Video Motion Analysis as a Quantitative Evaluation Tool for Essential Tremor during Magnetic Resonance-Guided Focused Ultrasound Thalamotomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRgFUS Thalamotomy Procedure
2.3. Clinical Rating Scale for Tremor
2.4. Videographic Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Test–Retest and Inter-Rater Reliabilities of the Videographic Parameters
3.3. Correlation between Videographic Parameters and Clinical Rating Scores
3.4. Videographic Parameters as an Assessment Tool to Evaluate Activities of Daily Living
3.5. Changes in Videographic Parameters by the MRgFUS Thalamotomy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Louis, E.D.; Ferreira, J.J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 2010, 25, 534–541. [Google Scholar] [CrossRef]
- Koller, W.C.; Vetere-Overfield, B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology 1989, 39, 1587–1588. [Google Scholar] [CrossRef]
- Diaz, N.L.; Louis, E.D. Survey of medication usage patterns among essential tremor patients: Movement disorder specialists vs. general neurologists. Park. Relat. Disord. 2010, 16, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Stacy, M.A.; Elble, R.J.; Ondo, W.G.; Wu, S.C.; Hulihan, J.; TRS study group. Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov. Disord. 2007, 22, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Chen, K.H.; Yang, B.S.; Chen, Y.J. A digital assessment system for evaluating kinetic tremor in essential tremor and Parkinson’s disease. BMC Neurol. 2018, 18, 25. [Google Scholar] [CrossRef]
- Legrand, A.P.; Rivals, I.; Richard, A.; Apartis, E.; Roze, E.; Vidailhet, M.; Meunier, S.; Hainque, E. New insight in spiral drawing analysis methods—Application to action tremor quantification. Clin. Neurophysiol. 2017, 128, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Elble, R.J.; Ellenbogen, A. Digitizing Tablet and Fahn-Tolosa-Marín Ratings of Archimedes Spirals have Comparable Minimum Detectable Change in Essential Tremor. Tremor Other Hyperkinetic Mov. 2017, 7, 481. [Google Scholar] [CrossRef]
- Elble, R.J.; McNames, J. Using Portable Transducers to Measure Tremor Severity. Tremor Other Hyperkinetic Mov. 2016, 6, 375. [Google Scholar] [CrossRef]
