Anti-MOG Positive Bilateral Optic Neuritis and Brainstem Encephalitis Secondary to COVID-19 Infection: A Case Report
Abstract
1. Introduction
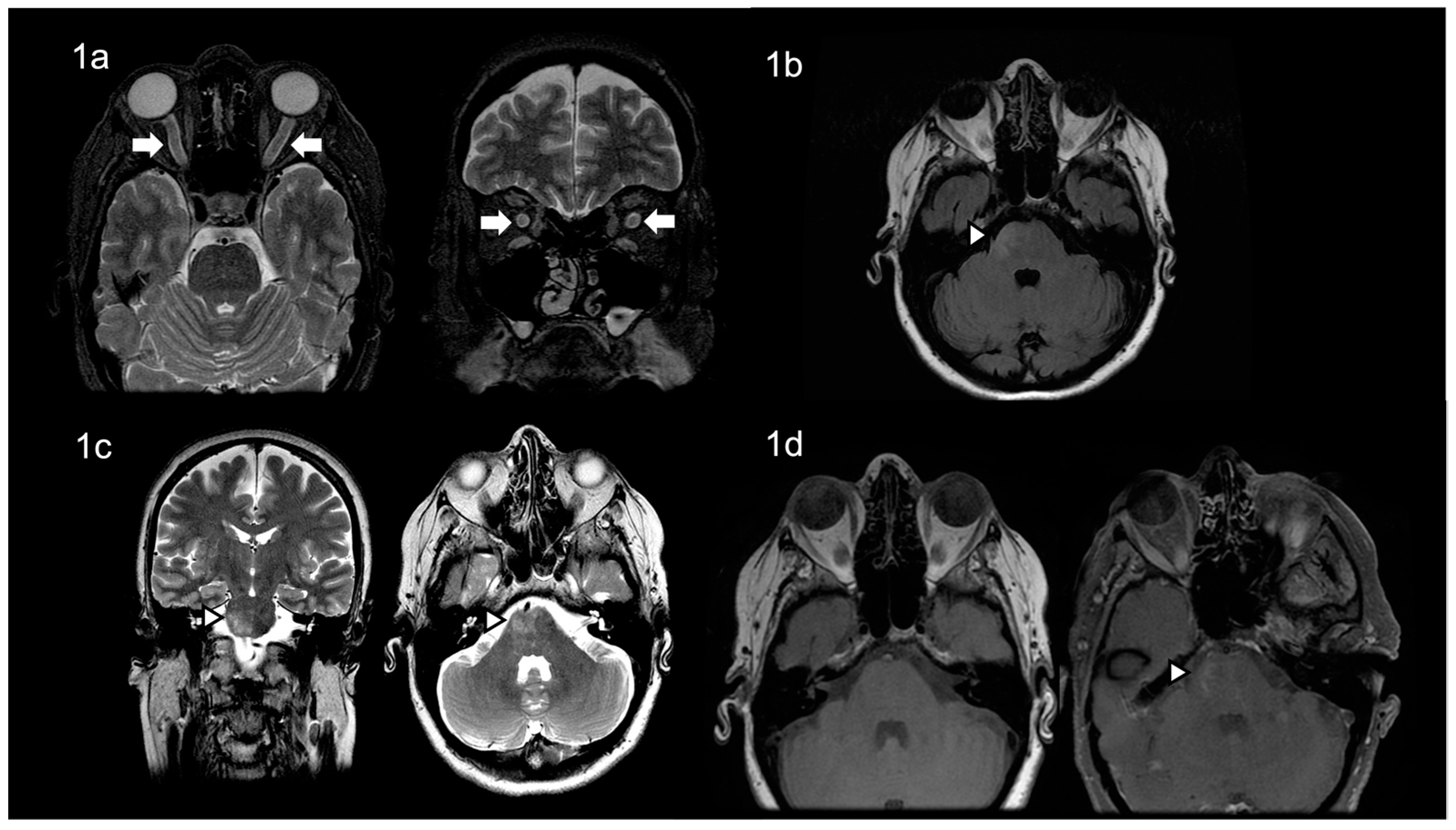
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, M.; Siokas, V.; Aloizou, A.M.; Liampas, I.; Mentis, A.A.; Tsouris, Z.; Papadimitriou, A.; Mitsias, P.D.; Tsatsakis, A.; Bogdanos, D.P.; et al. Unraveling the Possible Routes of SARS-CoV-2 Invasion into the Central Nervous System. Curr. Treat. Options Neurol. 2020, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Novi, G.; Rossi, T.; Pedemonte, E.; Saitta, L.; Rolla, C.; Roccatagliata, L.; Inglese, M.; Farinini, D. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e797. [Google Scholar] [CrossRef] [PubMed]
- Durovic, E.; Bien, C.; Bien, C.G.; Isenmann, S. MOG antibody-associated encephalitis secondary to Covid-19: Case report. BMC Neurol. 2021, 21, 414. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Alhasan, S.; Vogels, C.B.F.; Grubaugh, N.D.; Farhadian, S.; Longbrake, E.E. MOG-associated encephalitis following SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2021, 50, 102857. [Google Scholar] [CrossRef] [PubMed]
- de Ruijter, N.S.; Kramer, G.; Gons, R.A.R.; Hengstman, G.J.D. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: A case report. Mult. Scler. Relat. Disord. 2020, 46, 102474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jones-Lopez, E.C.; Soneji, D.J.; Azevedo, C.J.; Patel, V.R. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis and Myelitis in COVID-19. J. Neuroophthalmol. 2020, 40, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, K.; Adeodokun, S.; Kamoga, G.R. COVID-19-Induced Acute Bilateral Optic Neuritis. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620976018. [Google Scholar] [CrossRef] [PubMed]
- Zoric, L.; Rajovic-Mrkic, I.; Colak, E.; Miric, D.; Kisic, B. Optic Neuritis in a Patient with Seropositive Myelin Oligodendrocyte Glycoprotein Antibody During the Post-COVID-19 Period. Int. Med. Case Rep. J. 2021, 14, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Panwala, H.; Ramadoss, D.; Khubchandani, R. Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Disease in a 11 Year Old with COVID-19 Infection. Indian J. Pediatr. 2021, 88, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Kogure, C.; Kikushima, W.; Fukuda, Y.; Hasebe, Y.; Takahashi, T.; Shibuya, T.; Sakurada, Y.; Kashiwagi, K. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: A case report. Medicine 2021, 100, e25865. