1. Introduction
The battery industry is one of the fastest-growing industries in the world. In order to achieve a cost advantage over internal combustion engine vehicles, the manufacturing costs of the battery cells are a key factor since they account for 20% to 25% of the total battery cost and offer a lever for cost reduction [
1,
2]. The manufacturing process for lithium-ion batteries is subdivided into three main production areas as shown in
Figure 1: electrode manufacturing, cell assembly, and cell finishing. The challenges in cell finishing are the high dependency on previous production processes and the high number of possible process routes which are influenced by the material, cell format, and desired production as well as quality parameters. At the same time, cell finishing is a very time-consuming and therefore cost-intensive process step in the production of battery cells and thus an important factor in reducing production costs [
3,
4,
5,
6].
Current publications show that the share of production cost for cell finishing varies between 20% and 30% [
4,
7]. A large part of this is caused by just the formation process [
5]. The cost in the formation process is mainly driven by capital expenditures (CapEx) by the means of expensive equipment in large quantities as, due to high time consumption, high parallelization is required. High process times are partially related to unknown process quality relations, which are needed to control the process, and partially related to required innovative technologies. Another challenge is the high process time of aging as a part of the End-of-Line (EoL) test in which the cells are merely stored and tested over up to three weeks to measure the self-discharge. These are an extract of all challenges occurring in cell finishing. Since cell finishing is quite complex and not sufficiently researched, this paper attempts to give a detailed overview of the processes within cell finishing and their relationships as well as their respective pain points. It also presents optimization levers and research approaches that are being executed at the present moment to optimize cell finishing overall. Based on this, a technology roadmap for those developments is introduced in terms of the potential of the approach and the expected market readiness.
2. Cell Finishing Process Chain
To explain the function and tasks of the different production steps and technologies within cell finishing, this production area is divided into the following categories as shown in
Figure 2: pre-treatment, formation procedure, and quality testing. Pre-treatment focuses on the creation of optimal conditions for the formation itself. The formation procedure contains the formation cycles which describe the cell’s internal processes taking place during the first charging and discharging cycle(s) as well as auxiliary processes to either reduce process time or optimize the formation of the Solid Electrolyte Interphase (SEI) layer. Those auxiliary processes can contain high-temperature aging as well as degassing, sealing, and second filling. The last stage, quality testing, begins during room-temperature aging and closes with EoL testing and grading.
The pre-treatment can contain thermal or mechanical processes that improve or accelerate the wetting of the battery cell layers with electrolyte. Additionally, an electrical pre-treatment step (0.05 C, up to 0.8 V battery cell voltage) can be conducted that reduces copper foil corrosion and allows longer wetting times. Pre-treatment is particularly employed in the case of battery cells that are challenging for wetting such as large, densely packed battery cells, or designs that use low porosity cathodes and anodes.
Starting the formation procedure, a pre-charging step (0.05 C, up to 20% to 30% State of Charge (SOC)) can be conducted that allows the release of gas from the battery cell at an earlier process stage. This step is used for lager battery cells and materials that create a high amount of gas from side reactions such as high-nickel cathodes. The following main formation procedure triggers the development of protection layers on the surface of both electrodes. On the anode side this layer is called “Solid Electrolyte Interphase” (SEI), and its quality strongly influences the performance, quality, and safety of the battery cells. On the cathode side the layer is called “Cathode Electrolyte Interphase” (CEI). The conducted charging and discharging protocols aim to ensure stable and homogeneous electrolyte interphases [
3]. To complete the formation and ensure good quality, several auxiliary processes can be added in between or after the formation cycles. Degassing is necessary to release the gas that is produced as a side reaction during formation. A second filling which adds more electrolyte can further improve the wetting degree by compensating for the loss of electrolyte during the first cycle of the formation. Sealing ensures the air-tight and liquid-tight closing of the battery cell while aging ensures the completion of the remaining chemical side reactions and wetting processes taking place after formation. During aging, the open circuit voltage is monitored to detect voltage drops over an extended time which also acts as a first quality test of the battery cell.
In the End-of-Line test, the battery cell undergoes further quality checks for relevant parameters such as capacity, internal resistance, weight, and dimension. Pulse power or EIS are potential methods for quality testing, too. Afterwards the battery cells are sorted according to the quality level reached, which is known as “grading.”
Since there is no standardized process order in cell finishing and every cell manufacturer is developing their own production protocol to fit their individual requirements and cell characteristics, different process routes are possible.
Figure 2 shows different process routes for the existing prismatic, cylindrical, and pouch cell formats. The time required for each process step is displayed qualitatively in the figure as well. The process route of the cylindrical cell shown here has the shortest process time. Usually, smaller cells (cylindrical cells as well as small pouch and prismatic cells) have smaller energy content, and therefore the cell finishing process steps which are necessary for larger cells are shorter or sometimes even skipped. This results in shorter wetting times, limited pre-charging, and no second electrolyte filling or degassing. Formation and aging processes can be shortened due to the smaller energy content. Today’s prismatic and pouch cells are often much larger than cylindrical cells [
8]. Therefore, the pre-treatment process takes much longer for those cells. There are different options for how to shorten the pre-treatment time which will be introduced in detail in chapter 2.1.
2.1. Pre-Treatment
After the first electrolyte filling the cells are stored in racks and the wetting process starts immediately (
Figure 3) [
9]. As the first step in the cell finishing process chain, it serves to create a sufficient wetting degree for conducting the formation. This is usually achieved by a homogenous distribution of the electrolyte into the electrodes and separator [
10]. The penetration of the electrolyte into the pores of the electrodes and the separator establishes the performance of the ionic conductivity [
11].
A homogenous distribution of electrolyte is necessary as it has a crucial influence on the ionic conductivity and therefore on the distribution of current inside the cell. Only at fully wetted areas of the electrodes can ionic conductivity, and thus a current flow, be possible. Therefore, the wetting process has a high influence on cell quality, as a poor wetting degree can cause lithium plating on the surface of the electrode [
12,
13]. If the electrodes are not wetted equally, the risk of lithium plating on the surface of the electrodes is very high, and thus a crucial safety risk is introduced [
12].
The industrial standard is to conduct the filling process under negative pressure. Here, a vacuum down to 100 mbar is created in the lithium-ion cell so that the electrolyte can be filled into the cell quickly and efficiently [
10,
11]. The vacuum created is also beneficial for the wetting process since the compression forces allow the electrolyte to penetrate the electrode and separator structure more quickly. The pre-treatment process contains all processes, leading to a sufficient wetting degree for an optimal formation. The process can be subdivided into “thermal pre-treatment,” “mechanical pre-treatment,” and “electrical pre-treatment.” The order, the integration of the process steps, the process parameters, and their duration are dependent on the cell design and format.
2.1.1. Thermal Pre-Treatment
The term “thermal pre-treatment” summarizes everything that includes the storage of the lithium-ion battery cell under high-temperature conditions for a soaking step, called “high-temperature soaking.” By elevating the wetting temperature to 40 °C to 60 °C, the liquid electrolyte contact angle is reduced and the access of the electrolyte to the electrode mesopores is improved [
14,
15,
16]. This wetting/soaking process can last between 12 and 24 h for larger cells since it is related to the electrode surface area, electrode thickness, the porosity, separator, and cell geometry [
16]. The thermal pre-treatment is conducted either in larger climate chambers or climate rooms. The cells are therefore stored in rack systems [
17].
2.1.2. Mechanical Pre-Treatment
Another process type to reduce the wetting time and improve wetting quality are mechanical pre-treatments. After the first wetting phase, bubbles often still remain inside the battery cell. In particular, micro-sized bubbles existing inside the battery cell do not disappear for a long time, increasing the amount of dissolved oxygen and thus adversely affecting the capacity and aging behavior of the battery. There are two major approaches for the application of mechanical forces on the cells which will be introduced in the following: roll pressing and megasonic vibration. During roll pressing, the lithium-ion pouch cell is clamped in a special carrier with the help of a gripper while pressure is applied by two rollers. This step serves as preparation for the subsequent formation because electrochemically inactive areas are avoided due to the pressurization. This process step can only be applied for pouch cells, as cylindrical and prismatic cells cannot be handled like this because of their solid housing. By applying megasonic vibration to a preliminary battery cell, not only is it possible to increase a direct physical contact between an electrode assembly and the electrolyte due to vibration, thus enhancing the wettability of electrodes and separator, but it also enables the removal of small-sized bubbles and minimizes damage to the battery cell. Therefore, the time and cost required for manufacturing the battery cell is saved, and the safety of the battery cell is improved. To apply this technique, the battery cells are stored in a tray which is connected to an excitation unit [
18,
19].
2.1.3. Electrical Pre-Treatment
The industry also includes pre-charging steps with small currents prior to the formation process to improve the wetting degree and to avoid copper foil corrosion. By applying small C-rates up to 0.05 C, the soaking process can be prolonged without limited copper corrosion on the electrodes. The pre-charging can also be conducted as a part of the first charging cycle up to 20% to 30% SOC. Another reason for conducting a pre-charging lies in the huge gas development of high energy battery cells, which occurs mainly in the first 20% to 30% SOC in the first charging cycle, due to the SEI formation. Between pre-charging and formation, an initial degassing and optional second electrolyte filling are performed. Pre-charging results in higher cell quality due to the lower influence of gas bubbles between the electrodes for subsequent formation sequences and allows the formation of large-format cells. The downside is that two degassing and two formation steps are required [
3,
20,
21].
2.2. Formation Procedure
The formation procedures include all processes that serve the purpose of forming and stabilizing the SEI layer optimally. Therefore, this process includes not only the actual formation but also other auxiliary processes that either optimize the formation of the SEI in terms of quality and service life or accelerate the process. The main task of the formation procedure is the development of the passivation layers at both boundary layers of the electrodes to the electrolyte [
22]. Therefore, a strong dependence between the passivation layer quality and the formation process exists. The passivation layer at the anode is called “Solid Electrolyte Interphase” (SEI) layer, while the one on the cathode is known as the “Cathode Electrolyte Interphase” (CEI) layer. Both passivation layers protect the electrodes from further decomposition [
23,
24]. The SEI layer has a significantly greater influence on the safety and performance of the battery cell and is therefore primarily considered in this publication [
25,
26]. The aim of the formation process is the optimal composition and homogeneity of the protection layer, especially on the anode side. The ideal SEI contains an inorganic layer close to the electrode and a porous organic or polymeric layer close to the electrolyte [
25]. The layer should be 50 to 200 nm thin and homogeneously distributed on the surface of the anode [
27]. The formation of the SEI occurs by exceeding the stable potential range of the electrolyte, as the potential of graphite exceeds the stability window of the electrolyte during the charging process [
28]. As a by-product of the SEI formation reactions, gases are generated during formation. Commonly used electrolytes have reduction potentials of about 1 V, i.e., below this voltage they can be reductively decomposed (
Figure 4). The first step of the formation takes place between anode potential of 0.8 V and 0.25 V vs. Li
+. From 0.8 V, gas formation begins, which originates from decomposition reactions of the electrolyte solvent. One of the most dominant reactions involves the production of ethylene from electrolyte reduction, which occurs from 0.8 V until the charging cycle is completed. From 0.7 V, the surface morphology of the graphite anode changes [
29,
30,
31]. From a voltage of U ≤0.25 V, Li
+ ions are reversibly intercalated into the graphite anode. The resulting SEI layer consists of an inorganic inner layer near the electrode/SEI interface and an organic layer near the SEI/electrolyte interface. Components of the inorganic layer which allow Li
+ transport are, for example, Li
2CO
3, LiF, and Li
2O. On the other side, the organic layer is composed of products such as dilithium ethylene dicarbonate (Li
2EDC) and ROLi, where R depends on the solvent. Li+ but also electrolyte solvent molecules can penetrate the organic layer [
30,
32,
33,
34,
35].