- Zesiewicz, T.A.; Elble, R.J.; Louis, E.D.; Gronseth, G.S.; Ondo, W.G.; Dewey, R.B., Jr.; Okun, M.S.; Sullivan, K.L.; Weiner, W.J. Evidence-based guideline update: Treatment of essential tremor: Report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology 2011, 77, 1752–1755. [Google Scholar] [CrossRef]
- Iijima, K.; Yokota, H.; Yamaguchi, T.; Nakano, M.; Ouchi, T.; Maki, F.; Takasaki, M.; Shimizu, Y.; Hori, H.; Iwamuro, H.; et al. Predictors of thermal increase in magnetic resonance-guided focused ultrasound treatment for essential tremor: Histogram analysis of skull density ratio values for 1024 elements. J. Neurosurg. 2021, 136, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Elias, W.J.; Huss, D.; Voss, T.; Loomba, J.; Khaled, M.; Zadicario, E.; Frysinger, R.C.; Sperling, S.A.; Wylie, S.; Monteith, S.J.; et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 2013, 369, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Boutet, A.; Gwun, D.; Gramer, R.; Ranjan, M.; Elias, G.J.B.; Tilden, D.; Huang, Y.; Li, S.X.; Davidson, B.; Lu, H.; et al. The relevance of skull density ratio in selecting candidates for transcranial MR-guided focused ultrasound. J. Neurosurg. 2019, 132, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Sinai, A.; Nassar, M.; Eran, A.; Constantinescu, M.; Zaaroor, M.; Sprecher, E.; Schlesinger, I. Magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: A 5-year single-center experience. J. Neurosurg. 2019, 133, 417–424. [Google Scholar] [CrossRef]
- Segar, D.J.; Lak, A.M.; Lee, S.; Harary, M.; Chavakula, V.; Lauro, P.; McDannold, N.; White, J.; Cosgrove, G.R. Lesion location and lesion creation affect outcomes after focused ultrasound thalamotomy. Brain 2021, 144, 3089–3100. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ito, H.; Fukutake, S.; Kamei, T.; Yamaguchi, T.; Taira, T. Ventralis intermedius thalamotomy with focused ultrasound for patients with low skull density ratio. Mov. Disord. 2019, 34, 1239–1240. [Google Scholar] [CrossRef]
- Torii, J.; Maesawa, S.; Nakatsubo, D.; Tsugawa, T.; Kato, S.; Ishizaki, T.; Takai, S.; Shibata, M.; Wakabayashi, T.; Tsuboi, T.; et al. Cutoff values for the best management strategy for magnetic resonance-guided focused ultrasound ablation for essential tremor. J. Neurosurg. 2022, 138, 38–49. [Google Scholar] [CrossRef]
