Abstract
The relationship between nuclear factor erythroid 2-related factor 2 (Nrf2) and phytocannabinoids/endocannabinoids (pCBs/eCBs) has been investigated in a variety of models of peripheral illnesses, with little clarification on their interaction within the central nervous system (CNS). In this context, evidence suggests that the Nrf2-pCBs/eCBS interaction is relevant in modulating peroxidation processes and the antioxidant system. Nrf2, one of the regulators of cellular redox homeostasis, appears to have a protective role toward damaging insults to neurons and glia by enhancing those genes involved in the regulation of homeostatic processes. Specifically in microglia and macroglia cells, Nrf2 can be activated, and its signaling pathway modulated, by both pCBs and eCBs. However, the precise effects of pCBs and eCBs on the Nrf2 signaling pathway are not completely elucidated yet, making their potential clinical employment still not fully understood.
1. Introduction
The scientific community and general public are still debating whether the well-known beneficial effects of cannabis-based drugs could justify their clinical employment, despite the potential risks associated with the use of this class of compounds. Recently, after a revision of the scientific literature conducted by the Expert Committee on Drug Dependence (ECDD), an independent scientific advisory board to the World Health Organization (WHO), the positive effects of cannabis were clarified in comparison to the negative ones. As a result, the Commission on Narcotic Drugs (CND) of the United Nations removed cannabis and its derivatives from Schedule IV of the 1961 Single Convention on Narcotic Drugs in 2020 [1] and reclassified these substances as Schedule III. Although still considering cannabis and its derivates potentially harmful, cannabis’ medicinal and therapeutic potentials have been recognized. In 2024, the United States Drug Enforcement Administration has consented to reclassify cannabis as a lower risk drug [2]. The trichomes, specialized inflorescence structures of female cannabis plant, produce a family of terpenophenolic substances, called phytocannabinoids (pCBs), representing more than 110 of the nearly 500 compounds (terpenoids, flavonoids, sterols, and other non-pCB substances) found in all cannabis subspecies (Cannabis sativa, Cannabis indica, and Cannabis rudelalis) [3,4,5]. Among all pCBs, three molecules have garnered noteworthy attention: (-)-Δ9-tetrahydrocannabinol (THC), (-)-cannabidiol, (CBD) and cannabigerol (CBG). Initially, it was believed that THC’s effect, because of its lipophilic properties, was caused by a general disruption of cell membranes. Thereafter, it was proved that the pharmacological properties of THC were to be ascribed to its stereoselectivity, since synthetic (+)-Δ9-tetrahydrocannabinol enantiomer did not show the same effects, hence suggesting the existence of a putative receptor. Indeed, cannabinoid receptors 1 and 2 (CB1R and CB2R) were subsequently identified, and numerous ligands were synthesized, laying the foundations for extensive research on the potential use of cannabis-related substances in medicine [6]. Nowadays, many different CB1 and CB2 receptors ligands are available either directly extracted from the cannabis plant (pCBs) or manufactured in the laboratory (synthetic cannabinoids), with pharmacological characteristics such as agonists, partial agonists, antagonists, and inverse agonists [7]. Moreover, the molecular mechanism of action of pCBs on CB1R and CB2R, as well as the ability of some pCBs to bind non-CB1/CB2 receptors (i.e., GPR55, GPR18, 5-HT3, 5-HT1A, TRPV1, GPR119, GlyRs, and PPARs), have been extensively elucidated [6]. For example, THC behaves as an agonist while CBD as a negative allosteric modulator at CB1R [8]. The pCBs compounds are currently prescribed for the treatment of some pathological conditions. Sativex®, a 1:1 THC/CBD formulation available as mouth spray, has been approved for the treatment of multiple sclerosis symptoms, such as spasticity, neuropathic pain, and overactive bladder [9], while a CBD-based preparation has been approved for the treatment of the Lennox–Gastaut and Dravet epileptic syndromes, two types of childhood-onset epilepsies [10]. Furthermore, synthetic CB1R agonists are used in various medical conditions; for example, dronabinol and nabilone are prescribed to increase appetite or to reduce vomiting in patients receiving chemotherapy [9]. Unfortunately, a synthetic CB1R inverse agonist, Rimonabant, initially approved as an anti-obesity medication, was withdrawn globally in 2008 due to the serious psychiatric side effects reported [11]. The discovery of CB1R and CB2R prompted intense research into the identification of potential endogenous receptor ligands, with anandamide (N-arachidonoylethanolamine; AEA) [12] and 2-arachidonoylglycerol (2-AG) being identified [13,14] and collectively termed as endocannabinoids (eCBs). Subsequently, the enzymes responsible for eCBs synthesis and degradation were also discovered, leading to the complete identification and description of the termed endocannabinoid system. The possibility to modulate eCBs production by targeting metabolic enzymes has represented another potential clinical application. The inhibition of the fatty acid amide hydrolase (FAAH), the enzyme responsible for AEA degradation, was initially considered as beneficial for those conditions where reduced levels of eCBs had impairing effects. Although several studies demonstrated the potential benefits of eCB modulation using FAAH inhibitors (i.e., PF04457845, a highly selective and clinically tested FAAH inhibitor), the clinical application of such inhibitors was shelved following the tragic phase I clinical trial in which the purported FAAH inhibitor BIA 10-2474 was tested. The drug caused severe neurologic side effects leading to the death of a healthy volunteer [15]. Further investigations clearly demonstrated how the drug was able to inhibit not only FAAH, but also several other lipases, producing substantial alterations of lipid networks in human cortical neurons, highlighting how promiscuous lipase inhibitors, rather than authentic FAAH inhibitors, may cause severe metabolic dysregulation and neurotoxicity [16].
2. Cannabinoids and Endocannabinoids as Antioxidants
By looking at the chemical structures of THC, CBD, and other pCBs, it is evident that they all contain a phenolic group and double bonds (Figure 1).
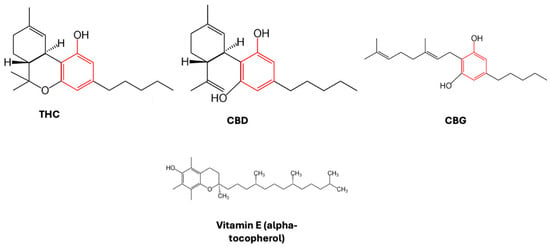
Figure 1.
Chemical structure of pCBs reported. The phenolic group is highlighted in red. As a comparison, the structure of a traditional antioxidant, vitamin E (alpha-tocopherol), is also shown.