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulos, R.; Hickman, S.J.; Toosy, A.; Sharrack, B.; Mallik, S.; Paling, D.; Altmann, D.R.; Yiannakas, M.C.; Malladi, P.; Sheridan, R.; et al. Phenytoin for neuroprotection in patients with acute optic neuritis: A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Calina, D.; Falzone, L.; Petrakis, D.; Mitrut, R.; Siokas, V.; Pennisi, M.; Lanza, G.; Libra, M.; Doukas, S.G.; et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020, 146, 111769. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Aloizou, A.M.; Siokas, V.; Bakirtzis, C.; Liampas, I.; Tsouris, Z.; Bogdanos, D.P.; Baloyannis, S.J.; Dardiotis, E. Coronaviruses and their relationship with multiple sclerosis: Is the prevalence of multiple sclerosis going to increase after the COVID-19 pandemia? Rev. Neurosci. 2022, 33, 703–720. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Age (Years)/Ethnicity/Sex (Male/Female) | Method of COVID-19 Diagnosis | COVID-19 Symptoms | Hospitalization (Yes/No) | Time of Onset of Neurological Manifestations from COVID-19 Diagnosis | Neurological Manifestations | MOG-Antibody Method of Detection | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| de Ruijter et al. (2020) | 15/Caucasian/Male | not confirmed | fever, nausea and cough | NM | Few weeks | Subacute vision loss with photopsias and frontal continuous headache | NM | IVMP 1 g/day for three days | Symptoms resolved |
| Zhou et al. (2020) | 26/Hispanic/Male | nasal and oropharyngeal swabs (RT-PCR) | dry cough | Yes | Few days | Bilateral, subacute, sequential vision loss first affecting the left eye, then the right eye 3 days later | MOG-IgG cell-based assays | IVMP 1 g/day for five days | Visual acuity improved rapidly |
| Sawalha et al. (2020) | 44/Hispanic/Male | nasopharyngeal swabs (RNA PCR) and serum (IgG abs) | shortness of breath and cough | No | Two weeks | Right eye pain that had progressed to his left eye along with bilateral blurring of vision leading to a complete vision loss | NM | IVMP 1 g/day for five days | Complete restoration of vision in the left eye with remarkable but not complete vision recovery in the right eye |
| Zoric et al. (2021) | 63/NM/Male | serology was positive for IgM and IgG antibodies against the virus | fatigue, shortness of breath, dry cough and fever | Yes | Four weeks | Right eye blurred vision | Indirect immunofluorescence (MOG antibodies) | IVMP 1 g/day for five days with prednisone tapering therapy for two weeks | Visual acuity was improved, and disk edema was resolved entirely |