It is possible to influence the ratio between organic and inorganic components and the amount of produced gases by changing the current density. Low current density leads to a larger proportion of organic components because more single electron reactions are carried out. This results in the formation of an excessively thick SEI layer, which leads to an increase in resistance and consequently to reduced cell performance. On the other side, high current density leads to a larger proportion of inorganic components because more electron pair reactions are favored due to a higher number of electrons reaching the anode. The result is a thinner, more uneven, and more porous (less resistant) SEI [
27]. Moreover, the components of the electrolyte can influence the SEI layer. Additives like FEC, DFEC, and VC assist in forming a more stable SEI due to their fast polymerization and leading to a controlled SEI growth as well as reducing the amount of formed gases [
27,
35,
36]. The quality of the SEI layer has a decisive influence on the cycle stability, lifetime, performance, and safety of lithium-ion cells [
37,
38,
39] and is strongly dependent on the previous process parameters, e.g., materials, electrode manufacturing parameters, and wetting degree/amount of electrolyte, and the settings of the formation parameters, especially the C-rate [
3,
40,
41,
42,
43,
44]. The choice of cell finishing process order design, especially in the formation step, varies from one manufacturer to the other, e.g., in C-rate and number of cycles as well as upper and lower voltage limits [
45]. Relevant process parameters in the formation are C-rate, number of cycles, temperature, and external pressure.
2.2.1. Pre-Charging and Formation
The formation process is defined as the first complete charge and discharge cycle(s) of the battery cell (
Figure 5). One option to allow the SEI layer to be formed homogeneously and with a robust, rather than porous, structure is to apply a low C-rate in the first charge cycle [
3,
45]. The C-rate is then continuously increased with each charging and discharging cycle [
3,
5,
45]. Another option is to integrate a pre-charging step up to 20% to 30% SOC. Due to the increased gas development of high-energy battery cells, which mainly occurs in the first 20% to 30% SOC in the first charging cycle, the SEI formation and formation time can be optimized by integrating a pre-charging step and thus a degassing and optional second electrolyte filling. Pre-charging results in higher cell quality due to less influence of gas bubbles between the electrodes for subsequent formation sequences and allows the formation of large-format cells. The downside is that two degassing and two formation steps are required. The formation procedure represents one of a cell manufacturer’s core competencies and can vary in terms of the sequences, included steps, and duration. Due to high competition between manufacturers, information about the exact formation procedure is not disclosed [
46]. For smaller cells, the formation in the industry usually contains only one cycle [
3,
39]. Due to higher gassing and safety requirements, larger cells contain two or more complete charge and discharge cycles [
13].
Research-based formation protocols currently apply C-rates between 0.05 C and 0.5 C or even higher, depending on the purpose and research goals of the formation. Depending on the C-rate, one to five full cycles are applied during formation. The ambient temperature is set between 20 °C to 50 °C. Especially for pouch cells, mechanical pressure is usually applied during formation [
16,
47,
48,
49].
2.2.2. Degassing and Second Filling
During the formation, gases are generated by electrolyte reduction reactions with the anode and cathode. Those gases must be removed from the battery cell (
Figure 6). The most commonly identified gases produced during the operation of LIBs are CO
2, C
2H
4, H
2, O
2, and CO. During the first formation cycles, H
2, CO, and C
2H
4 are the most typical gases formed. However, when the cell voltage is increased, CO
2 evolution at the cathode dominates. CO
2 and H
2 concentrations have been found to increase at elevated temperatures under open-circuit voltage due to chemical reactions of the electrolyte with the electrode materials [
50]. Nevertheless, the graphite of the anode can act as a sink for CO
2, which is generated through oxidation reactions at the cathode. Therefore, the CO
2 concentration is decreased in the exhaust gas during the degassing process [
51].
The gases that have been generated during the formation must be removed in the degassing process to prevent safety issues, reduced cell life, and performance losses. Gas bubbles are deposited in the porous material in addition to the emerging gas and prevent the penetration of the electrolyte for the reactions at these places. The degassing process takes place in a vacuum chamber and varies depending on the cell type. Pouch cells have a gas pocket where the gas is accumulated. The bag is subsequently punctured and the gases are vacuumed out. While still in the vacuum chamber, a final seal is applied to the cell. The empty pocket is then separated from the cell. In the process of degassing prismatic cells, the gases are drawn out through a small hole which afterwards is filled with a permanent seal, e.g., a metallic sphere [
12,
52]. Due to their size and therefore the small amount of formed gas, cylindrical cells normally do not have a degassing step. This applies also to the smaller pouch and prismatic cells. For large cells, it is necessary to first perform a pre-charging with subsequent degassing, because the amount of emerging gas increases proportionally to the amount of active material while the volume of the cell hardly increases. For highly gassing materials and large prismatic cells, there could also be more than one degassing step. As the size of battery cells for all formats has increased in the last years, the degassing step will also become more important for cylindrical cells. This results in a conflict between a desired cheap and fast process and the removal of gases which needs to be done more often. From a production safety standpoint, degassing should happen at a low state of charge (SOC) because the released energy in case of a short circuit is directly connected to the SOC, which is another conflict with the economic efficiency. After the degassing for prismatic and pouch cells, a second filling step can be included to refill the electrolyte consumed during the formation [
53].
2.2.3. High-Temperature Aging
The aging process can be divided into two different phases, namely high-temperature aging (HT aging) at a temperature of 30 °C to 50 °C and room-temperature aging (RT aging) with a temperature of approximately 20 °C to 25 °C. After formation, open-circuit voltage (OCV) measurements are performed to determine the self-discharge behavior of the battery cells. Unusual self-discharge rates indicate electrochemical defects and micro-shorts due to damaged separators. During the aging phase, there are also other reactions happening due to lithium-ion concentration balancing in the electrodes, final SEI stabilization reactions and final electrode wetting processes after a second filling. The described reactions can also lead to a drop in the OCV measures but are not related to any quality defects. These high-temperature phases can be integrated into the formation protocol or at the end of the formation [
11,
17].
2.3. Quality Testing
Quality testing processes include all processes for quality assurance and have no further influence on the battery cell quality itself. This includes room-temperature aging, End-of-Line (EoL) testing, and an optional grading of the battery cells.
2.3.1. Room-Temperature Aging
The room-temperature aging, unlike the high-temperature aging, is only performed for the purpose of quality assurance of the battery cell (Chapter 2.3.1.). Since the self-discharge rate is low for battery cells, the aging process can take up to three weeks for larger cells to be able to make valid statements about the quality. During this time the cells are stored in racks (
Figure 7).
The parameter that is mainly measured is the open-circuit-voltage (
OCV). Furthermore, parameters such as internal resistance and capacitance can also be measured during the aging process. By monitoring the
OCV, observations can be made on the self-discharge of the cell. In this process, the voltage loss must not exceed a defined value. The measured voltage must be divided by the cell resistance to obtain the self-discharge, which also causes an inaccurate self-discharge measurement. Another measurement principle based on
OCV is the
K-value. The
K-value is a physical quantity to describe the self-discharge rate of the cell. It is calculated by dividing the difference in
OCV between two measurements by the time interval between the measurements. Thus, it can be referred to as the rate of voltage change [
54].
The second reason for the time-consuming aging process is that reliable performance parameters can only be determined after a certain period of time. Battery cells are electrochemically unstable after electrolyte filling and formation. During storage, chemical reactions take place within the cells, such as the conversion of by-products and impurities, which serve for electrochemical stability and especially for SEI stabilization. These are called “self-discharge mechanisms.”
2.3.2. EoL Testing and Grading
During the EoL testing, various quality parameters are tested to ensure the function, quality, and safety of the battery cells (
Figure 8). These characteristics can be divided into four categories: performance, safety, lifetime, and mechanical properties. The quality characteristics in these categories are determined by various values, e.g., capacity, voltage range, internal resistance, self-discharge, and rate capability (the ratio of the possible power output in relation to energy content of the battery cell). The mechanical properties (e.g., deformed housings or tabs) can have little influence on cell functions, but influence the usability of the battery cells and are therefore required by certain customers. Technologies, the duration of the tests, and the test sequence are not specified or standardized in the literature and are dependent on the respective manufacturers of the cells.
The measurements that are predominantly reported in the literature are OCV, internal resistance, capacity, and pulse test. Additionally, the leak tightness is tested and an optical inspection is conducted. Some of these values can already be measured prior to EoL testing. For example, the OCV is tracked over an extended time during the aging process. During the formation process, a first indication on the capacity of the battery cell can be observed by looking at the data gathered for discharge capacity and charge efficiency (ratio of discharge capacity to charge capacity). The internal resistance is often measured between the different process steps of the formation procedure. Pulse testing is commonly used to assess battery cell characteristics by observing the voltage response of the battery cell during the pulse duration. This involves observing the dynamic response of the battery cell through loading and unloading pulses to determine its rate capability and available energy. Capacity tests are used to determine the energy capacity of the battery cell in its delivery state. Usually this can be done in a single dedicated station for electrical measurements [
55,
56].
The EoL testing results allow the sorting of the battery cells by quality in a downstream process called “grading” or “classification.” However, this process is optional and dependent on the manufacturer. In many cases, all battery cells that do not reach the desired quality characteristics are sorted out and not delivered to a customer but rather scrapped or recycled. If a grading process is conducted based on the data obtained during testing, cells are sorted into several classes according to their performance data. It is also common to sort and bundle cells with similar internal resistances into groups. This helps to minimize cell-to-cell variations within the battery modules and thus reduces the balancing effort [
56,
57].
3. Industrial Pain Points and Research-Based Solutions
Due to the high costs and process time within cell finishing, there is a high need from the industrial perspective to optimize cell finishing [
4]. In the following, the main pain points in the cell finishing process will be introduced and the related solutions and future concepts currently being researched will be described.
3.1. Wetting
Before the start of formation, the surface of the electrodes, in other words, the active materials, must be completely soaked. The main pain point is to find the trade-off between a sufficient wetting degree and the shortest possible process time and to be able to measure the wetting degree inline not only on a macroscopic but also on a microscopic level [
58]. To reduce the wetting time, the main solutions that are already applied are the elevation of the temperature during the soaking process and the application of vacuum during electrolyte filling. In order to detect when the optimal penetration degree is reached, new technologies such as chronoamperometric measurements, electrochemical impedance spectroscopy, ultrasonic measurements, and x-ray to analyze the wetting degree inline are currently being investigated. These technologies will be introduced in the following.
3.1.1. Chronoamperometric Measurements
During chronoamperometric measurements, a constant potential is applied to the cell immediately after the electrolyte wetting process. Once initiated, the current response is carefully analyzed as it reflects the progress of the electrolyte wetting process accompanied by SEI film formation. As a consequence, an electrochemical reaction is triggered at the electrode that is in contact with the electrolyte. The initial potential, which is applied during the chronoamperometric measurements, is an important parameter and has to be chosen carefully. To detect the electrolyte wetting degree, the potential of the anode has to be within 1.2 V and 200 mV to consider only the SEI film formation reaction. By applying this procedure, the influence of different separators, cell formats, and ambient temperatures on the wetting time can be investigated [
59].
3.1.2. Electrochemical Impedance Spectroscopy
Electrochemical Impedance Spectroscopy (EIS), a method for analyzing the processes in batteries, is increasingly being used not only in material development, but also in diagnostics and as a basis for modelling. In the meantime, EIS is finding its way into the production of battery cells, especially in the field of cell finishing [
60]. EIS is a non-destructive technique to determine the impedance of a lithium-ion battery working in two possible modes: galvanostatic and potentiostatic. Most commercial impedance spectroscopes use the potentiostatic method, which means that a voltage signal at a certain frequency is imposed and the current response is measured. The resulting response will have the same frequency, but a different phase angle and amplitude. The potentiostatic method is more common in battery cell analysis. From the measured complex cell impedance, it is possible to examine and qualitatively determine several processes of an electrochemical system. The time constants for these processes differ, so that their features show up at different frequencies in the EIS spectra [
61,
62,
63].