- Boutet, A.; Ranjan, M.; Zhong, J.; Germann, J.; Xu, D.; Schwartz, M.L.; Lipsman, N.; Hynynen, K.; Devenyi, G.A.; Chakravarty, M.; et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain 2018, 141, 3405–3414. [Google Scholar] [CrossRef]
- Ondo, W.; Hashem, V.; LeWitt, P.A.; Pahwa, R.; Shih, L.; Tarsy, D.; Zesiewicz, T.; Elble, R. Comparison of the Fahn-Tolosa-Marin Clinical Rating Scale and the Essential Tremor Rating Assessment Scale. Mov. Disord. Clin. Pract. 2017, 5, 60–65. [Google Scholar] [CrossRef]
- Elble, R.J.; Ondo, W. Tremor rating scales and laboratory tools for assessing tremor. J. Neurol. Sci. 2022, 435, 120202. [Google Scholar] [CrossRef]
- Elble, R.J.; Pullman, S.L.; Matsumoto, J.Y.; Raethjen, J.; Deuschl, G.; Tintner, R.; Tremor Research Group. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain 2006, 129 Pt 10, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Haubenberger, D.; Kalowitz, D.; Nahab, F.B.; Toro, C.; Ippolito, D.; Luckenbaugh, D.A.; Wittevrongel, L.; Hallett, M. Validation of digital spiral analysis as outcome parameter for clinical trials in essential tremor. Mov. Disord. 2011, 26, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Elble, R.J.; Hellriegel, H.; Raethjen, J.; Deuschl, G. Assessment of Head Tremor with Accelerometers Versus Gyroscopic Transducers. Mov. Disord. Clin. Pract. 2016, 4, 205–211. [Google Scholar] [CrossRef] [PubMed]
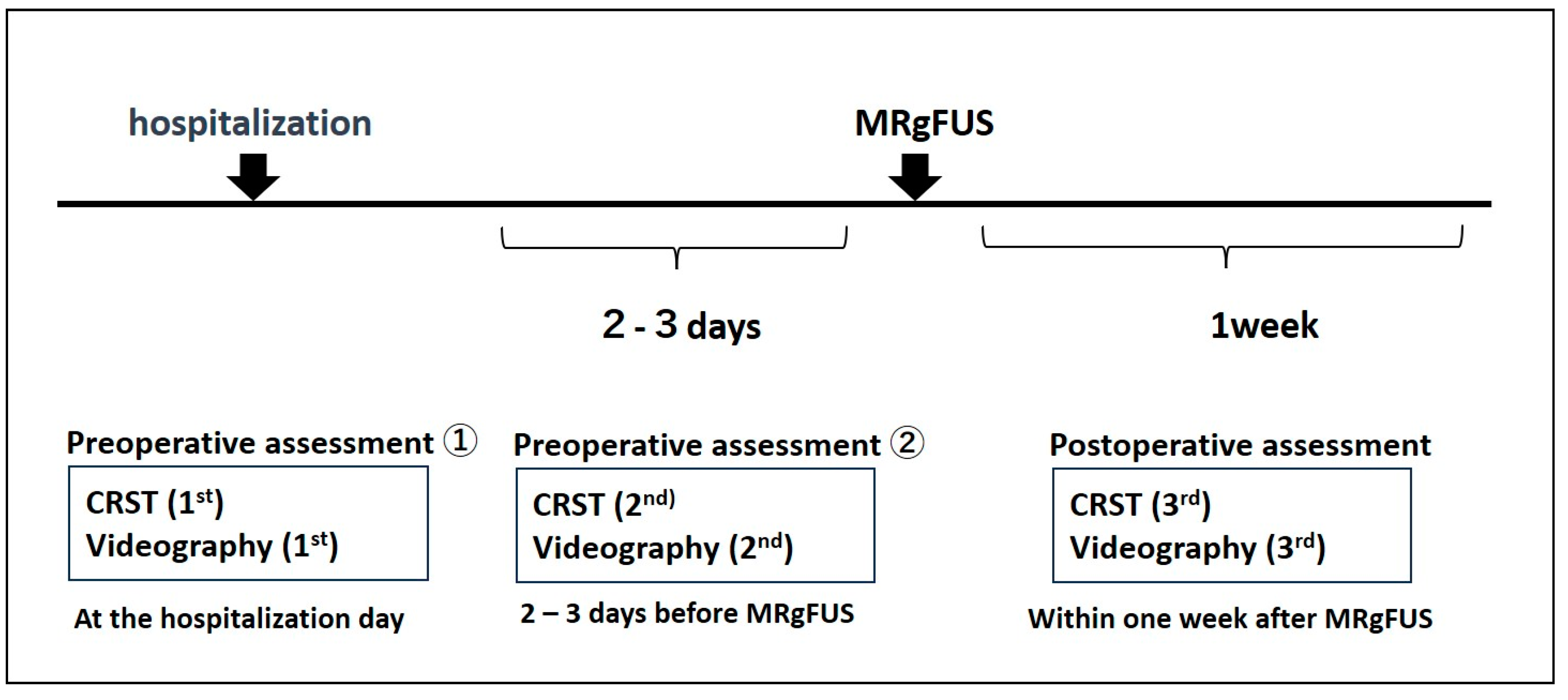
| Total | Reliability-Test Subgroup | p Value * | |
|---|---|---|---|
| Number of patients, n | 43 | 10 | |
| Age, mean (SD) | 69.9 (11.8) | 68.8 (10.0) | 0.144 |
| Male sex, n (%) | 29 (67.4) | 8 (80.0) | 0.876 |
| Disease duration, years (SD) | 27.7 (18.1) | 27.9 (17.8) | 0.437 |
| Family history, n (%) | 20 (46.5) | 8 (80) | 0.842 |
| Baseline tremor severity, median (min-max) | |||
| CRST total score | 36.0 (10–76) | 40.5 (14–70) | 0.536 |
| Part A score | 7.0 (1–33) | 10.0 (3–17) | 0.093 |
| Part B score, treatment side | 10.0 (2–20) | 11.5 (2–20) | 0.089 |
| Part B score, contralateral side | 7.0 (0–16) | 6.0 (1–14) | 0.336 |
| Part C score | 10.0 (1–26) | 11.0 (2–26) | 0.286 |
| Tremor score | 14.0 (3–28) | 14.5 (3–23) | 0.145 |
| Side of treatment, n (left:right) | 37:6 | 10:0 |