The phenolic group is prone to oxidation, as proven by a reddish-purple hue that appeared when methanolic KOH was applied to hashish fiber, with hydroxyquinone (cannabidiolquinone) being produced, for example, by the aerobic oxidation of cannabidiol [17]. Cannabidiolquinone’s distinct reddish color is a result of conjugated double bonds in its molecular structure, with delocalized electrons produced by alternating single and double bonds able to absorb visible light. When it comes to CBD-chinol, the conjugated double bond arrangement causes light to be absorbed in the blue-green portion of the spectrum; therefore, red light is reflected, giving the characteristic coloration (reddish-purple) of hashish fibers. In laboratory tests, the color shift of CBD to its oxidized form, CBD-chinol, is a visual indicator for the presence of cannabinoids in the specimens and that oxidation processes are taking place [18]. In this context, two mechanisms have been proposed to be responsible for the antioxidant effect of CBD. In the first one, an electron is transferred from the phenolic group of CBD to a free radical, whereas in the second one, a hydrogen atom is removed by a free radical from CBD. In both cases, to avoid chain radical reactions, the produced radicals need to be stable [19]. These chemical characteristics of cannabinoids make these compounds good antioxidants, able to scavenge free radicals, to protect against oxidation processes, and to reduce metal ions. Although the antioxidant activity is not the same for each cannabinoid compound, the activity is generally comparable to that of vitamin E [20,21]. In particular, since both THC- and CBD-cation-free radicals exhibit a number of resonance structures, with unpaired electrons primarily distributed on both the ether and alkyl moieties, as well as on the benzene ring, these compounds may potentially have antioxidant properties [19,21]. Indeed, in a landmark study conducted in rat cortical neurons, both CBD and THC were able to decrease glutamate toxicity and reactive oxygen species (ROS)-induced cell death [22]. These effects were proposed to be CBR-independent, since the neuroprotection caused by both compounds was still noticeable when CB1R were antagonized.
3. The Antioxidant Mechanisms
The role of pro-oxidants in the etiopathogenesis and development of various diseases has triggered an interest toward the antioxidant properties of pCBs. Generally, cellular metabolized ROS species are classified as pro-oxidants with a bell-shaped concentration profile. At lower concentrations, they maintain physiological cell processes, whereas at higher concentrations they cause harmful alterations to DNA, lipids, and proteins (for a detailed review, see [23]).
The transition from a lower to a higher concentration of cellular metabolized ROS species results in “oxidative stress”, a well-known factor involved in a variety of pathological conditions (i.e., neurological disorders such as Parkinson’s disease, Alzheimer’s disease, motor neuron disease [24]), with hydrogen peroxide, hydroxyl radicals, peroxyl radicals, hydroperoxyl radicals, and hypochlorous acid representing the primary endogenous oxidant species mainly contributing to ROS. On the other hand, the negative effects that ROS have on cellular metabolism are counteracted by an endogenous and integrated intracellular antioxidant mechanism composed of enzymatic and non-enzymatic antioxidants systems [25].
Specifically, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GTPx), thioredoxin (TRX), peroxidase, peroxiredoxin (PRX), NAD(P)H dehydrogenase (quinone)-1 (NQO-1), and heme oxygenase-1 (HO-1) are all examples of enzymatic systems, while β-carotene, vitamins A, E and C, as well as glutathione (GSH), are all examples of non-enzymatic systems. In this context, it is important to note that the genes coding for the enzymes responsible for the cellular control of GSH levels, such as glutathione reductase (GR), γ-glutamyl cysteine synthetase (GCL), and γ-glutamine cysteine synthase (GCS), are all regulated by the nuclear factor erythroid 2-related factor 2 (Nrf2), which is also involved in the genetic regulation of both NQO-1 and HO-1 [26].
By binding to the antioxidant response element (ARE) located in the promoter region of every detoxifying gene, the transcription factor Nrf2 is widely recognized as one of the regulators of cellular redox homeostasis, antioxidant defense, and detoxification [27].
4. Nrf2 Pathway
In homeostatic conditions within the cell cytoplasm, Nrf2 is normally bound to the Keap1-Cul3-RBX1 complex, composed of RING-box protein 1 (RBX1), Cullin-based (Cul3) E3 ligase, and Kelch-like ECH-associated protein 1 (Keap1). The complex, when bound to the Neh2 domain of Nrf2, facilitates its ubiquitination and proteasomal degradation [28,29,30,31,32,33,34,35].
In particular, the oxidative modification of cysteine residues in Keap1, induced by exposure to stressors such as electrophiles or excessive production of ROS, causes the dissociation of Nrf2 from the Keap1-Cul3-RBX1 complex and subsequently its migration into the nucleus. Here, heterodimerization with the small Maf protein occurs, leading to gene activation through binding to the ARE promoter region [35,36]. Other evidence suggests that Nrf2 activity can also be regulated by the beta-transducin repeats-containing protein (β-TrCP) and the Skp1-Cul1-Rbx1 ubiquitin ligase complex [26], while its proteasomal degradation could be initiated by the glycogen synthase kinase-3 (GSK3) through phosphorylation processes [26]. Furthermore, evidence of crosstalk between Nrf2 and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), this latter itself a redox-sensitive transcription factor, has been reported [37]. In particular, proposed mechanisms suggest that both transcriptional factors compete for the CREB binding protein (CBP) site in the nucleus, with NF-κB recruiting histone deacetylase 3 (HDAC3) and therefore inhibiting Nrf2 ARE-dependent gene expression. In contrast, Nrf2 could be indirectly activated by anti-inflammatory compounds that suppress NF-κB activity and vice versa, NF-κB could be indirectly activated by Nrf2 inhibitors [37].
Finally, in the brain, Nrf2 exerts its anti-inflammatory properties by reducing neuroinflammation brought on by damaging stimuli associated with neurodegenerative diseases. In this context, Nrf2 indirectly contribute to the formation of the multimeric protein complex, inflammasome NLRP3 [38], thus playing a critical central defensive role against oxidative stress in brain pathophysiology, such as gliosis, proteinopathy, oxidative and inflammatory stress [39].