The advantage of the potentiostatic method is that the amplitude is controlled, ensuring that the responses remain in the linear range when appropriately limited. The impedance spectroscope must adjust the current accordingly. No adjustments are required by the experimenter depending on the state of the battery [
60]. By applying EIS to the wetting process, the wetting degree of the LIB can be determined. At the beginning of the wetting process, the ohmic resistance of the LIB is very high since there is little ion conductivity inside the cell. With increasing wetting degree and thus ion conductivity, the ohmic part of the impedance decreases until a certain limit is reached. Once this limit is reached, a fully wetted battery cell can be assumed [
21].
3.1.3. Ultrasonic
Ultrasonic testing and acoustic emission (AE) are among the non-destructive testing (NDT) methods. They are cost-effective and offer a high degree of spatial and temporal resolution. AE records acoustic signals generated by the test specimen. In ultrasonic testing, an acoustic signal is generated and introduced into the test specimen. Due to the physical properties of sound waves, a fraction is reflected and recorded in time. There are various application-specific testing techniques to perform ultrasonic testing [
64]. During ultrasonic testing of lithium-ion batteries, a correlation between the “State of Health” (SoH), SOC, and the detected acoustic signal was proven. The cyclic charging and discharging of the battery lead to electrochemical changes in the active intercalation materials, which in turn leads to mechanical changes in these. The consequence is a shift of the detected ultrasonic signal at different SOC of the cell [
65,
66,
67,
68]. Imaging methods of ultrasonic testing provide a two-dimensional insight into the internal structure of the cell. Differences between solid, liquid, and gaseous structures can thus easily be examined, due to their different reflection behavior. This makes it easy to observe the wetting process, how the electrolyte is distributed, and which areas cannot be wetted well [
69].
3.1.4. Imaging Technologies (X-ray, Neutron Radiography, etc.)
With imaging technologies like X-ray and neutron radiography, it is possible to visualize different wetting behaviors inside the battery cell. This is primarily used for the process and product design to show exactly which areas are wetted first, which are wetted last, and which areas are not wetted at all. Therefore, the product and process design can be optimized to achieve optimal wetting [
70,
71,
72,
73,
74,
75].
3.2. Formation
Identifying the fastest possible formation protocol by simultaneously maintaining or increasing the quality of the SEI layer is the main pain point in the formation [
73]. Different studies estimate the formation process costs at up to 30% of the total production cost [
1,
74,
75]. In the formation process, not all dependencies and relations regarding the SEI layer formation have been identified and analyzed yet. Today’s research is focusing on identifying the relevant process parameters and analyzing their sensitivities on the cell quality and process impact. The possible combinations of parameter settings in terms of C-rate, protocol design, temperature, and pressure adjusted to the material combination and previous process steps lead to innumerable possibilities and directions to improve this process. A lot of development has also been undertaken regarding inline quality measurement technologies in the formation, but until today no technology can directly provide reliable information about the quality of the SEI and cell in the formation process. Main research areas are optimization of the formation protocol, thermal and mechanical influences, and the application of an artificial SEI layer.
3.2.1. Reduction of the Maximum Charging Cut-Off Voltage
The aim of the reduction of the maximum charging cut-off voltage is to dispense with the intercalation processes that take place from approx. 0.25 V to 0.2 V vs. Li/Li
+ onwards after the formation of a functional SEI layer and to terminate the loading process [
76]. Lee et al. (2004) [
77] showed with the help of a half-cell investigation of the graphite anode that the SEI layer forms sufficiently until the voltage of 0.3 V vs. Li/Li
+ is reached. It was observed that the cycling performance of the cells with final charge voltages of 3.7 V, 3.8 V, and 4.2 V were almost identical. Furthermore, impedance measurements did not show any major differences between the cells with end of charge voltages of 3.7 V and 4.2 V. On the other hand, higher capacity losses and poorer impedance results were found with a charge termination voltage of 3.6.
3.2.2. Dual-Current Protocol
With the dual-current protocol, the formation protocol is defined in a way that the intercalation ranging from approximately 0.2 V to 0.3 V vs. Li/Li
+ is not eliminated but passed through at a higher C-rate. Thus, the total duration of the formation can be reduced. Compared to the normal CCCV procedure, this protocol includes a two-step CC phase. At the beginning, the cell is charged with a low C-rate up to approximately 0.2 V to 0.3 V vs. Li/Li
+. Subsequently, the C-rate is increased as the intercalation processes of non-solvated lithium ions into the crystal structure of the graphite begin and the SEI layer is not subject to further changes due to decomposition reactions of the electrolyte under optimal conditions. The increase of the C-rate is done considering the SEI formation process, as the mere increase can lead to non-uniform passivation of the anode and to an inhomogeneous thickness of the SEI layer. Furthermore, high C-rates can lead to lithium plating, rapid resistance rise, and low capacitance retention. A typical dual-current formation protocol is shown in
Figure 9a [
78,
79].
3.2.3. Shallow-Cycling Protocol
The shallow-cycling formation protocol was derived based on the following assumptions:
Most SEI and CEI layers are formed in the higher SOC range because the electrolyte undergoes more reduction reactions at the anode and more oxidation reactions at the cathode in this range.
The SEI layer formed in the higher SOC region is more compact and stable than when formed in the lower SOC region. The potentials in the higher SOC lead to higher electrolyte stability, and more lithium is made available at the anode for reduction with the bulk compounds.
The SOC should be kept high for a long time and low for a short time, as this approach leads to a more compact and stable SEI layer. However, the SOC should not simply be kept constant in the high-voltage range, as this would cause the current flow to drop steadily to zero.
This formation protocol involves several repetitive charging and discharging cycles within a high SOC range. The full discharges between cycles are to be avoided, and instead more time is spent in the higher SOC range. There is only one full discharge in the last cycle. Avoiding the full discharges within the cycles results in a shortened formation time. The combination of higher C-rates in the low SOC range and the repetitive charging and discharging in the higher SOC range with lower C-rates can reduce the formation time. A typical shallow-cycling formation protocol is shown in
Figure 9b [
46,
48].
3.2.4. Pulse Formation
The charging profile in the pulse formation consists of a rest phase, a charge phase, and an optional discharge phase. In contrast to traditional processes, the current is not constant during formation. During the charging pulse, high C-rates (between C/2 and 3C) are applied which causes a delivery of lithium ions to the anode. At the resting phase, the lithium ions diffuse into the anode and oxidize. This creates an SEI layer which influences the performance of the battery. The optional discharge phase prevents an accumulation of lithium ions at the anode, the so-called lithium plating. A typical pulse formation protocol is shown in
Figure 9c, with variables that include resting duration between pulses (T
R), the magnitude of pulse current (C
P), and the pulsing time (T
P). For this case, C
P is set between 0.2C to 1C for different experiments, T
R is set to 1.5 ms, and T
P is set to 0.5 ms [
2].
3.2.5. Thermal Influence
Increasing the ambient temperature during formation can help reduce the formation time. The reaction rate temperature rule states that chemical processes take place disproportionately faster with increasing temperature [
49].
3.2.6. External Mechanical Pressure
The pressure causes the diffusion path to be reduced by decreasing the physical distance between the electrodes. Heimes et al. [
49] assume that this improves the diffusion of the electrolyte into the porous structure of the active material. The reduced diffusion path and the improved electrolyte diffusion accelerate the reaction rates and reduce the internal resistance. In addition, reducing the distance between the anode and the separator could reduce the risk of exfoliation and promote a thinner and more uniform SEI layer [
49,
80].
3.2.7. Pulse-Artificial SEI Layer by ALD Process
Another approach to accelerate the formation process is the application of an artificial SEI layer on the graphite anode by means of the so-called Atomic Layer Deposition (ALD) process. During the ALD process, starting materials, also called “precursors,” are sprayed alternately onto a surface. Chemical reactions take place on the surface of the material. Between the inlets of the precursors, the surface is flushed with inert gas. ALD technology enables homogeneous distribution and precise application of ultra-thin layers in the nanometre range [
81,
82].
3.2.8. Anode-Potential Controlled Formation Protocol
The potential of the graphite is close to the lithium-metal potential for a high degree of lithiation. Due to high currents or lower temperatures the overpotential of the cell rises. This results in a voltage drop and can lead to a negative potential of the anode. Negative anode potentials favor the formation of metallic lithium on the anode, known as “lithium plating.” Lithium plating reduces the capacity of the lithium-ion battery, because the lithium ions are deposited on the surface of the anode instead of intercalating into the anode structure, thus leading to capacity degradation. Furthermore, with increasing the number of cycles, lithium plating can lead to dendrite growth and therefore to an increase of the internal short circuit risk. Lithium plating is highly dependent on the charging rate as well as the used material combination and cell design [
83,
84].
For this formation strategy, a lithium reference inside the cell is required to monitor the anode potential. Since negative anode potentials favor lithium plating, this strategy aims to keep the anode potential higher than 0 V. The charging cycle starts with a CC phase limited due to the maximum allowed charging current, until the anode potential reaches theoretical 0 V. For tolerance and accuracy reasons, a potential higher than 0 V is set as a boundary, e.g., 10 mV. The CV phase is controlled by the anode potential rather than the cell voltage to ensure it will not drop below 0 V. The CV phase is performed until the current drops below the cut-off limit or the cell voltage reaches the upper limit [
85,
86,
87].
3.2.9. Electrochemical Impedance Spectroscopy
EIS measurements are usually performed when no load is applied to the cells, since the cell voltage is not allowed to change during the measurement. Studies on EIS in the formation process show that the impedance spectra highly change during the formation. Thus, there is always a pause in the formation process to perform the EIS measurement at a certain cell voltage.
Figure 10 shows the impedance spectra for several anode potentials. In most voltage ranges, the EIS is composed of two partially overlapped semicircles and a straight sloping line at the low frequency end [
88]. The influence of formation pauses to perform EIS on the cell quality is not clarified. Nevertheless, there are techniques to achieve a successful measurement under load with a so-called drift correction [
60]. The drift correction is an enabler for online EIS measurements, e.g., during the formation, and can deliver great information about cell quality and SEI status. So far, online EIS measurement during the formation process without pausing it has not been considered scientifically.
3.2.10. Ultrasonic
SEI formation and development are investigated with both AE and ultrasonic testing. In the AE investigation, a correlation between acoustic activity and the voltage range of initial SEI formation is observed. This correlation is attributed to the gas emissions and the formation of a solid layer on the anode material. Furthermore, it was found that the C-rate of the cell does not influence the amount of acoustically generated emission signals [
89,
90].
Ultrasonic testing is significantly hindered during initial SEI formation due to heavy gas formation. Subsequently, a correlation between Time-of-Flight (ToF) shift and capacity loss in the formation can be demonstrated. The ToF shift stagnates with flattening capacity loss. Chemical analyses of the pouch cells showed that the degree of passivation at the anode can be determined via in operando acoustic measurements [
91].
3.3. Aging
As described in the previous chapter, aging can last up to several weeks and therefore takes up most of the time in the cell finishing process. The reason for this is that the determination of reliable performance parameters is only possible once the chemical reactions which take place during storage have subsided and the quality of the cell can be determined with a high degree of probability using current technologies. The reduction or even elimination of the aging and EoL test for quality detection is one of the main pain points. The goal is to detect the cell quality directly after formation or to use predictive quality concepts in the battery cell production.
3.3.1. (Parallel) Potentiostatic Measurement of the Self-Discharge
This method is the direct measurement of the self-discharge current of a cell. It allows a faster analysis of the self-discharge rate of the battery cell. In the potentiostatic method, a low-noise, very stable DC source is set to match the OCV of the cell. The DC source is then connected to the cell via a micro-amperemeter to measure the current between the DC source and the cell. Now, as the cell continues to self-discharge, the DC source takes over and provides enough current to keep the cell at a constant voltage and SOC. When the DC source reaches equilibrium with the cell, the self-discharge current is no longer sourced internally, but entirely externally from the DC source. The self-discharge of the cell can then be measured directly with the micro-amperemeter [
92].