| Test–Retest Reliability | Inter-Rater Reliability | ||
|---|---|---|---|
| ICC (1,1) (95% CI) | MDC95 | ICC (2,3) (95% CI) | |
| Postural tremor, fingertip | |||
| Velocity | 0.985 (0.943–0.996) | 79.47 mm/s | 0.990 (0.970–0.997) |
| Acceleration | 0.999 (0.994–1.000) | 726.81 mm/s2 | 0.996 (0.989–0.999) |
| Frequency | 0.988 (0.967–0.997) | 0.46 Hz | 0.943 (0.833–0.985) |
| Amplitude | 0.999 (0.997–1.000) | 8.35 mm | 0.998 (0.995–1.000) |
| 15 cm Line-Drawing Task | |||
| Cumulative length | 0.999 (0.998–1.000) | 24.91 mm | 0.998 (0.996–1.000) |
| Velocity | 0.998 (0.995–1.000) | 40.65 mm/s | 0.997 (0.991–0.999) |
| Acceleration | 0.995 (0.986–0.999) | 1991.94 mm/s2 | 0.995 (0.986–0.999) |
| Frequency | 0.948 (0.802–0.987) | 0.88 Hz | 0.927 (0.787–0.980) |
| Amplitude | 0.997 (0.988–0.999) | 1.26 mm | 0.997 (0.992–0.999) |
| Postural Tremor, Fingertip | CRST Part A | TETRAS | Video-Amplitude | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Test 1–2 SDd | MDC% | Mean | Test 1–2 SDd | MDC% | Mean | Test 1–2 SDd | MDC% | |
| Test 1 | 2.7 | 0.823 | 89% | 1.75 | 0.408 | 84% | 13.95 | 0.211 | 61% |
| Test 2 | 3.4 | 1.75 | 14.03 | ||||||
| 15 cm Line-Drawing Task | CRST Part B | Video-amplitude | |||||||
| Mean | Test 1–2 SDd | MDC% | Mean | Test 1–2 SDd | MDC% | ||||
| Test 1 | 1.2 | −0.100 | 84% | 5.16 | 0.056 | 51% | |||
| Test 2 | 1.3 | 5.12 | |||||||
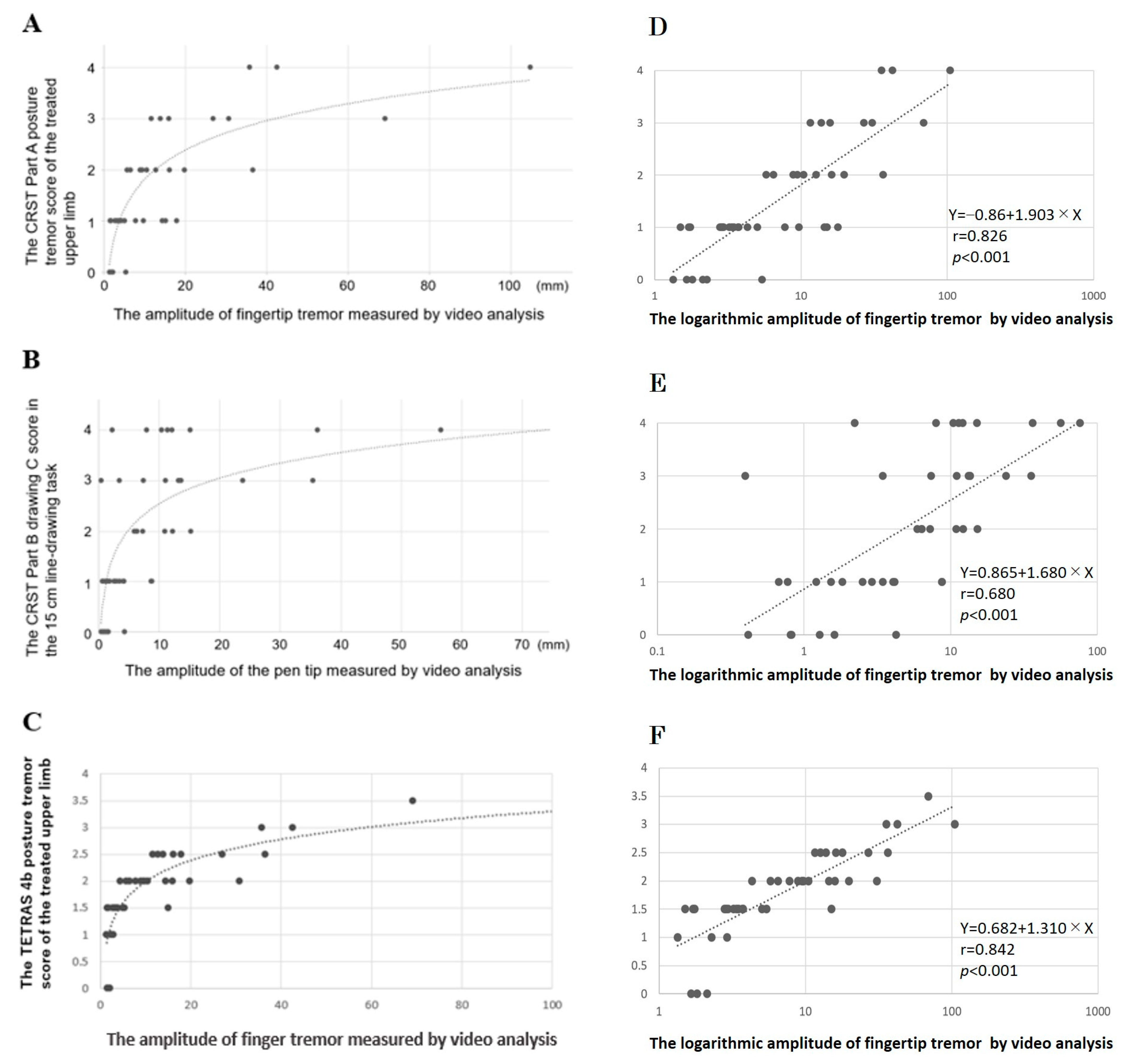
| Videographic Parameters | CRST | CRST | CRST | CRST | CRST |
|---|---|---|---|---|---|
| (logT1) | Total score | Part A | Part B | Part C | Tremor score |
| Postural tremor, fingertip | |||||
| Amplitude | 0.471 ** | 0.656 ** | 0.309 * | 0.389 ** | 0.507 ** |
| 15 cm Line-Drawing Task | |||||
| Cumulative length | 0.554 ** | 0.216 | 0.746 ** | 0.517 ** | 0.673 ** |
| Amplitude | 0.597 ** | 0.328 ** | 0.724 ** | 0.377 * | 0.682 ** |
| Before MRgFUS | After MRgFUS | p Value * | |
|---|---|---|---|
| CRST Score | median (min–max) | median (min–max) | |
| Total score | 36.0 (10–76) | 18.0 (2–48) | <0.001 |
| Part A | 7.0 (1–33) | 4.0 (0–20) | <0.001 |
| Part B treatment side | 10.0 (2–20) | 2.0 (0–10) | <0.001 |
| Contralateral side | 7.0 (0–16) | 6.0 (0–16) | 0.573 |
| Part C | 10.0 (1–26) | 4.0 (0–13) | <0.001 |