5. Effects of Phytocannabinoids and Endocannabinoids on Nrf2 Activity in the Central Nervous System (CNS)
It is not surprising that the relationship between pCBs/eCBs and Nrf2 has been first studied in brain cell models, given the well-known effect cannabinoids have on the CNS. The increased turnover occurring in lipid peroxidation processes and the central role played by the antioxidant system in the brain poses valid reasons for further investigation into the interaction between pCBs/eCBs and Nrf2. It has been demonstrated that THC causes glioma cells to undergo apoptosis [40], with a concomitant increase in intracellular ROS, as well as a decrease in GSH. In neonatal mice (post-natal day 10) exposed to a single dose of THC: (i) an increase in the Nrf2/Keap1 ratio was observed in both parietal cortex and hippocampus; (ii) increased levels of the apoptosis regulator BAX transcript were observed in the frontal cortex; (iii) increased levels of CB1R transcript were observed in the parietal cortex [41].
The Nrf2 and cannabinoid signaling pathways have been demonstrated to closely interact with each other in various models of neuropathic pain. For example, in male type 2 diabetic mice (BKS.Cg-m+/+Leprdb/J; db/db), the activation of the antioxidant Nrf2/HO-1 pathway amplified the antiallodynic effects of CB2R agonists JWH-015 and JWH-133 [42].
In addition, neuropathic mice treated with a combination of CBD and Δ9-tetrahydrocannabivarin (THCV) exhibited increased expressions of Nrf2, HO-1, and catalase in dorsal root ganglions.
It is noteworthy that the pCB THCV is a neutral, non-psychoactive CB1R antagonist/inverse agonist which, depending on the dose, can also behave as either an agonist or an antagonist at CB2R [43].
The combination of these pCBs resulted in a boost in their antioxidant potential, by lowering neuropathic pain and improving mitochondrial function, primarily acting on the activation of the AMPK-Nrf2-mitochondrial transcription factor A (TFAM) signaling cascade [44]. When phosphorylated, AMPK increases mitochondriogenesis and respiratory capacity through TFAM activation. Upon its activation, TFAM binds to the mitochondrial genome and regulates the transcription of mitochondrial subunit complexes, thereby decreasing the mitochondrial functional deficiencies observed, for example, in animal and cell culture models of neuropathic pain and diabetes [44,45].
Interesting studies were conducted on CB2Rs, whose activation does not result in psychoactive effects, to further elucidate their involvement in the regulation of Nrf2 within the CNS. These studies were conducted by using the CB2R selective agonist, β-caryophyllene (BCP) [46], a naturally occurring bicyclic sesquiterpene, classified as pCB, found in the essential oils of various species, such as Cannabis sativa, Piper nigrum, and Cinnamomum spp. [47].
When tested in the BV-2 cell line, BCP was able to regulate cellular antioxidant responses, primarily by preventing ROS production, restoring mitochondrial membrane potential, and protecting microglial cells from glutamate cytotoxicity. Moreover, BCP was also able to promote Nrf2 nuclear translocation, thereby enhancing the astrocytes’ cellular GSH antioxidant system [48]. Particularly, rat BV-2 cells were frequently used as an in vitro model to investigate the function of the endocannabinoid system in microglia, since this cell line, endocannabinoids, metabolic enzymes (i.e., FAAH), and CB2R are all constitutively expressed [49,50,51].
In oligodendrocytes (OLN-93), cells that also naturally express CB2Rs, BCP was able to prevent LPS-induced cytotoxicity, as well as ROS, TNF-α, and nitric oxide production. The protective effect of BCP was mediated by CB2R stimulation through the regulation of the Nrf2/HO-1/antioxidant axis and PPAR-γ pathways [52].
Similarly, classical pCBs were studied in experimental models of LPS-stimulated toxicity. For example, CBG, a non-psychoactive pCB, has shown protective effects on both RAW 264.7 (macrophages) and NSC-34 (motor neuron) cell lines, suggesting its possible application in the treatment of neurodegeneration, as well as in other pathological conditions, where oxidative stress and neuroinflammation are major factors, such as Huntington disease, Parkinson disease, multiple sclerosis [53]. Indeed, in an experimental model of neuroinflammation, pretreatment with CBG was observed to lower nitrotyrosine, SOD1, and inducible nitric oxide synthases (iNOS) protein levels, while increasing Nrf-2 levels and preventing apoptosis [54], with similar effects also observed in the presence of CBG-CBD co-administration [55].
Furthermore, in a mouse experimental model of chronic autoimmune encephalomyelitis (EAE) induced by myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35-55), CBD administration was observed to increase the expression of Nrf2 target genes, metallothioneins (Mt1 and Mt2), and Hmox-1 [56].
In BV-2 cells, CBD administration was able to stimulate those genes known to be involved in the regulation of inflammation and stress response, primarily through the Nrf2/Hmox1 axis and the ARE-Nrf2/ATF4 system [57,58]. In LPS-stimulated BV-2 cells, a particular repertoire of miRNAs was regulated by cannabinoids with TLR, Nrf2, and Notch crosstalk signaling, reported as responsible for the regulation of altered miRNAs of genes involved in the immune response, cell cycle regulation, cellular stress, and redox homeostasis [59].
The specific role played by CB2R in the above-mentioned mechanisms was further elucidated in other experimental models, such as primary cultures of rat microglia cells, where the administration of the synthetic CB2R selective agonist JWH133 was able to promote PI3K/Akt activation and therefore facilitate the nuclear translocation of Nrf2 [60].
Furthermore, in primary microglial cultures derived from Abcd1-null mice and from patients with X-linked adrenoleukodystrophy (X-ALD), the administration of JWH133 induced the Nrf2 antioxidant pathway and inhibited the ROS production elicited by excess very long-chain fatty acids [61].
The CNR2 gene encoding for CB2R contains a putative ARE motif that exhibits strong similarities to the consensus ARE sequence found in the Nrf2 promoter region [62]. In both hippocampal HT-22 cells and primary neonatal neurons from the mouse cortex, evidence suggested that Nrf2 was unable to control CB2R expression, while in microglial cells, the expression of the receptor was found to be Nrf2-dependent, suggesting that CNR2 gene activation could be mediated by different transcriptional factors in different cell types [63].
Collectively, the effects exerted by exogenous CB2R agonists on the Nrf2 pathway suggest a possible physiological role of eCBs in modulating/regulating the transcript activity.