3.3.2. Predictive Quality
Another concept to reduce aging time is predictive quality. According to Küpper et al. [
93], data collection along the process chain, together with advanced analytical tools, can be used to determine the risk of micro-shortages within the cell. Only the battery cells whose quality remains unclear need to go into the aging process. This approach known as “on-demand aging” can reduce the overall aging time in cell production by 80%.
3.4. Degassing
During formation, especially at the beginning of the process, a gas evolution occurs. The standard procedure is to separate this gas after formation as described in the previous chapter. In larger battery cells, the high pressure due to the gas evolution can cause damage during the formation process or inhibit faster procedures. But the integration of the degassing process into the formation process at a specific SOC also leads to a higher logistic effort. The trade-off between an optimized formation process regarding quality, time, and a lower process effort is the main pain point in the degassing process.
3.5. End-of-Line Testing
The major challenges in the End-of-Line testing are the time-consuming procedures and the expensive equipment. Testing can take up to several hours especially due to the capacity testing that requires a full charge-discharge cycle. At the same time, the increase of the average battery cell capacity requires equipment that can deliver higher currents to not further prolong the capacity testing. With rising cell sizes, the quality of the individual battery cell also becomes a more critical subject. This leads to the need for more accurate measurements in the testing procedure.
Integration of End-of-Line Testing into the Production Process
The most important solution to this problem is the increased use of inline measurements before the battery cell even reaches End-of-Line testing. This is also to be combined with the extensive use of the data collected during the production process. By collecting data in large amounts during production and by improving the evaluation models, certain aspects of the battery cell’s quality can be predicted before the End-of-Line testing. Currently, a large research effort is being made to better understand the relationships between inline parameters and the quality of the finished battery cell. On the one hand, this enables integrating quality gates into the production and filtering out the battery cells that do not meet the quality requirements before End-of-Line testing. On the other hand, simplified End-of-Line testing is made possible. For example, the depth of discharge for the capacity test can be much lower so that only a small section of a cycle has to be observed to complement the already existing data and to obtain a reliable image of the cell quality [
58,
94].
4. Research Roadmap
The research approaches listed here illustrate that there are many drivers for process optimization in cell finishing. In order to stress out the potential of the different approaches, in
Table 1 current publications were evaluated with regard to cost-saving and time-saving potentials as well as quality improvement potentials.
The results show that the approaches in the area of pre-treatment are mostly used to set the optimum time for complete wetting as quickly as possible or to be able to detect this using measurement technology. This leads to a reduction in time and, depending on the technology used, to a reduction in costs. However, this does not have a major impact on quality.
The optimization approaches in the formation process are mainly used to fully investigate the SEI formation and influencing parameters and then to apply them to make the formation process as fast as possible. On the one hand, this leads to a higher quality SEI layer, since it can be formed as needed. On the other hand, potentials for time reduction are also to be identified with the help of the approaches. In the area of formation, this means voltage ranges and cycles in which a faster current rate can be used for formation. These are areas that are less relevant for the formation of the SEI layer or have less influence on it. This leads to a reduction in both time and costs.
The approaches to optimization of EoL testing focus primarily on reducing or eliminating certain quality testing steps, e.g., the self-discharge test. In the long term, this leads to a reduction in time and costs, as the cells no longer have to be stored for several weeks, thus reducing the area or increasing the throughput of the factory.
Based on the research results to date, a roadmap for cell finalization has been drawn up. This roadmap shows when each optimization approach can be expected to be used in industry.
Figure 11 shows all approaches including a timeline until the technology can be expected to be used in an industrial application (reaching TRL 9). The integration of a pre-charging step, the application of temperature and pressure, and different formation strategies are already used but not yet fully understood for optimal use. In particular, with regard to material and certain process dependencies, there are still no precisely definable interactions with the settings in the formation step. The use of first simulation models and data-based optimization approaches is expected to make major progress in the next few years. Through a partially simulated SEI formation or through a data-based approach, such as machine-learning algorithms, certain correlations can be derived. These approaches are expected to support the development of a cell finalization concept in production labs from 2024/2025. Significantly later, the use of an artificial SEI layer will become relevant. Since all-solid-state battery (ASSB) manufacturing also requires the application of thin film interfaces (according to current product planning), this technology will also become possible and transferable for lithium-ion technology due to the demand in the ASSB area. These developments are expected on an industrial scale around 2028–2030. A fully digitized production which is capable of inline optimization is not expected until around 2030 at the earliest. With this production line, the formation could be adapted inline to the upstream process steps or a demand-oriented cell finalization could be created.
5. Conclusions and Perspective
The battery cell finishing process plays a crucial role in battery cell production. On the one hand, cell finishing accounts for 20% to 30 % of the entire battery production cost, and on the other hand it has great impact on the overall battery cell quality.
The battery cell finishing process comes with many different routes and process alternatives depending on the format, size, and chemistry of the battery cell produced. Therefore, this paper has analyzed the different processes and their routes. The cell finishing process was divided into three main areas: pre-treatment, formation procedure, and quality testing. Pre-treatment mainly takes the function of creating the optimal conditions for the formation process, such as the assurance of a fully wetted cell. The formation procedure deals with the creation of an optimal SEI layer to assure function and safety of the battery cell, with the formation as its core process. The after-treatment mainly covers quality assurance and has no direct impact on the battery quality itself. Up to now, there does not exist a standard cell finishing process dominating the industry, but many different procedures depending on the cell manufacturer.
Due to the long process times and expensive power electronics of the formation process, formation and aging take the greatest share of time and cost in battery cell finishing. Therefore, the industry is keen on shortening those process times to lower production costs. Several research-based solutions to this industrial pain point were addressed in this review. For example, alternative formation protocols, control of external pressure and temperature, and online measurement technologies can provide relief here. There are major pain points in the industry not only in formation and aging, but also in the other process steps of wetting, degassing, and EoL testing. Here, research already provides solutions as well, although they are not always industrialized.
Since not all technologies are available for series production today, a roadmap was elaborated to estimate when the technologies under research will be ready for application in series production. Up to this point, the battery cell finishing process remains a cost-intensive, quality-critical process chain.
Author Contributions
The conceptualization, methodology, validation, investigation, writing—original draft preparation, visualization, project administration, writing—review and editing was done by N.L., T.R. and S.W. The supervision was provided by A.K., H.H. and C.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the project “FormEL” (03XP0296C) as part of the competence cluster “ProZell” as well as the projects “InForm” (03XP0363B) and “OptiPro” (03XP0364A) as part of the competence cluster “InZePro,” funded by the German Federal Ministry of Education and Research (BMBF). The authors are solely responsible for the content of this contribution. Additional gratitude belongs to the project partners from the elenia Institute for High Voltage Technology and Power Systems of the Technical University of Braunschweig (elenia), the Münster Electrochemical Energy Technology (MEET) of the University of Münster, the Bavarian Center for Battery Technology (BayBatt) of the University of Bayreuth, the Institute for Electrical Energy Storage (EES) of the Technical University of Munich, the Center for Solar Energy and Hydrogen Research Baden-Württemberg (ZSW), the Helmholtz Institute Ulm (HIU), the Institute for Automotive Engineering (FTM) and the Institute for Machine Tools and Industrial Management (iwb) of the TU Munich, the Chair of Information Management in Mechanical Engineering & Institute for Enterprise Cybernetics (IMA & IfU Cybernetics Lab), the Institute for Power Electronics and Electrical Drives (iSEA), and the Chair of Production Engineering of E-Mobility Components (PEM) of RWTH Aachen University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The literature research and visualization work were supported by Ömer Arslan, Fares Ben Bennani, Michel Huneke, Diane Knödler, and Anika Paffhausen.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sakti, A.; Michalek, J.J.; Fuchs, E.R.H.; Whitacre, J.F. A techno-economic analysis and optimization of Li-ion batteries for light-duty passenger vehicle electrification. J. Power Sources 2015, 273, 966–980. [Google Scholar] [CrossRef]
- Patry, G.; Romagny, A.; Martinet, S.; Froelich, D. Cost modeling of lithium-ion battery cells for automotive applications. Energy Sci. Eng. 2015, 3, 71–82. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Duffner, F.; Mauler, L.; Wentker, M.; Leker, J.; Winter, M. Large-scale automotive battery cell manufacturing: Analyzing strategic and operational effects on manufacturing costs. Int. J. Prod. Econ. 2021, 232, 107982. [Google Scholar] [CrossRef]
- Kampker, A. Elektromobilproduktion; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-42021-4. [Google Scholar]
- Baumhöfer, T.; Brühl, M.; Rothgang, S.; Sauer, D.U. Production caused variation in capacity aging trend and correlation to initial cell performance. J. Power Sources 2014, 247, 332–338. [Google Scholar] [CrossRef]
- Degen, F.; Krätzig, O. Modelling Large Scale Manufacturing of Automotive Battery Cells–Impact of New Technologies on Production Economies. SSRN J. 2021, 1. [Google Scholar] [CrossRef]
- Löbberding, H.; Wessel, S.; Offermanns, C.; Kehrer, M.; Rother, J.; Heimes, H.; Kampker, A. From Cell to Battery System in BEVs: Analysis of System Packing Efficiency and Cell Types. World Electr. Veh. J. 2020, 11, 77. [Google Scholar] [CrossRef]
- Heimes, H.; Kampker, A.; Wennemar, S.; Plocher, L.; Bockey, G.; Michaelis, S.; Schütrumpf, J. Production Process of a Lithium-Ion Battery Cell; RWTH Aachen University: Aachen, Germany, 2023; ISBN 978-3-947920-26-6. [Google Scholar]
- Weydanz, W.J.; Reisenweber, H.; Gottschalk, A.; Schulz, M.; Knoche, T.; Reinhart, G.; Masuch, M.; Franke, J.; Gilles, R. Visualization of electrolyte filling process and influence of vacuum during filling for hard case prismatic lithium ion cells by neutron imaging to optimize the production process. J. Power Sources 2018, 380, 126–134. [Google Scholar] [CrossRef]
- Michaelis, S.; Rahimsei, E.; Kampker, A.; Heimes, H. Roadmap Batterie-Produktionsmittel 2030-Update 2020; VDMA: Frankfurt am Main, Germany, 2021. [Google Scholar]
- Yoshio, M. Lithium-Ion Batteries; Springer: Dordrecht, The Netherlands, 2010; ISBN 0387344446. [Google Scholar]
- Korthauer, R. Lithium-Ion Batteries: Basics and Applications; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-662-53069-6. [Google Scholar]
- Davoodabadi, A.; Li, J.; Liang, Y.; Wang, R.; Zhou, H.; Wood, D.L.; Singler, T.J.; Jin, C. Characterization of Surface Free Energy of Composite Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A2493–A2501. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Li, J.; Liang, Y.; Wood, D.L.; Singler, T.J.; Jin, C. Analysis of electrolyte imbibition through lithium-ion battery electrodes. J. Power Sources 2019, 424, 193–203. [Google Scholar] [CrossRef]
- Wood, D.L.; Li, J.; An, S.J. Formation Challenges of Lithium-Ion Battery Manufacturing. Joule 2019, 3, 2884–2888. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Kang, H.; Do, H.W.; Kim, G.; Kim, T.; Kim, S.; Choi, S.; Won, J.; Park, I.; Jung, K.; et al. Enhancing Li Ion Battery Performance by Mechanical Resonance. Nano Lett. 2021, 21, 5345–5352. [Google Scholar] [CrossRef]
- Kang, G.; Lee, Y.T.; Kim, J.; Ko, M.H. Electrolyte Wetting Device for Manufacturing Battery Cell by Using Vibration, and Battery cell manufacturing method using same. EU Patent 3800705 A1. PCT/KR2020/003473, 12 March 2020. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020189962 (accessed on 27 February 2023).
