. Activation of Cannabinoid Type 2 Receptor Protects Skeletal Muscle from Ischemia-Reperfusion Injury Partly via Nrf2 Signaling. Life Sci. 2019, 230, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, L.; Zhang, M.; Zhang, M.; Wang, C.; Zhao, R.; Guan, D. Cannabinoid Type 2 Receptor Manipulates Skeletal Muscle Regeneration Partly by Regulating Macrophage M1/M2 Polarization in IR Injury in Mice. Life Sci. 2020, 256, 117989. [Google Scholar] [CrossRef]
- Xu, A.; Yang, Y.; Shao, Y.; Wu, M.; Sun, Y. Activation of Cannabinoid Receptor Type 2-Induced Osteogenic Differentiation Involves Autophagy Induction and P62-Mediated Nrf2 Deactivation. Cell Commun. Signal. 2020, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y. Nrf2 Is Required for Suppressing Osteoclast RANKL-Induced Differentiation in RAW 264.7 Cells via Inactivating Cannabinoid Receptor Type 2 with AM630. Regen. Ther. 2020, 14, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, S.; Wang, Q.; Hu, W.; Wang, D.; Li, X.; Su, T.; Qin, X.; Zhang, X.; Ma, K.; et al. Effects of Cannabinoid Receptor Type 2 on Endogenous Myocardial Regeneration by Activating Cardiac Progenitor Cells in Mouse Infarcted Heart. Sci. China Life Sci. 2014, 57, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, D.; Tian, Z.; Gao, B.; Fan, M.; Li, C.; Li, X.; Wang, Y.; Ma, S.; Cao, F. Activation of Cannabinoid Receptor Type II by AM1241 Ameliorates Myocardial Fibrosis via Nrf2-Mediated Inhibition of TGF-Β1/Smad3 Pathway in Myocardial Infarction Mice. Cell. Physiol. Biochem. 2016, 39, 1521–1536. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ma, Y.; Liu, Y.; Wang, N.; Zhao, X.; Wen, D. CB2R Agonist JWH-133 Attenuates Chronic Inflammation by Restraining M1 Macrophage Polarization via Nrf2/HO-1 Pathway in Diet-Induced Obese Mice. Life Sci. 2020, 260, 118424. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.; Zhang, Z.; Zhao, M.; Tong, C.; Cong, P.; Mao, S.; Zhao, Y.; Hou, M.; Piao, Y.; et al. Protective Effect and Mechanism of Cannabidiol on Myocardial Injury in Exhaustive Exercise Training Mice. Chem. Biol. Interact. 2022, 365, 110079. [Google Scholar] [CrossRef]
- Li, H.; Wood, J.T.; Whitten, K.M.; Vadivel, S.K.; Seng, S.; Makriyannis, A.; Avraham, H.K. Inhibition of Fatty Acid Amide Hydrolase Activates Nrf2 Signalling and Induces Heme Oxygenase 1 Transcription in Breast Cancer Cells. Br. J. Pharmacol. 2013, 170, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, A.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds. Antioxidants 2023, 12, 2052. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Sirignano, C.; Benetti, E.; Marzaroli, V.; Collado, J.A.; de la Vega, L.; Appendino, G.; Muñoz, E.; Taglialatela-Scafati, O. A Nrf-2 Stimulatory Hydroxylated Cannabidiol Derivative from Hemp (Cannabis sativa). J. Nat. Prod. 2022, 85, 1089–1097. [Google Scholar] [CrossRef]
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-Related Factor-2-Dependent Genes Conferring Protection against Oxidative Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-Mediated Neuroprotection in the MPTP Mouse Model of Parkinson’s Disease: Critical Role for the Astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. The Molecular Activity of Cannabidiol in the Regulation of Nrf2 System Interacting with NF-κB Pathway under Oxidative Stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef]
| Cannabinoids | In Vitro Model | In Vivo Model | References |
|---|---|---|---|
| CBD | BV-2 cells (gene stimulation associated with Nrf2/Hmox1 axis and ARE-Nrf2/ATF4 system) | Mouse model of experimental autoimmune encephalomyelitis (increased the expression of Nrf2 target genes) | [56,57,58] |
| MOG35-55-specific T cell line (TMOG) (increased the expression of Nrf2 target genes) | [55,64] | ||
| HT-22 cells (activating the Nrf2-mediated antioxidant response) | |||
| THC | BV-2 cells (gene stimulation associated with Nrf2/Hmox1 axis and ARE-Nrf2/ATF4 system) | [41,57] | |
| Mice parietal cortex and Hippocampus (Nrf2/Keap1 ratio increase) | |||
| BCP | Oligodendrocytes (OLN-93) (Protective effect through the regulation of Nrf2/HO-1/antiodant axis mediated by CB2R) | [52] | |
| CBG | LPS-stimulated RAW 264.7 macrophages on NSC-34 motor neurons (neuroprotective effects increasing Nrf-2 levels) | [53,54] | |
| eCBs | Neurotoxic model of primary hippocampal neurons (neuroprotective effects increasing Nrf-2 levels) | [65] |
| Cannabinoids | In Vitro Model | In Vivo Model | References |
|---|---|---|---|
| CBD | Model of bladder pain syndrome and interstitial cystitis (Nrf2 enhancement) | Mice model of psoriasiform skin lesions (decreasing in plaque and epidermal thickness through CB2R activation and higher levels of Nrf2 and HO-1 protein expression | [66,67,68,69,71,72] |
| Mouse hepatocytes and L-02 cells exposed to α-amanitin (Nrf2 enhancement) | |||
| 5-fluorouracil-induced oral mucositis in mice and human oral keratinocytes (Nrf2 transcription increase; decrease in Keap1 activation) | |||
| Keratinocytes exposed to UVA and UVB radiation (activation of Nrf2 and suppression of NFκB transcription factors) | C57BL/6 male mice trained in rigorous exercise (protective effect against myocardial injury via Keap1/Nrf2/HO-1 pathway activation) | ||
| Primary and immortalized human keratinocytes (HaCaT cell line) (induction of expression of selected Nrf2 target genes) | |||
| eCBs | Breast cancer models (MCF-7 and MDA-MB-231 cell lines) (AEA and inhibition of FAAH activated Nrf2, resulting in HO-1 induction) | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, P.; Maccarrone, M.; Saso, L.; Tucci, P. The Effect of Phytocannabinoids and Endocannabinoids on Nrf2 Activity in the Central Nervous System and Periphery. Neurol. Int. 2024, 16, 776-789. https://doi.org/10.3390/neurolint16040057
Marini P, Maccarrone M, Saso L, Tucci P. The Effect of Phytocannabinoids and Endocannabinoids on Nrf2 Activity in the Central Nervous System and Periphery. Neurology International. 2024; 16(4):776-789. https://doi.org/10.3390/neurolint16040057
Chicago/Turabian StyleMarini, Pietro, Mauro Maccarrone, Luciano Saso, and Paolo Tucci. 2024. "The Effect of Phytocannabinoids and Endocannabinoids on Nrf2 Activity in the Central Nervous System and Periphery" Neurology International 16, no. 4: 776-789. https://doi.org/10.3390/neurolint16040057
APA StyleMarini, P., Maccarrone, M., Saso, L., & Tucci, P. (2024). The Effect of Phytocannabinoids and Endocannabinoids on Nrf2 Activity in the Central Nervous System and Periphery. Neurology International, 16(4), 776-789. https://doi.org/10.3390/neurolint16040057