For example, in HT-22 cells, those eCBs containing a phenolic moiety, such as N-acyl dopamines or N-arachidonoyl 5-HT, have shown an ability to exert antioxidant or anti-inflammatory actions through the activation of the Nrf2-mediated antioxidant response. Similar effects were also shown by the pCBs THC and CBD [64]. In a neurotoxic model of primary hippocampal hyperglycemic neurons and oligomeric amyloid β peptide (Aβ1-42), the involvement of eCBs in the regulation of Nrf2 activity has been demonstrated [65]. In these models, eCBs AEA and 2-AG, as well as the synthetic cannabinoids CP 55–940 and WIN 55,212–2, all reduced the assessed toxic endpoints. However, the strongest effect was observed in presence of URB597, an inhibitor of the FAAH enzyme responsible for AEA hydrolysis. This compound was the only one able to prevent the toxicity caused by high glucose and amyloid without raising Nrf2 and CREB phosphorylation [65]. The models used to evaluate the effects of pCBs and eCBs on central Nrf2 in vitro and in vivo are compiled in Table 1.

Table 1.
The in vivo and in vitro models used to investigate the effects of pCBs and eCBs on Nrf2 activation in the central nervous system are summarized in this table. These studies have mostly investigated the effect of the pCB CBD, which has been shown to modulate the Nrf2 pathway activation in different ways.
6. The Effects of Phytocannabinoids and Endocannabinoids on Nrf2 Activity in the Periphery
Outside of the CNS, the cannabinoid-mediated activation of Nrf2 was demonstrated in various models of peripheral illnesses. In experiments using SV-HUC1 cells (TNFα-stimulated normal human urothelial cells), an in vitro model of bladder pain syndrome and interstitial cystitis, administration of CBD was able to enhance the redox-sensitive transcription factor Nrf2 along with the expression of both the antioxidant enzymes, SOD 1 and 2 and the HO-1, potentially through the activation of the PPARγ receptor and attenuation of the NF-kB pathway [66]. Similarly, in mouse hepatocytes and L-02 cells exposed to α-amanitin, the lethal toxin of Amanita muscaria, CBD was able to upregulate either Nrf2 and both HO-1 and NADPH-Quinone Oxidoreductase1 (NQO1) antioxidant enzymes levels, thus attenuating the oxidative stress and apoptosis induced by the toxin [67].
Furthermore, CBD was found to reduce the severity of 5-fluorouracil-induced oral mucositis in mice and human oral keratinocytes by upregulating the expression level of antioxidant enzymes, such as HO-1 and NQO1, as well as by increasing the expression of Nrf2 and its nuclear translocation, all effects being concomitant with a decrease in Keap1 activation (Nrf2 suppressor). Both the Nrf2 inhibitor ML385 and Nrf2-siRNA transfection neutralized the protective effects of CBD, indicating the direct interaction between this cannabinoid compound and Nrf2 activation [68].
In many studies conducted on epidermal skin cell keratinocytes exposed to UVA and UVB radiation, CBD was observed to affect the interaction between Nrf2-NFκB transcription factors, promoting the activation of the former and suppressing the activation of the latter [69]. Further evidence of CBD ability to induce the expression of selected Nrf2 target genes was provided by experiments conducted on primary and immortalized human keratinocytes (HaCaT cell line). Interestingly, although CBD was significantly less effective than sulphoraphane (SFN) (a metabolite of glucoraphanin found in Brassica oleracea known to activate Nrf2 [70]) at inducing the expression of the main Nrf2 target genes aldo-ketoreductases AKR1B10 and AKR1C1, the compound was instead equally effective, or even more effective, at inducing the expression of specific Nrf2 subset target genes: HMOX1, glutamate-cysteine ligase catalytic subunit (GCLC), and p62 [71,72].
Moreover, in both keratinocytes and epidermal equivalents, CBD enhanced the expression of filaggrin, involucrin, Nrf2, and NQO1, as well as increased the expression of aryl hydrocarbon receptor target genes such as CYP1A1 and aryl hydrocarbon receptor repressor [73]. In experiments on skin rats exposed to UV light, topical application of CBD for four weeks induced a lower expression of SOD and a consequent decrease in the UV-enhanced levels of Nrf2, leading to an impaired cytoprotective effect in keratinocytes [74]. By considering that either activation or accumulation of Nrf2 could favor a suitable environment for the growing and proliferation of neoplastic, chemo- and radio-resistant cells, compounds such as CBD, with their ability to reduce Nrf2 levels, may provide a cellular protective effect [75].
In mice with imiquimod-induced experimental psoriasiform skin lesions, a significant improvement (decrease in both plaque and epidermal thickness) was observed upon CB2R activation. When compared to the control groups, the treated animals showed higher levels of Nrf2 and HO-1 protein expression, suggesting the involvement of the NF-κB and Keap1/Nrf2 pathways in CB2R’s downstream signaling [76].
It has been demonstrated that CB2R is involved in damaging H2O2-induced C2C12 myoblasts in vitro. In fact, pretreatment with the CB2R agonist AM1241 prevented the H2O2-induced reduction in C2C12 cell viability, reduced reactive oxygen species generation, and increased the expression of Nrf2 and its nuclear translocation [77].
This mechanism is further supported by evidence obtained in Nrf2 knockout mice, where degenerative oxidative damage, myogenesis, and skeletal muscle deterioration were observed upon AM1241 administration. Similarly, in C2C12 cells, the administration of the compound impaired differentiation [77]. The protective role of CB2R in its ability to promote skeletal muscle repair following ischemia-reperfusion injury has also been demonstrated [78], suggesting a significant role of CB2R in the musculoskeletal system. For example, hFOB 1.19 osteoblasts treated with the CB2R agonist HU308 showed decreased p62 expression and Nrf2 degradation [79]. In RAW 264.7 macrophage cells, osteoclast differentiation was stimulated by AM1241 and suppressed by the CB2R-selective antagonist, AM630. Although the expression of both HO-1 and Nrf2 was increased by AM1241 and AM630, only the CB2R selective antagonist was able to effectively activate the HO-1/Nrf2 pathway and to promote a decrease in osteoclast differentiation [80].
In contrast, in an experimental model of an infarcted heart, the administration of AM1241 improved the adverse oxidative stress and inflammation milieu through the upregulation of the fosfoinositide 3-chinasi (PI3K)/protein kinase B (Akt)/Nrf2 signal pathway [81], as well as reduced myocardial interstitial fibrosis through the Nrf2-mediated down-regulation of the transforming growth factor beta 1 (TGF-β1)/Smad3 pathway [82].
In the macrophage RAW264.7 cell line, administration of JWH-133 was able to polarize M1 macrophages via activation of the Nrf2/HO-1 pathway and the effect was reduced by the HO-1 inhibitor, Sn(IV) protoporphyrin IX dichloride [83].