- Guo, L.; Thornton, D.B.; Koronfel, M.A.; Stephens, I.E.L.; Ryan, M.P. Degradation in lithium ion battery current collectors. J. Phys. Energy 2021, 3, 32015. [Google Scholar] [CrossRef]
- Günter, F.J.; Habedank, J.B.; Schreiner, D.; Neuwirth, T.; Gilles, R.; Reinhart, G. Introduction to Electrochemical Impedance Spectroscopy as a Measurement Method for the Wetting Degree of Lithium-Ion Cells. J. Electrochem. Soc. 2018, 165, A3249–A3256. [Google Scholar] [CrossRef]
- Winter, M. The Solid Electrolyte Interphase—The Most Important and the Least Understood Solid Electrolyte in Rechargeable Li Batteries. Z. Phys. Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Arora, P.; White, R.E. Capacity Fade Mechanisms and Side Reactions in Lithium-Ion Batteries. J. Electrochem. Soc. 1998, 145, 3647–3667. [Google Scholar] [CrossRef]
- Edström, K.; Gustafsson, T.; Thomas, J. The Cathode-Electrolyte Interface in A Li-Ion Battery. In Lithium-Ion Batteries; Balbuena, P.B., Wang, Y., Eds.; Imperial College Press: London, UK, 2004; pp. 337–364. ISBN 978-1-86094-362-1. [Google Scholar]
- Liu, Y.-M.G.; Nicolau, B.; Esbenshade, J.L.; Gewirth, A.A. Characterization of the Cathode Electrolyte Interface in Lithium Ion Batteries by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 7171–7177. [Google Scholar] [CrossRef]
- Von Aspern, N.; Wölke, C.; Börner, M.; Winter, M.; Cekic-Laskovic, I. Impact of single vs. blended functional electrolyte additives on interphase formation and overall lithium ion battery performance. J. Solid State Electrochem. 2020, 24, 3145–3156. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Novák, P.; Joho, F.; Lanz, M.; Rykart, B.; Panitz, J.-C.; Alliata, D.; Kötz, R.; Haas, O. The complex electrochemistry of graphite electrodes in lithium-ion batteries. J. Power Sources 2001, 97–98, 39–46. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Understanding Formation of Solid Electrolyte Interface Film on LiMn2O4 Electrode. J. Electrochem. Soc. 2002, 149, A1521. [Google Scholar] [CrossRef]
- Peljo, P.; Girault, H.H. Electrochemical potential window of battery electrolytes: The HOMO–LUMO misconception. Energy Environ. Sci. 2018, 11, 2306–2309. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. A review of gas evolution in lithium ion batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]
- Metzger, M.; Strehle, B.; Solchenbach, S.; Gasteiger, H.A. Origin of H2 Evolution in LIBs: H2O Reduction vs. Electrolyte Oxidation. J. Electrochem. Soc. 2016, 163, A798–A809. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Penciner, J. The Anode/Electrolyte Interface. In Handbook of Battery Materials; Besenhard, J.O., Ed.; Wiley: Hoboken, NJ, USA, 1998; pp. 419–456. ISBN 9783527294695. [Google Scholar]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Xia, J.; Cameron, A.R.; Nie, M.; Botton, G.A.; Dahn, J.R. The Impact of Electrolyte Additives and Upper Cut-off Voltage on the Formation of a Rocksalt Surface Layer in LiNi0.8Mn0.1Co0.1O2 Electrodes. J. Electrochem. Soc. 2017, 164, A655–A665. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Kurzweil, P.; Dietlmeier, O.K. Elektrochemische Speicher; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2018; ISBN 978-3-658-21828-7. [Google Scholar]
- He, Y.-B.; Tang, Z.-Y.; Song, Q.-S.; Xie, H.; Liu, Y.-G.; Xu, Q. Effects of Temperature on the Formation of Graphite/LiCoO2 Batteries. J. Electrochem. Soc. 2008, 155, A481. [Google Scholar] [CrossRef]
- Günter, F.J.; Burgstaller, C.; Konwitschny, F.; Reinhart, G. Influence of the Electrolyte Quantity on Lithium-Ion Cells. J. Electrochem. Soc. 2019, 166, A1709–A1714. [Google Scholar] [CrossRef]
- Günter, F.J.; Rössler, S.; Schulz, M.; Braunwarth, W.; Gilles, R.; Reinhart, G. Influence of the Cell Format on the Electrolyte Filling Process of Lithium-Ion Cells. Energy Technol. 2020, 8, 1801108. [Google Scholar] [CrossRef]
- Tokranov, A.; Kumar, R.; Li, C.; Minne, S.; Xiao, X.; Sheldon, B.W. Control and Optimization of the Electrochemical and Mechanical Properties of the Solid Electrolyte Interphase on Silicon Electrodes in Lithium Ion Batteries. Adv. Energy Mater. 2016, 6, 1502302. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, G.; Song, X.; Ridgway, P.; Xun, S.; Battaglia, V.S. Cathode Performance as a Function of Inactive Material and Void Fractions. J. Electrochem. Soc. 2010, 157, A1060. [Google Scholar] [CrossRef]
- Rago, N.D.; Basco, J.K.; Vu, A.; Li, J.; Hays, K.; Sheng, Y.; Wood, D.L.; Bloom, I. Effect of formation protocol: Cells containing Si-Graphite composite electrodes. J. Power Sources 2019, 435, 126548. [Google Scholar] [CrossRef]
- Kampker, A.; Vallée, D.; Schnettler, A. Elektromobilität; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-31985-3. [Google Scholar]
- Sharova, V. Enhancing the Performance of Lithium Batteries through the Development of Improved Electrolyte Formulation, Formation Protocol and Graphite Surface Modification; KIT: Karlsruhe, Germany, 2018. [Google Scholar] [CrossRef]
- Mao, C.; An, S.J.; Meyer, H.M.; Li, J.; Wood, M.; Ruther, R.E.; Wood, D.L. Balancing formation time and electrochemical performance of high energy lithium-ion batteries. J. Power Sources 2018, 402, 107–115. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Du, Z.; Daniel, C.; Wood, D.L. Fast formation cycling for lithium ion batteries. J. Power Sources 2017, 342, 846–852. [Google Scholar] [CrossRef]
- Heimes, H.H.; Offermanns, C.; Mohsseni, A.; Laufen, H.; Westerhoff, U.; Hoffmann, L.; Niehoff, P.; Kurrat, M.; Winter, M.; Kampker, A. The Effects of Mechanical and Thermal Loads during Lithium-Ion Pouch Cell Formation and Their Impacts on Process Time. Energy Technol. 2020, 8, 1900118. [Google Scholar] [CrossRef]
- Berkes, B.B.; Schiele, A.; Sommer, H.; Brezesinski, T.; Janek, J. On the gassing behavior of lithium-ion batteries with NCM523 cathodes. J. Solid State Electrochem. 2016, 20, 2961–2967. [Google Scholar] [CrossRef]
- Xiong, D.J.; Petibon, R.; Nie, M.; Ma, L.; Xia, J.; Dahn, J.R. Interactions between Positive and Negative Electrodes in Li-Ion Cells Operated at High Temperature and High Voltage. J. Electrochem. Soc. 2016, 163, A546–A551. [Google Scholar] [CrossRef]
- Warner, J. Lithium-ion Battery Chemistries: A Primer; Elsevier: Amsterdam, The Netherland; Oxford, UK; Cambridge, MA, USA, 2019; ISBN 978-0-12-814778-8. [Google Scholar]
- Schreiner, D.; Zünd, T.; Günter, F.J.; Kraft, L.; Stumper, B.; Linsenmann, F.; Schüßler, M.; Wilhelm, R.; Jossen, A.; Reinhart, G.; et al. Comparative Evaluation of LMR-NCM and NCA Cathode Active Materials in Multilayer Lithium-Ion Pouch Cells: Part I. Production, Electrode Characterization, and Formation. J. Electrochem. Soc. 2021, 168, 30507. [Google Scholar] [CrossRef]
- Farmann, A.; Sauer, D.U. A study on the dependency of the open-circuit voltage on temperature and actual aging state of lithium-ion batteries. J. Power Sources 2017, 347, 1–13. [Google Scholar] [CrossRef]
- Wolter, M.; Fauser, G.; Bretthauer, C.; Roscher, M.A. End-of-line testing and formation process in Li-ion battery assembly lines. In Proceedings of the IEEE 9th International Multi-Conference on Systems, Signals and Devices (SSD), Chemnitz, Germany, 20–23 March 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 1–3, ISBN 978-1-4673-1590-6. [Google Scholar]
- R-Smith, N.A.-Z.; Ragulskis, M.; Kasper, M.; Wagner, S.; Pumsleitner, J.; Zollo, B.; Groebmeyer, A.; Kienberger, F. Multiplexed 16 × 16 Li-Ion Cell Measurements Including Internal Resistance for Quality Inspection and Classification. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Hoffmann, L.; Grathwol, J.-K.; Haselrieder, W.; Leithoff, R.; Jansen, T.; Dilger, K.; Dröder, K.; Kwade, A.; Kurrat, M. Capacity Distribution of Large Lithium-Ion Battery Pouch Cells in Context with Pilot Production Processes. Energy Technol. 2020, 8, 1900196. [Google Scholar] [CrossRef]
- Hagemeister, J.; Stock, S.; Linke, M.; Fischer, M.; Drees, R.; Kurrat, M.; Daub, R. Lean Cell Finalization in Lithium-Ion Battery Production: Determining the Required Electrolyte Wetting Degree to Begin the Formation. Energy Technol. 2022, 9, 2200686. [Google Scholar] [CrossRef]
- Peter, C.; Nikolowski, K.; Reuber, S.; Wolter, M.; Michaelis, A. Chronoamperometry as an electrochemical in situ approach to investigate the electrolyte wetting process of lithium-ion cells. J. Appl. Electrochem. 2020, 50, 295–309. [Google Scholar] [CrossRef]
- Sauer, D.U. Grundlagen der Impedanzspektroskopie für die Charakterisierung von Batterien. 2006. Available online: https://www.researchgate.net/publication/260020031_Grundlagen_der_Impedanzspektroskopie_fur_die_Charakterisierung_von_Batterien#fullTextFileContent (accessed on 8 February 2023).