| Khan et al. (2021) | 11/NM/Male | nasopharyngeal swab was positive by CBNAAT | redness and ophthalmodynia in both eyes four days after a brief febrile illness | Yes | Two weeks | Loss of vision in the right eye | NM | Pulse methylprednisolone with oral steroids continued and tapered over 12 weeks | Visual acuity was improved |
| Kogure et al. (2021) | 47/Japanese/Male | nasal and oropharyngeal swabs (PCR) | asymptomatic | Yes | N/A | Left eye pain and an upper-visual-field defect | MOG-immunoglobulin G (MOG-IgG) testing in blood | IVMP 1 g/day for a total of 3 days, followed by an oral prednisolone tape | Visual acuity subsequently improved |
| Peters et al. (2021) | 23/NM/Male | nasopharyngeal PCR testing | asymptomatic | Yes | N/A | Progressive headache associated with dysesthesias fatigue, inattention, cognitive slowing, fevers, generalized seizures | MOG-IgG via FACS | IVMP 1 g/day for five days | Cognitive improvement |
| Durovic et al. (2021) | 22/NM/Male | PCR testing | severe headache, fever, neck stiffness, general weakness, and a loss of smell and taste | Ten days | Headache, neck rigidity | Serum MOG-IgG (live-cell assay) * | IVMP 1 g/day for five days | Symptoms resolved |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsouris, Z.; Provatas, A.; Bakirtzis, C.; Aloizou, A.-M.; Siokas, V.; Tsimourtou, V.; Grigoriadis, N.; Hadjigeorgiou, G.M.; Dardiotis, E. Anti-MOG Positive Bilateral Optic Neuritis and Brainstem Encephalitis Secondary to COVID-19 Infection: A Case Report. Neurol. Int. 2022, 14, 991-996. https://doi.org/10.3390/neurolint14040078
Tsouris Z, Provatas A, Bakirtzis C, Aloizou A-M, Siokas V, Tsimourtou V, Grigoriadis N, Hadjigeorgiou GM, Dardiotis E. Anti-MOG Positive Bilateral Optic Neuritis and Brainstem Encephalitis Secondary to COVID-19 Infection: A Case Report. Neurology International. 2022; 14(4):991-996. https://doi.org/10.3390/neurolint14040078
Chicago/Turabian StyleTsouris, Zisis, Antonios Provatas, Christos Bakirtzis, Athina-Maria Aloizou, Vasileios Siokas, Vana Tsimourtou, Nikolaos Grigoriadis, Georgios M. Hadjigeorgiou, and Efthimios Dardiotis. 2022. "Anti-MOG Positive Bilateral Optic Neuritis and Brainstem Encephalitis Secondary to COVID-19 Infection: A Case Report" Neurology International 14, no. 4: 991-996. https://doi.org/10.3390/neurolint14040078
APA StyleTsouris, Z., Provatas, A., Bakirtzis, C., Aloizou, A.-M., Siokas, V., Tsimourtou, V., Grigoriadis, N., Hadjigeorgiou, G. M., & Dardiotis, E. (2022). Anti-MOG Positive Bilateral Optic Neuritis and Brainstem Encephalitis Secondary to COVID-19 Infection: A Case Report. Neurology International, 14(4), 991-996. https://doi.org/10.3390/neurolint14040078