In C57BL/6 male mice trained in rigorous exercise, CBD administration showed a protective effect against myocardial injury via the Keap1/Nrf2/HO-1 pathway activation, causing the down-regulation of Keap1 protein expression, increasing Nrf2 translocation into the nucleus, and therefore promoting the expression of the antioxidant protein HO-1 [84]. The strong CBD interaction with Keap1/Nrf2/HO-1 signaling pathway in myocardial injury induced by intensive exercise was further supported by molecular docking experiments [84].
Several studies using cancer cell models have also highlighted the involvement of eCBs in Nrf2 function and regulation. In breast cancer models, such as MCF-7 and MDA-MB-231 cell lines, independently of CBRs activation, administration of AEA or the suppression of its hydrolase FAAH, both activated Nrf2 and consequently induced HO-1 [85]. These findings suggest that in breast cancer cell survival, the Nrf2-HO-1 pathway may be directly activated by AEA in a non-receptor-mediated manner. The compound also appears able to target/modulate the function of endothelial cells, tumor macrophages, and tumor fibroblasts, all elements present in the microenvironment niche surrounding tumor cells in vivo [85]. This suggests a possible role played by this endocannabinoid in the Nrf2 pathway activation, as well as its potential involvement in chemotherapy resistance. Indeed, the role of cannabinoids on Nrf2-related factors has been recently reviewed in the context of cancer prevention and treatment [86], with an amount of evidence reporting the beneficial and protective role provided by the modulation of AEA/HO-1 signaling as a coadjutant pharmacological approach to reduce radio resistance and chemoresistance [85].
It is noteworthy that in HaCaT cells, hexocannabitriol, a hydroxylated CBD analogue isolated from hemp threshing residues, was recently shown to activate the Nrf2 pathway in a ROS-independent way, most likely as a result of direct Nrf2 stabilization [87]. Table 2 summarizes the models utilized to assess the effects of pCBs and eCBs on peripheral Nrf2 both in vitro and in vivo.

Table 2.
The in vitro and in vivo models used to assess the effects of pCBs and eCBs on peripheral Nrf2 activation are summarized in the table. The only pCB investigated in these models was CBD. The compound has shown the ability to activate the Nrf2 pathway in various ways. Moreover, this pathway can be activated by eCBs.
7. Conclusions
This literature review clearly highlights the antioxidant properties of pCBs and eCBs through their interaction with the Nrf2 pathway. However, we are still at a very early stage in the understanding of the pharmacological properties of cannabinoids in this area. Numerous genes that control homeostatic processes within the CNS, such as inflammation, naturally occurring redox metabolism, xenobiotic and carbohydrate metabolism, are enhanced by Nrf2, a transcription factor with crucial functions in defending neurons and glia against harmful insults [88,89]. Research findings show that both pCBs and eCBs can indeed trigger Nrf2 activation at different levels in different neuronal cells, with both micro and macroglia having been reported as the main cells where such interactions mostly occurred. Although pCBs and eCBs seem to be generally able to activate the Nrf2 pathway, further information is still needed to fully understand the modulatory mechanisms and the potential clinical applications of these compounds. In addition, the mechanism by which CB1R and CB2R stimulation can lead to Nrf2 activation (which under certain circumstances seems to be non-receptor mediated) is not fully understood yet. While the role of CB2R in activating the Nfr2 pathway has been established in peripheral tissues, the small number of CNS-focused experiments makes it difficult to fully elucidate the role of this receptor within the CNS. Convincing results of the ability of CB2R to activate the Nfr2 pathway have only been obtained from microglia and oligodendrocytes (Figure 2). Evidence that the pCD CBD may interact with the Nrf2 activation at various levels, including Keap1, different kinases, such as p38 mitogen-activated protein kinase, extracellular response kinase (ERK), c-Jun N-terminal kinase (JNK), and AKT, clearly highlight the complex network of signaling pathways activated by the compound. Moreover, by favoring Nrf2 activity, CBD could also reduce Bach1 expression (Nrf2 repressor), increase both p62 (Keap1 repressor) and sirtuin1 (SIRT1, Nrf2 activator) expression, as well as reduce glycogen synthase kinase-3 (GSK3, Nrf2 phosphorylation) [90]. Additional research is needed to determine the exact role played by eCBs at regulating Nrf2 activity within the brain. The possibility to regulate eCBs levels could represent a new potential therapeutic approach to tackle oxidative stress in CNS.
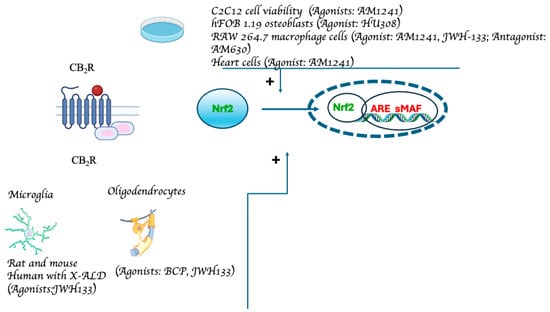
Figure 2.
Experimental models demonstrating CB2R-mediated Nfr2 pathway activation. The data from studies investigating the relationship between Nrf2 and cannabinoids via CB2R receptors are compiled in the figure. The receptor’s structure and the Nrf2 pathway have been simplified and schematized to aid in the understanding of the figure (from: [48,52,60,61,77,79,80,81]).
8. Literature Search Methods
For this review, we used data from peer-reviewed publications that were indexed in PubMed through 30 March 2024. For assessment and incorporation into this manuscript, only peer-reviewed original research articles and reviews in the English language were assessed.
Author Contributions
Conceptualization, L.S. and P.T.; methodology and literature search, P.M., M.M., L.S. and P.T.; writing—original draft preparation, P.M. and P.T.; writing—review and editing, P.M., M.M., L.S. and P.T.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union—Next Generation EU—NRRP M6C2—Investment 2.1 Enhancement and Strengthening of Biomedical Research in the NHS (project No. PNRR-MAD-2022-12376730 to MM).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- UN Commission Reclassifies Cannabis, yet Still Considered Harmful | UN News. Available online: https://news.un.org/en/story/2020/12/1079132 (accessed on 10 April 2024).
- U.S. Moves to Reclassify Cannabis as a Lower Risk Drug 2024. ScienceInsider, 1 May 2024.