- Koch, R. On-Line Electrochemical Impedance Spectroscopy for Lithium-Ion Battery Systems; Fakultät für Elektrotechnik und Informationstechnik: Vienna, Austria, 2017. [Google Scholar]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-ion Batteries. J. Electrochem. Sci. Technol 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and its Applications; Springer: New York, NY, USA, 2014; ISBN 978-1-4614-8932-0. [Google Scholar]
- Majasan, J.O.; Robinson, J.B.; Owen, R.E.; Maier, M.; Radhakrishnan, A.N.P.; Pham, M.; Tranter, T.G.; Zhang, Y.; Shearing, P.R.; Brett, D.J.L. Recent advances in acoustic diagnostics for electrochemical power systems. J. Phys. Energy 2021, 3, 32011. [Google Scholar] [CrossRef]
- Robinson, J.B.; Pham, M.; Kok, M.D.R.; Heenan, T.M.M.; Brett, D.J.L.; Shearing, P.R. Examining the Cycling Behaviour of Li-Ion Batteries Using Ultrasonic Time-of-Flight Measurements. J. Power Sources 2019, 444, 227318. [Google Scholar] [CrossRef]
- Robinson, J.B.; Maier, M.; Alster, G.; Compton, T.; Brett, D.J.L.; Shearing, P.R. Spatially resolved ultrasound diagnostics of Li-ion battery electrodes. Phys. Chem. Chem. Phys. 2019, 21, 6354–6361. [Google Scholar] [CrossRef]
- Ladpli, P.; Liu, C.; Kopsaftopoulos, F.; Chang, F.-K. Estimating Lithium-ion Battery State of Charge and Health with Ultrasonic Guided Waves Using an Efficient Matching Pursuit Technique. In Proceedings of the IEEE Transportation Electrification Conference and Expo, Asia-Pacific (ITEC Asia-Pacific), Bangkok, Thailand, 6–9 June 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–5, ISBN 978-1-5386-5782-9. [Google Scholar]
- Davies, G.; Knehr, K.W.; van Tassell, B.; Hodson, T.; Biswas, S.; Hsieh, A.G.; Steingart, D.A. State of Charge and State of Health Estimation Using Electrochemical Acoustic Time of Flight Analysis. J. Electrochem. Soc. 2017, 164, A2746–A2755. [Google Scholar] [CrossRef]
- Deng, Z.; Huang, Z.; Shen, Y.; Huang, Y.; Ding, H.; Luscombe, A.; Johnson, M.; Harlow, J.E.; Gauthier, R.; Dahn, J.R. Ultrasonic Scanning to Observe Wetting and “Unwetting” in Li-Ion Pouch Cells. Joule 2020, 4, 2017–2029. [Google Scholar] [CrossRef]
- Schilling, A.; Gümbel, P.; Möller, M.; Kalkan, F.; Dietrich, F.; Dröder, K. X-ray Based Visualization of the Electrolyte Filling Process of Lithium Ion Batteries. J. Electrochem. Soc. 2019, 166, A5163–A5167. [Google Scholar] [CrossRef]
- Habedank, J.B.; Günter, F.J.; Billot, N.; Gilles, R.; Neuwirth, T.; Reinhart, G.; Zaeh, M.F. Rapid electrolyte wetting of lithium-ion batteries containing laser structured electrodes: In situ visualization by neutron radiography. Int. J. Adv. Manuf. Technol. 2019, 102, 2769–2778. [Google Scholar] [CrossRef]
- Knoche, T.; Zinth, V.; Schulz, M.; Schnell, J.; Gilles, R.; Reinhart, G. In situ visualization of the electrolyte solvent filling process by neutron radiography. J. Power Sources 2016, 331, 267–276. [Google Scholar] [CrossRef]
- Wood, D.L.; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Nelson, P.; Gallagher, K.; Bloom, I.; Dees, D. Modeling the Performance and Cost of Lithium-ion Batteries for Electric-Drive Vehicles; U.S. Department of Energy: Washington, DC, USA, 2011. [Google Scholar]