| Tremor score | 14 (3–28) | 2 (0–11) | <0.001 |
| Videographic Parameters | mean (SD) | mean (SD) | |
| Postural tremor task, fingertip | |||
| Amplitude (mm) | 14.0 (19.7) | 2.7 (3.2) | <0.001 |
| 15 cm line-drawing task, mean | |||
| Cumulative length (mm) | 414.5 (578.1) | 159.0 (13.6) | <0.001 |
| Amplitude (mm) | 10.8 (15.0) | 2.5 (5.1) | <0.001 |
| Logarithmic Changes (logT2/T1) | Tremor Score (R2 − R1) | Part B Score (R2 − R1) | Part C Score (R2 − R1) |
|---|---|---|---|
| Postural tremor, fingertip | |||
| Amplitude | 0.488 ** | 0.357 ** | 0.434 ** |
| 15 cm line-drawing task | |||
| Cumulative length | 0.606 ** | 0.683 ** | 0.398 ** |
| Amplitude | 0.577 ** | 0.587 ** | 0.191 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaburagi, M.; Maki, F.; Hino, S.; Nakano, M.; Yamaguchi, T.; Takasaki, M.; Iwamuro, H.; Iijima, K.; Sasanuma, J.; Watanabe, K.; et al. Video Motion Analysis as a Quantitative Evaluation Tool for Essential Tremor during Magnetic Resonance-Guided Focused Ultrasound Thalamotomy. Neurol. Int. 2023, 15, 1411-1422. https://doi.org/10.3390/neurolint15040091
Kaburagi M, Maki F, Hino S, Nakano M, Yamaguchi T, Takasaki M, Iwamuro H, Iijima K, Sasanuma J, Watanabe K, et al. Video Motion Analysis as a Quantitative Evaluation Tool for Essential Tremor during Magnetic Resonance-Guided Focused Ultrasound Thalamotomy. Neurology International. 2023; 15(4):1411-1422. https://doi.org/10.3390/neurolint15040091
Chicago/Turabian StyleKaburagi, Mayumi, Futaba Maki, Sakae Hino, Masayuki Nakano, Toshio Yamaguchi, Masahito Takasaki, Hirokazu Iwamuro, Ken Iijima, Jinichi Sasanuma, Kazuo Watanabe, and et al. 2023. "Video Motion Analysis as a Quantitative Evaluation Tool for Essential Tremor during Magnetic Resonance-Guided Focused Ultrasound Thalamotomy" Neurology International 15, no. 4: 1411-1422. https://doi.org/10.3390/neurolint15040091
APA StyleKaburagi, M., Maki, F., Hino, S., Nakano, M., Yamaguchi, T., Takasaki, M., Iwamuro, H., Iijima, K., Sasanuma, J., Watanabe, K., Hasegawa, Y., & Yamano, Y. (2023). Video Motion Analysis as a Quantitative Evaluation Tool for Essential Tremor during Magnetic Resonance-Guided Focused Ultrasound Thalamotomy. Neurology International, 15(4), 1411-1422. https://doi.org/10.3390/neurolint15040091