- Procaccia, S.; Lewitus, G.M.; Lipson Feder, C.; Shapira, A.; Berman, P.; Meiri, D. Cannabis for Medical Use: Versatile Plant Rather Than a Single Drug. Front. Pharmacol. 2022, 13, 894960. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Köfalvi, A. Cannabis: A Treasure Trove or Pandora’s Box? Mini Rev. Med. Chem. 2017, 17, 1223–1291. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Marzo, V.D.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Voicu, V.; Brehar, F.-M.; Toader, C.; Covache-Busuioc, R.-A.; Corlatescu, A.D.; Bordeianu, A.; Costin, H.P.; Bratu, B.-G.; Glavan, L.-A.; Ciurea, A.V. Cannabinoids in Medicine: A Multifaceted Exploration of Types, Therapeutic Applications, and Emerging Opportunities in Neurodegenerative Diseases and Cancer Therapy. Biomolecules 2023, 13, 1388. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, Efficacy, and Mechanisms of Action of Cannabinoids in Neurological Disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Sam, A.H.; Salem, V.; Ghatei, M.A. Rimonabant: From RIO to Ban. J. Obes. 2011, 2011, 432607. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds to Cannabinoid Receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kerbrat, A.; Ferré, J.-C.; Fillatre, P.; Ronzière, T.; Vannier, S.; Carsin-Nicol, B.; Lavoué, S.; Vérin, M.; Gauvrit, J.-Y.; Le Tulzo, Y. Acute Neurologic Disorder from an Inhibitor of Fatty Acid Amide Hydrolase. N. Engl. J. Med. 2016, 375, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- van Esbroeck, A.C.M.; Janssen, A.P.A.; Cognetta, A.B.; Ogasawara, D.; Shpak, G.; van der Kroeg, M.; Kantae, V.; Baggelaar, M.P.; de Vrij, F.M.S.; Deng, H.; et al. Activity-Based Protein Profiling Reveals off-Target Proteins of the FAAH Inhibitor BIA 10-2474. Science 2017, 356, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, D.; Mattoteia, D.; Pollastro, F.; Negri, R.; Lopatriello, A.; Chianese, G.; Minassi, A.; Collado, J.A.; Munoz, E.; Taglialatela-Scafati, O. The Oxidation of Phytocannabinoids to Cannabinoquinoids. J. Nat. Prod. 2020, 83, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- UNODC—Bulletin on Narcotics—1962 Issue 3—004. Available online: https://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1962-01-01_3_page005.html (accessed on 27 June 2024).
- Borges, R.S.; Batista, J.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; Da Silva, A.B.F. Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as Antioxidant Agents and Their Intervention Abilities in Antioxidant Action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef]
- Borges, R.S.; da Silva, A.B.F. Chapter E12—Cannabidiol as an Antioxidant. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. e122–e130. ISBN 978-0-12-800756-3. [Google Scholar]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−) Δ9-Tetrahydrocannabinol Are Neuroprotective Antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Int. J. Mol. Sci. 2024, 25, 3360. [Google Scholar] [CrossRef]
- Suzen, S.; Tucci, P.; Profumo, E.; Buttari, B.; Saso, L. A Pivotal Role of Nrf2 in Neurodegenerative Disorders: A New Way for Therapeutic Strategies. Pharmaceuticals 2022, 15, 692. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Cuadrado, A. Structural and Functional Characterization of Nrf2 Degradation by Glycogen Synthase Kinase 3/β-TrCP. Free. Radic. Biol. Med. 2015, 88, 147–157. [Google Scholar] [CrossRef]
- Ma, Q.; He, X. Molecular Basis of Electrophilic and Oxidative Defense: Promises and Perils of Nrf2. Pharmacol. Rev. 2012, 64, 1055–1081. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Gordan, J.D.; Jin, J.; Harper, J.W.; Diehl, J.A. The Keap1-BTB Protein Is an Adaptor That Bridges Nrf2 to a Cul3-Based E3 Ligase: Oxidative Stress Sensing by a Cul3-Keap1 Ligase. Mol. Cell. Biol. 2004, 24, 8477–8486. [Google Scholar] [CrossRef]
- Furukawa, M.; Xiong, Y. BTB Protein Keap1 Targets Antioxidant Transcription Factor Nrf2 for Ubiquitination by the Cullin 3-Roc1 Ligase. Mol. Cell. Biol. 2005, 25, 162–171. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative Stress Sensor Keap1 Functions as an Adaptor for Cul3-Based E3 Ligase to Regulate Proteasomal Degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Zhang, D.D.; Lo, S.-C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 Is a Redox-Regulated Substrate Adaptor Protein for a Cul3-Dependent Ubiquitin Ligase Complex. Mol. Cell. Biol. 2004, 24, 10941–10953. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 Regulation of Cellular Defense Mechanisms against Electrophiles and Reactive Oxygen Species. Adv. Enzyme Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct Evidence That Sulfhydryl Groups of Keap1 Are the Sensors Regulating Induction of Phase 2 Enzymes That Protect against Carcinogens and Oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef]
- Tong, K.I.; Katoh, Y.; Kusunoki, H.; Itoh, K.; Tanaka, T.; Yamamoto, M. Keap1 Recruits Neh2 through Binding to ETGE and DLG Motifs: Characterization of the Two-Site Molecular Recognition Model. Mol. Cell. Biol. 2006, 26, 2887–2900. [Google Scholar] [CrossRef]
- Katsuoka, F.; Yamamoto, M. Small Maf Proteins (MafF, MafG, MafK): History, Structure and Function. Gene 2016, 586, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Sherratt, P.J.; Nioi, P.; Yang, C.S.; Pickett, C.B. Nrf2 Controls Constitutive and Inducible Expression of ARE-Driven Genes through a Dynamic Pathway Involving Nucleocytoplasmic Shuttling by Keap1. J. Biol. Chem. 2005, 280, 32485–32492. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef] [PubMed]
- Tucci, P.; Lattanzi, R.; Severini, C.; Saso, L. Nrf2 Pathway in Huntington’s Disease (HD): What Is Its Role? Int. J. Mol. Sci. 2022, 23, 15272. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A. NRF2 in Neurodegenerative Diseases. Curr. Opin. Toxicol. 2016, 2, 46–53. [Google Scholar] [CrossRef]