- Schünemann, J.-H. Modell zur Bewertung der Herstellkosten von Lithiumionenbatteriezellen; Braunschweig Technical University: Braunschweig, Germany; Sierke: Göttingen, Germany, 2015. [Google Scholar]
- German, F.; Hintennach, A.; Lacroix, A.; Thiemig, D.; Oswald, S.; Scheiba, F.; Hoffmann, M.J.; Ehrenberg, H. Influence of temperature and upper cut-off voltage on the formation of lithium-ion cells. J. Power Sources 2014, 264, 100–107. [Google Scholar] [CrossRef]
- Lee, H.-H.; Wang, Y.-Y.; Wan, C.-C.; Yang, M.-H.; Wu, H.-C.; Shieh, D.-T. A fast formation process for lithium batteries. J. Power Sources 2004, 134, 118–123. [Google Scholar] [CrossRef]
- Chiang, P.-C.J.; Wu, M.-S.; Lin, J.-C. A Novel Dual-Current Formation Process for Advanced Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2005, 8, A423. [Google Scholar] [CrossRef]
- Moretti, A.; Sharova, V.; Carvalho, D.V.; Boulineau, A.; Porcher, W.; de Meatza, I.; Passerini, S. A Comparison of Formation Methods for Graphite//LiFePO4 Cells. Batter. Supercaps 2019, 2, 240–247. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Stress evolution and capacity fade in constrained lithium-ion pouch cells. J. Power Sources 2014, 245, 745–751. [Google Scholar] [CrossRef]
- Li, F.-S.; Wu, Y.-S.; Chou, J.; Winter, M.; Wu, N.-L. A mechanically robust and highly ion-conductive polymer-blend coating for high-power and long-life lithium-ion battery anodes. Adv. Mater. 2015, 27, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, X. Elegant design of electrode and electrode/electrolyte interface in lithium-ion batteries by atomic layer deposition. Nanotechnology 2015, 26, 24001. [Google Scholar] [CrossRef] [PubMed]
- Drees, R.; Lienesch, F.; Kurrat, M. Fast charging lithium-ion battery formation based on simulations with an electrode equivalent circuit model. J. Energy Storage 2021, 36, 102345. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Liu, Q.; Du, C.; Shen, B.; Zuo, P.; Cheng, X.; Ma, Y.; Yin, G.; Gao, Y. Understanding undesirable anode lithium plating issues in lithium-ion batteries. RSC Adv. 2016, 6, 88683–88700. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. The Effects of Defects on Localized Plating in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A1365–A1373. [Google Scholar] [CrossRef]
- Tang, M.; Albertus, P.; Newman, J. Two-Dimensional Modeling of Lithium Deposition during Cell Charging. J. Electrochem. Soc. 2009, 156, A390. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Kircheva, N.; Genies, S.; Brun-Buisson, D.; Thivel, P.-X. Study of Solid Electrolyte Interface Formation and Lithium Intercalation in Li-Ion Batteries by Acoustic Emission. J. Electrochem. Soc. 2011, 159, A18–A25. [Google Scholar] [CrossRef]
- Villevieille, C.; Boinet, M.; Monconduit, L. Direct evidence of morphological changes in conversion type electrodes in Li-ion battery by acoustic emission. Electrochem. Commun. 2010, 12, 1336–1339. [Google Scholar] [CrossRef]
- Bommier, C.; Chang, W.; Li, J.; Biswas, S.; Davies, G.; Nanda, J.; Steingart, D. Operando Acoustic Monitoring of SEI Formation and Long-Term Cycling in NMC/SiGr Composite Pouch Cells. J. Electrochem. Soc. 2020, 167, 20517. [Google Scholar] [CrossRef]