- Goncharov, I.; Weiner, L.; Vogel, Z. Delta9-Tetrahydrocannabinol Increases C6 Glioma Cell Death Produced by Oxidative Stress. Neuroscience 2005, 134, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Philippot, G.; Forsberg, E.; Tahan, C.; Viberg, H.; Fredriksson, R. A Single Δ9-Tetrahydrocannabinol (THC) Dose During Brain Development Affects Markers of Neurotrophy, Oxidative Stress, and Apoptosis. Front. Pharmacol. 2019, 10, 1156. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.; Leánez, S.; Pol, O. The Inhibitory Effects of Cobalt Protoporphyrin IX and Cannabinoid 2 Receptor Agonists in Type 2 Diabetic Mice. Int. J. Mol. Sci. 2017, 18, 2268. [Google Scholar] [CrossRef]
- Abioye, A.; Ayodele, O.; Marinkovic, A.; Patidar, R.; Akinwekomi, A.; Sanyaolu, A. Δ9-Tetrahydrocannabivarin (THCV): A Commentary on Potential Therapeutic Benefit for the Management of Obesity and Diabetes. J. Cannabis Res. 2020, 2, 6. [Google Scholar] [CrossRef]
- Kumar Kalvala, A.; Bagde, A.; Arthur, P.; Kumar Surapaneni, S.; Ramesh, N.; Nathani, A.; Singh, M. Role of Cannabidiol and Tetrahydrocannabivarin on Paclitaxel-Induced Neuropathic Pain in Rodents. Int. Immunopharmacol. 2022, 107, 108693. [Google Scholar] [CrossRef]
- Yi, M.; Cruz Cisneros, L.; Cho, E.J.; Alexander, M.; Kimelman, F.A.; Swentek, L.; Ferrey, A.; Tantisattamo, E.; Ichii, H. Nrf2 Pathway and Oxidative Stress as a Common Target for Treatment of Diabetes and Its Comorbidities. Int. J. Mol. Sci. 2024, 25, 821. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Celenza, G.; Petricca, S. Multi-Target Effects of ß-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants 2022, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.C.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; de Bem, A.F. β-Caryophyllene Protects the C6 Glioma Cells against Glutamate-Induced Excitotoxicity through the Nrf2 Pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chu, S.; Yang, Y.; Zhang, Z.; Pang, Z.; Chen, N. Neuroinflammatory In Vitro Cell Culture Models and the Potential Applications for Neurological Disorders. Front. Pharmacol. 2021, 12, 671734. [Google Scholar] [CrossRef] [PubMed]
- Horvath, R.J.; Nutile-McMenemy, N.; Alkaitis, M.S.; DeLeo, J.A. Differential Migration, LPS-Induced Cytokine, Chemokine, and NO Expression in Immortalized BV-2 and HAPI Cell Lines and Primary Microglial Cultures. J. Neurochem. 2008, 107, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; De Caris, M.G.; Maggi, E.; Armeli, F.; Coccurello, R.; Bisogno, T.; D’Erme, M.; Maccarrone, M.; Mancini, P.; Businaro, R. Fatty Acid Amide Hydrolase (FAAH) Inhibition Modulates Amyloid-Beta-Induced Microglia Polarization. Int. J. Mol. Sci. 2021, 22, 7711. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. Promising Neuroprotective Effects of β-Caryophyllene against LPS-Induced Oligodendrocyte Toxicity: A Mechanistic Study. Biochem. Pharmacol. 2019, 159, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. [Google Scholar] [CrossRef]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Juknat, A.; Gao, F.; Kaushansky, N.; Coppola, G.; Vogel, Z. Pathways and Gene Networks Mediating the Regulatory Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, in Autoimmune T Cells. J. Neuroinflammation 2016, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Coppola, G.; Geschwind, D.; Vogel, Z. Differential Transcriptional Profiles Mediated by Exposure to the Cannabinoids Cannabidiol and Δ9-Tetrahydrocannabinol in BV-2 Microglial Cells. Br. J. Pharmacol. 2012, 165, 2512–2528. [Google Scholar] [CrossRef]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Gao, F.; Coppola, G.; Geschwind, D.; Vogel, Z. Microarray and Pathway Analysis Reveal Distinct Mechanisms Underlying Cannabinoid-Mediated Modulation of LPS-Induced Activation of BV-2 Microglial Cells. PLoS ONE 2013, 8, e61462. [Google Scholar] [CrossRef] [PubMed]
- Juknat, A.; Gao, F.; Coppola, G.; Vogel, Z.; Kozela, E. miRNA Expression Profiles and Molecular Networks in Resting and LPS-Activated BV-2 Microglia-Effect of Cannabinoids. PLoS ONE 2019, 14, e0212039. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, M.; Ma, Z. Cannabinoid Type 2 Receptor Activation Inhibits MPP+-Induced M1 Differentiation of Microglia through Activating PI3K/Akt/Nrf2 Signal Pathway. Mol. Biol. Rep. 2023, 50, 4423–4433. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, J.; Goicoechea, L.; Planas-Serra, L.; Pastor, A.; Ruiz, M.; Calingasan, N.Y.; Guilera, C.; Aso, E.; Boada, J.; Pamplona, R.; et al. Activating Cannabinoid Receptor 2 Preserves Axonal Health through GSK-3β/NRF2 Axis in Adrenoleukodystrophy. Acta Neuropathol. 2022, 144, 241–258. [Google Scholar] [CrossRef]
- Tadijan, A.; Vlašić, I.; Vlainić, J.; Đikić, D.; Oršolić, N.; Jazvinšćak Jembrek, M. Intracellular Molecular Targets and Signaling Pathways Involved in Antioxidative and Neuroprotective Effects of Cannabinoids in Neurodegenerative Conditions. Antioxidants 2022, 11, 2049. [Google Scholar] [CrossRef]
- Galán-Ganga, M.; Del Río, R.; Jiménez-Moreno, N.; Díaz-Guerra, M.; Lastres-Becker, I. Cannabinoid CB2 Receptor Modulation by the Transcription Factor NRF2 Is Specific in Microglial Cells. Cell. Mol. Neurobiol. 2020, 40, 167–177. [Google Scholar] [CrossRef]
- Jin, M.C.; Yoo, J.-M.; Sok, D.-E.; Kim, M.R. Neuroprotective Effect of N-Acyl 5-Hydroxytryptamines on Glutamate-Induced Cytotoxicity in HT-22 Cells. Neurochem. Res. 2014, 39, 2440–2451. [Google Scholar] [CrossRef]
- Elmazoglu, Z.; Rangel-López, E.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Túnez, I.; Aschner, M.; Santamaría, A.; Karasu, Ç. Cannabinoid-Profiled Agents Improve Cell Survival via Reduction of Oxidative Stress and Inflammation, and Nrf2 Activation in a Toxic Model Combining hyperglycemia+Aβ1-42 Peptide in Rat Hippocampal Neurons. Neurochem. Int. 2020, 140, 104817. [Google Scholar] [CrossRef]
- Kuret, T.; Kreft, M.E.; Romih, R.; Veranič, P. Cannabidiol as a Promising Therapeutic Option in IC/BPS: In Vitro Evaluation of Its Protective Effects against Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 5055. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.; Zhang, X.; Zhang, H.; Liu, Y.; Wang, C.; Miao, L.; Li, Y.; Huang, Y.; Teng, H.; et al. Cannabidiol Protects the Liver from α-Amanitin-Induced Apoptosis and Oxidative Stress through the Regulation of Nrf2. Food Chem. Toxicol. 2023, 182, 114196. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xuan, Y.; Zhu, B.; Wang, X.; Tian, X.; Zhao, L.; Wang, Y.; Jiang, X.; Wen, N. Protective Effects of Cannabidiol on Chemotherapy-Induced Oral Mucositis via the Nrf2/Keap1/ARE Signaling Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 4619760. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol Induces Antioxidant Pathways in Keratinocytes by Targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef]
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A Review of Its Therapeutic Potentials, Advances in Its Nanodelivery, Recent Patents, and Clinical Trials. Phytother. Res. 2021, 35, 5440–5458. [Google Scholar] [CrossRef]
- Jang, Y.S.; Jeong, S.; Kim, A.-R.; Mok, B.R.; Son, S.J.; Ryu, J.-S.; Son, W.S.; Yun, S.K.; Kang, S.; Kim, H.J.; et al. Cannabidiol Mediates Epidermal Terminal Differentiation and Redox Homeostasis through Aryl Hydrocarbon Receptor (AhR)-Dependent Signaling. J. Dermatol. Sci. 2023, 109, 61–70. [Google Scholar] [CrossRef]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic Application of Cannabidiol on UVA and UVB Irradiated Rat Skin. A Proteomic Study. J. Pharm. Biomed. Anal. 2021, 192, 113656. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jia, H.; Yang, Q.; Shan, W.; Chen, X.; Huang, X.; Liu, T.; Sun, R. Specific Activation of CB2R Ameliorates Psoriasis-Like Skin Lesions by Inhibiting Inflammation and Oxidative Stress. Inflammation 2023, 46, 1255–1271. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, M.; Wang, L.; Yu, T.; Jiang, S.; Jiang, P.; Sun, Y.; Pi, J.; Zhao, R.; Guan, D. Activation of Cannabinoid Type 2 Receptor Protects Skeletal Muscle from Ischemia-Reperfusion Injury Partly via Nrf2 Signaling. Life Sci. 2019, 230, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, L.; Zhang, M.; Zhang, M.; Wang, C.; Zhao, R.; Guan, D. Cannabinoid Type 2 Receptor Manipulates Skeletal Muscle Regeneration Partly by Regulating Macrophage M1/M2 Polarization in IR Injury in Mice. Life Sci. 2020, 256, 117989. [Google Scholar] [CrossRef]
- Xu, A.; Yang, Y.; Shao, Y.; Wu, M.; Sun, Y. Activation of Cannabinoid Receptor Type 2-Induced Osteogenic Differentiation Involves Autophagy Induction and P62-Mediated Nrf2 Deactivation. Cell Commun. Signal. 2020, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y. Nrf2 Is Required for Suppressing Osteoclast RANKL-Induced Differentiation in RAW 264.7 Cells via Inactivating Cannabinoid Receptor Type 2 with AM630. Regen. Ther. 2020, 14, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, S.; Wang, Q.; Hu, W.; Wang, D.; Li, X.; Su, T.; Qin, X.; Zhang, X.; Ma, K.; et al. Effects of Cannabinoid Receptor Type 2 on Endogenous Myocardial Regeneration by Activating Cardiac Progenitor Cells in Mouse Infarcted Heart. Sci. China Life Sci. 2014, 57, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, D.; Tian, Z.; Gao, B.; Fan, M.; Li, C.; Li, X.; Wang, Y.; Ma, S.; Cao, F. Activation of Cannabinoid Receptor Type II by AM1241 Ameliorates Myocardial Fibrosis via Nrf2-Mediated Inhibition of TGF-Β1/Smad3 Pathway in Myocardial Infarction Mice. Cell. Physiol. Biochem. 2016, 39, 1521–1536. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ma, Y.; Liu, Y.; Wang, N.; Zhao, X.; Wen, D. CB2R Agonist JWH-133 Attenuates Chronic Inflammation by Restraining M1 Macrophage Polarization via Nrf2/HO-1 Pathway in Diet-Induced Obese Mice. Life Sci. 2020, 260, 118424. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.; Zhang, Z.; Zhao, M.; Tong, C.; Cong, P.; Mao, S.; Zhao, Y.; Hou, M.; Piao, Y.; et al. Protective Effect and Mechanism of Cannabidiol on Myocardial Injury in Exhaustive Exercise Training Mice. Chem. Biol. Interact. 2022, 365, 110079. [Google Scholar] [CrossRef]
- Li, H.; Wood, J.T.; Whitten, K.M.; Vadivel, S.K.; Seng, S.; Makriyannis, A.; Avraham, H.K. Inhibition of Fatty Acid Amide Hydrolase Activates Nrf2 Signalling and Induces Heme Oxygenase 1 Transcription in Breast Cancer Cells. Br. J. Pharmacol. 2013, 170, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, A.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds. Antioxidants 2023, 12, 2052. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Sirignano, C.; Benetti, E.; Marzaroli, V.; Collado, J.A.; de la Vega, L.; Appendino, G.; Muñoz, E.; Taglialatela-Scafati, O. A Nrf-2 Stimulatory Hydroxylated Cannabidiol Derivative from Hemp (Cannabis sativa). J. Nat. Prod. 2022, 85, 1089–1097. [Google Scholar] [CrossRef]
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-Related Factor-2-Dependent Genes Conferring Protection against Oxidative Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-Mediated Neuroprotection in the MPTP Mouse Model of Parkinson’s Disease: Critical Role for the Astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. The Molecular Activity of Cannabidiol in the Regulation of Nrf2 System Interacting with NF-κB Pathway under Oxidative Stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).