- Schulze, M.C.; Rodrigues, M.-T.F.; McBrayer, J.D.; Abraham, D.P.; Apblett, C.A.; Bloom, I.; Chen, Z.; Colclasure, A.M.; Dunlop, A.R.; Fang, C.; et al. Critical Evaluation of Potentiostatic Holds as Accelerated Predictors of Capacity Fade during Calendar Aging. J. Electrochem. Soc. 2022, 169, 50531. [Google Scholar] [CrossRef]
- Küpper, D.; Kuhlmann, C.; Wolf, S.; Pieper, C.; Xu, G.; Ahmad, J. The Future of Battery Production for Electric Vehicles; BCG: Boston, MA, USA, 2018. [Google Scholar]
- Turetskyy, A.; Wessel, J.; Herrmann, C.; Thiede, S. Data-driven cyber-physical System for Quality Gates in Lithium-ion Battery Cell Manufacturing. Proc. CIRP 2020, 93, 168–173. [Google Scholar] [CrossRef]
- Antonopoulos, B.K.; Stock, C.; Maglia, F.; Hoster, H.E. Solid electrolyte interphase: Can faster formation at lower potentials yield better performance? Electrochim. Acta 2018, 269, 331–339. [Google Scholar] [CrossRef]
- Lee, S.-B.; Pyun, S.-I. The effect of electrolyte temperature on the passivity of solid electrolyte interphase formed on a graphite electrode. Carbon 2002, 40, 2333–2339. [Google Scholar] [CrossRef]
- Huang, C.; Huang, K.; Wang, H.; Liu, S.; Zeng, Y. The effect of solid electrolyte interface formation conditions on the aging performance of Li-ion cells. J. Solid State Electrochem. 2011, 15, 1987–1995. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Riahi, A.R.; Alpas, A.T. Thermal cycling induced capacity enhancement of graphite anodes in lithium-ion cells. Carbon 2014, 67, 592–606. [Google Scholar] [CrossRef]
- Zimmerman, A.H. Self-discharge losses in lithium-ion cells. IEEE Aerosp. Electron. Syst. Mag. 2004, 19, 19–24. [Google Scholar] [CrossRef]
- Turetskyy, A.; Laue, V.; Lamprecht, R.; Thiede, S.; Krewer, U.; Herrmann, C. Artificial Neural Network enabled P2D Model Deployment for End-of-Line Battery Cell Characterization. In Artificial Neural Network Enabled P2D Model Deployment for End-of-Line Battery Cell Characterization, Proceedings of the IEEE 17th International Conference on Industrial Informatics (INDIN), Helsinki, Finland, 22–25 July 2019; Turetskyy, A., Laue, V., Lamprecht, R., Thiede, S., Krewer, U., Herrmann, C., Eds.; IEEE: Piscataway, NJ, USA, 2019; pp. 53–58. ISBN 978-1-7281-2927-3. [Google Scholar]
- Turetskyy, A.; Wessel, J.; Herrmann, C.; Thiede, S. Battery production design using multi-output machine learning models. Energy Storage Mater. 2021, 38, 93–112. [Google Scholar] [CrossRef]
- Vadivel, N.R.; Ha, S.; He, M.; Dees, D.; Trask, S.; Polzin, B.; Gallagher, K.G. On Leakage Current Measured at High Cell Voltages in Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A508–A517. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

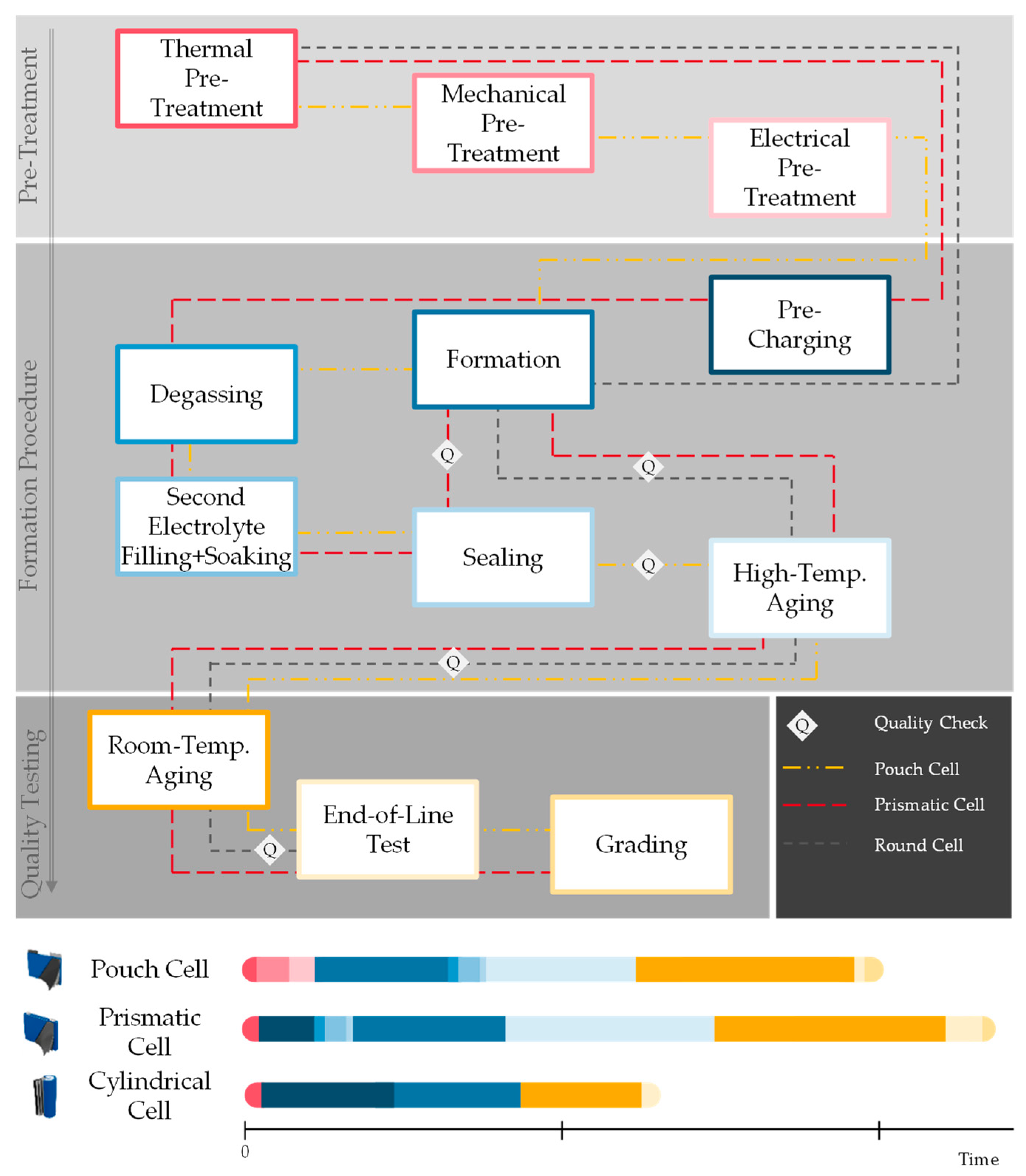
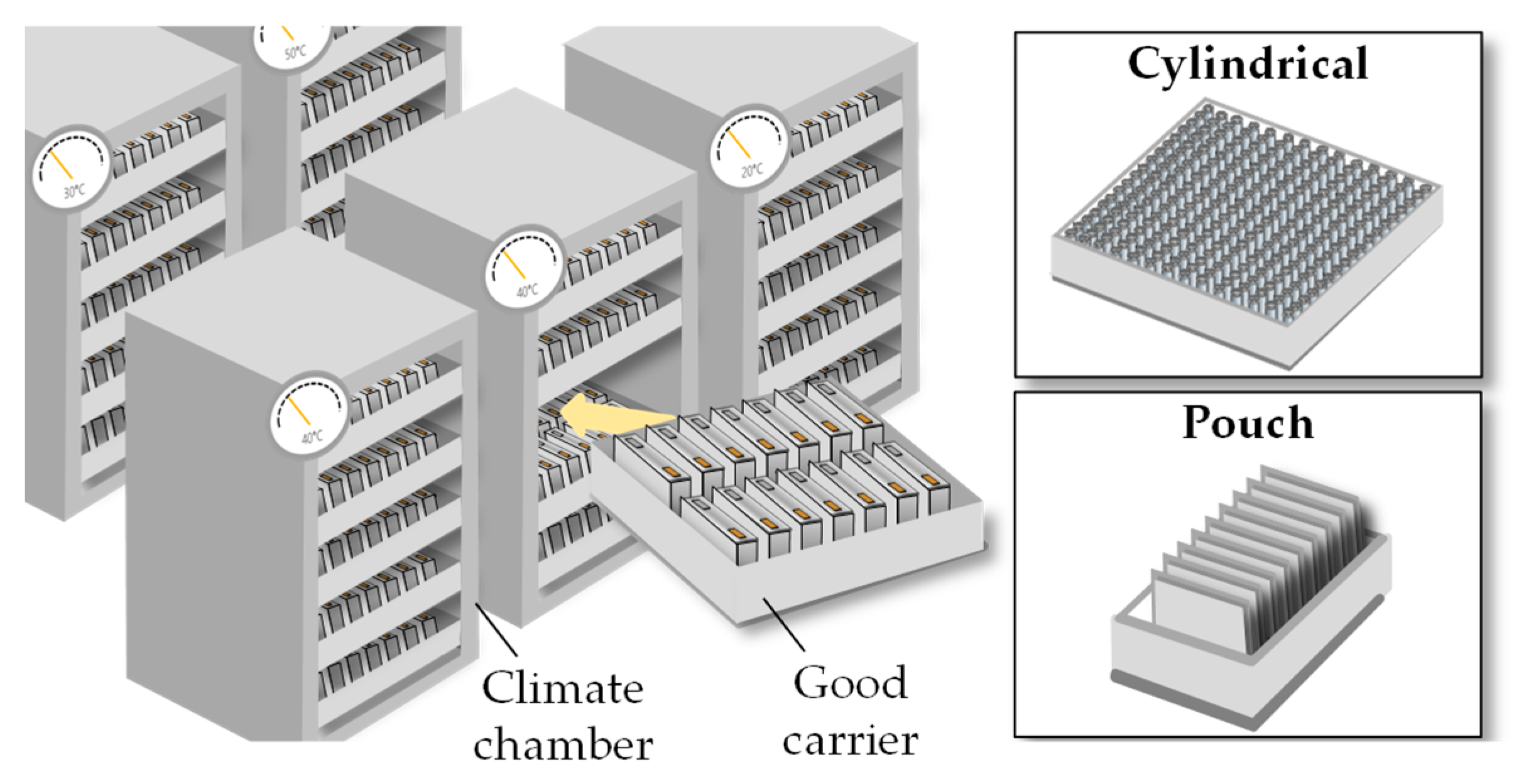
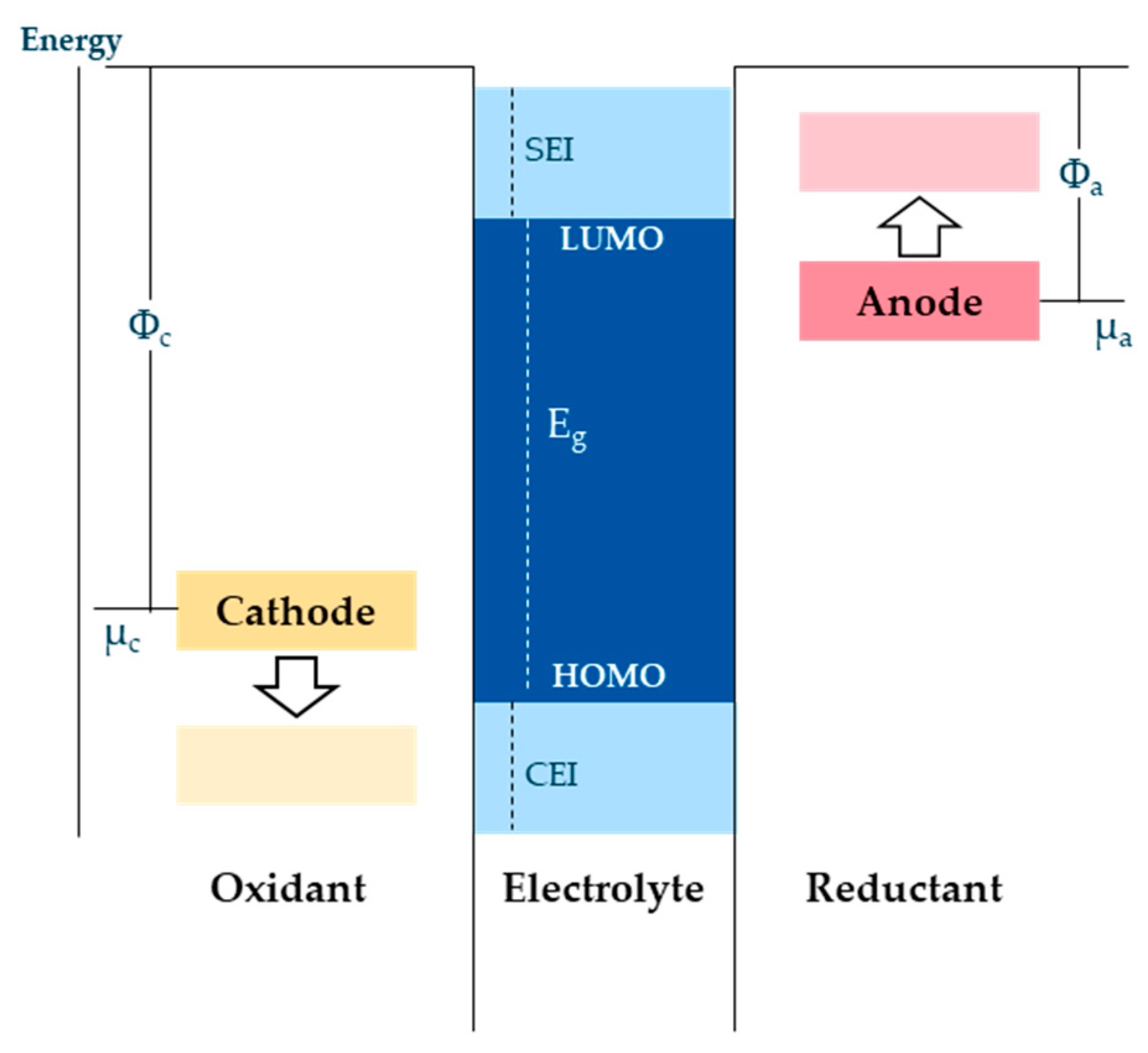

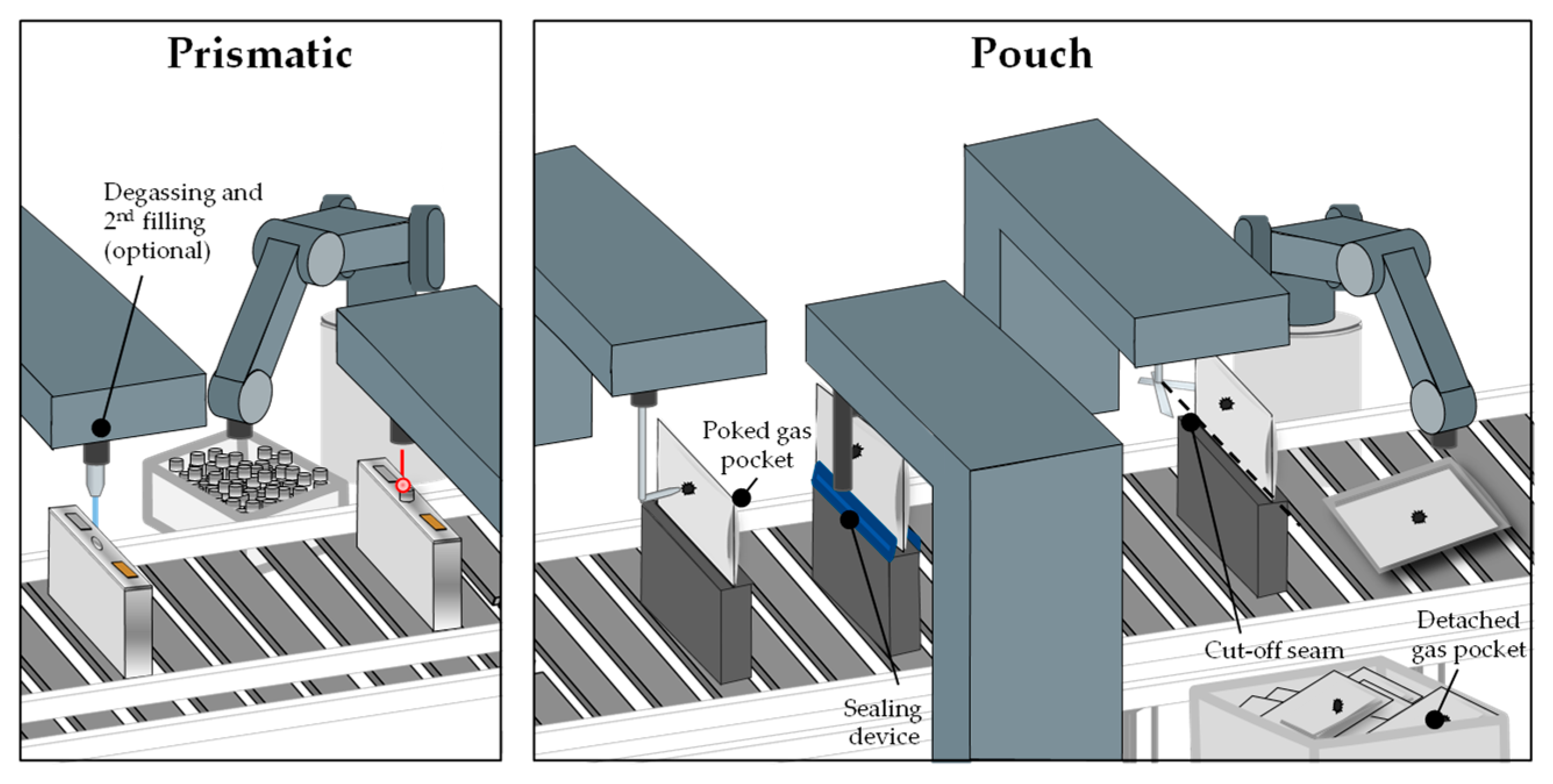
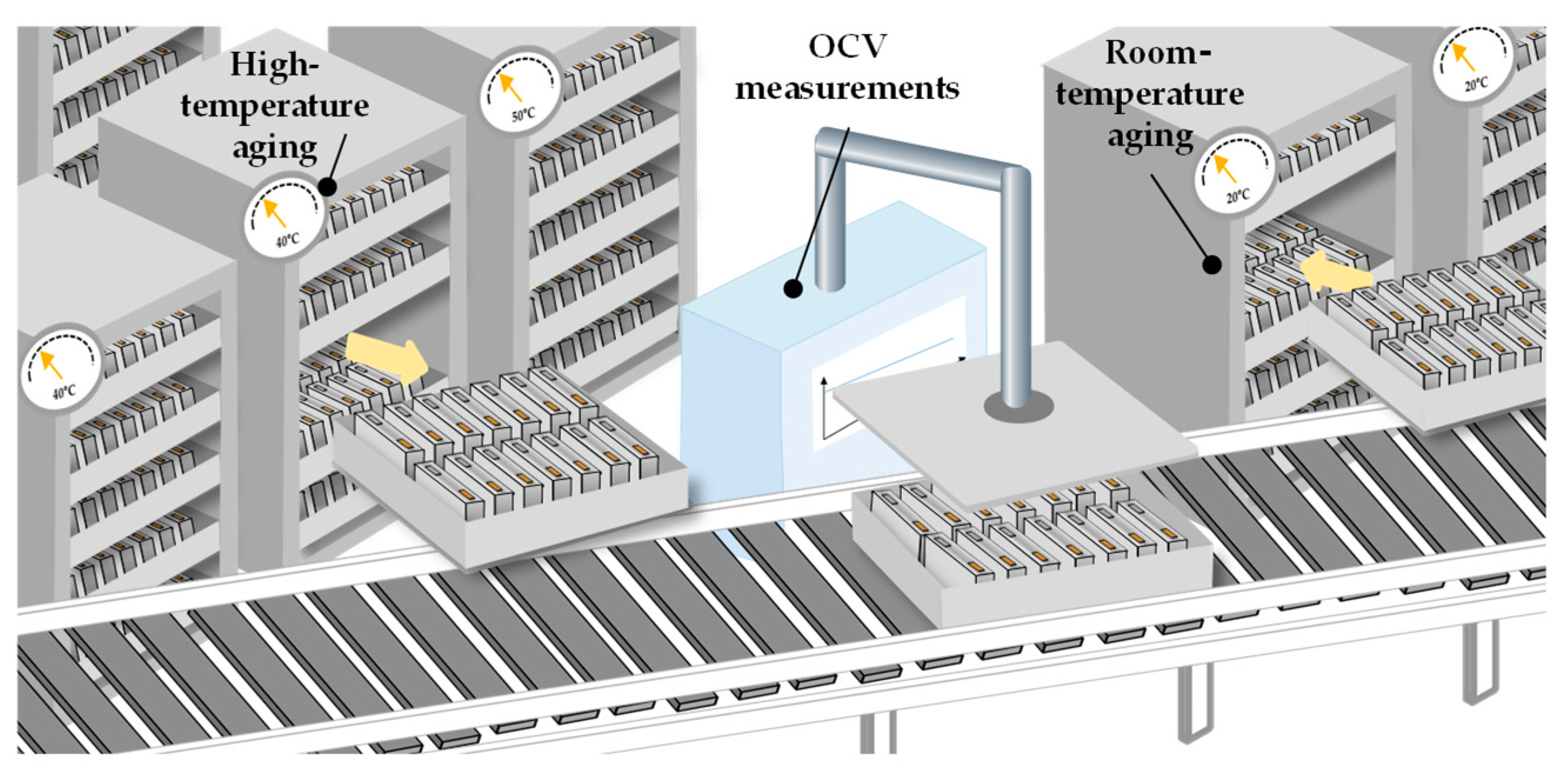
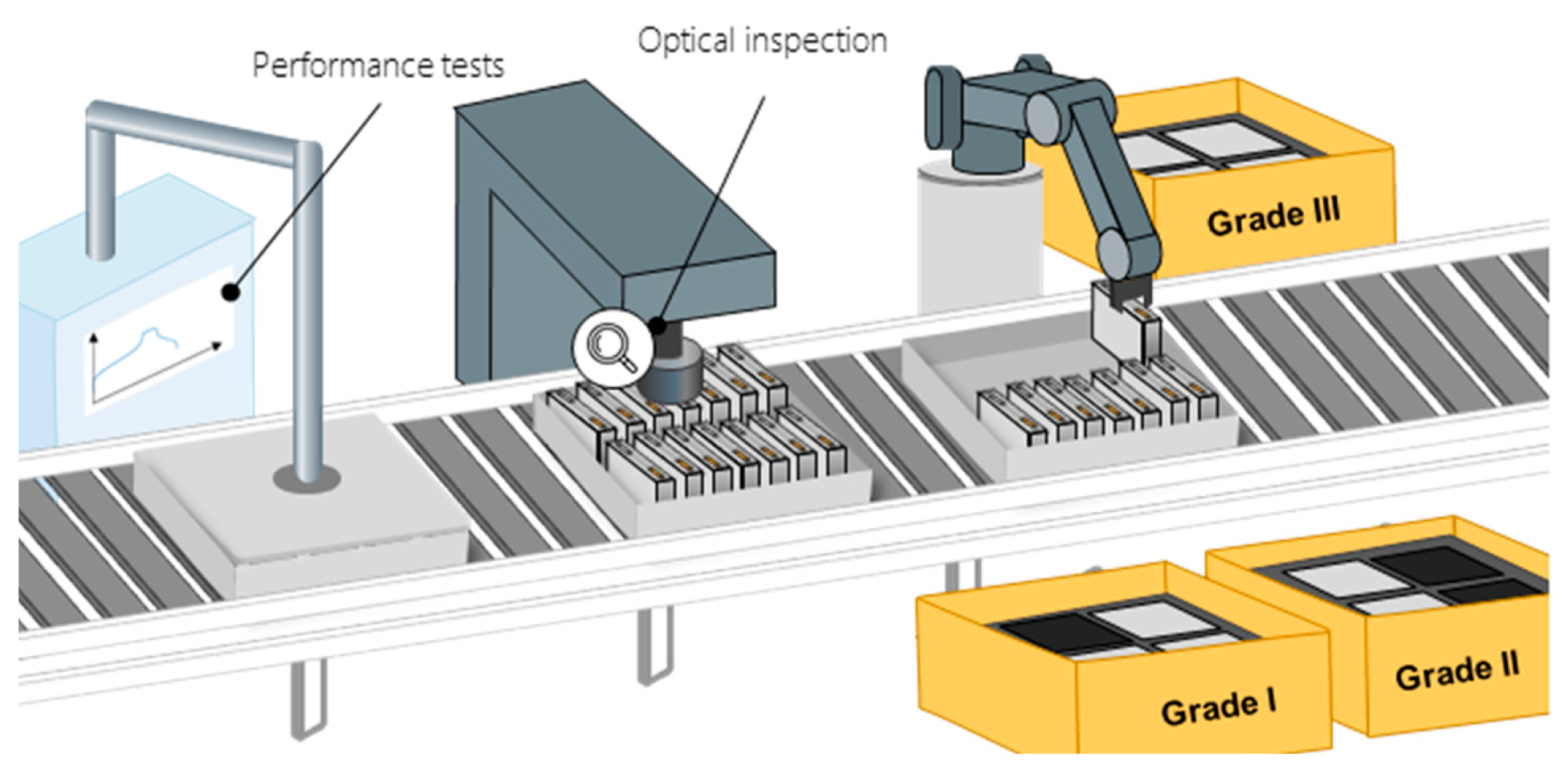


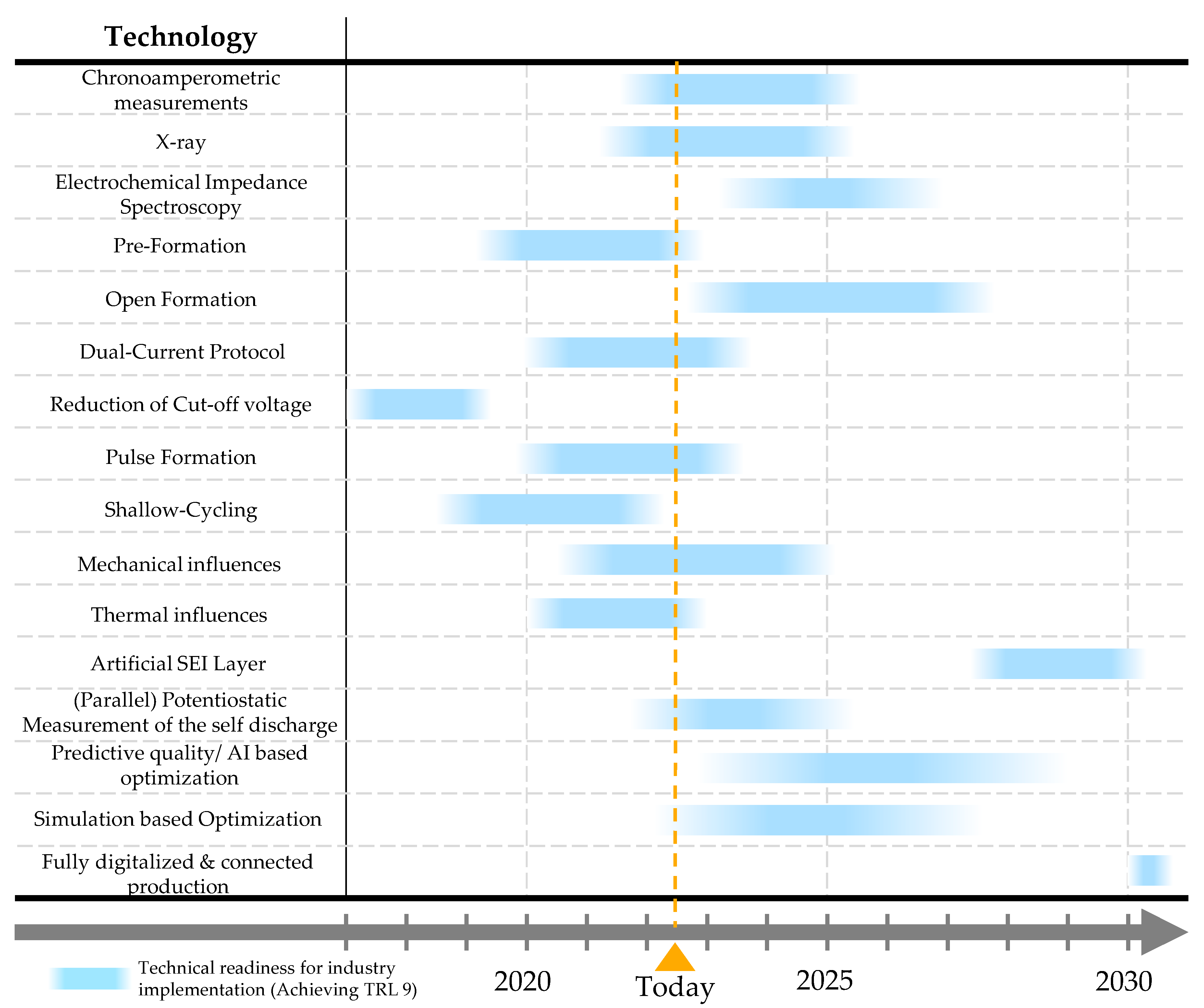










































 Significantly worsening
Significantly worsening Partly worsening
Partly worsening Constant
Constant Partly optimized
Partly optimized Significantly optimized
Significantly optimized








